Abstract
Previously, our laboratory reported that weight-cycled mice outlive their obese counterparts. To gain a better mechanistic understanding of these results, we evaluated cellular senescence in white adipose tissue (WAT) of lean, obese, and weight cycled mice. Our results show that at the end of a 28 day weight loss cycle cellular senescence is significantly reduced in multiple WAT depots compared to obese mice, which also corresponds to a reduction in circulating activin A (a marker of senescence). These findings suggest that a previously undescribed benefit to weight loss may be a reduction of cellular senescence in WAT.
Keywords: White adipose tissue, cellular senescence, obesity, weight loss, weight cycling, yo-yo diet, activin A
1. Introduction
With obesity rates on the rise, more individuals are attempting to lose weight for improved health. Unfortunately, the vast majority of weight loss attempts are short-lived and are followed by weight gain. That is, for individuals that successfully achieve weight loss of at least 10%, approximately 80% will regain the weight in the first year alone [1, 2]. Repeated attempts at weight loss results in a phenomenon referred to as weight cycling (also known colloquially as “yo-yo dieting”). As global rates of obesity increase, weight cycling is becoming increasingly common [3–6]. Unfortunately, clinical studies have produced conflicting results with some studies suggesting that weight cycling may decrease lifespan [7–12] while others suggest that weight cycling has no negative effect [4, 5, 13–17]. Review of these clinical studies suggests that inclusion of confounding factors, such as unintentional weight loss, likely accounts for the discrepancies and that further research is needed [18, 19]. Additionally, these authors indicate that when intentional weight loss is evaluated, little to no evidence exists for an adverse effect of weight cycling; however, better controlled studies are needed [18, 19]. In attempts to perform a controlled animal study, our laboratory set out to evaluate the impact of lifelong weight cycling on longevity in mice [20]. Results of this study showed that weight-cycled mice lived significantly longer than obese mice (801 vs 544 days), suggesting that periodic, repeated, weight loss attempts were preferable to no weight loss attempts in obese mice. To better understand the molecular changes that occur during weight cycling, we analyzed cellular senescence via senescence-associated β-galactosidase staining in white adipose tissue (WAT) and circulating levels of activin A, a recently identified marker of cellular senescence [21].
2. Methods and materials
A total of 130 male C57BL/6J mice were placed on one of four diets at 4 weeks of age: 1) a high-fat (HF) diet (n = 40; D12492; Research Diets; 60% of energy from fat, 20% from carbohydrates, and 20% from protein); 2) a standard chow (LF) diet (n = 40; ProLab RMH 3000, PMI Nutrition International; 14% of energy from fat, 60% from carbohydrates, and 26% from protein); 3) a cycled diet in which mice alternated between 4 weeks on the LF diet and 4 weeks on the HF diet (n = 40) and were sacrificed at the end of a HF cycle (YoyoHF); and 4) a smaller group on a cycled diet (n = 10), which were identical to group 3 except the cycles were offset in order to provide WAT from cycled mice at the end of a LF cycle (YoyoLF). Body weight and fat mass were determined as previously described [20]. Plasma was collected from groups 1–3 at 12 and 13 months of age to determine the effects of cycling on activin A levels in the same group of mice at the end of a HF and a LF cycle. Plasma levels of activin A were measured using Activin A ELISA kits (DAC00B, R&D Systems) according to manufacturer’s instructions. All procedures were approved by the Ohio University Institutional Animal Care and Use Committee and fully complied with all federal, state and local policies.
Forty mice were sacrificed at 15 months of age (n = 10 per diet group). Four WAT depots (inguinal subcutaneous, mesenteric, epididymal, and retroperitoneal) were collected from each mouse, and senescence-associated β-galactosidase assays were performed to determine senescent cell numbers as previously described [22]. Stained samples were analyzed under a Nikon Eclipse E600 fluorescent microscope. The number of senescent cells (X-gal stained cells) and DAPI stained nuclei were used to determine percentages of senescent cells per nuclei. Comparisons were made by one-way ANOVA across all four groups and post-hoc Tukey analysis. Differences were considered significant at p < 0.05.
