Abstract
As many organic molecules, formic acid (HCOOH) has two conformers (trans and cis). The energy barrier to internal conversion from trans to cis is much higher than the thermal energy available in molecular clouds. Thus, only the most stable conformer (trans) is expected to exist in detectable amounts. We report the first interstellar detection of cis-HCOOH. Its presence in ultraviolet (UV) irradiated gas exclusively (the Orion Bar photodissociation region), with a low trans-to-cis abundance ratio of 2.8 ± 1.0, supports a photoswitching mechanism: a given conformer absorbs a stellar photon that radiatively excites the molecule to electronic states above the interconversion barrier. Subsequent fluorescent decay leaves the molecule in a different conformer form. This mechanism, which we specifically study with ab initio quantum calculations, was not considered in Space before but likely induces structural changes of a variety of interstellar molecules submitted to UV radiation.
Keywords: Astrochemistry, Line: identification, ISM: clouds, ISM: molecules, Photon-dominated region (PDR)
1. Introduction
Conformational isomerism refers to isomers (molecules with the same formula but different chemical structure) having the same chemical bonds but different geometrical orientations around a single bond. Such isomers are called conformers. An energy barrier often limits the isomerization. This barrier can be overcome by light. Photoisomerization (or photoswitching) has been studied in ice IR-irradiation experiments (e.g. Maçôas et al. 2004), in biological processes, and, for large polyatomic molecules, also in gas-phase experiments (Ryan & Levy 2001). HCOOH is the simplest organic acid and has two conformers (trans and cis) depending on the orientation of the hydrogen single bond. The most stable trans conformer was the first acid detected in the interstellar medium, ISM (Zuckerman et al. 1971). Gas-phase trans-HCOOH shows moderate abundances towards hot cores (Liu et al. 2001) and hot corinos (Cazaux et al. 2003), in cold dark clouds (Cernicharo et al. 2012), and in cometary coma (Bockelée-Morvan et al. 2000). Solid HCOOH is present in interstellar ices (Keane et al. 2001) and in chondritic meteorites (Briscoe & Moore 1993).
The ground-vibrational state of cis-HCOOH is 1365 ± 30 cm−1 higher in energy than the trans conformer (Hocking 1976). The energy barrier to internal rotation (the conversion from trans to cis) is about 4827 cm−1 (Hocking 1976), approximately 7000 K in temperature units. This is much higher than the thermal energy available in molecular clouds (having typical temperatures from about 10 to 300 K). Generalizing this reasoning, only the most stable conformer of a given species would be expected in such clouds. Photoswitching, however, may be a viable mechanism producing the less stable conformers in detectable amounts: a given conformer absorbs a high-energy photon that radiatively excites the molecule to electronic states above the interconversion energy barrier. Subsequent radiative decay to the ground-state would leave the molecule in a different conformer.
In this work we have searched for pure rotational lines of the trans- and cis-HCOOH conformers in the 3 millimetre spectral band. We observed three prototypical interstellar sources known to display a very rich chemistry and bright molecular line emission: (i) the Orion Bar photodissociation region (PDR): the edge of the Orion cloud irradiated by ultraviolet (UV) photons from nearby massive stars (e.g. Goicoechea et al. 2016), (ii) the Orion hot core: warm gas around massive protostars (e.g. Tercero et al. 2010), and (iii) Barnard 1-b (B1-b): a cold dark cloud (e.g. Cernicharo et al. 2012). The two latter sources are shielded from strong UV radiation fields. We only detect cis-HCOOH towards the Orion Bar. This represents the first interstellar detection of the conformer.
2. Source selection and observations
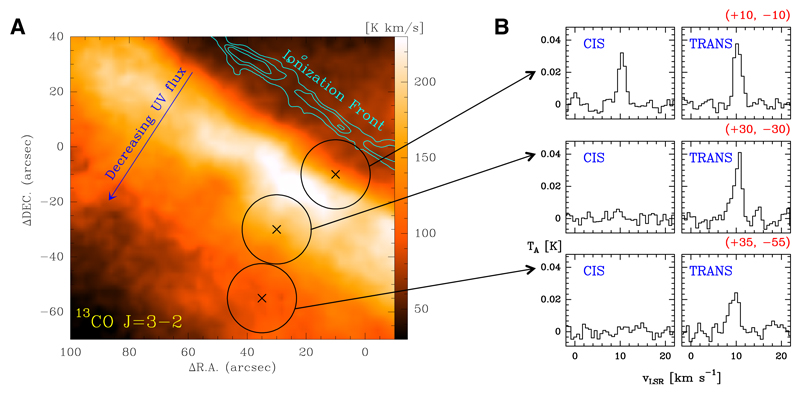
Because of its nearly edge-on orientation, the Orion Bar PDR is a template source to study the molecular content as the far-UV radiation field (FUV; stellar photons with energies below 13.6 eV, or wavelengths (λ) longer than 911 Å, the hydrogen atom ionisation threshold) is attenuated from the cloud edge to the interior (Hollenbach & Tielens 1999). The impinging FUV radiation field at the edge of the Bar is about 4 × 104 times the mean interstellar radiation field (e.g. Goicoechea et al. 2016, and references therein). We observed three positions of the Bar characterized by a decreasing FUV photon flux.
