Abstract
Background
Fronto-limbic regions of the brain including the sub-genual (sgPFC) and medial prefrontal (mPFC) cortices are central to processing emotionally salient and hedonic stimuli (Mayberg, 2009; Sescousse et al., 2010) and implicated in depression. The relevance of cortico-limbic models of emotion and reward processing in children with genetic risk for psychiatric disorders has not been assessed.
Methods
Here we studied adolescents at risk for schizophrenia (HRS) and controls (HC) using an event-related fMRI continuous affective appraisal task. HRS were divided into sub-groups based on the presence or absence of negative symptoms (Miller et al., 2003), HRS_NS+ and HRS_NS− respectively. Brain responses to positive, negative and neutral emotional stimuli were estimated.
Results
Consistent with observations in the depressive phenotype, for positively valenced stimuli, HRS_NS+ (relative to HC and HRS_NS−) were characterized by hypo-responsivity of the sgPFC and the mPFC, but hyper-responsivity of the mid-brain. sgPFC and mPFC signals were coupled across groups.
Limitations
Such studies can benefit from larger sample sizes, though our observed effect sizes were in the moderate to large range.
Conclusions
Children and adolescents at risk for psychiatric illness and who evince reliably present negative symptoms show brain responses to socially rewarding stimuli similar to those observed in depression. Studies in at-risk children and adolescents may be important in understanding how early manifestations of depression-like characteristics impact brain function.
Keywords: Adolescence, fMRI, Risk, Negative Symptoms, Social Reward
INTRODUCTION
Behavioral phenotypes associated with negative symptoms and anhedonia cut across diagnostic boundaries and have been associated with both depression and negative symptom related schizophrenia (Bottlender et al., 2003; Kitamura and Suga, 1991; Kulhara and Chadda, 1987). In the adolescent pre-morbid phase, sub-groups at-risk for schizophrenia such as offspring of schizophrenia patients (HRS) show an increased incidence of anhedonia and negative symptoms often leading to an impaired ability to feel pleasure. In general, anhedonia predicts schizophrenia-spectrum disorders and social dysfunction (Kwapil, 1998), and is thought to lead to social withdrawal. In turn, social withdrawal during adolescence is an important predictor of the eventual emergence of schizophrenia in young adulthood (Cannon et al., 2008).
Though the psychosocial results are compelling, the neural correlates of anhedonia and negative symptoms in at risk adolescents have not generally been investigated. Studying the relationship between negative symptoms and the fMRI response to socially rewarding stimuli in HRS can prove informative, providing plausible insights into the impact of genetic risk in neurodevelopment (Lewis and Levitt, 2002), and the relationship between clinical features and brain responses in the domain of social reward processing. Recent work has highlighted the importance of linking emergent clinical symptoms with fMRI markers in HRS: for example, it is specifically HRS with deficits in pre-morbid function who show impairments in the engagement of cortico-striatal attention systems relative to HRS who do not show clinical deficits (Diwadkar et al., In Press-c). Thus, if fMRI is sensitive to pre-morbid clinical symptoms, it may help identify emergent dysfunction in mood circuitry in HRS with (or without) negative symptoms. Furthermore, by linking clinical presentation with fMRI data, such approaches can further sharpen neurodevelopmental hypotheses of schizophrenia (Keshavan et al., 2005; Murray et al., 2004).
Negative Symptoms in the Schizophrenia and Mood Diathesis
Negative symptoms comprising of asociality, avolition, flat affect, anhedonia, and alogia are prevalent among relatives of schizophrenia patients and adolescent schizophrenia probands (Dworkin and Lenzenweger, 1983, 1984; Kendler et al., 1982; Tsuang et al., 1999). Negative symptoms appear to manifest early and may reflect an inherited vulnerability to schizophrenia and schizophrenia spectrum disorders. They predict poorer quality of life, social functioning, interpersonal relationships, work performance, and overall outcome in patients, but also appear early in the premorbid phase and predict a poorer illness course (Stahl and Buckley, 2007).
In general, studies of the relevance of negative symptoms in the schizophrenia diathesis are related to studies in depression (Kitamura and Suga, 1991). Thus 32-77% of patients with major depression have negatives symptoms (Winograd-Gurvich et al., 2006) with withdrawal, altered emotional experience, anhedonia, avolition, and deteriorated role functioning particularly characteristic of depression. The shared quality of negative symptoms in depression and schizophrenia may relate to an inability to effectively process rewarding stimuli such as positively valenced faces. Regions of the brain including the sub-genual and medial prefrontal cortices display functional abnormalities in depressed patients with anhedonia (Davidson et al., 2002; Drevets et al., 2008; Lemogne et al., 2010; Ongur et al., 1998; Pizzagalli et al., 2004). Many of these critical regions evince a pattern of dense connectivity that sub-serves their roles in affective processing. For example, the subgenual prefrontal cortex connects with emotion and reward processing areas such as the amygdala, dorsal raphe, orbitofrontal, cingulate cortices, amygdala, nucleus accumbens, and hippocampus (Freedman et al., 2000; Mayberg, 2009). Additionally, this region receives rich dopamanergic innervations from the ventral tegmental area (VTA), a reward processing center (Pizzagalli et al., 2004), and the failure to maintain the tonic dopamine related activity in this area is thought to underlie anhedonia (Drevets et al., 1997).
Many of these interactions have been captured in well articulated models of cognitive and emotional dysfunction seen in depression (Mayberg, 2009; Mayberg et al., 1999)(See Supplementary Figure 1). In this framework, dorsal-cortical areas correlate with cognitive and attention tasks, whereas the ventral-limbic areas are associated with the generation of mood states, and influence autonomic responses to affective stimuli. Interacting with the dorsal and ventral areas are sub-cortical and medial frontal regions. The sub-cortical regions act primarily to process salience, and the medial prefrontal regions modulate regulation of mood states. Disruption of one node within the circuit can result in secondary adaptive or maladaptive changes occur in other nodes comprising the circuit. Altered activity within the circuit leads to abnormal mood states and behavior associated with depression. Further, functional deficits in one region of the circuit might lead to dysmodulation and/or compensatory changes in other regions of the circuit.
