Abstract
Individuals with paraplegia due to spinal cord injury rank restoration of walking high on the list of priorities to improving their quality of life. Powered lower-limb exoskeleton technology provides the ability to restore standing up, sitting down, and walking movements for individuals with paraplegia. The robotic exoskeletons generally have electrical motors located at the hip and knee joint centers, which move the wearers' lower limbs through the appropriate range of motion for gait according to control systems using either trajectory control or impedance control. Users of exoskeletons are able to walk at average gait speeds of 0.26 m/s and distances ranging between 121-171 m. However, the achieved gait speeds and distances fall short of those required for full community ambulation (0.8 m/s and at least 230 m), restricting use of the devices to limited community use with stand-by assist or supervised rehabilitation settings. Improvement in the gait speed and distance may be achievable by combining a specially designed powered exoskeleton with neuromuscular stimulation technologies resulting in a hybrid system that fully engages the user and achieves the necessary requirements to ambulate in the community environment with benefits of muscle contraction.
Between 270,000 and 1.275 million individuals in the United States are living with spinal cord injury (SCI), where approximately 37% of SCI result in paraplegia1,2. Among individuals with paraplegia, 38% rank restoration of walking as first or second priorities to improve their quality of life3. Restoring gait can improve overall health including increased cardiovascular fitness, better bone density, improved bladder and bowel function, reduced spasticity, and reduced onset of pressure sores4-6. Gait for individuals with SCI can be restored using neuromuscular stimulation, orthotic braces, robotic exoskeletons, and hybrid neuroprostheses which combine stimulation and orthoses. In this review paper, we will address the existing powered exoskeleton technology that is used to restore gait for individuals with paraplegia and their mobility outcomes.
Several exoskeletons are in the process of being commercialized or are still only found in laboratory research or rehabilitation center environments. Motorized exoskeletal devices developed to aid walking disabilities include ‘Rex’ (Rex Bionics, New Zealand)7-8, ‘ReWalk™’ (ReWalk Robotics Ltd., Israel)9-13, ‘HAL’ (Cyberdyne, Inc., Japan)14-16, ‘Ekso™’ (Ekso™ Bionics, USA)17-18, ‘Indego®’ (Parker Hannifin, USA)19-24 (Figure 1). There is also a research grade powered exoskeleton called the H2 developed to assist individuals post-stroke but also claimed to be capable of assisting individuals with SCI25. Robotic exoskeletons generally use battery-powered electric motors at each hip and knee joint to move the lower extremities through the proper trajectory to produce ambulation. A joystick, control pad, wrist watch, or preprogrammed motions such as a weight shift from forward lean are used to perform the user-selected activity.
Figure 1.
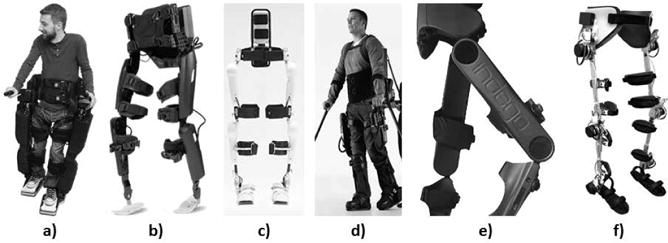
a) Rex Bionics, b) ReWalk™, c) HAL, d) Ekso™, e) Indego®, f) H2.
Rex
Rex is a robotic walking device that is self-supporting and independently controlled to enable a user to perform basic functions such as stand up, walk, sit down, ascend and descend stairs, and turn without the need for crutches or a walker (Figure 1a)7. During static standing, the hands can be free for the user to perform tasks such as reaching at a counter. The robotic device can be used by individuals with a complete SCI up to the C4/C5 level. Rex has not undergone clinical trials but preliminary research has been performed to combine the Rex robotic device with electroencephalography signals. By combining Rex with the electroencephalography-based brain machine interface capabilities, researchers aim to interpret user intent to assist an impaired individual to walk independently without the need for external control8. There has not been any published research on gait outcomes from the Timed Up and Go (TUG) test, 10-meter walk test (10MWT), or 6-minute walk test (6MWT).