We have used the IRAM-30 m telescope (Pico Veleta, Spain) and the 90 GHz EMIR receiver. We employed the Fast Fourier Transform Spectrometer (FFTS) backend at 200 kHz spectral resolution (0.7 km s−1 at 90 GHz). Observations towards the Orion Bar are part of a complete millimetre (mm) line survey (80 − 360 GHz, Cuadrado et al. 2015). They include specific deep searches for HCOOH lines in the 3 mm band towards three different positions located at a distance of 14″, 40″, and 65″ from the ionisation front (Fig. 1A). Their offset coordinates with respect to the α2000 = 05h 35m 20.1s , δ2000 = − 05°25′07.0″ position at the ionisation front are (+10″, −10″), (+30″, -30″’), and (+35″, -55″). The observing procedure was position switching with a reference position at (−600″, 0″) to avoid the extended emission from the Orion molecular cloud. The half power beam width (HPBW) at 3 mm ranges from ∼30.8″ to 21.0″. We reduced and analysed the data using the GILDAS software as described in Cuadrado et al. (2015). The antenna temperature, , was converted to the main beam temperature, TMB, using where ηMB is the antenna efficiency (ηMB = 0.87 − 0.82 at 3 mm). The rms noise obtained after 5 h integration is ∼1 − 5 mK per resolution channel.
Fig. 1.
Detection of cis-HCOOH towards the FUV-illuminated edge of the Orion Bar. Left: 13CO J = 3 → 2 integrated emission image with a HPBW of 8′ obtained with the IRAM-30 m telescope (Cuadrado et al. in prep.). The cyan contour marks the position of neutral cloud boundary traced by the O i 1.317 µm fluorescent line emission (in contours from 3 to 7 by 2 × 10−4 erg s−1 cm−2 sr−1; Walmsley et al. 2000). Right: Cis- and trans-HCOOH stacked spectra towards the observed positions.
We also searched for HCOOH in regions shielded from strong FUV radiation fields (see Appendix E). We selected two chemically rich sources for which we have also carried out deep mm-line surveys with the IRAM-30m telescope: towards the hot core in Orion BN/KL (Tercero et al. 2010) and towards the quiescent dark cloud Barnard 1-b (B1-b; Cernicharo et al. 2012).
3. Results
3.1. Line identification
We specifically computed the cis-HCOOH rotational lines frequencies by fitting the available laboratory data (Winnewisser et al. 2002) with our own spectroscopic code, MADEX (Cernicharo 2012). The standard deviation of the fit is 60 kHz. For the trans conformer, higher frequency laboratory data (Cazzoli et al. 2010) were also used in a separate fit. The standard deviation of the fit for trans-HCOOH is 42 kHz. These deviations are smaller than the frequency resolution of the spectrometer we used to carry out the astronomical observations. Formic acid is a near prolate symmetric molecule with rotational levels distributed in different Ka rotational ladders (Ka = 0, 1, 2…). Both a- and b-components of its electric dipole moment µ exist (Winnewisser et al. 2002). The dipole moments of the cis conformer (µa = 2.650 D and µb = 2.710 D, Hocking 1976) are stronger than those of the trans conformer (µa = 1.421 D and µb = 0.210 D, Kuze et al. 1982).
