Abstract
Background: The Explorys Enterprise Performance Management (EPM) database contains de-identified clinical data for 50 million patients. Multiple sclerosis (MS) disease-modifying therapies (DMTs), specifically interferon beta (IFNβ) treatments, may potentiate depression. Conflicting data have emerged, and a large-scale claims-based study by Patten et al. did not support such an association. This study compares the results of Patten et al. with those using the EPM database.
Methods: “Power searches” were built to test the relationship between antidepressant drug use and DMT in the MS population. Searches were built to produce a cohort of individuals diagnosed as having MS in the past 3 years taking a specific DMT who were then given any antidepressant drug. The antidepressant drug therapy prevalence was tested in the MS population on the following DMTs: IFNβ-1a, IFNβ-1b, combined IFNβ, glatiramer acetate, natalizumab, fingolimod, and dimethyl fumarate.
Results: In patients with MS, the rate of antidepressant drug use in those receiving DMTs was 40.60% to 44.57%. The rate of antidepressant drug use for combined IFNβ DMTs was 41.61% (males: 31.25%–39.62%; females: 43.10%–47.33%). Antidepressant drug use peaked in the group aged 45 to 54 years for five of six DMTs.
Conclusions: We found no association between IFNβ treatment and antidepressant drug use in the MS population compared with other DMTs. The EPM database has been validated against the Patten et al. data for future use in the MS population.
Multiple sclerosis (MS) is a chronic immune-mediated disease of the central nervous system. It has an incidence of 0.005% and a prevalence of 0.1%, with variations depending on geographic and population parameters.1 Depressive disorder is the most common MS psychiatric comorbidity,2 with prevalence estimates ranging from 15.7%3 to 50.3%.4 This equates to three times the prevalence of depressive disorder in the general population.5 In the MS population, depression is more common in women and patients younger than 45 years.6
Disease-modifying therapies (DMTs) have been instrumental in the care of patients with MS since 1993. However, early in the DMT era, interferon beta-1b (IFNβ-1b; 0.25 mg subcutaneously every other day) was linked to depression7 and suicide attempts.8,9 Subsequent studies regarding this issue have produced inconsistent findings. Goeb et al.10 published a review in 2006 of 16 smaller observational studies (n < 361) testing depression or suicide in the MS population taking IFNβ. Fourteen of these studies showed a lack of association between IFNβ therapy and depression. A prospective study performed by Feinstein et al.11 showed an association between depression and IFNβ therapy in those with a history of psychiatric illness before treatment initiation. Patten et al.12 compared concomitant antidepressant drug use in people receiving IFNβ or another MS DMT, glatiramer acetate, using the IMS Health Canada database Therapy Dynamics in 2008. Antidepressant drug use was substituted as a proxy for depressive disorders, and the data were stratified by age and sex. The study found no evidence of increased antidepressant drug treatment in the IFNβ-treated MS population compared with those receiving glatiramer acetate. Given these inconsistencies, we sought to further study this issue but to expand the scope to compare the risk of depression after IFNβ treatment with the risk of depression after treatment with non-interferon DMTs.
As the use of electronic medical records (EMRs) grows in the United States, so too does their potential utility in the clinical research realm. Explorys Enterprise Performance Management (EPM) is a Health Insurance Portability and Accountability Act–compliant database containing de-identified clinical data for 50 million patients.13 It has been externally validated in large retrospective studies characterizing venous thromboembolic events,14 the effect of obesity on total knee arthroplasty,15 and secondary malignancies.16 We aimed to use the EPM database as a novel approach to assessing the association between the use of DMTs, including interferon, and depression in the MS population, comparing the results with those from the 2008 study by Patten et al.12
Methods
Data Source
The Explorys EPM database spans 26 different health-care systems, including Cleveland Clinic, Trinity Health, St. Joseph Health System, Mercy Health, and Adventist Health System, and it is updated daily from EMRs. Such a large sample is especially useful for investigating diseases with low prevalence. It does not require institutional review board (IRB) approval and, thus, can be tailored to the needs of the researcher, particularly as an exploratory tool.