ReWalk™
ReWalk™ is a powered exoskeleton that can restore independent gait with the use of forearm crutches for individuals with thoracic-level (T7) to lumbar-level (L5) SCI (Figure 1b)9. ReWalk™ is the only exoskeleton currently approved by the FDA for use at home and in the community with a trained companion, and one of the two exoskeletons approved by the FDA for therapeutic purposes in medical settings under close supervision. The exoskeleton includes motors at each hip and knee joint, a battery unit, computer-based closed-loop controller, and various sensors to measure upper-body tilt angle and joint angles. The hip and knee joints are hinged and the ankle joints are articulated with a spring-assisted dorsiflexion. Activity modes of stand, sit, or start walking can be selected with a wristwatch controller by the user. During walking, the user initiates a step with the forward upper-body movement detected by a tilt sensor, at which point the joint motors will move through a predefined joint trajectory to complete the step.
Clinical trials have evaluated the mobility outcomes such as gait speed, maximum walking distance, Timed Up and Go (TUG) test, 10-meter walk test (10MWT), and 6-minute walk test (6MWT) when using the ReWalk™. Ten male participants with injury levels ranging from cervical level 8 (C8) to lumbar level 1 (L1) have been trained to walk with the ReWalk™ exoskeleton10. After one hour of exoskeleton training session twice a week, for a 10-week training period, the majority of participants were able to walk at gait speeds ranging from 0.25-0.48 m/s, walk longer distances ranging from 91-170 m (increased by an approximate average of 23 m from mid-training), and be quicker in standing up, rotating and sitting down (decreased TUG test time by an approximate average of 9s). Twelve individuals with SCI have been trained to walk without human assistance using the ReWalk™ and evaluated with the mobility measures. Gait speeds ranged from 0.03 to 0.45 m/s (average of 0.25 m/s) and walking distances during the 6MWT ranged from 10.8 to 150.4 m [12-13].
HAL
The Hybrid Assistive Limb (HAL) is a powered exoskeleton that was designed to augment nondisabled individuals in their activities, physically support users performing heavy work, and assist gait for individuals with incomplete SCI or who have paralysis due to a stroke (Figure 1c) 14. The robot suit HAL includes motors at each hip and knee joint, a passive spring at each ankle for dorsiflexion bias, controller computer unit, batteries, bioelectrical sensors, angular sensors, acceleration sensors, and floor reaction force sensors. The bioelectrical sensors detect minimal electromyography signals from the extensor and flexor muscles of the hip and knee, which can be used to indicate a user's intent to take a step when walking with HAL.
For users who have impaired walking, HAL uses an autonomous controller based on healthy walking to provide the necessary assistance at the hip and knee joints to move the lower extremities through the appropriate trajectory for ambulation. There is also a cable connection between the exoskeleton and the user, which would allow voluntary robotic supported range of motion. HAL has been evaluated as a tool for rehabilitation for those with chronic incomplete SCI15. Eight participants (injury levels ranging from T7-L3) were trained with the exoskeleton HAL for body weight supported treadmill walking at variable gait speeds and with varying levels of body weight support. After 90 days (five days a week) of training, individuals with incomplete SCI walked at an average speed of 0.50 ± 0.34 m/s with the device as compared to 0.28 ± 0.28 m/s before the training. Walking distances achieved using HAL in the 6MWT before the training was 70.1 ± 130 m and after the training was 163.3 ± 160.6 m, where the distance after training was significantly different from the distance before training15.
Ekso™ Bionics
The Ekso™ Bionics exoskeleton enables individuals with weakened or impaired lower extremities to stand up and walk over ground using an assistive device such as forearm crutches or a walker (Figure 1d)17. The Ekso™ is the second of the two exoskeletons approved by the FDA for therapeutic purposes in medical settings under close supervision. The exoskeleton has battery-powered motors at each hip and knee joint that drives the legs through the proper step pattern and a passive spring for dorsiflexion at the ankle joint, which can provide rehabilitation, over ground gait training, and upright, weight-bearing exercise. Walking is initiated by the user appropriately shifting the upper body. After 24 weekly sessions of training, seven participants with SCI (two with tetraplegia and five with motor-complete injuries) were able to stand, walk, and sit using the Ekso™ 18. The participants were able to walk with average speeds ranging from 0.11 to 0.21 m/s and were able to walk for times ranging from 28 to 94 minutes.