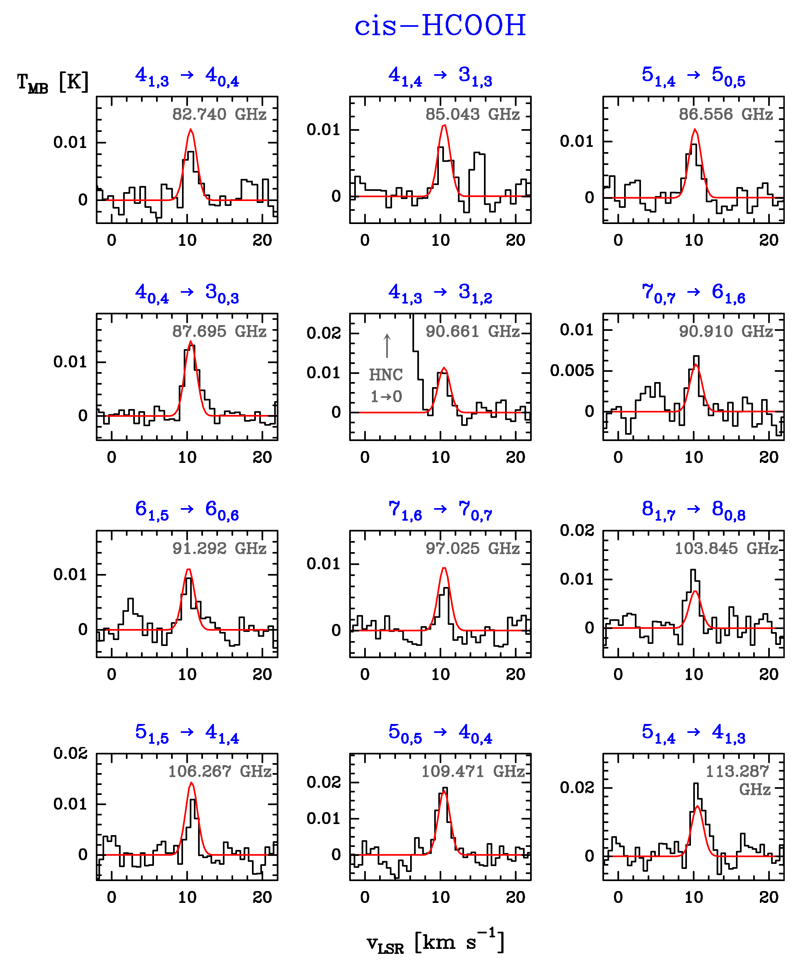
In total, we identify 12 rotational lines of cis-HCOOH and 10 of trans-HCOOH above 3σ towards the FUV-illuminated edge of the Orion Bar, (+10″, -10″) position. The detected lines from the cis- and trans-HCOOH are shown in Figs. 2 and D.1, respectively. Lines attributed to HCOOH show a Gaussian line profile centred at the systemic velocity of the Orion Bar (10.4 ± 0.3 km s−1). Lines are narrow, with linewidths of 1.9 ± 0.3 km s−1. The large number of detected lines, and the fact that none of the lines correspond to transitions of abundant molecules known to be present in the Bar or in spectroscopic line catalogues, represents a robust detection of the cis conformer. The observational parameters and Gaussian fit results are tabulated in Tables F.1 and F.2 for the cis and trans conformer, respectively.
Fig. 2.
Detected cis-HCOOH rotational lines towards the Orion Bar, (+10″, -10″) position. The ordinate refers to the intensity scale in main beam temperature units, and the abscissa to the LSR velocity. Line frequencies (in GHz) are indicated at the top-right of each panel together with the rotational quantum numbers (in blue). The red curve shows an excitation model that reproduces the observations.
3.2. Line stacking analysis
Complex organic molecules have relatively low abundances in FUV-irradiated interstellar gas (Guzmán et al. 2014). Indeed, detected trans-HCOOH lines are faint. To improve the statistical significance of our search towards the positions inside the Bar, we performed a “line stacking” analysis. For each observed position, we added spectra at the expected frequency of several HCOOH lines that could be present within the noise level (sharing similar rotational level energies and Einstein coefficients). The spectra in frequency scale were first converted to local standard of rest (LSR) velocity scale and resampled to the same velocity channel resolution before stacking. We repeated this procedure for trans-HCOOH lines. This method allows us to search for any weak line signal from the two conformers that could not be detected individually.
Figure 1B shows a comparison of the stacking results for cis and trans-HCOOH lines towards the three target positions in the Bar. Although we detect trans-HCOOH in all positions, emission from the cis conformer is only detected towards the position located closer to the cloud edge, (+10″, -10″). They demonstrate that cis-HCOOH is detected close the FUV-illuminated edge of the Bar, but the emission disappears towards the more shielded cloud interior.