Using this model as an exploratory framework, we investigated the response of these key cortico-limbic regions during an affective appraisal paradigm. Differences in the fMRI response to positive, negative and neutrally valenced stimuli were examined between HRS with negative symptoms (henceforth, HRS_NS+), without negative symptoms (henceforth, HRS_NS−) and controls with no family history of psychiatric illness to the 2nd degree (HC). Given the known processing bias toward socially rewarding stimuli that is associated with healthy subjects, and typically reversed in the context of negative symptoms and depression (Adolphs, 2009; Victor et al., 2010), we expected abnormal activity particularly to socially rewarding stimuli in HRS_NS+ in areas shown to be central to reward and affective processing. These regions included the sub-genual prefrontal cortex (Drevets et al., 1998), the medial prefrontal cortex (Lemogne et al., 2010), and the midbrain (Kumar et al., 2008).
METHODS
Subjects
Forty one subjects gave informed consent to participate in the fMRI studies. MRI and behavioral protocols were approved by the Human Investigative Committee (HIC) of Wayne State University. Subjects received monetary compensation for their participation. The twenty two HC (age:10-20, mean=14.76 yrs; 8 females) had no family history of psychiatric illness to the 2nd degree. Nineteen HRS had at least one parent with schizophrenia (age:10-20, mean=14.71 yrs; 5 females). Subjects were recruited from the greater Detroit area through advertisements and through in patient services at the University Physicians Center, Wayne State University School of Medicine. Rule outs were achieved through telephone and personal interview, and screening questionnaires, to ascertain if subjects had a history of psychotic illness in first-degree relatives. Diagnoses for parents of offspring were reached using the Structured Clinical Interview for DSM-IV schizophrenia (First et al., 1997). Subjects younger than 15 years were clinically evaluated using the Schedule for Affective Disorders and Schizophrenia -Child Version (K-SADS)(Kaufman et al., 1997); those aged 15 years or above were assessed using the SCID. Assessments were administered by trained interviewers under the supervision of a child psychiatrist.
Behavioral Task
During fMRI subjects observed sequentially presented normatively rated (Ekman and Oster, 1979) photographs depicting positive (happy), negative (angry, fearful, sad) and neutral emotions. To ensure that subjects processed the stimuli (and did not simply view them passively), they were required to appraise the affective consistency between successively presented stimuli. Stimuli were presented (3s/stimulus) in pseudo-random order with a randomly jittered inter-stimulus interval (3-5 s. in 0.5s increments) . Control stimuli were created by inverting and pixilating face pictures; these stimuli were used to provide a baseline to assess the effects of visual stimulation.
Functional MRI
Gradient echo EPI was acquired over an a full body Bruker MedSpec 4.0T system (TR: 2s, TE: 30 ms, matrix: 64x64, 24 slices, FOV: 240 mm, voxel size 3.8×3.8×4.0 mm) with an 8 channel head coil. Functional images were preprocessed using a standard protocol in SPM5. Raw images were realigned with the AC-PC orientation following which images in the series were co-registered with the first image and correction for susceptibility-by-movement interactions. Co-registered images were normalized to a standard EPI template (Montreal Neurological Institute) using 12-point affine transformation. Individual stimulus presentations were modeled as 3s box-car events convolved with a canonical hemodynamic reference wave form. For random fields analyses, normalized images were smoothed with an 8 mm full width at half maximum (FWHM) isotropic Gaussian kernel. Individual first-level contrasts were employed to examine main effects of valence (Positive, Neutral, Negative vs. Distorted images). First level contrasts were submitted to second level analyses of covariance with group (see below) as single factor, and age and gender as covariates. Cluster level significance (p<.05)(Ward, 2000) was employed to identify clusters with significant differences in fMRI responses.
Assessing Negative Symptoms
Each subject was evaluated using the structured interview for prodromal syndromes/scale of prodromal symptoms (SIPS/SOPS)(Miller et al., 2003). The SOPS negative symptoms sub-scale consisting of six items (social isolation/withdrawal, avolition, decreased expression of emotion, decreased experience of emotion and self, decreased ideational richness, and deterioration in role functioning) was used to identify subjects with negative symptoms. Each item was scored based on symptom severity (0-6). The subscale rating is based on confidence that the subject displays symptoms (0: absent to 6:extreme)(Miller et al., 1999). To conservatively characterize negative symptoms, we only considered scores (across sub-scales) for which the rater unambiguously detected symptoms (i.e., excluding “absent” or “questionably present” ratings). Thus for any given sub-scale, a rating of “2” or greater (“Mild Symptoms” to more severe symptoms) was considered. As a result, schizophrenia offspring were divided into two sub-groups based on reliably present (n=7, HRS_NS+) or absent (n=12, HRS-NS−) negative symptoms. The sub-group assignments depicted in Figure 1a. The two HRS sub-groups and HC constituted the three levels of Group for the resultant fMRI analyses.
Figure 1.
(a) Pie-chart shows group membership across each of the HC, HRS_NS− and HRS_NS+ groups in the analyzed data. (b) As seen, sensitivity to task (assessed with d’) did not differ across groups (p>.5) indicating that groups remained on-task and inter-group differences were not driven by differences in cognitive performance.
