Abstract
Human organic anion transporter 4 (hOAT4) belongs to a family of organic anion transporters that play critical roles in the body disposition of clinically important drugs, including anti-viral therapeutics, anti-cancer drugs, antibiotics, antihypertensives, and anti-inflammatories. hOAT4 is abundantly expressed in the kidney and placenta. In the current study, we examined the regulation of hOAT4 by serum- and glucocorticoid-inducible kinase 2 (sgk2) in the kidney COS-7 cells. We showed that sgk2 stimulated hOAT4 transport activity. Such stimulation mainly resulted from an increased cell surface expression of the transporter, kinetically revealed as an increased maximal transport velocity Vmax without significant change in substrate-binding affinity Km. We further showed that regulation of hOAT4 activity by sgk2 was mediated by ubiquitin ligase Nedd4-2. Overexpression of Nedd4-2 enhanced hOAT4 ubiquitination, and inhibited hOAT4 transport activity, whereas overexpression of ubiquitin ligase-dead mutant Nedd4-2/C821A or siRNA knockdown of endogenous Nedd4-2 had opposite effects on hOAT4. Our co-immunoprecipitation experiment revealed that sgk2 weakened the association between hOAT4 and Nedd4-2. In conclusion, our study demonstrated for the first time that sgk2 stimulated hOAT4 transport activity by abrogating the inhibition effect of Nedd4-2 on the transporter.
Keywords: Organic Anion Transporter, Drug Transport, Regulation, Serum and Glucocorticoid-Inducible Kinase, Ubiquitin Ligase, Ubiquitination
1. Introduction
Human organic anion transporter 4 (hOAT4) belongs to a family of organic anion transporters, which play critical roles in the body disposition of clinically important drugs, including anti-human immunodeficiency virus therapeutics, anti-tumor drugs, antibiotics, antihypertensives, and antiinflammatories. The activity of these transporters is subjected to the regulation at multiple levels such as transcription, mRNA stability, translation, and posttranslational modification [1-6]. hOAT4 is abundantly expressed in the kidney and placenta [7]. In the kidney, hOAT4 localizes at the apical membrane of the proximal tubule cells, and is involved in renal secretion and reabsorption of endogenous substances as well as many drugs and xenobiotics. In the placenta, hOAT4 is localized to the basolateral membrane of syncytiotrophoblasts [8]. In the placenta, estrogen biosynthesis uses dehydroepiandrosterone sulfate (DHEAS), a precursor produced by the fetal adrenals. Accumulation of excess DHEAS is associated with intrauterine growth retardation [9]. DHEAS is a hOAT4 substrate. Therefore, hOAT4 may play an important role in efficient uptake of DHEAS by the placenta for estrogens production and for the protection of fetus from the cytotoxicity of DHEAS.
Given such an important role, understanding the regulation of hOAT4 has profound clinical significance. We previously demonstrated that members of OAT family undergo constitutive internalization from and recycling back to cell surface and OAT transport activity can be regulated by altering the trafficking kinetics and stability of these transporters. For example, activation of protein kinase C (PKC) inhibits OAT transport activity through accelerating the internalization rate of the transporter [10, 11]. Prolonged activation of PKC leads to the degradation of the internalized OAT [11]. We further demonstrated that modification of OAT by ubiquitin conjugation is an important step that precedes OAT internalization [12].
Recently, modification of receptors and channels by ubiquitin conjugation has emerged as the major regulatory mechanism of cell surface internalization, intracellular sorting, and turnover of these membrane proteins [13, 14]. Ubiquitin is a highly conserved 76-amino-acid protein that forms an isopeptide bond between its C-terminal glycine and a lysine (K) residue on the target protein. Each ubiquitin moiety itself harbors seven lysine residues, allowing for the formation of ubiquitin chains linked through its internal lysine residues. Therefore, a substrate can be modified by different types of ubiquitin conjugation: monoubiquitination (conjugation of one single ubiquitin to one single lysine on the substrate), or polyubiquitination (extended polyubiquitin chain). More and more evidence indicates that ubiquitination serves as a major signal in PKC-regulated cellular trafficking of transporters, including dopamine transporter (DAT) [15], cationic amino acid transporter (CAT-1) [16], and glutamate transporter (GLT-1) [17].
The serum- and glucocorticoid-inducible kinases (sgk) are involved in controlling diverse cellular processes including sodium Na+ homeostasis, osmoregulation, cell survival, and cell proliferation [18-23]. The sgk family of protein kinases has three isoforms: sgk1, sgk2 and sgk3. It has been shown that the expression, regulation, and role of sgk2 within the mammalian kidney are distinct from sgk1 and sgk3 [24]. Sgk1 and sgk3 are expressed in every tissue, whereas sgk2 seems to be present primarily in the liver, kidney, pancreas, and brain. Unlike sgk1 and sgk3, sgk2 expression in the kidney was not subjected to the regulation by aldosterone. Immunochemical characterization localized sgk1 protein to distal convoluted tubule, cortical and medullary collecting duct, whereas sgk2 protein was highly expressed in kidney proximal tubule cells, where it modulates the function of membrane proteins such as Na+/H+ exchanger [24]. Based on the distinct characteristics of sgk2, we investigated whether hOAT4, also highly expressed in proximal tubule cells, is regulated by sgk2. We demonstrated a new regulatory mechanism that sgk2 modulates hOAT4 expression and function through an ubiquitin ligase Nedd4-2.
2. Materials and Methods
2.1 Materials
COS-7 cells were purchased from American Type Culture Collection (Manassas, VA). [3H]-labeled estrone sulfate was purchased from PerkinElmer (Waltham, MA). Membrane-impermeable biotinylation reagent NHS-SS-biotin, streptavidin-agarose beads and protein G-agarose beads were purchased from Pierce (Rockford, IL). cDNAs for mouse sgk2 (wild-type sgk2 and constitutive active sgk2 (CA-sgk2)) were generously provided by Dr. Alan C. Pao from Department of Medicine, Stanford University (Stanford, CA). cDNA for human Nedd4-2 was generously provided by Dr. Peter M. Snyder of the College of Medicine, University of Iowa (Iowa City, IA). Mouse anti-myc antibody (9E10) was purchased from Roche (Indianapolis, IN). Rabbit anti-sgk2 antibody was purchased from Cell signaling (Danvers, MA). Nedd4-2-specific siRNA oligonucleotides (Silencer® Select, identification number s23570) and negative control siRNA oligonucleotides (Silencer® Select, catalog number 4390843) were purchased from Ambion (Grand Island, NY). All other reagents were from Sigma-Aldrich (St. Louis, MO).