The EPM database allows each participating health-care system to access its own information as well as data composing the Explorys universe. In this study, all the data encompass the EPM universe. The EPM database's “power search” tool provides the ability to create refined cohorts with specific temporal relationships among criteria. Power searches were built between July 7, 2015, and July 13, 2015, to test the relationship between antidepressant drug use and DMT use in the MS population. Given that clinicians may not always code comorbidities such as depression when these are not the chief reason for the visit,17 and the improved sensitivity of case definitions that use administrative health claims data when antidepressant drugs are included, we chose the more sensitive measure of antidepressant drug use instead of depressive disorder for this study.18 Although IRB approval was not necessary, it was received on September 4, 2015, from the Cleveland Clinic IRB.
Study Population
Each power search began with an index event of an MS diagnosis within the past 3 years. In the EPM database universe, drug ingredient served as a proxy for each DMT. A temporal attribute was created for each specific drug ingredient, which included the following: IFNβ-1a (RxNorm C0254119), IFNβ-1b (RxNorm C0244713), interferon, glatiramer (RxNorm C0717787), natalizumab (RxNorm C1172734), fingolimod (RxNorm C1699926), and dimethyl fumarate (RxNorm C0058218). (RxNorm is a normalized naming system for generic and branded drugs produced by the National Library of Medicine.) A second temporal attribute was created in which antidepressant as a “drug pharm class” was coded, with an added qualifier of occurring within 1095 days of initiating a DMT. The goal of this second attribute was to eliminate individuals who were taking antidepressant drugs before beginning treatment with a DMT. This search produced a cohort of individuals diagnosed as having MS in the past 3 years who were taking a specific DMT and were then given any antidepressant drug. Stratification data on specific antidepressant drug use frequencies were not produced. This search was repeated without antidepressant drugs as a way to generate the total population of patients with MS taking a specific DMT.
The EPM database power search cohorts were produced as described previously herein, substituting the “drug brand name” for DMTs in the first temporal attribute. In addition, cohorts were produced substituting a diagnosis of depressive disorder for antidepressant drug use. Finally, the search was performed without DMTs to show antidepressant drug use in the MS population not treated with a DMT. Each of these searches provided demographic data based on sex and age, with age categorized in 10-year increments beginning with 15 to 24 years.
Because standard deviations or raw population data were not available, statistical analysis of data was not possible in this study.
Results
At the time of data evaluation (July 23, 2015), the EPM database included 58,820 unique patients with MS diagnosed in the United States in the 3 years before data evaluation. Of the 58,820 patients in the EPM database, 16,960 were treated with a DMT. The MS population taking a DMT was 75.24% female (n = 12,760) and 24.76% male (n = 4200); 370 were aged 15 to 24 years, 1980 were aged 25 to 34 years, 3970 were aged 35 to 44 years, 4950 were aged 45 to 54 years, and 5670 (33.43%) were 55 years and older. Differences in total number of patients when broken down by age or sex were accounted for by EPM rounding errors when dealing with small cohorts.
According to our search, the rate of antidepressant drug use in the MS population not treated with a DMT was 32.72%. The most common DMT was any IFNβ (n = 4710), followed by glatiramer acetate (n = 4550), IFNβ-1a (n = 4050), dimethyl fumarate (n = 3990), fingolimod (n = 1870), natalizumab (n = 1840), and IFNβ-1b (n = 680). The rate of antidepressant drug use alongside DMTs ranged from 40.60% to 44.57%. The rates of antidepressant drug use were highest for natalizumab (44.57%), followed by fingolimod (42.78%), IFNβ-1b (42.65%), glatiramer acetate (41.76%), IFNβ-1a (41.73%), combined IFNβ (41.61%), and dimethyl fumarate (40.60%). When depressive disorder was substituted for antidepressant drug use, the data were uniformly lower, with rates highest for natalizumab (25.54%), followed by IFNβ-1b (25.00%), glatiramer acetate (23.74%), combined IFNβ (23.14%), fingolimod (22.99%), IFNβ-1a (22.96%), and dimethyl fumarate (21.30%). A side-by-side comparison of antidepressant drug use versus depressive disorder stratified by DMT is shown in Figure 1.
Figure 1.