RESULTS
Behavioral
Discrimination sensitivity on the task was assessed using d’ (Macmillan and Creelman, 2005). A univariate analyses of covariance with group as single factor and age and gender as covariates, revealed no significant differences in performance on the task between the three groups, F2,37=.62, p>.5, MSe=.408. A marginally significant effect of age was observed, F1,37=3.30, p<.05, one-tailed, driven by age-related increases in discrimination performance across groups.
fMRI
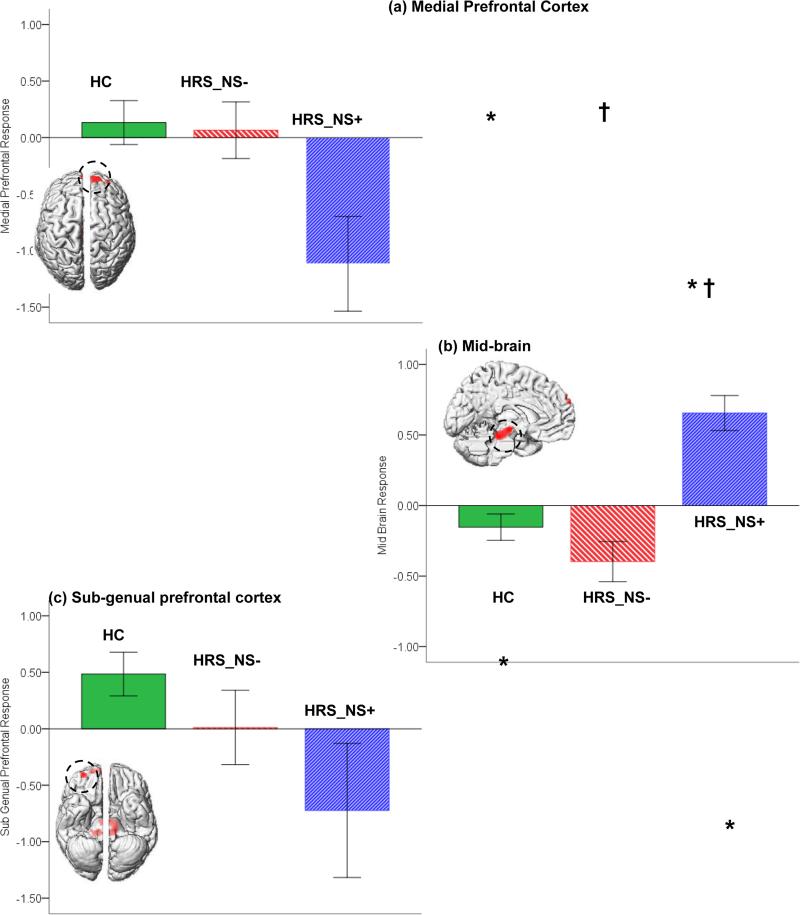
No significant clusters under the main effect of group were observed for the neutral or negatively valenced faces. For the positively valenced faces, three clusters of significance were observed in the mid-brain, the sgPFC and the medial frontal cortex (see Table 1). To investigate the direction of inter-group differences in these, we conducted analyses of co-variance on the estimated fMRI response under the significance peaks in each. The significant clusters under the main effect and the graphs depicting fMRI responses in each group are depicted in Figure 2 (a-c). As seen, markedly different trends were observed across the regions and across groups. In both sgPFC and the medial frontal cortex, HRS_NS+ evinced reduced responses relative to both HC and to HRS_NS−. By comparison, in the mid-brain cluster, HRS_NS+ with negative symptoms evinced increased responses to stimuli compared to controls and non-symptomatic offspring. These observations were confirmed by analyses of covariance in the mid-brain, F2,37 =12.81, p<.01, MSe=.19, the sgPFC, F2,37 =3.48, p<.05, MSe=1.24, and the medial prefrontal cortex, F2,37 =4.64, p<.02, MSe=.89. The differential pattern of responses across regions were confirmed in a significant Group x Region interaction in an omnibus repeated measures analysis of covariance (with Region of Interest as repeated measure), F4,74=7.6, p<.001, MSe=.546. The data in Figure 2 are visualized in a composite figure (Supplementary Figure 2) to emphasizethe highly complementary pattern of activation across regions and groups.
Table 1.
Significant clusters (Ward, 2000) under the overall main effect of group are listed. Results are pictorially depicted in Figure 2.
| Region | F statistic | p | Coordinates |
|---|---|---|---|
| Midbrain | 13.00 | 0.001 | −6, −36, −16 |
| Middle Prefrontal Cortex | 12.60 | 0.001 | 4, 60, 36 |
| Subgenual Prefrontal Cortex | 9.96 | 0.001 | 30, 52, −14 |
Figure 2.
Significant clusters under the overall main effect of group are depicted for responses to positively valenced stimuli. Clusters are projected to the dorsal surface for the medial prefrontal cortex (a), the medial surface for the mid-brain (b) and the ventral surface for the sub-genual prefrontal cortex (c)(see Table 1 for significance). Adjoining graphs depict estimated BOLD (% signal change) under the clusters of significance for each of the groups and each of the main effects (error bars are ± sem). As indicated, significant effects were observed in analyses of signal change data (See Results). Post-hoc comparisons (Sidak, 1967)(p<.05) revealed significant differences between HRS_NS+ and HC (mPFC, mid-brain and sgPFC; * in the Figure) and HRS_NS+ and HRS_NS− (mPFC and mid-brain; † in the Figure).
Across groups and subjects (n=41), statistical relationships between the BOLD response across regions were explored using parametric linear regression (Cohen, 1988). The only significant regression observed was between the mPFC and the sgPFC signal, (Pearson's r=.58, p<.001)(Table 2).
Table 2.