3. Results
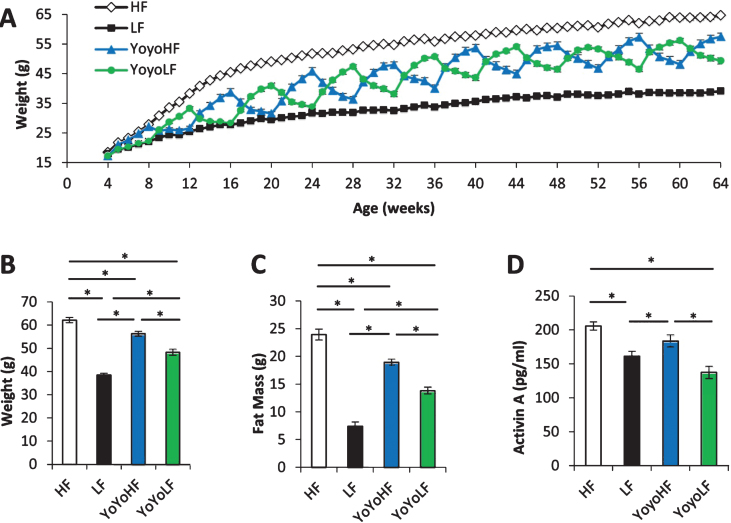
Longitudinal body weight of the 4 diet groups are shown in Fig. 1A while body weight and fat mass at the time of the dissection are shown in Fig. 1B & C, respectively. As expected, body weight and fat mass fluctuated dramatically in cycled mice with each diet transition. These results help confirm that the current dietary regimen replicates the weight cycling observed in our initial longevity study published in 2013 [20]. Circulating levels of activin A (Fig. 1D) were highest in and did not differ between HF mice and weight-cycled mice at the end of a 4 week HF cycle. Activin A levels were significantly lower in LF and cycled mice at the end of a 4 week LF cycle.
Fig.1.
Physiological data from weight-cycled mice. (A) Body weight over time (n = 10–40). (B) Body weight at 15mo just prior to dissection (n = 10/group). (C) Fat mass at 15mo just prior to dissection (n = 10). (D) Circulating plasma levels of activin A (n = 26–35). HF fed mice (white), LF fed mice (black), YoyoHF (blue or dark grey), YoyoLF (green or light grey). Data are expressed as mean±SEM. *Indicates p < 0.05.
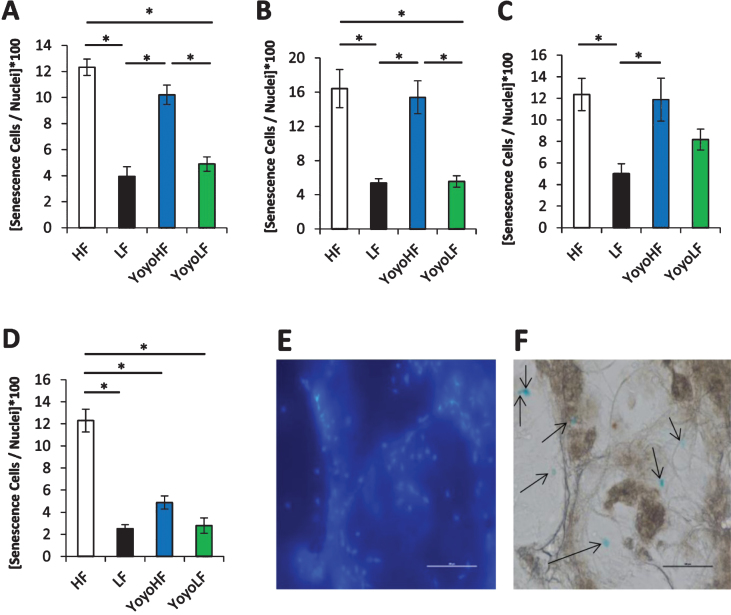
The number of senescent positive cells in the four WAT depots is shown in Fig. 2. In all WAT depots, HF fed mice had a significantly higher percentage of senescent cells than LF mice. In inguinal and retroperitoneal WAT, YoyoLF had significantly less senescent cells than the YoyoHF, but there was no significant difference in senescent cells between HF and YoyoHF groups or between LF controls and YoyoLF groups. While the trend for the other two WAT depots was similar, statistical significance was not reached for all comparisons. For example, in epididymal WAT, YoyoHF had a higher percentage of senescent cells compared to YoyoLF, but none of the other group comparisons reached statistical significance. Likewise, in mesenteric WAT, no statistical difference was seen between LF, YoyoHF, and YoyoLF groups.
Fig.2.