A similar stacking analysis was carried out for the Orion hot core and B1-b spectra. Although we detect several trans-HCOOH lines, the cis conformer was not detected towards the hot core and the cold dark cloud (see Appendix E).
3.3. Trans-to-cis abundance ratios
Given the number of HCOOH lines detected towards the Bar, we can determine the column density and rotational temperatures of both conformers accurately (see Appendix D). In particular, we infer a low trans-to-cis abundance ratio of 2.8 ± 1.0. The nondetection of cis-HCOOH towards the Orion hot core and B1-b (see Appendix E) provides much higher trans-to-cis limits (>100 and >60, respectively). This suggests that the presence of cis-HCOOH in the Orion Bar PDR is related to the strong FUV field permeating the region.
4. Photoisomerization rates and discussion
Photolysis of HCOOH has been widely studied, both experimentally (Sugarman 1943; Ioannoni et al. 1990; Brouard & Wang 1992; Su et al. 2000) and theoretically (Beaty-Travis et al. 2002; He & Fang 2003; Maeda et al. 2015). Dissociation of HCOOH takes place after absorption of FUV photons with energies greater than ∼5 eV (λ < 2500 Å). Recently, Maeda et al. (2015) determined that this dissociation threshold coincides with the crossing of the S0 and T1 electronic states of the molecule. The specific products of the photofragmentation process (of the different photodissociation channels) depend on the specific energy of the FUV photons and on the initial HCOOH conformer. Interestingly, absorption of lower energy photons does not dissociate the molecule but induces fluorescent emission. In particular, HCOOH fluorescence from the S1 excited electronic state has been observed in laser-induced experiments performed in the λ = 2500 − 2700 Å range (Ioannoni et al. 1990; Brouard & Wang 1992). These studies indicate that the geometrical configuration of the two hydrogen atoms is different in the S0 and S1 states. The fluorescence mechanism from the S1 state is a likely route for the trans → cis isomerization. In addition, the isomerization barrier from the S1 state (∼1400 cm−1) is much lower than from the ground.
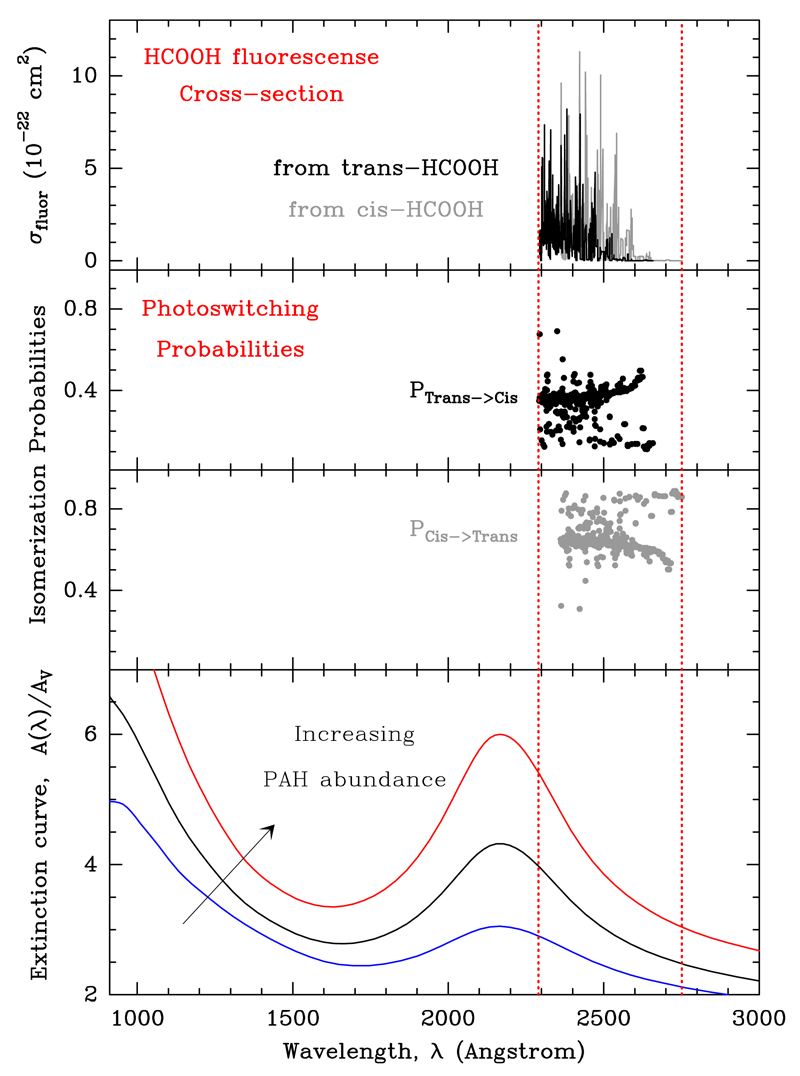
In order to quantify the role of the photoswitching mechanism, we carried out ab initio quantum calculations and determined the HCOOH potential energy surfaces of the S0 and S1 electronic states as a function of the two most relevant degrees of freedom, ϕ1 the torsional angle of OH and ϕ2, the torsional angle of CH (see Appendix A and Fig. A.1). With this calculation we can compute the position of the photon absorptions leading to fluorescence (those in the approximate λ = 2300 − 2800 Å range), and the probabilities to fluoresce from one conformer to the other (the trans-to-cis and cis-to-trans photoswitching cross-sections and probabilities, see Fig. 3).
Fig. 3.
Ab initio absorption cross-sections and photoisomerization probabilities computed in this work. Top panel: trans- and cis-HCOOH absorption cross-sections for photons with E < 5 eV (those leading to fluorescence). Middle panels: normalized probabilities of bound-bound decays producing isomerization (trans → cis and cis → trans). Bottom panel: standard interstellar dust extinction curve (blue). Black and red curves show the effect of an increased PAH abundance.
With a knowledge of Nph(λ), the FUV photon flux in units of photon cm−2 s−1 Å−1, we can calculate the number of trans-to-cis and cis-to-trans photoisomerizations per second (ξtc and ξct, respectively. See Appendix B). In the absence of any other mechanism destroying HCOOH, the ξct/ξtc ratio provides the trans-to-cis abundance ratio in equilibrium. The time needed to reach the equilibrium ratio is then (ξtc + ξct)−1. Nph(λ), and thus ξtc and ξct, depend on the FUV radiation sources (type of star) and on the cloud position. Describing the cloud depth position in terms of the visual extinction into the cloud (AV), one magnitude of extinction is equivalent to a column density of about 1021 H2 molecules per cm−2 in the line-of-sight.
In general (for a flat, wavelength-independent FUV radiation field), HCOOH photodissociation will always dominate over fluorescence (photodissociation cross-sections are larger and the relevant photons can be absorbed over a broader energy range, E > 5 eV). The strength and shape of the interstellar FUV radiation field, however, is a strong function of AV and is very sensitive to the dust and gas absorption properties. Because of the wavelength-dependence of the FUV-absorption process, Nph(λ) drastically changes as one moves from the cloud edge to the shielded interior. In particular, the number of low-energy FUV photons (e.g. below 5 eV) relative to the high-energy photons (e.g. those above 11 eV dissociating molecules such as CO and ionising atoms such as carbon) increases with AV. In this work we used a FUV radiative transfer and thermo-chemical model (Le Petit et al. 2006; Goicoechea & Le Bourlot 2007) to estimate Nph(λ) at different positions of the Orion Bar. The well-known “2175 Å bump” of the dust extinction curve (absorption of λ = 1700 − 2500 Å photons by PAHs and small carbonaceous grains, Cardelli et al. 1989; Joblin et al. 1992) greatly reduces the number of HCOOH dissociating photons relative to those producing HCOOH fluorescence (Fig. 3, bottom panel). The resulting FUV radiation spectrum, Nph(λ), at different AV is used to calculate ξtc and ξct (Table B.1). We determine that at a cloud depth of about AV = 2 − 3 mag, and if the number of HCOOH dissociating photons is small compared to the number of photons producing photoisomerization (i.e. most photons with E > 5 eV have been absorbed), the cis conformer should be detectable with a trans-to-cis abundance ratio of about 3.5 − 4.1. These values are remarkably close to the trans-to-cis ratio inferred from our observations of the Bar.
Closer to the irradiated cloud edge (AV = 0 − 2 mag), photodissociation destroys the molecule much faster than the time needed for the trans-to-cis isomerization. On the other hand, too deep inside the cloud, the flux of E > 5 eV photons decreases to values for which the isomerization equilibrium would take an unrealistic amount of time (>106 years for AV = 5 mag). Therefore, our detection of cis-HCOOH in irradiated cloud layers where CO becomes the dominant carbon carrier (a signature of decreasing flux of high-energy FUV photons) agrees with the photoswitching scenario.
For standard grain properties and neglecting HCOOH photodissociation, we calculate that the time needed to achieve a low trans-to-cis abundance ratio and make cis-HCOOH detectable at AV = 2 − 3 mag is 104 − 105 years (see Table B.1). This is reasonably fast, and shorter than the cloud lifetime. In practice, it is not straightforward to quantify the exact contribution of HCOOH photodissociation and photoisomerization at different cloud positions. The above time-scales require that the flux of E > 5 eV dissociating photons is small compared to those producing fluorescence. This depends on the specific dust absorption properties, that sharply change with AV as dust populations evolve (Draine 2003), on the strength and width of the 2175 Å extinction bump, and on the role of molecular electronic transitions blanketing the FUV spectrum. The similar trans-HCOOH line intensities observed towards the three positions of the Bar (Fig. 1) suggest that even if the HCOOH photodestruction rate increases at the irradiated cloud edge, the HCOOH formation rate (from gas-phase reactions or desorbing directly from grain surfaces, Garrod et al. 2008) must increase as well. The inferred HCOOH abundances are not particularly high, (0.6 − 3.0) × 10−10 with respect to H. Hence, modest HCOOH photodestruction and formation rates are compatible with the photoswitching mechanism occurring in realistic times.
Although the observed abundances of trans- and cis-HCOOH in the Orion Bar are compatible with gas-phase photoisomerization, we note that photoswitching may also occur on the surface of grains covered by HCOOH ices. In a similar way, solid HCOOH (mostly trans) can absorb FUV photons that switch the molecule to the cis form before being desorbed. Once in the gas, both conformers will continue their photoisomerization following absorption of λ ≳ 2500 Å photons. Laboratory experiments are needed to quantify the mechanisms leading to HCOOH ice photoswitching by FUV photon absorption.
Searching for further support to the FUV photoswitching scenario, we qualitatively explored two other possibilities for the trans-to-cis conversion. First, the isomerization of solid HCOOH after IR irradiation of icy grain surfaces (as observed in the laboratory, Maçôas et al. 2004; Olbert-Majkut et al. 2008) and subsequent desorption to the gas-phase. Second, the gas-phase isomerization by collisions of HCOOH with energetic electrons (∼0.5 eV). We concluded that if these were the dominant isomerization mechanisms, emission lines from cis-HCOOH would have been detected in other interstellar sources (see Appendix C).
Isomerization by absorption of UV photons was not considered as a possible mechanism to induce structural changes of molecules in interstellar gas. The detection of cis-HCOOH towards the Orion Bar opens new avenues to detect a variety of less stable conformers in Space. This can have broad implications in astrochemistry and astrobiology.
Supplementary Material
Acknowledgements
We thank N. Marcelino for helping with the observations of B1-b. We thank the ERC for support under grant ERC-2013-Syg-610256-NANOCOSMOS. We also thank Spanish MINECO for funding support under grants AYA2012-32032 and FIS2014-52172-C2, and from the CONSOLIDER-Ingenio program “ASTROMOL” CSD 2009-00038. IRAM is supported by INSU/CNRS (France), MPG (Germany), and IGN (Spain).
References
- Beaty-Travis LM, Moule DC, Lim EC, Judge RH. J Chem Phys. 2002;117:4831. [Google Scholar]
- Blake GA, Sutton EC, Masson CR, Phillips TG. ApJ. 1987;315:621. [Google Scholar]
- Bockelée-Morvan D, Lis DC, Wink JE, et al. A&A. 2000;353:1101. [Google Scholar]
- Briscoe JF, Moore CB. Meteoritics. 1993;28:330. [Google Scholar]
- Brouard M, Wang J-X. J Chem Soc Faraday Trans. 1992;88:3511. [Google Scholar]
- Cardelli JA, Clayton GC, Mathis JS. ApJ. 1989;345:245. [Google Scholar]
- Cazaux S, Tielens AGGM, Ceccarelli C, et al. ApJ. 2003;593:L51. [Google Scholar]
- Cazzoli G, Puzzarini C, Stopkowicz S, Gauss J. A&A. 2010;520:A64. [Google Scholar]
- Cernicharo J. Vol. 58. EAS Publications Series; 2012. pp. 251–26. [Google Scholar]
- Cernicharo J, Kisiel Z, Tercero B, et al. A&A. 2016;587:L4. doi: 10.1051/0004-6361/201527531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernicharo J, Marcelino N, Roueff E, et al. ApJ. 2012;759:L43. [Google Scholar]
- Cuadrado S, Goicoechea JR, