Abstract
Objective
To assess whether an abnormality in cholesterol absorption or synthesis may be associated with hypocholesterolemia in patients with single ventricle anatomy following Fontan palliation.
Study design
This is a cross-sectional study of 21 patients with hypocholesterolemia receiving Fontan and age-sex matched healthy controls, with median age of 13.4 (IQR 10.6–16.1) years. Laboratory values of several biomarkers, including phytosterols and 5-α-cholestanol (for cholesterol absorption) and lathosterol (for cholesterol biosynthesis), as well as cholesterol levels, inflammatory markers, and indices of liver function were compared between patients receiving Fontan and controls.
Results
The group receiving Fontan had significantly lower total cholesterol (Mean 117± SD 13.9, vs. 128±19.2 mg/dL, p= 0.03) and free cholesterol (35.5±4.5 vs. 39.2±5.4 mg/dl, p= 0.02) compared with control patients. There was an increase in normalized 5-α-cholestanol (1.51±0.6 vs. 1.14±0.37 μg/ml, p= 0.02), and a significantly lower lathosterol/5-α-cholestanol ratio (0.70±0.38 vs. 1.11±0.76, p= 0.04). There was a strong correlation (r=0.78, p<0.0001) between lathosterol and cholesterol levels in the group receiving Fontan, not seen in controls (r=0.47, p=0.04). The group receiving Fontan also had significantly higher C-reactive protein, transaminases, total bilirubin, and gamma-glutamyl transferase levels.
Conclusions
Patients with hypocholesterolemia receiving Fontan have evidence of increased cholesterol absorption and decreased cholesterol synthesis. As cholesterol absorption efficiency is a regulated process, this finding suggests an up-regulation of cholesterol absorption as a result of decreased cholesterol production. In the setting of elevated liver indices and possible inflammation, this finding supports a growing body of data suggesting development of liver disease in patients receiving Fontan.
Keywords: lipids, congenital heart disease, liver disease, inflammation, cholesterol metabolism
As advances in the surgical palliation of complex single ventricle congenital heart lesions have progressed, there is a larger cohort of patients surviving longer with the unique physiology of a Fontan circulation. The Fontan procedure routes systemic venous blood passively into the pulmonary arterial circulations and utilizes the single ventricle as the systemic pump. This circulation results most notably in a state of decreased cardiac output and chronically elevated central venous pressure, as the pulmonary arterial pressures are transmitted into the systemic venous circulation. There has been more evidence that this chronic physiologic state can affect numerous organ systems, in particular it is increasingly appreciated that the liver is largely affected 1.
We recently reported that patients receiving Fontan have significantly lower serum total, low density lipoprotein cholesterol (LDL-C) and high density lipoprotein cholesterol (HDL-C) levels than age-sex matched normalized data from NHANES III 2, however the etiology, significance, or implications of this finding remain unknown. Cholesterol levels are regulated by a balance of cholesterol absorption and synthesis and the liver plays a large role in the latter process. We therefore sought to find an etiology for the decreased cholesterol levels found in a population of patients receiving Fontan by investigating whether an abnormality in cholesterol absorption and/or synthesis occurred in these patients. As cholesterol absorption and synthesis are difficult to measure directly, we measured circulating concentrations of cholesterol metabolites and precursors (5-α-cholestanol and lathosterol) and plant sterols (sitosterol, campesterol, stigmasterol), which have been shown to serve as good biomarkers of these processes 3–5. The identification of an abnormality in cholesterol synthesis may provide more evidence of abnormalities of liver function in patients receiving Fontan.
METHODS
This study is a cross-sectional study comparing patients receiving Fontan with hypocholesterolemia with age-sex (frequency) matched healthy patients. Patients receiving Fontan between 7 and 21 years of age, who had been previously identified as having low total cholesterol levels, were approached to participate in the study. Patients were excluded if they had known protein losing enteropathy (PLE) or plastic bronchitis. Healthy patients were chosen from a cohort of patients without known structural heart disease presenting to the University of Michigan C.S Mott Children’s Hospital for other appointments or procedures. These patients included those with isolated supraventricular tachycardia presenting for an ablation procedure, as well as other healthy patients presenting for same-day surgical procedures and clinic visits outside the Congenital Heart Center. All potential participants were approached by phone, participation confirmed, and appropriate study materials provided. All participants were asked to complete a diet log for the 3 days prior to the research visit, fast for 8 hours prior to the blood draw, and complete a survey regarding family history of dyslipidemias or premature coronary artery disease. Informed consent and assent, when appropriate, for the study were obtained from all participants. The study was approved by the University of Michigan Institutional Review Board.
Following an 8-hour fast, patients were seen by a research study representative (research coordinator or principal investigator) either in a dedicated research visit or prior to start of a scheduled procedure. To assure no significant difference in the diet of all participants, 3-day diet logs were collected and analyzed for daily total calories, carbohydrates, protein, and fat. A family history survey was completed by all participants to identify any family history of high or low cholesterol, high or low triglycerides, any family members on lipid-altering medications, and any family history of premature coronary artery disease (at less than 55 years of age in men and less than 65 years of age in women). All patients were included in the study irrespective of family history.
A single fasting blood draw was then performed to obtain the following laboratory values: serum lipid profile (total cholesterol, HDL-C, LDL-C, and triglycerides), apolipoprotein B and apolipoprotein A1 levels, free and total cholesterol levels, plasma insulin and glucose levels, inflammatory markers including high-sensitivity C-reactive protein (hsCRP), Interleukin-6 (IL-6), and Tumor necrosis factor-alpha (TNF-α), complete blood count, complete metabolic panel, and gamma-glutamyl transferase (GGT). Additional blood was obtained for analysis of plasma sterols (sitosterol, campesterol, stigmasterol), the cholesterol metabolite 5-α-cholestanol, and cholesterol precursor lathosterol. These samples were processed, serum stored, and samples later sent to Washington University in St. Louis for processing by gas-chromatography-mass spectrometry 6.
Additional chart review was performed to obtain demographic information, clinical information regarding initial cardiac anatomy, date and type of Fontan procedure, and current medication use in patients receiving Fontan, and additional past medical history and medication use in healthy control patients.
Statistical analyses
Data are presented as frequency with percentage for categorical variables and mean ± standard deviation or median with interquartile range (IQR), as appropriate, for continuous variables. Patient and laboratory data were compared between the patients receiving Fontan and healthy controls using Chi-square test or Fisher exact test for categorical variables and t-test or Wilcoxon rank sum test for continuous variables. Pearson correlation coefficient, r, was used to evaluate an association between the selected laboratory variables. All analyses were performed using SAS Version 9.3 (SAS Institute Inc., Cary, NC), with statistical significance set at a p-value <0.05 using a two-sided test.
RESULTS
Twenty-one patients receiving Fontan and 21 healthy control patients were enrolled in the study (Table I). Control patients consisted of 17 patients (81%) with the primary diagnosis of arrhythmia and a structurally normal heart, who were enrolled at the time of an electrophysiology procedure, and 3 patients (14%) without a cardiac diagnosis, who were enrolled at the time of a same day surgical procedure or clinic visit. One patient was the healthy twin sibling of an enrolled patient receiving Fontan. Patients receiving Fontan were a median of 11.4 years (IQR 8.5–14.2) from their Fontan procedure. Twelve patients (57.1%) had a systemic right ventricle and 19 (90.5%) had undergone a fenestrated lateral tunnel Fontan with the remaining two patients having undergone an extracardiac Fontan.
Table 1.
Demographic characteristics of Fontan and healthy control patients (N=42)
| Characteristics | Fontan (n=21) | Controls (n=21) | P-value |
|---|---|---|---|
| Male sex | 13 (61.9) | 13 (61.9) | 1.00 |
| Caucasian race | 20 (95.2) | 16 (76.2) | 0.18 |
| Age at lab draw, years | 13.6 (11.4–17.3) | 12.5 (10.5–15.6) | 0.36 |
| Weight, kg | 51.3 ± 24.6 | 53.8 ± 18.7 | 0.71 |
| Body mass index (BMI), kg/m2 | 20.8 ± 5.2 | 20.5 ± 4.3 | 0.83 |
Data are presented as N (%) for categorical variables and Median (IQR) or Mean ± SD for continuous variables.
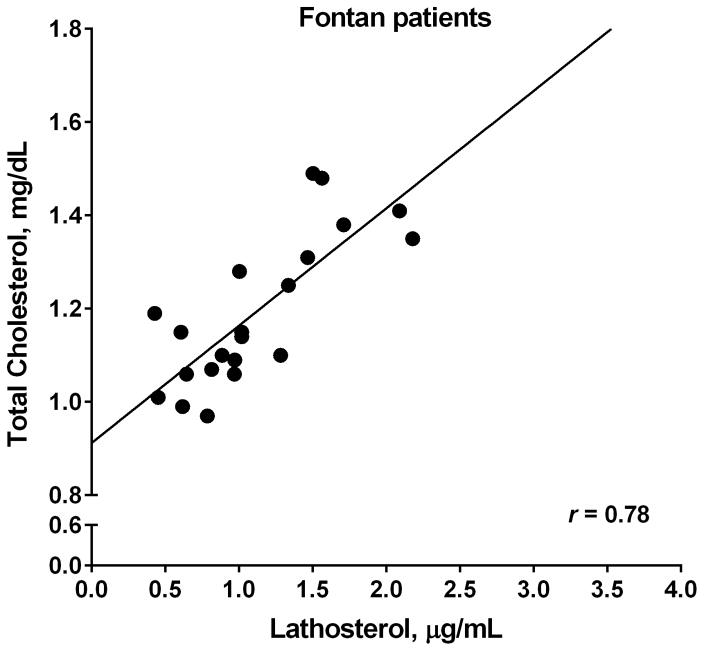
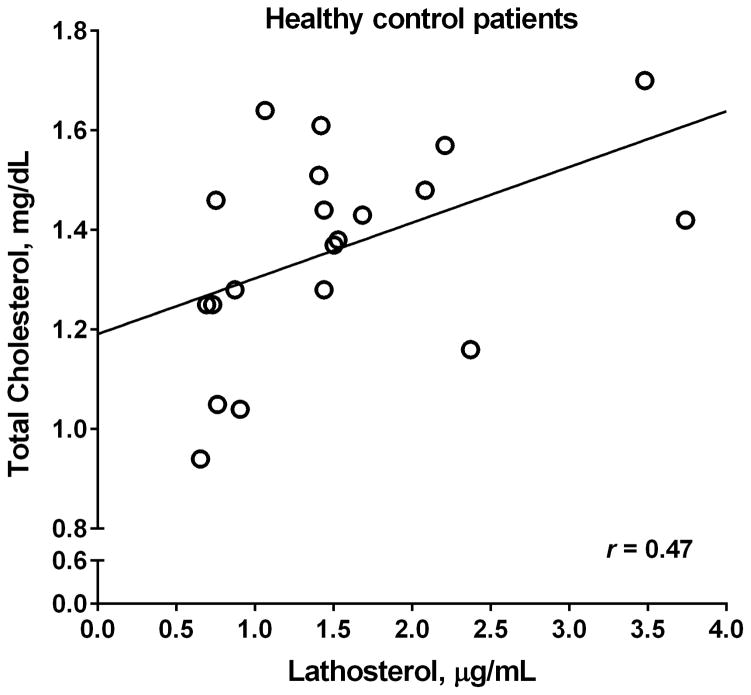
As was expected from previous results, the patients receiving Fontan had a significantly lower total cholesterol, free cholesterol, and triglyceride concentration than healthy controls (Table II). Patients receiving Fontan had a significantly higher normalized 5-α-cholestanol level, a marker of cholesterol absorption, and a trend towards a lower lathosterol level, a marker of cholesterol synthesis (Table II). The ratio of lathosterol/5-α-cholestanol, representing the ratio of cholesterol biosynthesis to cholesterol absorption, is also significantly reduced in the group receiving Fontan with no significant difference between the ratio of campesterol/5-α-cholestanol levels between groups to suggest a difference in intake of dietary plant sterols. There was also no significant difference in the composition of diets between the Fontan and healthy groups (as measured by comparison of total calories, total carbohydrates, total protein and total fat intake). Lathosterol and total cholesterol levels correlated strongly in patients receiving Fontan (r=0.78, p<0.0001), but not in controls (r=0.47, p=0.04; Figure).
Table 2.
Cholesterol levels and markers of cholesterol absorption and synthesis
| Characteristics | Fontan (n=21) | Controls (n=21) | P-value§ |
|---|---|---|---|
| Total cholesterol, mg/dL | 117 ± 13.9 | 128 ± 19.2 | 0.03 |
| HDL-C, mg/dL | 40.0 ± 8.2 | 45.1 ± 13.3 | 0.14 |
| Non-HDL-C, mg/dL | 77.0 ± 9.6 | 83.2 ± 19.3 | 0.19 |
| LDL-C, mg/dL | 63.9 ± 10.2 | 62.5 ± 15.7 | 0.73 |
| Triglycerides, mg/dL | 65.5 ± 18.9 | 105 ± 60.8 | 0.01 |
| Free cholesterol, mg/dL | 35.5 ± 4.5 | 39.2 ± 5.4 | 0.02 |
| Apolipoprotein A-1, mg/dL | 117 ± 18.0 | 119 ± 23.3 | 0.72 |
| Apolipoprotein B, mg/dL | 64.0 ± 8.3 | 66.1 ± 14.5 | 0.58 |
| Normalized 5-α-cholestanol, μg/mg | 1.51 ± 0.60 | 1.14 ± 0.37 | 0.02 |
| Lathosterol, μg/mL | 1.11 ± 0.50 | 1.54 ± 0.88 | 0.07 |
| Campesterol, μg/mL | 3.66 ± 1.77 | 3.50 ± 1.26 | 0.74 |
| Stigmasterol, μg/mL | 0.10 ± 0.05 | 0.16 ± 0.14 | 0.08 |
| Sitosterol, μg/mL | 2.69 ± 1.33 | 2.72 ± 1.04 | 0.94 |
| Lathosterol/5-α-cholestanol | 0.70 ± 0.38 | 1.11 ± 0.76 | 0.04 |
| Campesterol/5-α-cholestanol | 2.33 ± 1.26 | 2.61 ± 1.31 | 0.49 |
Data are presented as Mean ± SD.
P-value from t-test on comparison of each laboratory value between Fontan patients and healthy controls.
Figure.
Association between total cholesterol and lathosterol for Fontan vs healthy control patients. A, Strong correlation between total cholesterol and lathosterol in patients receiving Fontan, r= 0.78. B, The relationship between total cholesterol and lathosterol levels in healthy control patients, which shows a weaker correlation, r= 0.47.
Patients receiving Fontan had higher transaminases, GGT, and total bilirubin levels than healthy patients (Table III) and there was a negative association between high total bilirubin levels and low cholesterol (r= −0.44, p= 0.003) in the overall patients. Patients receiving Fontan had significantly higher hsCRP level than healthy patients (p=0.01) however a similar association was not found with the other inflammatory markers measured. There was no significant association of hsCRP level with total cholesterol levels, lathosterol, or lathosterol/5-α-cholestanol level; however those with a normal or high hsCRP had a significantly higher normalized 5-α-cholestanol level than those with a low CRP level (1.62 ± 0.63 vs 1.14 ± 0.34, p=0.01). Patients receiving Fontan also had higher fasting plasma insulin levels and significantly lower fasting glucose levels without a significant difference in the Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) ratio (Table III).
Table 3.
Other laboratory data
| Characteristics | Fontan (n=21) | Controls (n=21) | P-value§ |
|---|---|---|---|
| Plasma insulin, μU/mL | 6.9 (3–13.4) | 4.6 (1.9–6.3) | 0.05 |
| High-sensitivity CRP, mg/L | 0.7 (0.3–1.0) | 0.3 (0.2–0.4) | 0.01 |
| TNF-α, highly sensitive, pg/mL | 1.9 (1.7–2.4) | 2.2 (1.7–2.8) | 0.30 |
| Hematocrit, % | 44.1 ± 2.5 | 36.3 ± 4.0 | <.0001 |
| Platelet count, K/μL | 189 ± 80.3 | 245 ± 47.5 | 0.01 |
| Fasting glucose | 81.1 ±6.2 | 90.2 ± 10.5 | 0.002 |
| HOMA-IR | 1.3 (0.6–2.6) | 1.1 (0.4–1.5) | 0.15 |
| Albumin, g/dL | 4.6 ± 0.3 | 4.0 ± 0.3 | <.0001 |
| AST, IU/L | 31.8 ± 6.3 | 23.9 ± 5.7 | 0.0001 |
| ALT, IU/L | 29.8 ± 8.1 | 17.5 ± 6.4 | <.0001 |
| Total bilirubin, mg/dL | 0.99 ± 0.41 | 0.49 ± 0.23 | <.0001 |
| GGTP, IU/L | 54.2 ± 24.3 | 10.8 ± 7.0 | <.0001 |
Data are presented as N (%) for categorical variables and Median (IQR) or Mean ± SD for continuous variables.
P-value from Wilcoxon rank sum test or t-test, as appropriate, on comparison of each laboratory value between Fontan patients and healthy controls.
The family histories between Fontan and control groups showed no significant differences in lipid abnormalities or history of premature coronary artery disease between groups. Patients with a family history of high cholesterol showed a higher total cholesterol (126 ± 18.1 with family history vs 118 ± 13.4 without a family history, p= 0.27) however this difference was not significant.
DISCUSSION
This study extends our previous study in patients with hypocholesterolemia receiving Fontan 2, by showing the pattern of increased normalized 5-α-cholestanol and decreased lathosterol/5-α-cholestanol ratios to suggest significantly increased cholesterol absorption efficiency. There may be an association of this finding with increased inflammatory markers and with elevated indices of liver function and liver inflammation.
The use of non-cholesterol sterols as markers of cholesterol synthesis and percent cholesterol absorption has become widely accepted. Lathosterol, a precursor of cholesterol synthesis, is a good indicator of cholesterol synthesis and levels of 5-α-cholestanol, a metabolite of endogenous cholesterol, and plant sterols (e.g. sitosterol, campesterol, stigmasterol), which are not synthesized by the human body and only absorbed, are reliable indicators of cholesterol absorption efficiency 3, 7–9. Serum levels of plant sterols to cholesterol depend on dietary intake, absorption efficiency, and biliary secretion. Cholesterol metabolism as a whole is a regulated process, with an ongoing balance between cholesterol absorption and synthesis 10. In our study, we demonstrated that patients with hypocholesterolemia receiving Fontan have increased cholesterol absorption efficiency likely in response to decreased cholesterol synthesis.
As the liver plays a large role in cholesterol metabolism, an abnormality in cholesterol synthesis in patients receiving Fontan may well fit within the growing body of evidence for the presence of liver abnormalities following Fontan palliation. Patients receiving Fontan have been shown to develop coagulation disorders, cholestasis, liver fibrosis, cirrhosis, and hepatocellular carcinoma over time 11–15. The etiology for these findings is postulated to be multifactorial, including chronically elevated systemic venous pressures, low cardiac output, and multiple peri-operative insults to the liver. There is evidence of structural abnormalities consistent with congestive hepatopathy, including sinusoidal fibrosis and sinusoidal dilation 14, 16. Generally, however, there is little if any elevation in liver enzymes or in other indices of hepatic function due to the lack of significant inflammation and cell death 12. Guha et al assessed liver function by indocyanine green clearance, and liver fibrosis through serum fibrosis markers, and showed that fibrosis in patients receiving Fontan was not well correlated with global markers of liver function 17. It may be that dysregulation of cholesterol metabolism in the liver, as manifest by low cholesterol levels, may serve as another marker of the extent of liver injury in patients receiving Fontan.
Another potential etiology contributing to the low cholesterol levels observed in patients receiving Fontan may involve increased inflammation. Elevation of inflammatory markers (including TNF-α and CRP) has been demonstrated in over 30% of patients receiving Fontan studied by Ostrow et al. 18 In patients receiving Fontan, this finding has received the most attention in the setting of PLE. It has been hypothesized that the combined effects of an alteration in mesenteric hemodynamics and intestinal inflammation may play important roles 19. In adults, chronic congestive heart failure and a chronically low-output state, results in stimulation of the inflammatory system 20 and therefore even in the absence of overt symptoms of PLE, patients receiving Fontan may live in a state of chronically elevated inflammation due to limited cardiac output. Interestingly, patients with rheumatoid arthritis who exist in a chronic inflammatory state, exhibit a very similar lipid profile with low total, LDL and HDL cholesterol 21. These levels have been demonstrated even during the 5 years prior to development of rheumatoid arthritis and this cholesterol panel has been associated with an increased risk of cardiovascular disease development 22. Our small sample size limited our ability to find a difference between the inflammatory markers tested (TNF-α, IL-6) however elevated hsCRP levels found in these patients does make this etiology an interesting one in need of additional evaluation in a larger population receiving Fontan.
It is clear that detailed evaluation of the liver is becoming more and more important in the long term care of the growing population receiving Fontan. The addition of a lipid panel to the evaluation of these patients may be of value. In many populations, including cirrhosis, end stage renal disease, chronic heart failure, and sepsis, low cholesterol levels have been associated with poor prognosis 23–25. Detection of a low cholesterol level may serve as a biomarker of impaired liver function, beyond that appreciated by other invasive and non-invasive measures. Establishing a relationship between this finding and measures of clinical status will be important in determining the utility of this finding in the overall care of patients receiving Fontan.
This study does have several limitations. The sample size was small and data obtained represent only a single cross-sectional sample. Long term follow-up of these patients, changes in cholesterol levels over time, indices of current and future clinical status, and relation to intracardiac hemodynamics are unavailable. The use of non-cholesterol sterols as markers of cholesterol absorption and synthesis is also limited by being an indirect measure of these processes and has not been validated in pediatric patients. Providing control values from healthy patients was therefore undertaken to allow for comparison with an age-sex matched pediatric population. Future studies in larger populations with long-term follow-up and in association with other invasive or non-invasive evaluation of both liver and cardiac function, may help to establish the significance of this finding in the health of patients receiving Fontan over time. Future studies also should focus on comparing patients receiving Fontan with and without low cholesterol values.
These results add to the growing body of literature implicating impaired liver function in patients receiving Fontan. Further study to determine an association with other markers of liver health, further elucidation of potential etiologies, and relation to clinical outcomes in a larger sample is necessary to determine the clinical importance of these results.
Acknowledgments
Supported by the National Institutes of Health (R01 108160 [to R.O.]). Internal institutional funding provided by Michigan Congenital Heart Outcomes Research and Discovery Program (Griese-Hutchinson-Woodson Award M-CHORD [to W.W.]).
We acknowledge Gina Willis, research coordinator, for her assistance with subject recruitment and enrollment.
ABBREVIATIONS
- LDL-C
low density lipoprotein cholesterol
- HDL-C
high density lipoprotein cholesterol
- GGT
gamma-glutamyl transferase
- PLE
protein losing enteropathy
- hsCRP
high-sensitivity C-reactive protein
- IL-6
Interleukin-6
- and TNF-α
Tumor necrosis factor-alpha
- IQR
interquartile range
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rychik J, Goldberg DJ. Late Consequences of the Fontan Operation. Circulation. 2014;130:1525–8. doi: 10.1161/CIRCULATIONAHA.114.005341. [DOI] [PubMed] [Google Scholar]
- 2.Whiteside W, Tan M, Yu S, Rocchini A. Low Total, Low-Density Lipoprotein, High-Density Lipoprotein, and Non–High-Density Lipoprotein Cholesterol Levels in Patients with Complex Congenital Heart Disease after Fontan Palliation. J Pediatr. 2013;162:1199–204.15. doi: 10.1016/j.jpeds.2012.11.073. [DOI] [PubMed] [Google Scholar]
- 3.Miettinen TA. Serum plant sterols and cholesterol precursors reflect cholesterol absorption and synthesis in volunteers of a randomly selected male population. Am J Epidemiol. 1990;131:20–31. doi: 10.1093/oxfordjournals.aje.a115479. [DOI] [PubMed] [Google Scholar]
- 4.Miettinen TA, Gylling H, Nissinen MJ. The role of serum non-cholesterol sterols as surrogate markers of absolute cholesterol synthesis and absorption. Nutr Metab Cardiovasc Dis. 2011;21:765–9. doi: 10.1016/j.numecd.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Tilvis RS, Miettinen TA. Serum plant sterols and their relation to cholesterol absorption. Am J Clin Nutr. 1986;43:92–7. doi: 10.1093/ajcn/43.1.92. [DOI] [PubMed] [Google Scholar]
- 6.Lin X, Racette SB, Ma L, Wallendorf M, Spearie CA, Ostlund RE., Jr Plasma Biomarker of Dietary Phytosterol Intake. PLoS ONE. 2015;10:e0116912. doi: 10.1371/journal.pone.0116912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miettinen TA, Tilvis RS, Kesaniemi YA. Serum cholestanol and plant sterol levels in relation to cholesterol metabolism in middle-aged men. Metabolism. 1989;38:136–40. doi: 10.1016/0026-0495(89)90252-7. [DOI] [PubMed] [Google Scholar]
- 8.Simonen P, Gylling H, Miettinen TA. The validity of serum squalene and non-cholesterol sterols as surrogate markers of cholesterol synthesis and absorption in type 2 diabetes. Atherosclerosis. 2008;197:883–8. doi: 10.1016/j.atherosclerosis.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Nissinen MJ. Responses of surrogate markers of cholesterol absorption and synthesis to changes in cholesterol metabolism during various amounts of fat and cholesterol feeding among healthy men. Br J Nutr. 2008;99:370–8. doi: 10.1017/S0007114507811998. [DOI] [PubMed] [Google Scholar]
- 10.Grundy SM, Ahrens EH, Davignon J. The interaction of cholesterol absorption and cholesterol synthesis in man. J Lipid Res. 1969;10:304–15. [PubMed] [Google Scholar]
- 11.Wu FM, Ukomadu C, Odze RD, Valente AM, Mayer JJE, Earing MG. Liver Disease in the Patient with Fontan Circulation. Congenit Heart Dis. 2011;6:190–201. doi: 10.1111/j.1747-0803.2011.00504.x. [DOI] [PubMed] [Google Scholar]
- 12.van Nieuwenhuizen RC, Peters M, Lubbers LJ, Trip MD, Tijssen JG, Mulder BJ. Abnormalities in liver function and coagulation profile following the Fontan procedure. Heart. 1999;82:40–6. doi: 10.1136/hrt.82.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baek JS, Bae EJ, Ko JS, Kim GB, Kwon BS, Lee SY, et al. Late hepatic complications after Fontan operation; non-invasive markers of hepatic fibrosis and risk factors. Heart. 2010;96:1750–5. doi: 10.1136/hrt.2010.201772. [DOI] [PubMed] [Google Scholar]