Senescent cell content in various WAT depots. Senescent cell number per nuclei is shown for four distinct WAT depots. (A) Inguinal subcutaneous, (B) retroperitoneal, (C) epididymal, and (D) mesenteric WAT depots were analyzed. (E-F) Sample DAPI stained images (E) and X-gal stained images (F) from the senescence-associated beta-galactosidase assay. Scale bar = 500 μm. Senescent cells are marked by arrows. HF fed mice (white), LF fed mice (black), YoyoHF (blue or dark grey), YoyoLF (green or light grey). Data are expressed as mean±SEM. *Indicatesp < 0.05.
4. Discussion
In this study and in agreement with other studies [23–26], we show that obesity induced by a HF diet results in a significant increase in senescent cells in WAT compared to LF controls. Circulating activin A levels were also increased in the HF group compared to the LF controls. Importantly, our data indicate that 28 days of weight loss are sufficient to significantly reduce the number of senescent cells as shown by significantly reduced activin A levels and a significant reduction in senescent beta-galactosidase stained cells in inguinal and retroperitoneal WAT depots. Of note, since inguinal and retroperitoneal WAT were the most responsive to the weight loss, there appears to be a depot specific difference in cellular senescence in response to this dietary manipulation.
Recently, the Kirkland laboratory at Mayo Clinic published a comprehensive study identifying activin A as a marker for cellular senescence in humans and mice [21]. In this study, it was determined that i) human senescent fat cell progenitors release activin A, ii) activin A impedes the normal function of stem cells and fat tissue, iii) older mice have higher levels of activin A in both their blood and fat tissue than young mice, and iv) eliminating senescent cells from mice leads to lower levels of activin A. Since most procedures used to determine senescent cell accumulation require tissue collection, the discovery of a circulating marker of cellular senescence represents an important step for detection of senescent-related disease. This is particularly important in a clinical setting since blood is relatively easy to collect. Research has shown there is a correlation between obesity and increased cellular senescence [23–26], which may account for increased mortality and progression of age-related diseases. Thus, the possibility of senolytic treatment (agents that clear senescent cells), particularly in WAT, has been suggested as a potential therapeutic target [27]. Duel treatment with dasatinib (an anti-cancer drug) and Quercetin (a flavonol found in fruits and vegetables) has been shown to reduce the amount of senescent cells and improve vasculature dysfunction in aging mice and mice with atherosclerosis [28]. Ruxolitinib (an FDA approved JAK 1/2 inhibitor) has also been shown to eliminate senescent cells and reduce activin A, which results in improved adipogenesis and insulin sensitivity [21]. For those reasons, clearance of senescent cells in WAT with senolytic agents or, as we show here, with dietary manipulation, may be a promising approach for treatment of metabolic syndrome, type 2 diabetes, and other age-related complications [27]. In conclusion, data from the current study shows that weight loss due to diet can decreases cellular senescence in WAT.
Acknowledgments
This work was supported by the State of Ohio’s Eminent Scholar Program that includes a gift from Milton and Lawrence Goll to JJK, the AMVETS, and by the Diabetes Institute at Ohio University.
REFERENCES
- [1]. Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr. 2001;21:323–41. [DOI] [PubMed] [Google Scholar]
- [2]. Kraschnewski JL, Boan J, Esposito J, et al. Long-term weight loss maintenance in the United States. Int J Obes. 2010;34:1644–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. French SA, Jeffery RW, Folsom AR, et al. Weight variability in a population-based sample of older women: Reliability and intercorrelation of measures. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 1995;19:22–9. [PubMed] [Google Scholar]
- [4]. Field AE, Malspeis S, Willett WC. Weight cycling and mortality among middle-aged or older women. Arch Intern Med. 2009;169:881–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Stevens VL, Jacobs EJ, Sun J, et al. Weight cycling and mortality in a large prospective US study. Am J Epidemiol. 2012;175:785–92. [DOI] [PubMed] [Google Scholar]
- [6]. Stevens VL, Jacobs EJ, Sun J, et al. Weight cycling and risk of endometrial cancer. Cancer Epidemiology, Biomarkers & Prevention: A publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology. 2012;21:747–52. [DOI] [PubMed] [Google Scholar]