2.2 Cell culture and Transfection
Parental COS-7 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum at 37 °C in 5% CO2. Transfection with plasmids was carried out for 48 hours using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions Transfected cells were starved with serum free medium for 2-4 hours for further experiments. Cells stably expressing hOAT4 were maintained in DMEM medium supplemented with 0.2mg/ml G418 (Invitrogen, Carlsbad, CA), 10% fetal bovine serum.
2.3 Transport Measurements
Cells were plated in 48-well plates. For each well, uptake solution was added. The uptake solution consisted of phosphate-buffered saline (PBS)/Ca2+/Mg2+ (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4, 0.1 mM CaCl2, and 1 mM MgCl2, pH 7.3) and [3H]ES (100 nM). At the times indicated, uptake process was stopped by aspirating the uptake solution and rapidly washing the cells with ice-cold PBS solution. The cells were then solubilized in 0.2 N NaOH, neutralized in 0.2 N HCl, and aliquotted for liquid scintillation counting.
2.4 Cell Surface Biotinylation
Cell surface expression level of hOAT4 was examined using the membrane-impermeable biotinylation reagent, NHS-SS-biotin. Cells were seeded in 6-well plates. Each well of cells was incubated with 1 ml of freshly made NHS-SS-biotin (0.5 mg/ml in PBS/CM) in two successive 20 min incubations on ice with very gentle shaking. After biotinylation, each well was briefly rinsed with 3 ml of PBS/CM containing 100 mM glycine then incubated with the same solution for 20 min on ice, to ensure complete quenching of the unreacted NHS-SS-biotin. The cells were then lysed on ice for 30 min in 400 ml of lysis buffer (10 mM Tris/HCl, 150 mM NaCl, 1 mM EDTA, 0.1% SDS, 1% Triton X-100 with 1/100 protease inhibitor mixture). The cell lysates were cleared by centrifugation at 16,000g at 4 °C. 40 μl of streptavidin-agarose beads was then added to the supernatant to isolate cell membrane proteins. hOAT4 was detected in the pool of surface proteins by SDS-PAGE and immunoblotting using an anti-myc antibody 9E10.
2.5 Degradation Assay
We followed the procedure previously established in our laboratory [11]. Cells, co-transfected with hOAT4 and control vector, or with hOAT4 and CA-sgk2, underwent biotinylation with 0.5 mg/ml sulfo-NHS-SS-biotin at 4 °C. After biotinylation, each dish was rinsed with 2ml PBS containing 100mM glycine and then incubated with the same solution for 20 minutes on ice, to ensure complete quenching of the unreacted NHS-SS-biotin. The biotin-labeled cells were incubated in DMEM at 37 °C. Cells were collected at 0, 2, 4, and 6 hours and lysed in lysis buffer with protease inhibitor cocktail. The cell lysates were cleared by centrifugation at 16,000 × g at 4 °C. 40 μl of streptavidin agarose beads were then added to the supernatant to isolate cell membrane proteins, followed by immunoblotting with anti-myc antibody.
2.6 Immunoprecipitation
Cells were lysed with lysis buffer (20 mM Tris/HCl, pH 7.5, 1% Triton X-100, 2 mM EDTA, and 25 mM NaF), freshly added with 1% of proteinase inhibitor cocktail. Cell lysates were precleared with protein G-agarose beads to reduce nonspecific binding at 4 °C for 1.5 hours. Anti-myc antibody (1:100) was incubated with appropriate volume of protein G-agarose beads at 4 °C for 1.5 hours. The precleared protein sample was then mixed with antibody-bound protein G-agarose beads and underwent end-over-end rotating at 4 °C overnight. Proteins bound to the protein G-agarose beads were eluted with Urea buffer containing β-mecaptoethanol and analyzed by immunoblotting with indicated antibodies.
2.7 Electrophoresis and Immunoblotting
Protein samples were resolved on 7.5% SDS-PAGE minigels and electroblotted on to polyvinylidene difluoride membranes. The blots were blocked for 1 hour with 5% nonfat dry milk in PBS-0.05% Tween 20, washed, and incubated overnight at 4 °C with appropriate primary antibodies, followed by horseradish peroxidase-conjugated secondary antibodies. The signals were detected by SuperSignal West Dura Extended Duration Substrate kit (Pierce, Rockford, IL). Nonsaturating, immunoreactive protein bands were quantified by scanning densitometry with the FluorChem 8000 imaging system (Alpha Innotech Corp., San Leandro, CA).
2.8 Data Analysis
Each experiment was repeated a minimum of three times. The statistical analysis was from multiple experiments. Statistical analysis was performed using Student's paired t-tests. A p-value of <0.05 was considered significant.
3. Results
3.1 Effect of sgk2 on hOAT4 transport activity
To explore the role of sgk2 in hOAT4 function, we transfected COS-7 cells with wild type sgk2 (WT-sgk2) or with its constitutive active form CA-sgk2. hOAT4-mediated uptake of [3H] estrone sulfate was then measured. As shown in Fig. 1, wild type sgk2 stimulated ~ 40% increase in the uptake as compared to that in control cells, and CA-sgk2 significantly augmented the effect of sgk2, resulting in an additional 30% increase in the uptake. To examine the mechanism of sgk2-induced stimulation of hOAT4 activity, we determined hOAT4-mediated [3H] estrone sulfate uptake at different substrate concentrations. An Eadie-Hofstee analysis of the derived data (Fig. 2) showed that transfection of CA-sgk2 resulted in an increased maximal transport velocity Vmax of hOAT4 (48.66 ± 1.53 pmol·mg−1·3min−1 with control cells and 80.59 ± 4.74 pmol·mg−1·3min−1 with cells transfected with CA-sgk2) with no significant change in the substrate-binding affinity Km of the transporter (5.32 ± 0.32 μM with control cells and 5.43 ± 0.46 μM with cells transfected with CA-sgk2).
Fig. 1. Effect of sgk2 on hOAT4 transport activity.