- 14.Kiesewetter C, Sheron N, Vettukattill J, Hacking N, Stedman B, Millward-Sadler H, et al. Hepatic changes in the failing Fontan circulation. Heart. 2007;93:579–84. doi: 10.1136/hrt.2006.094516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rychik J, Veldtman G, Rand E, Russo P, Rome J, Krok K, et al. The Precarious State of the Liver After a Fontan Operation: Summary of a Multidisciplinary Symposium. Pediatr Cardiol. 2012;33:1001–12. doi: 10.1007/s00246-012-0315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kendall TJ, Stedman B, Hacking N, Haw M, Vettukattill JJ, Salmon AP, et al. Hepatic fibrosis and cirrhosis in the Fontan circulation: a detailed morphological study. J Clin Pathol. 2008;61:504–8. doi: 10.1136/jcp.2007.052365. [DOI] [PubMed] [Google Scholar]
- 17.Guha IN, Bokhandi S, Ahmad Z, Sheron N, Cope R, Marshall C, et al. Structural and functional uncoupling of liver performance in the Fontan circulation. Int J Cardiol. 2013;164:77–81. doi: 10.1016/j.ijcard.2011.06.062. [DOI] [PubMed] [Google Scholar]
- 18.Ostrow A, Freeze H, Rychik J. Protein-losing enteropathy after fontan operation: investigations into possible pathophysiologic mechanisms. Ann Thorac Surg. 2006;82:695–700. doi: 10.1016/j.athoracsur.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 19.Rychik J, Goldberg D, Rand E, Semeao E, Russo P, Dori Y, et al. End-organ consequences of the Fontan operation: liver fibrosis, protein-losing enteropathy and plastic bronchitis. Cardiol Young. 2013;23:831–40. doi: 10.1017/S1047951113001650. [DOI] [PubMed] [Google Scholar]
- 20.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated Circulating Levels of Tumor Necrosis Factor in Severe Chronic Heart Failure. N Engl J Med. 1990;323:236–41. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 21.Amezaga Urruela M, Suarez-Almazor M. Lipid Paradox in Rheumatoid Arthritis: Changes With Rheumatoid Arthritis Therapies. Curr Rheumatol Rep. 2012;14:428–37. doi: 10.1007/s11926-012-0269-z. [DOI] [PubMed] [Google Scholar]
- 22.Choy E, Sattar N. Interpreting lipid levels in the context of high-grade inflammatory states with a focus on rheumatoid arthritis: a challenge to conventional cardiovascular risk actions. Ann Rheum Dis. 2009;68:460–9. doi: 10.1136/ard.2008.101964. [DOI] [PubMed] [Google Scholar]
- 23.Habib A, Mihas AA, Abou-Assi SG, Williams LM, Gavis E, Pandak WM, et al. High-density lipoprotein cholesterol as an indicator of liver function and prognosis in noncholestatic cirrhotics. Clin Gastroenterol Hepatol. 2005;3:286–91. doi: 10.1016/s1542-3565(04)00622-6. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Coresh J, Eustace JA, Longenecker JC, Jaar B, Fink NE, et al. Association Between Cholesterol Level and Mortality in Dialysis Patients. JAMA. 2004;291:451–9. doi: 10.1001/jama.291.4.451. [DOI] [PubMed] [Google Scholar]
- 25.Horwich TB, Hamilton MA, MacLellan WR, Fonarow GC. Low serum total cholesterol is associated with marked increase in mortality in advanced heart failure. J Card Fail. 2002;8:216–24. doi: 10.1054/jcaf.2002.0804216. [DOI] [PubMed] [Google Scholar]