Indego®
Indego® is a powered lower extremity orthosis that uses motors at the hip and knee joints to move the user's joints through a prescribed range of motion for walking based on a set of normal biomechanical walking trajectories (Figure 1e)19. The knee motors have electrically controllable normally locked brakes that will lock the knee joints in the event of a power failure. Standard ankle-foot orthoses can be worn with the Indego® exoskeleton. An assistive device, like forearm crutches or a walker, is used for balance and stability. Joint angle sensors are included at the hip and knee joints, and accelerometers are located in each thigh segment. A tilt sensor in the thoracic piece determines whether the user wants to stand up, sit down, or initiate walking by leaning forward or leaning backward. The Indego® is currently undergoing clinical trials.
Participants trained in walking with the Indego® exoskeleton have been shown to have a mean walking speed of 0.22 m/s, with speeds ranging from 0.22-0.45 m/s depending on the participants' level of injury20-22. Based on the 6MWT, individuals using the Indego® have been shown to have walking distances ranging from 64-121 m depending on the participants' level of injury, where participants with higher levels of injury walked the shorter distances and participants with lower levels of injury walked the longer distances20. Indego® also estimates that a user would be able to walk a range of 800 m if walking at 0.22 m/s, based on the electrical power measurements of the batteries21-22. TUG test measures have not been reported for the Indego®.
The Indego® has been evaluated for stair ascent and descent with one individual with paraplegia (T10 complete injury level), who was able to successfully ascend and descend a set of steps while using upper body effort on handrails23. Research has also been performed on cooperative control of neuromuscular stimulation with the Indego® powered exoskeleton24. Three subjects with paraplegia (T6-T10 complete) walked with stimulation of the hip and knee extensors while the exoskeleton motors generated hip and knee flexion at the appropriate time during the gait cycle. The cooperative control of neuromuscular stimulation with the powered exoskeleton showed consistent and repeatable gait trajectories, as well as reduced the required torque and power output of the motors compared to walking without neuromuscular stimulation.
H2
H2 is a lower limb exoskeleton that can be used for over ground gait rehabilitation training with an assistive device such as forearm crutches (Figure 1f)25. The device weighs 12kg and has six actuated joints, with the hip, knee, and ankle joints all fitted with electric motors, a battery pack, joint angle and velocity sensors, and sensors to measure force and torque interaction between the user's limbs and the exoskeleton, and foot switches to measure contact between the user's feet and the ground. Unlike the other exoskeletons that use trajectory control for the strategy to restore the user's gait, the H2 algorithm uses a combination of trajectory control and an interaction torque between the subject and the exoskeleton to generate an adaptive reference for the gait assistance that only assists as needed25. The H2 system has been tested with three participants with post-stroke, though the researchers claim that the system can also be used for individuals with incomplete SCI. Two of the three subjects showed slight improvements in the 6MWT and TUG test.
Challenges
While these commercially available and research grade powered exoskeletons are able to restore walking motion at speeds effective for household ambulation, walking speeds and distances when using these devices are still less than what is defined as community ambulation. A full community ambulator is defined as someone able to maintain a speed of 0.8 m/s, while a limited community ambulator is able to walk a speed of 0.4 m/s26. The required velocity to cross a road safely is considered to be approximately 1.06 m/s27-28. It is estimated that the walking distance of 230-342 m for some activities such as supermarket shopping is necessary for full community ambulation28.