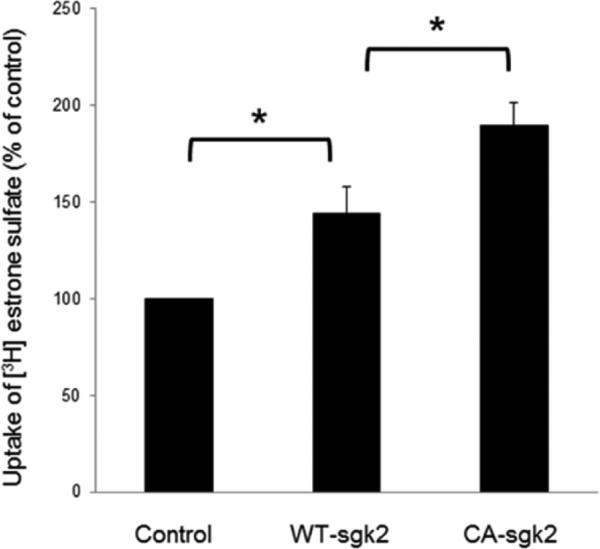
(a) COS-7 cells were co-transfected with hOAT4 and control vector, or with hOAT4 and wild type sgk2 (WT-sgk2), or hOAT4 and the constitutive active form of sgk2 (CA-sgk2). 3-min uptake of [3H]-estrone sulfate (0.1 μM) was then measured. Uptake activity was expressed as a percentage of the uptake measured in control cells. The data represent uptake into hOAT4-transfected cells minus uptake into mock cells (parental COS-7 cells). Values are mean ± S.E. (n = 3). *P<0.05
Fig. 2. Effect of sgk2 on the kinetics of hOAT4-mediated estrone sulfate transport.
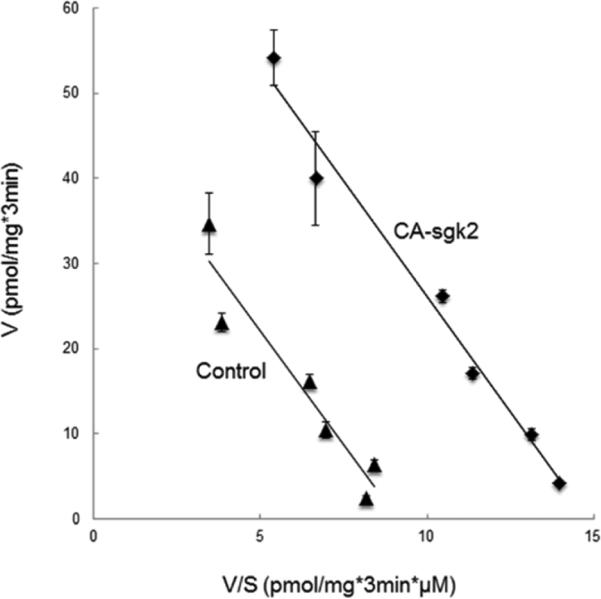
COS-7 cells were co-transfected with hOAT4 and the constitutive active form of sgk2 (CA-sgk2), or with hOAT4 and control vector. Initial uptake (3 min) of [3H] estrone sulfate was measured at the concentration of 0.1–10 μM. The data represent uptake into hOAT4-transfected cells minus uptake into mock cells (parental COS-7 cells). Values are means ± S.E. (n = 3). V, velocity; S, substrate concentration.
3.2 Effect of sgk2 on hOAT4 Expression
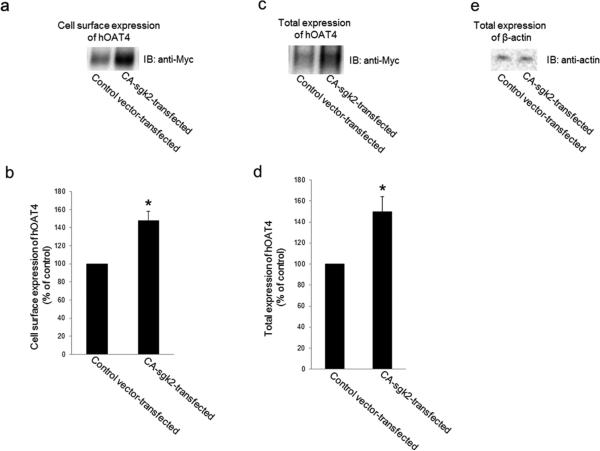
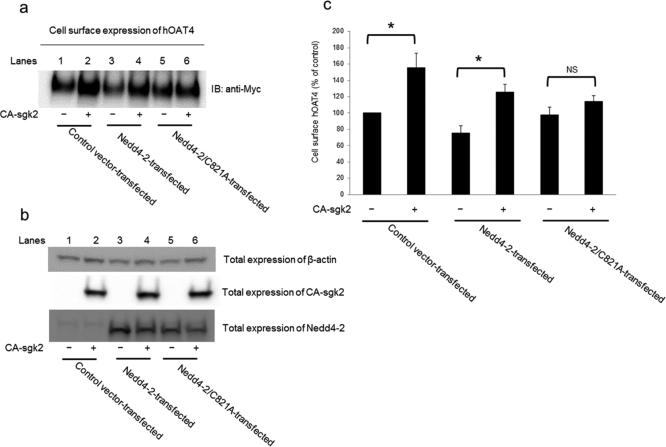
An increased maximal transport velocity Vmax of hOAT4 shown above could be affected by either an increased number of the transporter at the cell surface or an increased transporter turnover rate. We performed experiments that differentiate between these possibilities by measuring transporter expression both at the cell surface and in the total cell lysates. We showed that overexpression of sgk2 resulted in an increase of hOAT4 expression both at the cell surface (Fig. 3a) and in the total cell lysate (Fig. 3c). Such a change in hOAT4 expression was not due to the general perturbation of cellular proteins as the expression of the house-keeping protein β-actin was not affected under these conditions (Fig. 3e).
Fig. 3. Effect of sgk2 on hOAT4 expression.
(a). Cell surface expression of hOAT4. COS-7 cells were co-transfected with hOAT4 and control vector or with hOAT4 and the constitutive active form of sgk2 (CA-sgk2). Transfected cells were labeled with biotin. Biotinylated/cell surface proteins were separated with streptavidin beads, followed by immunoblotting (IB) with an anti-myc antibody. (b). Densitometry plot of results from Fig. 3a, as well as from other experiments. The values are mean ± S.E. (n = 3). *P<0.05. (c). Total cell expression of hOAT4. COS-7 cells were co-transfected with hOAT4 and control vector or with hOAT4 and the constitutive active form of sgk2 (CA-sgk2). Transfected cells were lysed, followed by immunoblotting (IB) with an anti-myc antibody. (d). Densitometry plot of results from Fig. 3c, as well as from other experiments. The values are mean ± S.E. (n = 3). *P<0.05. (e). Total cell expression of β-actin. COS-7 cells were co-transfected with hOAT4 and control vector or with hOAT4 and the constitutive active form of sgk2 (CA-sgk2). Transfected cells were lysed, followed by immunoblotting (IB) with an anti-actin antibody.