Parametric regression analyses between the fMRI response across the significant clusters in mPFC, the sgPFC and the midbrain are indicated. The linear regression analyses between the mPFC and the sgPFC signal was highly significant (see Figure 3a).
| sgPFC | Midbrain | |
|---|---|---|
| mPFC | r=.58, F1,39=19.27, MSe=.93, p<.001* | r=.13, F1,39=.64, MSe=.31, p>.4 |
| sgPFC | r=.04, F1,39=.06, MSe=.32, p>.75 |
This relationship between the mPFC and the sgPFC response was further investigated using spline interpolation (Hastie et al., 2001). The aim of these analyses was to characterize signal relationships across nodes irrespective of group membership using local curve-fitting methods. Spline interpolation is a useful approach as it provides a measure of local curve fitting across the entire range of data using piecewise polynomial fits thereby minimizing local interpolation error. The graph in Figure 3a represents the relationship between the mPFC and the sgPFC with the fitted curve representing the interpolated spline. As seen, a largely monotonically increasing relationship between the mPFC and the sgPFC response was observed across groups (r2=.37, SSe=34.75). The overlaid bivariate normal density ellipses to higjlight the densest portions of the intra-group distributions (p=.95)(SAS, 2009) on each group's data provide characterization of the form of the mPFC and sgPFC relationship across groups. As seen from the shaded density contours, this form is consistent across groups suggesting mPFC-sgPFC signal coupling, and consistent with the inter-digitated roles of these regions in the affective system. Juxtaposed against group differences (Fig 2a & 2c), these analyses suggest that the mPFC-sgPFC coupling in HRS_NS+ is intact but shifted to the left suggesting that at-risk adolescents with reliably present negative symptoms show hypo-functionality within both nodes in this circuit.
Figure 3.
fMRI response (across all subjects) in each of the mPFC and sgPFC (a) and mPFC and the midbrain (b) are plotted in scatterplots. As seen, across groups, the mPFC-sgPFC response is highly coupled (confirmed by significant parametric regression). Across groups, the mPFC-midbrain response is not statistically coupled. Within HRS, a marginally significant negative coupling was observed suggesting that within the risk group, decreases in the mPFC response were associated with increases in the midbrain response. The overlaid density ellipses denote the density of data within each group. The dotted lines represent the results of spline interpolation (see Methods).
Non-parametric correlations (Spearman's ρ) within HRS alone (n=19) were used to supplement parametric regression and spline interpolation across the entire sample, Within the HRS, these exploratory analyses provided intriguing evidence of complementary relationships between the mPFC and sgPFC, and the mPFC and the midbrain response. As with the overall parametric analyses, the mPFC – sgPFC was positively coupled (Spearman's ρ = .77, p<.001). By comparison, a marginally significant negative coupling between the mPFC and the midbrain was observed (Spearman's ρ = −.41, p<.05, one-tailed) suggesting that within HRS, the responses of these areas were negatively coupled. Both trends are discernable under the points subsumed by the density ellipses imposed on the HRS sub-groups in both Figures 3a and 3b (where the regression in the combined analyses, Table 2 was not significant).
DISCUSSION
The observed results from these analyses were:
-
a)
In response to a positive emotional probes (but not negative or neutral), at-risk children and adolescents with negative symptoms showed significantly decreased activation in the mPFC (Fig 2a) and the sgPFC (Fig 2c), but significantly increased activation in the midbrain (Fig 2b).
-
b)
Across groups, the mPFC and the sgPFC response was highly correlated with HRS_NS+ showing correlated by reduced responses in each region (Fig 3a).
-
c)
These results were independent of sensitivity to the primary task (Figure 1b), suggesting that the observed results are plausibly specific to the task's affective component.
To our knowledge this is the first study to demonstrate that the fMRI response in young subjects at risk for psychiatric disorders and who present with negative symptoms evince compelling similarities to depression. The data also provide suggestive evidence in support of the hypothesized modulatory effects of the mPFC on the sgPFC, and evidence of compensatory or aberrant hypo-activation of the mid-brain in the context of negative symptoms. The specific linkage between negative (or anhedonic symptoms) and positively valence or socially rewarding stimuli is particular compelling. The interpretation of these results (and any results of healthy subjects in a “risk” state) constitutes a conceptual challenge, as their relevance must be considered based on dimensional relevance, as opposed to categorically defined phenotypes (Diwadkar et al., In Press-a; Diwadkar et al., In Press-c). Nevertheless, the response of neural systems in depression to positive reward assumes particular developmental relevance (Forbes and Dahl, 2005).
Frontal and midbrain contributions to affective processing
The mPFC, the sgPFC, and the midbrain are contextually related to the domains of both reward processing, and the experience of affective states (Bunzeck et al., In Press; Martin-Soelch, 2009; Mayberg, 2009). Recent evidence suggests a high degree of convergence in brain function in these parallel domains; that is, reward and affective circuits are largely overlapping (Hahn et al., 2010). In schizophrenia itself, increased anhedonia has been linked to decrease responsivity of the mPFC and the sgPFC (Harvey et al., 2010), suggesting a lack of sensitivity to emotional cues.
Medial Prefrontal Cortex: Function in high-risk offspring with negative symptoms
The mPFC has been studied in schizophrenia and depression with regards to its role in mentalizing and in emotional stimuli processing. According to the simulationist perspectives on theory of mind and mentalizing, the interpretation of exogenous mental states is in part driven by reflections on intra-endogenous experiences (Amodio and Frith, 2006; Saxe and Baron-Cohen, 2006). The mPFC contributes to this by subserving the ability to mentalize, or interpret the mental state of the self and others (Lane et al., 1997; Lee and Siegle, 2009; Peelen et al., 2010; Zaki et al., 2010). In HRS_NS+, the reduced responsivity of the mPFC may in part be linked to the reduced ability to use emotional expressions and social cues to infer positive affective states in others (Goghari et al., 2010). This ability may be particularly affected in the context of negative symptoms, and dysfunction in this area may contribute to the asociality component of negative symptoms. By extension, the hypo- and hyper-responsivity observed across these regions in HRS_NS+ specifically to socially rewarding stimuli is suggestive of reward-related deficits associated with negative symptoms in the risk state.