The walking speeds reported for Ekso™ (0.11-0.21 m/s), ReWalk™ (0.25-0.48 m/s), HAL (0.50 ± 0.34 m/s), and Indego® (0.22-0.51 m/s) are all on average (0.26 m/s) less than half the established and commonly accepted threshold of 0.8 m/s for full community ambulation26, 29. Based on the 6MWT, ReWalk™ has been shown to have walking distances ranging from 10.8-170 m10-13. Indego® has been shown to have walking distances ranging from 64-121 m depending on the participants' level of injury20. Indego® also estimates that a user would be able to walk a range of 800m if walking at 0.22 m/s, based on the electrical power measurements of the batteries21-22. Ekso™ does not report any walking distances. Indego® has the potential to achieve the 342.0 m for community ambulation because of the battery life and assuming the user is conditioned to complete the distance. Maximum walking distances reported for these powered exoskeletons range from 121 to 171 m, approximately half the distances assumed to be functional in the community, where functional distances can be over 500 m for certain tasks27, 28. The robotic exoskeletons can be effective at helping “non-ambulators” become “household ambulators”, but are inadequate for unstructured community environments that involve other pedestrians or automobile traffic.
Alternative Approaches
An alternative approach taken to restore walking for individuals with paraplegia is neuromuscular stimulation. Stimulation can produce a majority of the torque required to move or stabilize the lower extremities against collapse, enabling most users of a surface stimulation system or an implanted neuroprosthesis to stand and initiate stepping. There have been anecdotal and subjective improvements in pain, spasticity, and bowel and bladder function when assuming an upright posture, passively moving the joints, and exercising the arms and torso with the powered exoskeletons30-31. However, this does not take advantage of the individual's own muscle power and the added exercise benefits that can be gained by stimulating the paralyzed muscles. Use of a stimulation system (Parastep®) has been shown to reduce muscle spasticity, increase muscle mass and blood flow in the lower extremities, and result in psychological benefits such as enhanced self-image and decreased incidence of depression4, 6, 32. With the implanted neuroprostheses developed in our laboratory applying neuromuscular stimulation to the appropriate lower extremity muscles, gait speeds can range from 0.5 to 0.9 m/s over maximal distances of 300-400 m33-35. Gait speeds and distances vary between subjects, but approach the accepted benchmark for unrestricted community ambulation of 0.8 m/s26 and the 1.06 m/s gait speed considered necessary to safely cross an intersection, as well as the 230-342 m distance for activities such as supermarket shopping27, 28.
Even though walking with neuromuscular stimulation approaches the accepted gait speeds and distances required for full community ambulation, the lower extremity muscles of the neuroprosthesis users can rapidly fatigue. Implanted neuromuscular stimulation that actively generates joint moments by contracting the otherwise paralyzed muscles has been successfully integrated with passive hydraulic exoskeleton orthotic constraints to mechanically support the user's body weight in a hybrid neuroprosthesis (HNP) that enables individuals with SCI to stand, walk, negotiate stairs, and perform a controlled stand-to-sit transition (Figure 2). In one design, a variable hip constraint mechanism can lock, free, or reciprocally couple the hips, while a dual state knee mechanism locks the knee during stance and frees the knee during swing phase36-38. State-based control of the mechanisms and modulation of neuromuscular stimulation patterns targeting hip and knee muscles during gait have been developed that use pressure and position sensors to implement real-time control of the HNP39.
Figure 2.
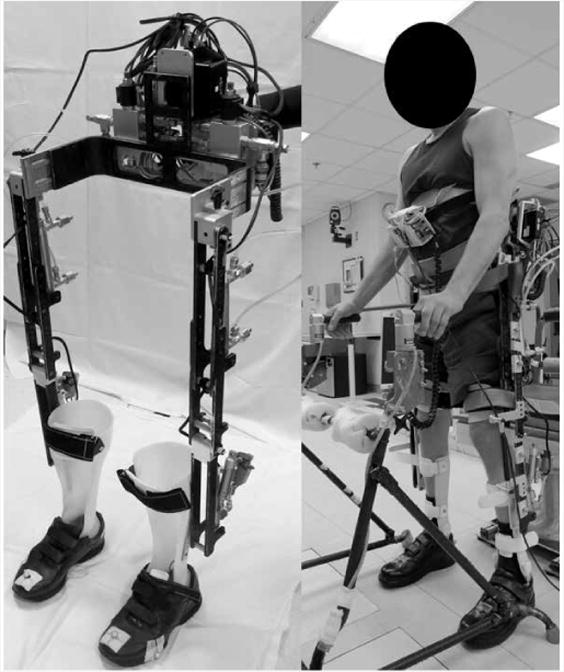
Hybrid neuroprosthesis (HNP) that coordinates neuromuscular stimulation with passive orthotic constraints.
In preliminary clinical tests, the combination of neuromuscular stimulation and orthotic constraints increased walking speed by almost 15% compared to conventional reciprocal gait orthoses. The hybrid approach also reduced stimulation duty cycle by more than two thirds as compared to walking with stimulation only, potentially delaying the onset of fatigue and extending walking distance36-38. A variable impedance knee mechanism combined a fluid damper with a linkage transmission to provide sufficient knee stiffness to support a user and substitute for eccentric contractions of the knee extensor muscles during stance phase of gait, while minimizing knee impedance during swing. Damping the knee during stance phase reduces impact at loading and maintains forward progression during gait40-42. When negotiating stairs, the damper assisted in regulating lowering speeds during descent and reduced the reliance on upper limbs to approximately 40-45% body weight as compared to 70% body weight measured when descending stairs with stimulation alone43.
Control of the knee during the stand-to-sit transition for individuals with paraplegia has been improved with the implementation of kinematic and kinetic orthotic constraints. A coupling mechanism was designed to coordinate the hip and knee joints, and a damping mechanism was designed to keep a constant knee angular velocity during the transition. Use of these orthotic mechanisms improved the overall coordination between the hip and knee joints for individuals with paraplegia, causing the joints to approach the 1:1 coupling ratio seen in nondisabled individuals. The upper limb forces on the walker were reduced by 70% when sitting down with both the coupling and damping mechanisms as compared to sitting with only stimulation. Similarly, the impact force when making contact with the seating surface was reduced by half for individuals with SCI sitting down with the coupling and damping mechanisms as compared to sitting with stimulation alone44-45. By reducing upper limb and impact forces with the orthotic mechanisms, the potential for injuries during the stand-to-sit transition can be decreased.
Conclusion
The ability to restore gait for individuals with paraplegia has improved with progress in various powered exoskeletons and neuromuscular stimulation technologies. The powered exoskeletons are able to restore the stand up, sit down, and walking motions. However, they have limits in achievable gait speeds and distances. Neuromuscular stimulation has been shown to allow users to approach the gait speeds and distances for full community ambulation. As advancements through research in these technologies continue to be made, the intersection of powered exoskeletons and neuromuscular stimulation is foreseeable in the next steps to creating a commercial-grade ambulatory assist system that requires less effort of the user and provides more consistent results, while capable of interacting in the home and community environments and at the same time providing tremendous health benefits to the user.
Acknowledgments
S.R. Chang was supported by the Training Program in Musculoskeletal Research Grant 5T32AR007505-28.
References
- 1.National Spinal Cord Injury Statistical Center; Birmingham, Alabama: Feb, 2013. Spinal cord injury facts and figures at a glance. < https://www.nscisc.uab.edu/PublicDocuments/fact_figures_docs/Facts%202013.pdf>. [Google Scholar]
- 2.Christopher and Dana Reeve Foundation; Short Hills, New Jersey: One degree of separation: paralysis and spinal cord injury in the United States. < http://www.christopherreeve.org/site/c.mtKZKgMWKwG/b.5184255/k.6D74/Prevalence_of_Paralysis.htm>. [Google Scholar]
- 3.Anderson KD. Targeting Recovery: Priorities of the Spinal Cord-Injured Population. J of Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- 4.Nash MS, Jacobs PL, Montalvo BM, Klose KJ, Guest RS, Needham-Shropshire BM. Evaluation of a training program for persons with SCI paraplegia using the Parastep 1 ambulation system: part 5. Lower extremity blood flow and hyperemic response to occlusion are augmented by ambulation training. Arch Phys Med Rehabil. 1997;78(8):808–814. doi: 10.1016/s0003-9993(97)90192-1. [DOI] [PubMed] [Google Scholar]
- 5.Graupe D, Kohn KH. Functional neuromuscular stimulator for shortdistance ambulation by certain thoracic-level spinal-cord-injured paraplegics. Surg Neurol. 1998;50(3):202–207. doi: 10.1016/s0090-3019(98)00074-3. [DOI] [PubMed] [Google Scholar]
- 6.Guest RS, Klose KJ, Needham-Shropshire BM, Jacobs PL. Evaluation of a training program for persons with SCI paraplegia using the Parastep 1 ambulation system: part 4. Effect on physical self-concept and depression. Arch Phys Med Rehabil. 1997;78(8):804–807. doi: 10.1016/s0003-9993(97)90191-x. [DOI] [PubMed] [Google Scholar]
- 7.Rex Bionics – Step into the Future. 2015 Nov 18; < http://www.rexbionics.com/>.
- 8.Contreras-Vidal JL, Grossman RG. NeuroRex: a clinical neural interface roadmap for EEG-based brain machine interfaces to a lower body robotic exoskeleton. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:1579–1582. doi: 10.1109/EMBC.2013.6609816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ReWalk™. 2015 Nov 18; < http://rewalk.com/>.
- 10.Benson I, Hart K, Tussler D, van Middendorp JJ. Lower-limb exoskeletons for individuals with chronic spinal cord injury: Findings from a feasibility study. Clin Rehabil. 2015:1–12. doi: 10.1177/0269215515575166. [DOI] [PubMed] [Google Scholar]
- 11.Yang A, Asselin P, Knezevic S, Kornfeld S, Spungen AM. Assessment of In-Hospital Walking Velocity and Level of Assistance in a Powered Exoskeleton in Persons with Spinal Cord Injury. Top Spinal Cord Inj Rehabil. 2015;21(2):100–109. doi: 10.1310/sci2102-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esquenazi A, Talaty M, Packel A, Saulino M. The ReWalk powered exoskeleton to restore ambulatory function to individuals with thoracic-level motor-complete spinal cord injury. Am J Phys Med Rehabil. 2012;91(11):911–921. doi: 10.1097/PHM.0b013e318269d9a3. [DOI] [PubMed] [Google Scholar]
- 13.Zeilig G, Weingarden H, Zwecker, Dudkiewicz I, Bloch A, Esquenazi A. Safety and tolerance of the ReWalk™ exoskeleton suit for ambulation by people with complete spinal cord injury: A pilot study. J Spinal Cord Med. 2012;35(2):96–101. doi: 10.1179/2045772312Y.0000000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.HAL – Cyberdyne. 2015 Nov 18; < http://www.cyberdyne.jp/english/products/HAL/>.
- 15.Aach M, Cruciger O, Sczesny-Kaiser M, Höffken O, Meindl RCh, Tegenthoff M, Schwenkreis P, Sankai Y, Schildhauer TA. Voluntary driven exoskeleton as a new tool for rehabilitation in chronic spinal cord injury: a pilot study. Spine. 2014;14(12):2847–2853. doi: 10.1016/j.spinee.2014.03.042. [DOI] [PubMed] [Google Scholar]
- 16.Sankai Y. HAL: Hybrid Assistive Limb Based on Cybernics. Springer Tracts in Advanced Robotics. In: Kaneko M, Nakamura Y, editors. Robotics Research. Vol. 66. 2011. pp. 25–34. [Google Scholar]
- 17.Ekso™ Bionics. 2015 Nov 18; < http://eksobionics.com/>.
- 18.Kozlowski AJ, Bryce TN, Dijkers MP. Time and Effort Required by Persons with Spinal Cord Injury to Learn to Use a Powered Exoskeleton for Assisted Walking. Top Spinal Cord Inj Rehabil. 2015;21(2):110–121. doi: 10.1310/sci2102-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Indego®. 2015 Nov 18; < http://www.indego.com/indego/en/home>.
- 20.Hartigan C, Kandilakis C, Dalley S, Clausen M, Wilson E, Morrison S, Etheridge S, Farris R. Mobility outcomes following five training sessions with a powered exoskeleton. Top Spinal Cord Inj Rehabil. 2015;21(2):93–99. doi: 10.1310/sci2102-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quintero H, Farris R, Goldfarb M. Control and Implementation of a Powered Lower Limb Orthosis to Aid Walking in Paraplegic Individuals. IEEE Int Conf Rehabil Robot. 2011 doi: 10.1109/ICORR.2011.5975481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farris R, Quintero H, Goldfarb M. Preliminary Evaluation of a Powered Lower Limb Orthosis to Aid Walking in Paraplegic Individuals. IEEE Trans Neural Syst Rehabil Eng. 2011 Dec;19(6):652–659. doi: 10.1109/TNSRE.2011.2163083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farris RJ, Quintero HA, Goldfarb M. Performance evaluation of a lower limb exoskeleton for stair ascent and descent with paraplegia. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:1908–1911. doi: 10.1109/EMBC.2012.6346326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ha KH, Murray SA, Goldfarb M. An approach for the cooperative control of FES with a powered exoskeleton during level walking for persons with paraplegia. IEEE Trans Neural Syst Rehabil Eng. 2015;99:1–12. doi: 10.1109/TNSRE.2015.2421052. [DOI] [PubMed] [Google Scholar]
- 25.Bortole M, Venkatakrishnan A, Fangshi Z, Moreno JC, Francisco GE, Pons JL, Contreras-Vidal JL. The H2 robotic exoskeleton for gait rehabilitation after stroke: early findings from a clinical study. J NeuroEng Rehabil. 2015;12:54. doi: 10.1186/s12984-015-0048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perry J, Garrett, Gronley J, Mulroy S. Classification of Walking Handicap in the Stroke Population. Stroke. 1995;26:982–989. doi: 10.1161/01.str.26.6.982. [DOI] [PubMed] [Google Scholar]
- 27.Lapointe R, Lajoie Y, Serresse O, Barbeau H. Functional community ambulation requirements in incomplete spinal cord injured subjects. Spinal Cord. 2001;39(6):327–335. doi: 10.1038/sj.sc.3101167. [DOI] [PubMed] [Google Scholar]
- 28.Robinett CS, Vondran MA. Functional ambulation velocity and distance requirements in rural and urban communities. A clinical report. Phys Ther. 1988;68(9):1371–1373. doi: 10.1093/ptj/68.9.1371. [DOI] [PubMed] [Google Scholar]
- 29.Louie DR, Eng JJ, Lam T. Gait speed using powered robotic exoskeletons after spinal cord injury: a systematic review and correlational study. J Neuroeng Rehabil. 2015;12:82. doi: 10.1186/s12984-015-0074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolakowsky-Hayner SA, Crew J, Moran S, Shah A. Safety and feasibility of using the Ekso™ bionic exoskeleton to aid ambulation after spinal cord injury. J Spine. 2013;S4:003. [Google Scholar]
- 31.Kressler J, Thomas CK, Field-Fote EC, Sanchez JC, Widerstrom-Noga E, Cilien DC, Gant K, Ginnety K, Gonzalez H, Martinez A, Anderson KD, Nash MS. Lower limb bionic exoskeleton for rehabilitation, exercise or mobility? Exploratory case series in persons with chronic, complete spinal cord injury; American Spinal Cord Injury (ASIA) Annual Scientific Meeting; San Antonio, TX. 2014; [DOI] [PubMed] [Google Scholar]
- 32.Brissot R, Gallien P, Le Bot MP, Beaubras A, Laisne D, Beillot J, Dassonville J. Clinical experience with functional electrical stimulation-assisted gait with Parastep in spinal cord-injured patients. Spine. 2000;25(4):501–508. doi: 10.1097/00007632-200002150-00018. [DOI] [PubMed] [Google Scholar]