3.3 Effect of sgk2 on hOAT4 stability
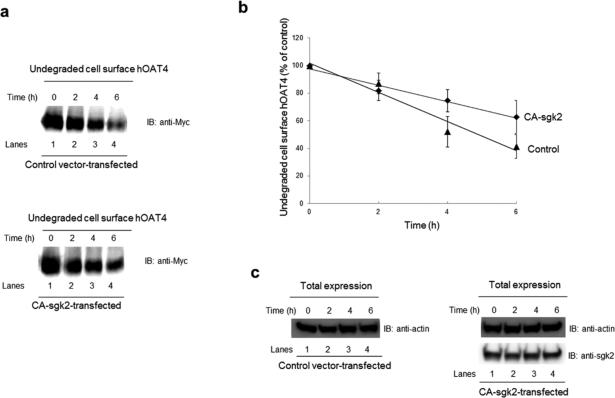
Sgk2-induced increase in hOAT4 expression may reflect an increased stability of the transporter. In this experiment, we examined such possibility by measuring the degradation rate of cell surface hOAT4 in the presence or absence of sgk2. COS-7 cells were co-transfected with hOAT4 and control vector or with hOAT4 and CA-sgk2 for 48 hours and then biotinylated with membrane impermeable biotinylation reagent sulfo-NHSSS-biotin. Biotin-labeled cells were lysed at 2, 4, and 6 hour time point after the biotinylation, and cell surface proteins were isolated using streptavidin-agarose beads, followed by immunoblotting with anti-myc antibody (hOAT4 was tagged with myc at its carboxyl terminus). Our results (Figs. 4a, 4b) showed that the degradation rate of cell surface hOAT4 decreased significantly in the presence of sgk2 as compared to that in control cells. The degradation rate change was not due to the general perturbation of the cellular proteins as evident when we measured the expression level of the house-keeping protein β–actin. β–actin was equally expressed in all samples tested (Fig. 4c).
Fig. 4. Effect of sgk2 on the degradation of cell surface hOAT4.
(a) COS-7 cells were cotransfected with hOAT4 and control vector (top panel) or with hOAT4 and the constitutive active form of sgk2 (CA-sgk2) (bottom panel). Cell surface hOAT4 degradation was then analyzed as described in “Materials and Methods” section followed by immunoblotting (IB) using anti-myc antibody. (b) Densitometry plot of results from Fig. 4a as well as from other experiments. The amount of undegraded cell surface hOAT4 was expressed as % of total initial cell surface hOAT4 pool. Values are mean ± S.E. (n = 3). (c). Total expression of sgk2 and house-keeping protein β-actin. COS-7 cells were co-transfected with hOAT4 and control vector or with hOAT4 and the constitutive active form of sgk2 (CA-sgk2). Transfected cells were lysed, followed by immunoblotting (IB) with anti-sgk2 antibody or anti-actin antibody.
3.4 Effect of Nedd4-2 on hOAT4 ubiquitination and transport activity
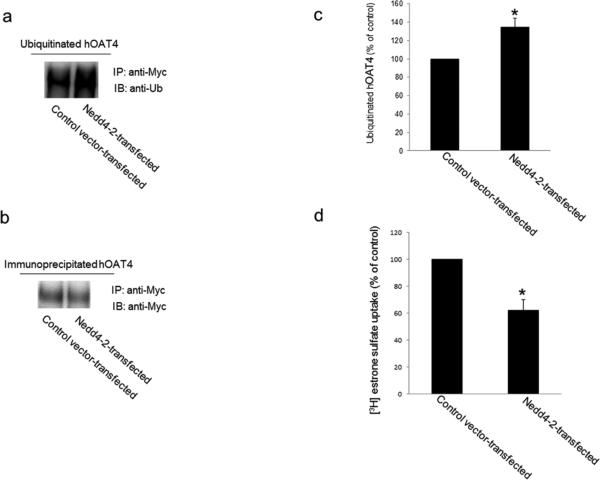
It has been reported that one of the mediators for sgk regulation of membrane proteins is the ubiquitin ligase Nedd4-2 [25, 26]. Nedd4-2 promotes the ubiquitination of many channels and transporters, and leads these membrane proteins to internalize from the cell surface and subsequently to be degraded [15, 17, 27]. As a result, the expression of these membrane proteins at the cell surface is reduced, and their function is decreased. To examine whether Nedd4-2 is an ubiquitin ligase for hOAT4, we transfected Nedd4-2 into hOAT4-expressing COS-7 cells. Transfected cells were then lysed and hOAT4 was immunoprecipitated with anti-myc antibody (myc was tagged to hOAT4), followed by immunoblotting with anti-ubiquitin antibody. As shown in Fig. 5a, hOAT4 ubiquitination was significantly enhanced in cells transfected with Nedd4-2 as compared to that in control cells. Moreover, the differences in ubiquitination were not due to the differences in the amount of hOAT4 immunoprecipitated as evident when the same immunoblot was reprobed with anti-myc antibody. Similar amount of hOAT4 was immunoprecipitated in all samples under these conditions (Fig. 5b). We then examined the effect of Nedd4-2 on hOAT4 transport activity. As shown in Fig. 5d, hOAT4 transport activity was decreased ~40% in cells transfected with Nedd4-2 as compared to that in control cells.
Fig. 5. Effect of Nedd4-2 on hOAT4 ubiquitination and transport activity.
(a) hOAT4-expressing cells were transfected with or without wild type Nedd4-2. Transfected cells were then lysed, and hOAT4 was immunoprecipitated (IP) with anti-myc antibody (myc was tagged to hOAT4), followed by immunoblotting (IB) with anti-ubiquitin antibody (anti-Ub). (b) The same immunoblot from Fig. 5a was reprobed by anti-myc antibody. (c) Densitometry plot of results from Fig. 5a, as well as from other experiments. The values are mean ± S.E. (n = 3). *P<0.05. (d) COS-7 cells were co-transfected with hOAT4 and control vector or with hOAT4 and wild type Nedd4-2. 3-min uptake of [3H]-estrone sulfate (0.1 μM) was then measured in these cells. The data represent uptake into hOAT4-transfected cells minus uptake into mock cells (parental COS-7 cells). Values are mean ± S.E. (n = 3). *P<0.05
3.5 Effect of sgk2 on Nedd4-2 interaction with hOAT4
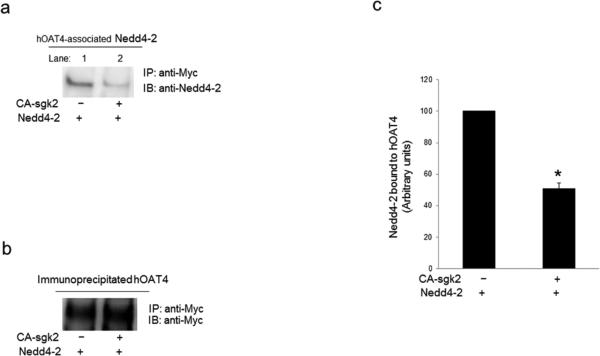
Nedd4-2 has been reported to interact either directly or indirectly with its target proteins [15, 17, 28, 29], and thus, we assessed whether there is an association between Nedd4-2 and hOAT4 through co-immunoprecipitation assay. As shown in Fig. 6a, when COS-7 cells were transfected with hOAT4, Nedd4-2 and control vector, significant amount of Nedd4-2 was detected in hOAT4 immunoprecipitates (lane 1), suggesting a direct association between these two proteins. However, in cells triple-transfected with hOAT4, Nedd4-2 and sgk2 (lane 2), the amount of Nedd4-2 detected in hOAT4 immunoprecipitates was much less than that in cells without sgk2. Furthermore, the differences in the amount of hOAT4 detected were not due to the differences in the amount of hOAT4 immunoprecipitated as evident when the same immunoblot was reprobed with anti-myc antibody. Similar amount of hOAT4 was immunoprecipitated in all samples under these conditions (Fig. 6b). These data suggest that sgk2 decreased the interaction between hOAT4 and Nedd4-2.
Fig. 6. Effect of sgk2 on the interaction between Nedd4-2 and hOAT4.
(a) COS-7 cells were transfected with hOAT4, Nedd4-2 and control vector or with hOAT4, Nedd4-2 and the constitutive active form of sgk2 (CA-sgk2). Transfected cells were then lysed, and hOAT4 was immunoprecipitated (IP) with anti-myc antibody, followed by immunoblotting (IB) with anti-Nedd4-2 antibody. (b) The same immunoblot from Fig. 6a was reprobed by anti-myc antibody. (c) Densitometry plot of results from Fig. 6a as well as from other experiments. The values are mean ± S.E. (n = 3). *P<0.05
3.6 Effect of sgk2 on Nedd4-2-mediated cell surface expression of hOAT4
As mentioned above, Nedd4-2 ubiquitinates many membrane proteins, which leads these proteins to internalize from the cell surface. As a result, the amount of these proteins at the cell surface is reduced. In our current experiment, we further explored the relationship between sgk2 and Nedd4-2 in hOAT4 expression. We showed (Fig. 7a) that sgk2 significantly increased hOAT4 expression at the cell surface (lane 2 as compared to lane 1) in control cells. In cells transfected with Nedd4-2 for 48 hours, the initial amount of hOAT4 at the cell surface (lane 3) was already significantly reduced as compared to that in control cells, suggesting that a portion of hOAT4 was already ubiquitinated and therefore internalized from the cell surface during the 48-hour period of Nedd4-2 transfection. Such Nedd4-2-induced reduction of hOAT4 at the cell surface was abrogated by additional expression of sgk2 (lane 4). However, an ubiquitin ligase-dead mutant of Nedd4-2 (Nedd4-2/C821A) failed to reduce hOAT4 expression at the cell surface (lane 5), and furthermore, sgk2 was unable to enhance hOAT4 expression in the presence of Nedd4-2/C821A (lane 6). Therefore, these results suggest that stimulation of hOAT4 expression by sgk2 was via the ubiquitin ligase activity of Nedd4-2.
Fig. 7. Effect of Nedd4-2 ligase activity on sgk2 regulation of hOAT4 cell surface expression.
(a). COS-7 cells were transfected with hOAT4, CA-sgk2 and control vector, or with hOAT4, CA-sgk2 and Nedd4-2, or with hOAT4, CA-sgk2 and ubiquitin ligase-dead Nedd4-2 mutant (Nedd4-2/C821A). Transfected cells were labeled with biotin. Biotinylated/cell surface proteins were separated with streptavidin beads, followed by immunoblotting (IB) with an anti-myc antibody. (b) COS-7 cells were transfected with hOAT4, CA-sgk2 and control vector, or with hOAT4, CA-sgk2 and Nedd4-2, or with hOAT4, CA-sgk2 and ubiquitin ligase-dead Nedd4-2 mutant (Nedd4-2/C821A). Transfected cells were then lysed, followed by immunoblotting with anti-actin antibody, anti-sgk2 antibody or anti-Nedd4-2 antibody, respectively. (c). Densitometry plot of results from Fig. 7a, as well as from other experiments. The values are mean ± S.E. (n = 3). *P<0.05, NS: statistically not significant.
3.7 Effect of sgk2 on Nedd4-2-mediated transport activity of hOAT4
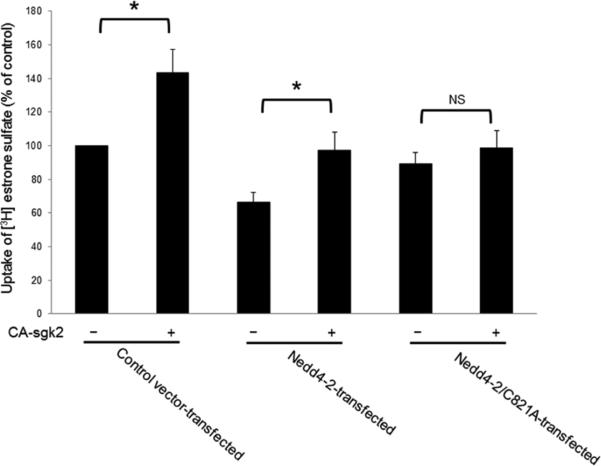
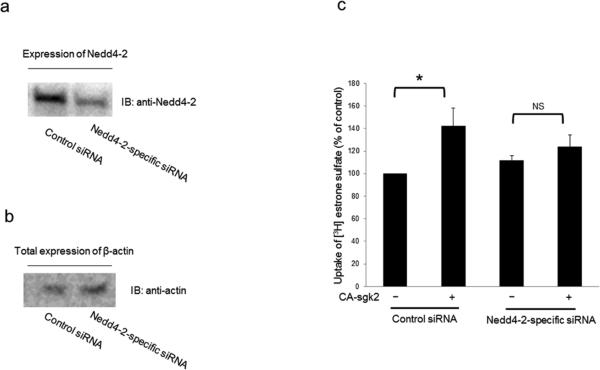
In our experiments described above, we showed that sgk2 and Nedd4-2 modulated hOAT4 expression at the cell surface. Therefore, in this experiment, we investigated whether the altered surface expression will translate into hOAT4 functional change. As shown in Fig. 8, sgk2 stimulated hOAT4 transport activity by 44% in control cells. In cells transfected with Nedd4-2 for 48 hours, the initial hOAT4 transport activity was already considerably decreased as compared to that in control cells. This was consistent with the inhibition effect of Nedd4-2 on hOAT4 function (Fig. 5d). Additional expression of sgk2 abrogated the inhibition effect of Nedd4-2 on hOAT4 transport activity. However, in cells transfected with an ubiquitin ligase-dead mutant of Nedd4-2 (Nedd4-2/C821A), sgk2 was unable to enhance hOAT4 activity. Similar results were also observed in cells transfected with Nedd4-2-specific siRNA to knock down the endogenous Nedd4-2 (Fig. 9). As shown in Fig. 9a, Nedd4-2-specific siRNA effectively reduced the expression of endogenous Nedd4-2 without interference with the expression of the house-keeping protein β-actin was (Fig. 9b). Under such condition, sgk2 failed to significantly stimulate hOAT4-mediated transport activity in cells transfected with Nedd4-2-specific siRNA as compared to that in control cells (Fig. 9c).
Fig. 8. Effects of Nedd4-2 ligase activity on sgk2 regulation of hOAT4 transport function.
COS-7 cells were transfected with hOAT4, CA-sgk2 and control vector, or with hOAT4, CA-sgk2 and Nedd4-2, or with hOAT4, CA-sgk2 and ubiquitin ligase-dead Nedd4-2 mutant (Nedd4-2/C821A). Transfected cells were then measured for the uptake of [3H]-estrone sulfate (3-min uptake and 0.1 μM estrone sulfate). The data represent uptake into hOAT4-transfected cells minus uptake into mock cells (parental COS-7 cells). Values are mean ± S.E. (n = 3). *P<0.05, NS: statistically not significant.
Fig 9. Effect of Nedd4-2-specifc siRNA-knock down of endogenous Nedd4-2 on sgk2 regulation of hOAT4 transport activity.
(a) COS-7 cells were co-transfected with hOAT4 and scrambled control siRNA or with hOAT4 and Nedd4-2-specific siRNA. The effectiveness of Nedd4-2-specific siRNA was tested by probing the lysis sample with anti-Nedd4-2 antibody. (b) COS-7 cells were co-transfected with hOAT4 and scrambled control siRNA or with hOAT4 and Nedd4-2-specific siRNA. The expression of the house-keeping protein β-actin was determined by probing the lysis sample with anti-actin antibody. (c) COS-7 cells were co-transfected with hOAT4 and scrambled control siRNA or with hOAT4 and Nedd4-2-specific siRNA. Transfected cells were then measured for the uptake of [3H]-estrone sulfate (3-min uptake and 0.1 μM estrone sulfate). The data represent uptake into hOAT4-transfected cells minus uptake into mock cells (parental COS-7 cells). Values are mean ± S.E. (n = 3). *P<0.05, NS: statistically not significant.
4. Discussion
Active organic anion transport mediated by organic anion transporters (OATs) is a major determinant of the effects of therapeutics and toxic chemicals. Therefore, understanding the molecular and cellular mechanisms underlying OAT regulation is of clinical and pharmacological importance. The present study revealed a new regulatory mechanism for hOAT4-mediated organic anion/drug transport, namely, sgk2 regulated hOAT4 transport activity through modulating the inhibition of the transporter by Nedd4-2.
Our current studies were carried out in a heterologous cell system - COS-7 cells. COS-7 cells offer several useful advantages for study of the cloned organic anion transporter. (i) These cells are kidney origin. Studies in these cells have led to the understanding of other renal transport processes, including organic cation transport [30, 31]. (ii) This cell line does not express endogenous OATs. Therefore, expression of hOAT4 in these cells will allow us to dissect the transport characteristics of hOAT4 without the interference of other organic anion transporters. (iii) The multiple signaling pathways in these cells provide a good experimental model system for delineating the underlying regulatory mechanisms of many transport processes [32, 33]. (iv) The previously published work showed that the transport characteristics of OATs in these cells were in a good agreement with that observed in other systems [34-38].Our studies in COS-7 cells will pave the way for the future work focusing on determining whether the same mechanisms are operative in native epithelia.
In our current study, we first established that sgk2 stimulated hOAT4-mediated estrone sulfate uptake (Fig. 1) as a result of an enhanced maximum transport velocity (Vmax) (Fig. 2), an increased hOAT4 expression (Fig. 3), and a decreased degradation rate (Fig. 4).
One of the mechanisms in which sgk kinases modulate their targets is by interaction with cytosolic mediators. Among these mediators is the ubiquitin ligase Nedd4-2, which has been shown to play a key role in the regulation of many membrane proteins such as epithelial sodium channel ENaC, potassium channel hERG, and the human excitatory amino acid transporter EAAT2 [17, 27, 39]. Nedd4-2 catalyzes the conjugation of ubiquitin molecules to its target membrane proteins, which leads to the internalization of these membrane proteins from cell surface and subsequent degradation. As a result, the amount of these membrane proteins at the cell surface is reduced and their function is decreased. Our current studies identified Nedd4-2 as an important regulator for hOAT4. Nedd4-2 enhanced hOAT4 ubiquitination (Figs. 5a and 5c), and inhibited hOAT4 transport activity (Fig. 5d).
Our current studies also suggest that the effect of Nedd4-2 on hOAT4 occurs through a direct interaction between these two proteins as co-immunoprecipitation experiments detected an association between them (Fig. 6). Nedd4-2 has 4 protein-protein interacting WW domains. These WW domains can bind with the proline-rich PY motif (PPxY) of its substrates such as in the cases of the epithelial sodium channel ENaC [28, 29]. Although hOAT4 sequence does not contain the classic PY motif, it does have a PY-like motif (a proline-containing motif [PLSV]Px[YF]) VPFF in its sequence [40]. On the other hand, it was also reported that Nedd4-2 participates in the regulation of substrates that do not bear PY motifs, such as in the cases of DAT [15] and GLT-1 [16], where the physical association was found for both of them with Nedd4-2.
Our finding that Nedd4-2 was a crucial mediator for sgk2 regulation of hOAT4 expression and transport activity was supported by several lines of evidence: first, our coimmunoprecipitation experiments showed that sgk2 weakened the interaction between Nedd4-2 and hOAT4 (Fig. 6). Secondly, sgk2 abrogated inhibition effect of Nedd4-2 on hOAT4 cell surface expression. However, sgk2 was without any influence on hOAT4 expression in the presence of an ubiquitin ligase-dead Nedd4-2 mutant (Nedd4-2/C821A) (Fig. 7). Thirdly, sgk2 abolished inhibition effect of Nedd4-2 on hOAT4 transport activity. However, sgk2 was without any influence on hOAT4 activity in cells transfected with an ubiquitin ligase-dead Nedd4-2 mutant (Nedd4-2/C821A) (Fig. 8) or in cells transfected with Nedd4-2-specific siRNA to knock down the endogenous Nedd4-2 (Fig. 9).
Sgks, like other protein kinases, exert their effects through phosphorylating their target substrates. The hOAT4 sequence does not bear any putative sgk phosphorylation consensus sites. Therefore, sgk2 may modulate hOAT4 expression and function by phosphorylating an unconventional site(s) in hOAT4 sequence. On the other hand, it has been reported that several transporters and channels are modulated by sgk1, an isoform of sgk2, not directly through phosphorylating the transporters themselves but rather indirectly through phosphorylating the ubiquitin ligase Nedd4-2 [27, 39, 41]. The work aiming at identifying the sgk2-specific phosphorylation sites on hOAT4 and/or Nedd4-2 is currently being carried out in our lab.
In conclusion, this is the first demonstration of a new regulatory mechanism of hOAT4 transport activity: sgk2 stimulates hOAT4 transport activity by abrogating the inhibition effect of Nedd4-2 on the transporter. Our study provides important insight into the understanding of the molecular and cellular bases of hOAT4 regulation.
Acknowledgement
This work was supported by grants (to Dr. Guofeng You) from National Institute of General Medical Sciences (R01-GM079123 and R01-GM097000).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.You G. Structure, function, and regulation of renal organic anion transporters. Medicinal research reviews. 2002;22:602–16. doi: 10.1002/med.10019. [DOI] [PubMed] [Google Scholar]
- 2.Srimaroeng C, Perry JL, Pritchard JB. Physiology, structure, and regulation of the cloned organic anion transporters. Xenobiotica; the fate of foreign compounds in biological systems. 2008;38:889–935. doi: 10.1080/00498250801927435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dantzler WH, Wright SH. The molecular and cellular physiology of basolateral organic anion transport in mammalian renal tubules. Biochimica et biophysica acta. 2003;1618:185–93. doi: 10.1016/j.bbamem.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 4.VanWert AL, Gionfriddo MR, Sweet DH. Organic anion transporters: discovery, pharmacology, regulation and roles in pathophysiology. Biopharmaceutics & drug disposition. 2010;31:1–71. doi: 10.1002/bdd.693. [DOI] [PubMed] [Google Scholar]
- 5.Ahn SY, Nigam SK. Toward a systems level understanding of organic anion and other multispecific drug transporters: a remote sensing and signaling hypothesis. Molecular pharmacology. 2009;76:481–90. doi: 10.1124/mol.109.056564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terada T, Inui K. Gene expression and regulation of drug transporters in the intestine and kidney. Biochemical pharmacology. 2007;73:440–9. doi: 10.1016/j.bcp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Cha SH, Sekine T, Kusuhara H, Yu E, Kim JY, Kim DK, et al. Molecular cloning and characterization of multispecific organic anion transporter 4 expressed in the placenta. The Journal of biological chemistry. 2000;275:4507–12. doi: 10.1074/jbc.275.6.4507. [DOI] [PubMed] [Google Scholar]
- 8.Ugele B, St-Pierre MV, Pihusch M, Bahn A, Hantschmann P. Characterization and identification of steroid sulfate transporters of human placenta. American journal of physiology Endocrinology and metabolism. 2003;284:E390–8. doi: 10.1152/ajpendo.00257.2002. [DOI] [PubMed] [Google Scholar]
- 9.Rabe T, Hosch R, Runnebaum B. Diagnosis of intrauterine fetal growth retardation (IUGR) and placental insufficiency by a dehydroepiandrosterone sulfate (DHAS) loading test. Biological research in pregnancy and perinatology. 1983;4:130–6. [PubMed] [Google Scholar]
- 10.Zhang Q, Hong M, Duan P, Pan Z, Ma J, You G. Organic anion transporter OAT1 undergoes constitutive and protein kinase C-regulated trafficking through a dynamin- and clathrin-dependent pathway. The Journal of biological chemistry. 2008;283:32570–9. doi: 10.1074/jbc.M800298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Q, Suh W, Pan Z, You G. Short-term and long-term effects of protein kinase C on the trafficking and stability of human organic anion transporter 3. International journal of biochemistry and molecular biology. 2012;3:242–9. [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Q, Li S, Patterson C, You G. Lysine 48-linked polyubiquitination of organic anion transporter-1 is essential for its protein kinase C-regulated endocytosis. Molecular pharmacology. 2013;83:217–24. doi: 10.1124/mol.112.082065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miranda M, Sorkin A. Regulation of receptors and transporters by ubiquitination: new insights into surprisingly similar mechanisms. Molecular interventions. 2007;7:157–67. doi: 10.1124/mi.7.3.7. [DOI] [PubMed] [Google Scholar]
- 14.Staub O, Rotin D. Role of ubiquitylation in cellular membrane transport. Physiological reviews. 2006;86:669–707. doi: 10.1152/physrev.00020.2005. [DOI] [PubMed] [Google Scholar]
- 15.Sorkina T, Miranda M, Dionne KR, Hoover BR, Zahniser NR, Sorkin A. RNA interference screen reveals an essential role of Nedd4-2 in dopamine transporter ubiquitination and endocytosis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:8195–205. doi: 10.1523/JNEUROSCI.1301-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vina-Vilaseca A, Bender-Sigel J, Sorkina T, Closs EI, Sorkin A. Protein kinase C-dependent ubiquitination and clathrin-mediated endocytosis of the cationic amino acid transporter CAT-1. The Journal of biological chemistry. 2011;286:8697–706. doi: 10.1074/jbc.M110.186858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Tardon N, Gonzalez-Gonzalez IM, Martinez-Villarreal J, Fernandez-Sanchez E, Gimenez C, Zafra F. Protein kinase C (PKC)-promoted endocytosis of glutamate transporter GLT-1 requires ubiquitin ligase Nedd4-2-dependent ubiquitination but not phosphorylation. The Journal of biological chemistry. 2012;287:19177–87. doi: 10.1074/jbc.M112.355909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen SY, Bhargava A, Mastroberardino L, Meijer OC, Wang J, Buse P, et al. Epithelial sodium channel regulated by aldosterone-induced protein sgk. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:2514–9. doi: 10.1073/pnas.96.5.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naray-Fejes-Toth A, Canessa C, Cleaveland ES, Aldrich G, Fejes-Toth G. sgk is an aldosterone-induced kinase in the renal collecting duct. Effects on epithelial na+ channels. The Journal of biological chemistry. 1999;274:16973–8. doi: 10.1074/jbc.274.24.16973. [DOI] [PubMed] [Google Scholar]