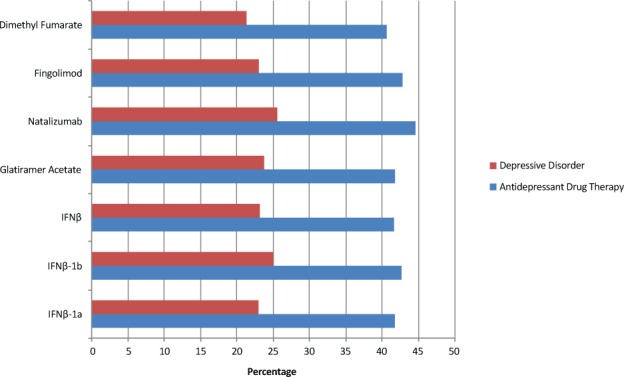
Antidepressant drug use versus depressive disorder stratified by multiple sclerosis disease-modifying therapy
IFN, interferon.
All search cohorts produced information stratified by sex and age. In females, the frequency of antidepressant drug use was highest for natalizumab (47.33%) and lowest for dimethyl fumarate (43.10%). Interestingly, combined IFNβ produced the lowest antidepressant drug frequency (32.14%) in males (Figure 2). Antidepressant drug use peaked in the group aged 45 to 54 years in five of the six DMTs, with only natalizumab peaking in the group aged 35 to 44 years (Figure 3).
Figure 2.
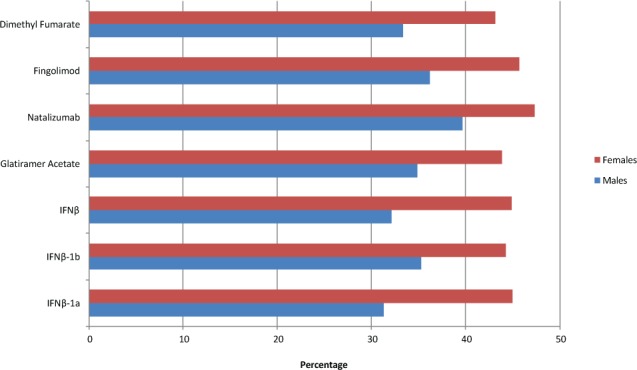
Antidepressant drug use among disease-modifying therapies stratified by sex
IFN, interferon.
Figure 3.
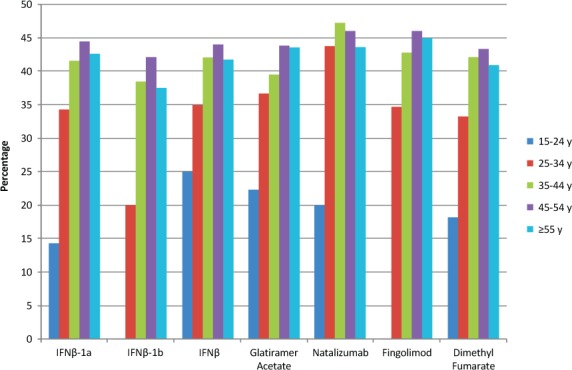
Antidepressant drug use among disease-modifying therapies stratified by age
IFN, interferon.
Compared with the data from Patten et al.12 (39.0%–43.1%), our observation that antidepressant drug use with combined IFNβ is 41.61% matches up closely. In addition, the present data confirm that this use is not more than with other DMTs used in MS. Figure 4 represents a side-by-side comparison between the Patten et al. data and ours. Stratified by sex, the Patten et al. data are within range for both males and females, and both data sets showed an increased frequency of antidepressant drug use in females. When broken down by age, both data sets showed an increase in antidepressant drug use through at least the first three age cohorts.
Figure 4.
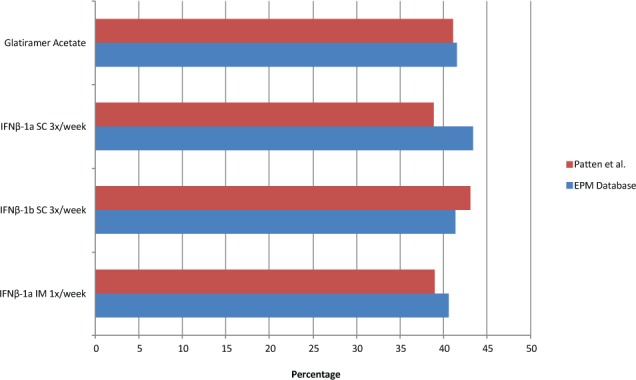
Side-by-side comparison of antidepressant drug use between the study by Patten et al.12 and the Explorys Enterprise Performance Management (EPM) database
IFN, interferon; IM, intramuscularly; SC, subcutaneously.
Discussion
Whereas previous studies focused on the association between depression and the use of one or two DMTs, this study evaluated IFNβ in addition to multiple other DMTs using a contemporary data source. According to the Explorys EPM database, there did not seem to be an increased frequency of antidepressant drug use after IFNβ treatment in MS compared with the use of other DMTs for MS. The present findings regarding the frequency of depression and antidepressant drug use correspond closely to the data from the study by Patten et al.12 Although there seems to be no increased risk of depression in IFNβ treatment, the rate of antidepressant drug use is greater than 40% for all DMTs, emphasizing the importance of depression in the MS population. Note that another interferon, IFNα, has been positively linked to depression19 when used to treat hepatitis C, which might have led to increased vigilance for depression emergence in patients receiving IFNβ. The fact that antidepressant drug use in patients with MS not treated with a DMT was 7.88% lower than the bottom of the DMT range is interesting and worthy of investigation in the future.
Although the EPM database is a potential research tool, there are some limitations to be aware of. The EPM database is built in such a way that any statistical measures or odds ratios cannot be produced on qualitative variables. In addition, all patient age cohorts are rounded to the nearest 10 years for the purposes of de-identification. The EPM database does not provide information about patients who switched DMTs or did not persist with treatment throughout the study period. Last, the EPM database did not allow for the following risks to be searched: remote history of depression, previous use of antidepressant drugs, and family history of depression. A potential critique of this article is that clinicians may not prescribe IFNβ in depressed patients knowing the previous association studies. Parameters such as this might assist in determining the presence of previous disease affecting prescribing practices.
Note that the study by Patten et al.12 defined age cohorts in 10-year increments beginning with 18 to 25 years, whereas the EPM database provided cohorts in 10-year increments beginning with 15 to 24 years. One limitation of both studies was that antidepressant drugs are sometimes prescribed for chronic pain, migraines, and sleep disorders. Although we took this into consideration, the data when depressive disorder was used instead of antidepressant drug use showed the same pattern. We were more worried about the scenario of depressive disorder being underrepresented by EPM database coding.
One of the central challenges in the current environment is harnessing EMR data for research purposes. The Explorys EPM database contains 58,820 unique patients with MS active within the past 3 years, and it is a dynamic database. If validated for use, such a large cohort provides a potentially rich archive for investigation into diseases of low prevalence. This study substantiates the external validation of the EPM database from previous studies,14–16 clearing the way for the EPM database to be used to test other hypotheses in the MS population.
In summary, the present data do not demonstrate any association between IFNβ use and depression in the MS population over and above that of other DMTs used in MS. Depression rates were high in the MS population overall, with antidepressant drug use of greater than 40% for all DMTs included. Antidepressant drug use was higher in females than in males, and it peaked in those aged 45 to 54 years for five of the six DMTs studied. The Explorys EPM database is a powerful tool capable of using EMR data for clinical research in the MS world in the future.
PracticePoints
Using the Explorys Enterprise Performance Management (EPM) database containing de-identified clinical data from 58,800 patients diagnosed as having MS in the past 3 years, we found no association between antidepressant drug use and interferon beta treatment in the MS population over and above other disease-modifying therapies used in MS.
The Explorys EPM database has been validated for use in the MS population.
Footnotes
Financial Disclosures: Dr. Rae-Grant is a local principal investigator for an MS clinical trial with Novartis, but he receives no personal compensation or time support for his work. Dr. Marrie and Mr. Mirsky have no conflicts of interest to disclose.
Funding/Support: This research was supported through the National Multiple Sclerosis Society's Ohio Buckeye Chapter Medical Student Fellowship.
References
- 1. Koch MW. Epidemiology and natural history of multiple sclerosis. : Rae-Grant A, Fox R, Bethoux F, et al. Multiple Sclerosis and Related Disorders: Diagnosis, Medical Management, and Rehabilitation. New York, NY: Demos Medical Publishing; 2013: 21. [Google Scholar]
- 2. Khawam EA. Emotional disorders in multiple sclerosis. : Rae-Grant A, Fox R, Bethoux F, et al. Multiple Sclerosis and Related Disorders: Diagnosis, Medical Management, and Rehabilitation. New York, NY: Demos Medical Publishing; 2013: 156. [Google Scholar]
- 3. Patten SB, Beck CA, Williams JV, Barbui C, Metz LM.. Major depression in multiple sclerosis: a population-based perspective. Neurology. 2003; 61: 1524– 1527. [DOI] [PubMed] [Google Scholar]
- 4. Sadovnick AD, Remick RA, Allen J, . et al. Depression and multiple sclerosis. Neurology. 1996; 46: 628– 632. [DOI] [PubMed] [Google Scholar]
- 5. Kessler RC, McGonagle KA, Zhao S, . et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994; 51: 8– 19. [DOI] [PubMed] [Google Scholar]
- 6. Patten SB, Francis G, Metz LM, Lopez-Bresnahan M, Chang P, Curtin F.. The relationship between depression and interferon beta-1a therapy in patients with multiple sclerosis. Mult Scler. 2005; 11: 175– 181. [DOI] [PubMed] [Google Scholar]
- 7. The IFNB Multiple Sclerosis Study Group. . Interferon beta-1b is effective in relapsing-remitting multiple sclerosis, I: clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993; 43: 655– 661. [DOI] [PubMed] [Google Scholar]
- 8. The IFNB Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group. . Interferon beta-1b in the treatment of multiple sclerosis: final outcome of the randomized controlled trial. Neurology. 1995; 45: 1277– 1285. [PubMed] [Google Scholar]
- 9. Stenager EN, Jensen B, Stenager M, Stenager K, Stenager E.. Suicide attempts in multiple sclerosis. Mult Scler. 2011; 17: 1265– 1268. [DOI] [PubMed] [Google Scholar]
- 10. Goeb JL, Even C, Nicolas G, Gohier B, Dubas F, Garré JB.. Psychiatric side effects of interferon-beta in multiple sclerosis. Eur Psychiatry. 2006; 21: 186– 193. [DOI] [PubMed] [Google Scholar]
- 11. Feinstein A, O'Connor P, Feinstein K.. Multiple sclerosis, interferon beta-1b and depression: a prospective investigation. J Neurol. 2002; 249: 815– 820. [DOI] [PubMed] [Google Scholar]
- 12. Patten SB, Williams JV, Metz LM.. Anti-depressant use in association with interferon and glatiramer acetate treatment in multiple sclerosis. Mult Scler. 2008; 14: 406– 411. [DOI] [PubMed] [Google Scholar]
- 13. About us: our purpose. Explorys website. https://www.explorys.com/about-us.html. Accessed October 4, 2016. [Google Scholar]
- 14. Kaelber DC, Foster W, Gilder J, Love TE, Jain AK.. Patient characteristics associated with venous thromboembolic events: a cohort study using pooled electronic health record data. J Am Med Inform Assoc. 2012; 19: 965– 972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pfefferle KJ, Gil KM, Fening SD, Dilisio MF.. Validation study of a pooled electronic healthcare database: the effect of obesity on the revision rate of total knee arthroplasty. Eur J Orthop Surg Traumatol. 2014; 24: 1625– 1628. [DOI] [PubMed] [Google Scholar]
- 16. Van Fossen VL, Wilhelm SM, Eaton JL, McHenry CR.. Association of thyroid, breast and renal cell cancer: a population-based study of the prevalence of second malignancies. Ann Surg Oncol. 2013; 20: 1341– 1347. [DOI] [PubMed] [Google Scholar]
- 17. Levy AR, Tamblyn RM, Fitchett D, McLeod PJ, Hanley JA.. Coding accuracy of hospital discharge data for elderly survivors of myocardial infarction. Can J Cardiol. 1999; 15: 1277– 1282. [PubMed] [Google Scholar]
- 18. Marrie RA, Fisk JD, Yu BN, . et al. Mental comorbidity and multiple sclerosis: validating administrative data to support population-based surveillance. BMC Neurol. 2013; 13: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dieperink E, Willenbring M, Ho SB.. Neuropsychiatric symptoms associated with hepatitis C and interferon alpha: a review. Am J Psychiatry. 2000; 157: 867– 876. [DOI] [PubMed] [Google Scholar]