- 33.Kobetic R, Marsolais EB. Synthesis of paraplegic gait with multichannel functional neuromuscular stimulation. IEEE Trans Rehabil Eng. 1994;2(2):66–79. [Google Scholar]
- 34.Marsolais EB, Kobetic R. Development of a practical electrical stimulation system for restoring gait in the paralyzed patient. Clin Orthop Relat Res. 1988;(233):64–74. [PubMed] [Google Scholar]
- 35.Kobetic R, Triolo R, Marsolais EB. Muscle Selection and Walking Performance of Multichannel FES Systems for Ambulation in Paraplegia. IEEE Trans Rehabil Eng. 1997;5(1):23–29. doi: 10.1109/86.559346. [DOI] [PubMed] [Google Scholar]
- 36.To C, Kobetic R, Bulea TC, Audu ML, Schnellenberger J, Pinault GC, Triolo R. Sensor-based hip control with a hybrid neuroprosthesis for walking in paraplegia. J Rehabil Res Dev. 2014;51(2):229–244. doi: 10.1682/JRRD.2012.10.0190. [DOI] [PubMed] [Google Scholar]
- 37.To C, Kobetic R, Bulea TC, Audu ML, Schnellenberger JR, Pinault G, Triolo RJ. Sensor-based stance control with orthosis and functional neuromuscular stimulation for walking after spinal cord injury. Journal of Prosthetics and Orthotics. 2012;24(3):124–132. [Google Scholar]
- 38.To CS, Kobetic R, Bulea T, Audu M, Schnellenberger J, Pinault G, Triolo RJ. Stance control knee mechanism for lower extremity support in a hybrid neuroprosthesis. J Rehabil Res Dev. 2011;48(7):839–850. doi: 10.1682/jrrd.2010.07.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kobetic R, To CS, Schnellenberger JR, Audu ML, Bulea TC, Gaudio R, Pinault G, Tashman S, Triolo RJ. Development of hybrid orthosis for standing, walking, and stair climbing after spinal cord injury. J Rehabil Res Dev. 2009;46(3):447–462. [PubMed] [Google Scholar]
- 40.Bulea TC, Kobetic R, Audu ML, Schnellenberger JR, Pinault G, Triolo RJ. Stance phase knee flexion improves stimulation driven walking after spinal cord injury. J Neuroeng Rehabil. 2013;10(68) doi: 10.1186/1743-0003-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bulea TC, Kobetic R, Audu ML, Schnellenberger JR, Triolo RJ. Finite state control of a variable impedance hybrid neuroprosthesis for locomotion after paralysis. IEEE Trans Neural Syst Rehabil Eng. 2013;21(1):141–151. doi: 10.1109/TNSRE.2012.2227124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bulea TC, Kobetic R, To CS, Audu M, Schnellenberger J, Triolo RJ. A variable impedance knee mechanism for controlled stance flexion during pathological gait. IEEE/ASME Transactions on Mechatronics. 2012;17(5):822–832. [Google Scholar]
- 43.Bulea TC, Kobetic R, Audu MS, Schnellenberger JR, Pinault G, Triolo RJ. Forward stair descent with a hybrid neuroprosthesis after paralysis: a single case study demonstrating feasibility. J Rehabil Res Dev. 2014;51(7):1077–1094. doi: 10.1682/JRRD.2013.12.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang SR, Kobetic R, Triolo RJ. Understanding stand-to-sit maneuver: implications for motor system neuroprostheses after paralysis. J Rehabil Res Dev. 2014;51(9):1339–1351. doi: 10.1682/JRRD.2013.12.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang SR, Nandor MJ, Kobetic R, Foglyano KM, Quinn RD, Triolo RJ. Improving stand-to-sit maneuver for individuals with spinal cord injury by controlling the knee with a hybrid neuroprosthesis. J Neuroeng Rehabil. 2015 Oct; doi: 10.1186/s12984-016-0137-6. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]