- 20.Rozansky DJ, Wang J, Doan N, Purdy T, Faulk T, Bhargava A, et al. Hypotonic induction of SGK1 and Na+ transport in A6 cells. American journal of physiology Renal physiology. 2002;283:F105–13. doi: 10.1152/ajprenal.00176.2001. [DOI] [PubMed] [Google Scholar]
- 21.Waldegger S, Barth P, Forrest JN, Jr., Greger R, Lang F. Cloning of sgk serine-threonine protein kinase from shark rectal gland - a gene induced by hypertonicity and secretagogues. Pflugers Archiv : European journal of physiology. 1998;436:575–80. doi: 10.1007/s004240050674. [DOI] [PubMed] [Google Scholar]
- 22.Leong ML, Maiyar AC, Kim B, O'Keeffe BA, Firestone GL. Expression of the serum- and glucocorticoid-inducible protein kinase, Sgk, is a cell survival response to multiple types of environmental stress stimuli in mammary epithelial cells. The Journal of biological chemistry. 2003;278:5871–82. doi: 10.1074/jbc.M211649200. [DOI] [PubMed] [Google Scholar]
- 23.Buse P, Tran SH, Luther E, Phu PT, Aponte GW, Firestone GL. Cell cycle and hormonal control of nuclear-cytoplasmic localization of the serum- and glucocorticoid-inducible protein kinase, Sgk, in mammary tumor cells. A novel convergence point of anti-proliferative and proliferative cell signaling pathways. The Journal of biological chemistry. 1999;274:7253–63. doi: 10.1074/jbc.274.11.7253. [DOI] [PubMed] [Google Scholar]
- 24.Pao AC, Bhargava A, Di Sole F, Quigley R, Shao X, Wang J, et al. Expression and role of serum and glucocorticoid-regulated kinase 2 in the regulation of Na+/H+ exchanger 3 in the mammalian kidney. American journal of physiology Renal physiology. 2010;299:F1496–506. doi: 10.1152/ajprenal.00075.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rotin D, Staub O. Nedd4-2 and the regulation of epithelial sodium transport. Frontiers in physiology. 2012;3:212. doi: 10.3389/fphys.2012.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang B, Kumar S. Nedd4 and Nedd4-2: closely related ubiquitin-protein ligases with distinct physiological functions. Cell death and differentiation. 2010;17:68–77. doi: 10.1038/cdd.2009.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naray-Fejes-Toth A, Snyder PM, Fejes-Toth G. The kidney-specific WNK1 isoform is induced by aldosterone and stimulates epithelial sodium channel-mediated Na+ transport. Proc Natl Acad Sci U S A. 2004;101:17434–9. doi: 10.1073/pnas.0408146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macias MJ, Hyvonen M, Baraldi E, Schultz J, Sudol M, Saraste M, et al. Structure of the WW domain of a kinase-associated protein complexed with a proline-rich peptide. Nature. 1996;382:646–9. doi: 10.1038/382646a0. [DOI] [PubMed] [Google Scholar]
- 29.Staub O, Dho S, Henry P, Correa J, Ishikawa T, McGlade J, et al. WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle's syndrome. The EMBO journal. 1996;15:2371–80. [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X, Evans KK, Wright SH. Molecular cloning of rabbit organic cation transporter rbOCT2 and functional comparisons with rbOCT1. American journal of physiology Renal physiology. 2002;283:F124–33. doi: 10.1152/ajprenal.00367.2001. [DOI] [PubMed] [Google Scholar]
- 31.Nagai K, Takikawa O, Kawakami N, Fukao M, Soma T, Oda A, et al. Cloning and functional characterization of a novel up-regulator, cartregulin, of carnitine transporter, OCTN2. Archives of biochemistry and biophysics. 2006;452:29–37. doi: 10.1016/j.abb.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Kazanietz MG, Caloca MJ, Aizman O, Nowicki S. Phosphorylation of the catalytic subunit of rat renal Na+, K+-ATPase by classical PKC isoforms. Archives of biochemistry and biophysics. 2001;388:74–80. doi: 10.1006/abbi.2000.2264. [DOI] [PubMed] [Google Scholar]
- 33.Cobb BR, Ruiz F, King CM, Fortenberry J, Greer H, Kovacs T, et al. A(2) adenosine receptors regulate CFTR through PKA and PLA(2). American journal of physiology Lung cellular and molecular physiology. 2002;282:L12–25. doi: 10.1152/ajplung.2002.282.1.L12. [DOI] [PubMed] [Google Scholar]
- 34.Wolff NA, Thies K, Kuhnke N, Reid G, Friedrich B, Lang F, et al. Protein kinase C activation downregulates human organic anion transporter 1-mediated transport through carrier internalization. Journal of the American Society of Nephrology : JASN. 2003;14:1959–68. doi: 10.1097/01.asn.0000079040.55124.25. [DOI] [PubMed] [Google Scholar]
- 35.Zhou F, Hong M, You G. Regulation of human organic anion transporter 4 by progesterone and protein kinase C in human placental BeWo cells. American journal of physiology Endocrinology and metabolism. 2007;293:E57–61. doi: 10.1152/ajpendo.00696.2006. [DOI] [PubMed] [Google Scholar]
- 36.Zhou F, Illsley NP, You G. Functional characterization of a human organic anion transporter hOAT4 in placental BeWo cells. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 2006;27:518–23. doi: 10.1016/j.ejps.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Shuprisha A, Lynch RM, Wright SH, Dantzler WH. PKC regulation of organic anion secretion in perfused S2 segments of rabbit proximal tubules. American journal of physiology Renal physiology. 2000;278:F104–9. doi: 10.1152/ajprenal.2000.278.1.F104. [DOI] [PubMed] [Google Scholar]
- 38.Gekle M, Mildenberger S, Sauvant C, Bednarczyk D, Wright SH, Dantzler WH. Inhibition of initial transport rate of basolateral organic anion carrier in renal PT by BK and phenylephrine. The American journal of physiology. 1999;277:F251–6. doi: 10.1152/ajprenal.1999.277.2.F251. [DOI] [PubMed] [Google Scholar]
- 39.Lamothe SM, Zhang S. The serum- and glucocorticoid-inducible kinases SGK1 and SGK3 regulate hERG channel expression via ubiquitin ligase Nedd4-2 and GTPase Rab11. The Journal of biological chemistry. 2013;288:15075–84. doi: 10.1074/jbc.M113.453670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fang NN, Chan GT, Zhu M, Comyn SA, Persaud A, Deshaies RJ, et al. Rsp5/Nedd4 is the main ubiquitin ligase that targets cytosolic misfolded proteins following heat stress. Nature cell biology. 2014;16:1227–37. doi: 10.1038/ncb3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boehmer C, Henke G, Schniepp R, Palmada M, Rothstein JD, Broer S, et al. Regulation of the glutamate transporter EAAT1 by the ubiquitin ligase Nedd4-2 and the serum and glucocorticoid inducible kinase isoforms SGK1/3 and protein kinase B. Journal of neurochemistry. 2003;86:1181–8. doi: 10.1046/j.1471-4159.2003.01937.x. [DOI] [PubMed] [Google Scholar]