- [7]. Lissner L, Odell PM, D’Agostino RB, et al. Variability of body weight and health outcomes in the Framingham population. The New England Journal of Medicine. 1991;324:1839–44. [DOI] [PubMed] [Google Scholar]
- [8]. Blair SN, Shaten J, Brownell K, et al. Body weight change, all-cause mortality, and cause-specific mortality in the Multiple Risk Factor Intervention Trial. Ann Intern Med. 1993;119:749–57. [DOI] [PubMed] [Google Scholar]
- [9]. Folsom AR, French SA, Zheng W, et al. Weight variability and mortality: The Iowa Women’s Health Study. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 1996;20:704–9. [PubMed] [Google Scholar]
- [10]. Mikkelsen KL, Heitmann BL, Keiding N, Sorensen TI. Independent effects of stable and changing body weight on total mortality. Epidemiology. 1999;10:671–8. [PubMed] [Google Scholar]
- [11]. Diaz VA, Mainous AG 3rd, Everett CJ. The association between weight fluctuation and mortality: Results from a population-based cohort study. J Community Health. 2005;30:153–65. [DOI] [PubMed] [Google Scholar]
- [12]. Rzehak P, Meisinger C, Woelke G, et al. Weight change, weight cycling and mortality in the ERFORT Male Cohort Study. Eur J Epidemiol. 2007;22:665–73. [DOI] [PubMed] [Google Scholar]
- [13]. Lissner L, Andres R, Muller DC, Shimokata H. Body weight variability in men: Metabolic rate, health and longevity. Int J Obes. 1990;14:373–83. [PubMed] [Google Scholar]
- [14]. Dyer AR, Stamler J, Greenland P. Associations of weight change and weight variability with cardiovascular and all-cause mortality in the Chicago Western Electric Company Study. Am J Epidemiol. 2000;152:324–33. [DOI] [PubMed] [Google Scholar]
- [15]. Wannamethee SG, Shaper AG, Walker M. Weight change, weight fluctuation, and mortality. Arch Intern Med. 2002;162:2575–80. [DOI] [PubMed] [Google Scholar]
- [16]. Gregg EW, Gerzoff RB, Thompson TJ, Williamson DF. Intentional weight loss and death in overweight and obese U.S. adults 35 years of age and older. Ann Intern Med. 2003;138:383–9. [DOI] [PubMed] [Google Scholar]
- [17]. Shea MK, Houston DK, Nicklas BJ, et al. The effect of randomization to weight loss on total mortality in older overweight and obese adults: The ADAPT Study. The journals of gerontology. Series A, Biological Sciences and Medical Sciences. 2010;65:519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Weight cycling. National Task Force on the Prevention and Treatment of Obesity. JAMA. 1994;272:1196–202. [PubMed] [Google Scholar]
- [19]. Mehta T, Smith DL Jr., Muhammad J, Casazza K. Impact of weight cycling on risk of morbidity and mortality. Obesity Reviews: An Official Journal of the International Association for the Study of Obesity. 2014;15:870–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. List EO, Berryman DE, Wright-Piekarski J, et al. The effects of weight cycling on lifespan in male C57BL/6J mice. Int J Obes (Lond). 2013;37:1088–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Xu M, Palmer AK, Ding H, et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife. 2015;4:e12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Stout MB, Tchkonia T, Pirtskhalava T, et al. Growth hormone action predicts age-related white adipose tissue dysfunction and senescent cell burden in mice. Aging (Albany NY). 2014;6:575–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Zhang X, Zhou D, Strakovsky R, et al. Hepatic cellular senescence pathway genes are induced through histone modifications in a diet-induced obese rat model. Am J Physiol Gastrointest Liver Physiol. 2012;302:G558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Minamino T, Orimo M, Shimizu I, et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med. 2009;15:1082–7. [DOI] [PubMed] [Google Scholar]
- [25]. Sikora E, Arendt T, Bennett M, Narita M. Impact of cellular senescence signature on ageing research. Ageing Res Rev. 2011;10:146–52. [DOI] [PubMed] [Google Scholar]
- [26]. Villaret A, Galitzky J, Decaunes P, et al. Adipose tissue endothelial cells from obese human subjects: Differences among depots in angiogenic, metabolic, and inflammatory gene expression and cellular senescence. Diabetes. 2010;59:2755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Palmer AK, Kirkland JL. Aging and adipose tissue: Potential interventions for diabetes and regenerative medicine Exp Gerontol; 2016. Feb 26. pii: S0531-5565(16)30054-7. doi: 10.1016/j.exger.2016.02.013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, Hagler M, Jurk D, Smith LA, Casaclang-Verzosa G, Zhu Y, Schafer MJ, Tchkonia T, Kirkland JL, Miller JD. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice Aging Cell; 2016;15(5):973–7. [DOI] [PMC free article] [PubMed] [Google Scholar]