Sub-genual Prefrontal Cortex: Function in high-risk offspring with negative symptoms
Investigations on sgPFC activity in depression point to its role in reward processing, producing affective states, and in autonomic responses to affective stimuli. The sgPFC plays a central role in mood induction (Drevets et al., 1997), and its response to emotional stimuli such as faces may in part reflect the inability of depressed subjects to experience positive social reward (Lawrence et al., 2004), that is typically associated with positively valenced faces (Adolphs, 2009; Todorov et al., 2008). Studies examining melancholic depression, that is depression with marked negative symptoms, find decreased glucose metabolism in the sgPFC (BA 25) compared to non-melancholic depressives (Pizzagalli et al., 2004; Wacker et al., 2009) suggesting that this hypo-responsivity is an essential correlate of anhedonia. Thus, the observed sgPFC reductions may be related to relative inhibition of rewarding experiences in HRS_NS+, that result in blunting of the response to rewarding social cues. A plausible psycho-social correlate of these impairments is deficits in social interaction and isolation that have been documented as eventual predictors of psychosis (Cannon et al., 1997). The current results provide suggestive evidence that this path may be manifest early in life in vulnerable children and adolescents.
Midbrain: Function in high-risk offspring with negative symptoms
To our knowledge, increased activity within the midbrain has not been previously reported in depression or patients with negative symptoms, and our findings in fact stand in contraposition to findings of mid-brain function in the depressive phenotype. In vivo imaging studies have documented abnormalities in the raphe nuclei and the brain stem in clinical depression (Cannon et al., 2007; Supprian et al., 2004), with evidence of reduced serotonin transporter binding in this area, and these results are linked to the loss of reward experience associated with depression. The serotonergic system of the mid-brain is linked to reward processing, with both dopaminergic and serotonergic contributions being vital (Kranz et al., 2010; Ziauddeen and Murray, 2010). fMRI studies indicate that the midbrain is highly response to monetary reward (Elliott et al., 2000), providing convergence with the neurochemical and functional roles of this structure in the reward system (Duzel et al., 2009). Furthermore, this responsivity is also predictive of how positive or emotionally rewarding stimuli are more likely to be recalled in longer term memory (Wittmann et al., 2008; Wittmann et al., 2005). The robust response of the midbrain in HRS_NS+ suggests at least two plausible reasons for why this structure behaves as it does in the context of at-risk adolescents with negative symptoms: a) even though the higher order frontal emotional responses are blunted in adolescents with signs of negative symptoms, the mid-brain response remains intact or b) the mid-brain response constitutes an aberrant pattern of hyper-activity with unclear compensatory implications. We believe that the data (particularly Figure 3) argue against a). The response of the mid-brain in controls and HRS_NS− appears close to the overall mean (baseline), whereas HRS_NS+ are robustly hyper-active. This suggests that in the context of this paradigm (a conscious affective continuous appraisal task), early reward related circuitry to socially rewarding stimuli is not relevant in control subjects, who instead rely on prefrontal regions more associated with the mentational responses to affect (Anticevic et al., 2011). Therefore the robust mid-brain hyperactivity in HRS_NC+ may reflect a disengagement of prefrontal region in favor of compensatory responses of the mid-brain. The current evidence also only weakly supports the idea that the mid-brain hyper-response in this context results from disordered “top-down” modulation from the mPFC. The prefrontal and the brain's limbic regions share reciprocal connections (Morgane et al., 2005; Roberts, 2011) and such modulation has been elegantly demonstrated in depression (Anand et al., 2005), yet evidence for inverse coupling between the mPFC and the midbrain responses is relatively weak (only revealed in non-parametric regression analyses).
In general, the prefrontal results provide fairly striking similarities with the literature on depression. In addition to previously cited studies, recent evidence suggests that sub-genual prefrontal responses to negative social emotional outcomes (e.g. simulation of peer rejection) is increased in adolescents with increased risk for depression (Masten et al., 2011). Seen collectively, these results suggest that the “negative-bias” or the bias against reward is observed not only in depression, but also in the risk state for depressive phenotypes. Whereas research on biological precursors of the depressive phenotype do not offer clear indications (Wals and Verhulst, 2005), analyses of psycho-social functioning in longitudinal birth cohorts indicates that impaired premorbid social functioning in adolescence is predictive of the eventual emergence of schizophrenia or mood disorders in adulthood (Cannon et al., 1997).
Limitations
The study of at-risk populations in adolescence is limited by significant conceptual challenges. Though HRS are at higher risk to develop schizophrenia compared to the general population (30-40%), the majority of subjects do not present with a psychiatric disorder in their lifetime. Thus, corrective developmental plasticity during critical periods of adolescence may characterize a large percentage of children at risk for psychiatric illness (Lewis and Levitt, 2002), not dissimilar to mechanisms proposed in children and adolescents with ADHD (Shaw et al., 2007). In this context, it is untenable to think of in vivo imaging measures as being able to forecast the eventual emergence of complex illnesses like schizophrenia or bipolar disorder. Rather, fMRI offers the opportunity of imaging the state of the brain in a risk state; in turn, risk for illness confers a propensity for disordered regional or network function (Shim et al., 2010). A conceptual lattice linking brain function with clinical features in the risk state assumes particular importance for what have been deemed as “close in” strategies (Diwadkar et al., 2006; McGorry et al., 2003). The advantage of using functional neuroimaging is that fMRI signals offer greater power for detecting differences than structural MRI measures (Diwadkar et al., In Press-b; Friston et al., 1999). Therefore, even with relatively small sample sizes in each of the HRS sub-groups, the observed effect sizes on the main effect (Cohen, 1988) in each of the mPFC (Figure 2a), the mid-brain (Figure 2b) and the sgPFC (Figure 2c) were moderate to large: partial η2=.22, η2=.41 and η2=.15 respectively.
Furthermore, by comparing these findings in the context of models of explicit phenotypes such as depression (Mayberg, 2009), it is possible to identify potentially deviant developmental pathways in risk for phenotypes or dimensions of symptoms. Reliably characterizing the state of brain function in the risk state may prove vital in informing strategies of early intervention and ultimately the prevention of symptom progression (McGorry, 1998). Such strategies appear to be viable in the prodromal state (in schizophrenia) but patients with prodromal symptoms are already in advanced stages of psychiatric disease. By comparison, understanding at risk children and adolescents with clinical characteristics affords the opportunity of studying the vulnerable brain in a “pre-morbid” and neuroleptic naïve state, and may identify early developmental deviations in the functional response of the brain.
Supplementary Material
Acknowledgements
We thank M. S. Keshavan, A. Amirsadri, R. Rajarathinem, L. Haddad, U. Rajan, A. Jenrow, D. Khatib and C. Zajac-Benitez for assistance in data collection and subject characterization. We also thank E. Murphy, V. Gumenyuk. K. Sundaresan and S. Fedorov for general assistance, and J. Stanley for helpful discussions.
Role of Funding Source
This work was supported by grants to VAD from the National Institute of Mental Health (MH 68680), an Experienced Investigator Award from the Children's Research Center of Michigan (CRCM), and the Elizabeth Elser Doolittle Investigatorship from the National Alliance for Research on Schizophrenia and Depression (NARSAD). Additional support was provided by the Joe Young Sr. Fund to the Dept of Psychiatry & Behavioral Neurosciences. The funding sources played no role in the preparation of this manuscript and in the analyses of the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions
TB was involved in the conceptual approach to the analyses, image analyses and manuscript preparation. PP was involved in data acquisition and data analyses. VAD was involved in study conceptualization, design, analyses and manuscript preparation.
REFERENCES
- Adolphs R. The social brain: neural basis of social knowledge. Ann. Rev. Psychol. 2009;60:693–716. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, Mathews VP, Kalnin A, Lowe MJ. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol. Psychiatry. 2005;57:1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Anticevic A, Repovs G, Barch DM. Emotion Effects on Attention, Amygdala Activation, and Functional Connectivity in Schizophrenia. Schizophr. Bull. 2011 doi: 10.1093/schbul/sbq168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottlender R, Sato T, Groll C, Jager M, Kunze I, Moller HJ. Negative symptoms in depressed and schizophrenic patients: how do they differ? The J. Clin. Psychiatry. 2003;64:954–958. doi: 10.4088/jcp.v64n0816. [DOI] [PubMed] [Google Scholar]
- Bunzeck N, Doeller CF, Dolan RJ, Duzel E. In Press. Contextual interaction between novelty and reward processing within the mesolimbic system. Hum. Brain Mapp. doi: 10.1002/hbm.21288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon DM, Ichise M, Rollis D, Klaver JM, Gandhi SK, Charney DS, Manji HK, Drevets WC. Elevated serotonin transporter binding in major depressive disorder assessed using positron emission tomography and [11C]DASB; comparison with bipolar disorder. Biol. Psychiatry. 2007;62:870–877. doi: 10.1016/j.biopsych.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Cannon M, Jones P, Gilvarry C, Rifkin L, McKenzie K, Foerster A, Murray RM. Premorbid social functioning in schizophrenia and bipolar disorder: similarities and differences. Am. J. Psychiatry. 1997;154:1544–1550. doi: 10.1176/ajp.154.11.1544. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, Seidman LJ, Perkins D, Tsuang M, McGlashan T, Heinssen R. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch. Gen. Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for behavioral sciences. Laurence Erlbaum Associates; Hillsdale, NJ.: 1988. [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Ann. Rev. Psychology. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA, Goradia D, Hosanagar A, Mermon D, Montrose DM, Birmaher B, Axelson D, Rajarathinem R, Haddad L, Amirsadri A, Zajac-Benitez C, Rajan U, Keshavan MS. In Press-a. Working memory and attention deficits in adolescent offspring of schizophrenia or bipolar patients: Comparing vulnerability markers. Prog. Neuro-psychopharmacol. Biol. Psychiatry. doi: 10.1016/j.pnpbp.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA, Montrose DM, Dworakowski D, Sweeney JA, Keshavan MS. Genetically predisposed offspring with schizotypal features: an ultra high-risk group for schizophrenia? Prog. Neuro-psychopharmacol. Biol. Psychiatry. 2006;30:230–238. doi: 10.1016/j.pnpbp.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA, Pruitt P, Goradia D, Murphy ER, Bakshi N, Keshavan MS, Rajan U, Reid A, Zajac-Benitez C. In Press-b. Fronto-parietal hypo-activation during working memory independent of structural abnormalities: Conjoint fMRI and sMRI analyses in adolescent offspring of schizophrenia patients. NeuroImage. doi: 10.1016/j.neuroimage.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA, Segel J, Pruitt P, Murphy ER, Keshavan MS, Radwan J, Rajan U, Zajac-Benitez C. In Press-c. Hypo-activation in the executive core of the sustained attention network in adolescent offspring of schizophrenia patients mediated by pre-morbid functional deficits. J. Psychiatr. Res. Neuroimaging. doi: 10.1016/j.pscychresns.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Ongur D, Price JL. Neuroimaging abnormalities in the subgenual prefrontal cortex: implications for the pathophysiology of familial mood disorders. Mol. Psychiatry. 1998;3:220–226. 190–221. doi: 10.1038/sj.mp.4000370. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct. Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr., Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Duzel E, Bunzeck N, Guitart-Masip M, Wittmann B, Schott BH, Tobler PN. Functional imaging of the human dopaminergic midbrain. Trends Neurosci. 2009;32:321–328. doi: 10.1016/j.tins.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Lenzenweger MF. DSM III and the genetics of schizophrenia. Am. J. Psychiatry. 1983;140:646. doi: 10.1176/ajp.140.5.646a. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Lenzenweger MF. Symptoms and the genetics of schizophrenia: implications for diagnosis. Am. J. Psychiatry. 1984;141:1541–1546. doi: 10.1176/ajp.141.12.1541. [DOI] [PubMed] [Google Scholar]
- Ekman P, Oster H. Facial expressions of emotion. Ann. Rev. Psychology. 1979;30:527–554. [Google Scholar]
- Elliott R, Friston KJ, Dolan RJ. Dissociable neural responses in human reward systems. J. Neurosci. 2000;20:6159–6165. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MD, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured clinical interview for DSM-IV Axis II personality disorders. Biometrics Research Department, NYSPI; New York: 1997. [Google Scholar]
- Forbes EE, Dahl RE. Neural systems of positive affect: relevance to understanding child and adolescent depression? Develop. Psychopathol. 2005;17:827–850. doi: 10.1017/S095457940505039X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman LJ, Insel TR, Smith Y. Subcortical projections of area 25 (subgenual cortex) of the macaque monkey. J. Comp. Neurol. 2000;421:172–188. [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ. How many subjects constitute a study? NeuroImage. 1999;10:1–5. doi: 10.1006/nimg.1999.0439. [DOI] [PubMed] [Google Scholar]
- Goghari VM, Sponheim SR, MacDonald AW., 3rd The functional neuroanatomy of symptom dimensions in schizophrenia: a qualitative and quantitative review of a persistent question. Neurosci. Biobehav. Rev. 2010;34:468–486. doi: 10.1016/j.neubiorev.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn T, Marquand AF, Ehlis AC, Dresler T, Kittel-Schneider S, Jarczok TA, Lesch KP, Jakob PM, Mourao-Miranda J, Brammer MJ, Fallgatter AJ. Integrating neurobiological markers of depression. Arch. Gen. Psychiatry. 2010;68:361–368. doi: 10.1001/archgenpsychiatry.2010.178. [DOI] [PubMed] [Google Scholar]
- Harvey PO, Armony J, Malla A, Lepage M. Functional neural substrates of self-reported physical anhedonia in non-clinical individuals and in patients with schizophrenia. J. Psychiatr. Res. 2010 doi: 10.1016/j.jpsychires.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Hastie T, Tibshirani R, Friedman J. The elements of statistical learning: Data mining, inference and prediction. Springer-Verlag; New York: 2001. [Google Scholar]
- Josephs O, Henson RN. Event-related functional magnetic resonance imaging: modelling, inference and optimization. Phil. Trans. Royal Soc. London. 1999;354:1215–1228. doi: 10.1098/rstb.1999.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adol. Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gruenberg AM, Strauss JS. An independent analysis of the Copenhagen sample of the Danish Adoption Study of Schizophrenia. V. The relationship between childhood social withdrawal and adult schizophrenia. Arch. Gen. Psychiatry. 1982;39:1257–1261. doi: 10.1001/archpsyc.1982.04290110023004. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar V, Rosenberg DR. Developmental biomarkers in schizophrenia and other psychiatric disorders: common origins, different trajectories? Epidemiol. Psichiatr. Soc. 2005;14:188–193. doi: 10.1017/s1121189x00007934. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Suga R. Depressive and negative symptoms in major psychiatric disorders. Compr. Psychiatry. 1991;32:88–94. doi: 10.1016/0010-440x(91)90074-m. [DOI] [PubMed] [Google Scholar]
- Kranz GS, Kasper S, Lanzenberger R. Reward and the serotonergic system. Neurosci. 2010;166:1023–1035. doi: 10.1016/j.neuroscience.2010.01.036. [DOI] [PubMed] [Google Scholar]
- Kulhara P, Chadda R. A study of negative symptoms in schizophrenia and depression. Compr. Psychiatry. 1987;28:229–235. doi: 10.1016/0010-440x(87)90029-0. [DOI] [PubMed] [Google Scholar]
- Kumar P, Waiter G, Ahearn T, Milders M, Reid I, Steele JD. Abnormal temporal difference reward-learning signals in major depression. Brain. 2008;131:2084–2093. doi: 10.1093/brain/awn136. [DOI] [PubMed] [Google Scholar]
- Kwapil TR. Social anhedonia as a predictor of the development of schizophrenia-spectrum disorders. J. Abnorm. Psychol. 1998;107:558–565. doi: 10.1037//0021-843x.107.4.558. [DOI] [PubMed] [Google Scholar]
- Lane RD, Fink GR, Chau PM, Dolan RJ. Neural activation during selective attention to subjective emotional responses. Neuroreport. 1997;8:3969–3972. doi: 10.1097/00001756-199712220-00024. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, Frangou S, Ecker C, Phillips ML. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol. Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Lee KH, Siegle GJ. Common and distinct brain networks underlying explicit emotional evaluation: a meta-analytic study. Soc. Cogn. Affect. Neurosci. 2009 doi: 10.1093/scan/nsp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemogne C, Delaveau P, Freton M, Guionnet S, Fossati P. Medial prefrontal cortex and the self in major depression. Journal of affective disorders. 2010 doi: 10.1016/j.jad.2010.11.034. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Ann. Rev. Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection Theory: A Users Guide. Lawrence Erlbaum Associates; Mahwah, NJ.: 2005. [Google Scholar]
- Martin-Soelch C. Is depression associated with dysfunction of the central reward system? Biochem. Soc. Trans. 2009;37:313–317. doi: 10.1042/BST0370313. [DOI] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, McNealy K, Pfeifer JH, Dapretto M. Subgenual anterior cingulate responses to peer rejection: a marker of adolescents' risk for depression. Develop. Psychopathol. 2011;23:283–292. doi: 10.1017/S0954579410000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS. Targeted electrode-based modulation of neural circuits for depression. J. Clin. Invest. 2009;119:717–725. doi: 10.1172/JCI38454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am. J. Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- McGorry PD. “A stitch in time”...the scope for preventive strategies in early psychosis. Europ. Arch. Psychiatry Clin. Neurosci. 1998;248:22–31. doi: 10.1007/s004060050014. [DOI] [PubMed] [Google Scholar]
- McGorry PD, Yung AR, Phillips LJ. The “close-in” or ultra high-risk model: a safe and effective strategy for research and clinical intervention in prepsychotic mental disorder. Schizophr. Bull. 2003;29:771–790. doi: 10.1093/oxfordjournals.schbul.a007046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, McFarlane W, Perkins DO, Pearlson GD, Woods SW. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr. Bull. 2003;29:703–715. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Woods SW, Stein K, Driesen N, Corcoran CM, Hoffman R, Davidson L. Symptom assessment in schizophrenic prodromal states. Psychiatr. Q. 1999;70:273–287. doi: 10.1023/a:1022034115078. [DOI] [PubMed] [Google Scholar]
- Morgane PJ, Galler JR, Mokler DJ. A review of systems and networks of the limbic forebrain/limbic midbrain. Prog. Neurobiol. 2005;75:143–160. doi: 10.1016/j.pneurobio.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Murray RM, Sham P, Van Os J, Zanelli J, Cannon M, McDonald C. A developmental model for similarities and dissimilarities between schizophrenia and bipolar disorder. Schizophr. Res. 2004;71:405–416. doi: 10.1016/j.schres.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc. Natl. Acad. Sci. USA. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelen MV, Atkinson AP, Vuilleumier P. Supramodal representations of perceived emotions in the human brain. J. Neurosci. 2010;30:10127–10134. doi: 10.1523/JNEUROSCI.2161-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Oakes TR, Fox AS, Chung MK, Larson CL, Abercrombie HC, Schaefer SM, Benca RM, Davidson RJ. Functional but not structural subgenual prefrontal cortex abnormalities in melancholia. Mol. Psychiatry. 2004;9:325, 393–405. doi: 10.1038/sj.mp.4001501. [DOI] [PubMed] [Google Scholar]
- Roberts AC. The importance of serotonin for orbitofrontal function. Biol. Psychiatry. 2011;69:1185–1191. doi: 10.1016/j.biopsych.2010.12.037. [DOI] [PubMed] [Google Scholar]
- SAS, I. JMP 8.02. Cary, N.C.: 2009. [Google Scholar]
- Saxe R, Baron-Cohen S. The neuroscience of theory of mind. Soc. Neurosci. 2006;1:i–ix. doi: 10.1080/17470910601117463. [DOI] [PubMed] [Google Scholar]
- Sescousse G, Redoute J, Dreher JC. The architecture of reward value coding in the human orbitofrontal cortex. J. Neurosci. 2010;30:13095–13104. doi: 10.1523/JNEUROSCI.3501-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, Clasen L, Evans A, Giedd J, Rapoport JL. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc. Natl. Acad. Sci. USA. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim G, Oh JS, Jung WH, Jang JH, Choi CH, Kim E, Park HY, Choi JS, Jung MH, Kwon JS. Altered resting-state connectivity in subjects at ultra-high risk for psychosis: an fMRI study. Behav. Brain Funct. 2010;6:58. doi: 10.1186/1744-9081-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidak Z. Rectangular confidence regions for the means of multivariate normal distributions. J. Am. Stat. Assoc. 1967;62:626–633. [Google Scholar]
- Stahl SM, Buckley PF. Negative symptoms of schizophrenia: a problem that will not go away. Acta Psychiatr. Scand. 2007;115:4–11. doi: 10.1111/j.1600-0447.2006.00947.x. [DOI] [PubMed] [Google Scholar]
- Supprian T, Reiche W, Schmitz B, Grunwald I, Backens M, Hofmann E, Georg T, Falkai P, Reith W. MRI of the brainstem in patients with major depression, bipolar affective disorder and normal controls. Psychiatr. Res. 2004;131:269–276. doi: 10.1016/j.pscychresns.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Todorov A, Baron SG, Oosterhof NN. Evaluating face trustworthiness: a model based approach. Soc. Cogn. Affect. Neurosci. 2008;3:119–127. doi: 10.1093/scan/nsn009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang MT, Stone WS, Faraone SV. The genetics of schizophrenia. Curr. Psychiatry Reports. 1999;1:20–24. doi: 10.1007/s11920-999-0006-0. [DOI] [PubMed] [Google Scholar]
- Victor TA, Furey ML, Fromm SJ, Ohman A, Drevets WC. Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Arch. Gen. Psychiatry. 2010;67:1128–1138. doi: 10.1001/archgenpsychiatry.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker J, Dillon DG, Pizzagalli DA. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, fMRI, and volumetric techniques. NeuroImage. 2009;46:327–337. doi: 10.1016/j.neuroimage.2009.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wals M, Verhulst F. Child and adolescent antecedents of adult mood disorders. Curr. Opin. Psychiatry. 2005;18:15–19. [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for fMRI data. Medical College of Wisconsin; Milwaukee, WI.: 2000. [Google Scholar]
- Winograd-Gurvich C, Fitzgerald PB, Georgiou-Karistianis N, Bradshaw JL, White OB. Negative symptoms: A review of schizophrenia, melancholic depression and Parkinson's disease. Brain Res. Bull. 2006;70:312–321. doi: 10.1016/j.brainresbull.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Schiltz K, Boehler CN, Duzel E. Mesolimbic interaction of emotional valence and reward improves memory formation. Neuropsychologia. 2008;46:1000–1008. doi: 10.1016/j.neuropsychologia.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Schott BH, Guderian S, Frey JU, Heinze HJ, Duzel E. Reward-related FMRI activation of dopaminergic midbrain is associated with enhanced hippocampus-dependent long-term memory formation. Neuron. 2005;45:459–467. doi: 10.1016/j.neuron.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Zaki J, Hennigan K, Weber J, Ochsner KN. Social cognitive conflict resolution: contributions of domain-general and domain-specific neural systems. J. Neurosci. 2010;30:8481–8488. doi: 10.1523/JNEUROSCI.0382-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziauddeen H, Murray GK. The relevance of reward pathways for schizophrenia. Curr. Opin. Psychiatry. 2010;23:91–96. doi: 10.1097/YCO.0b013e328336661b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.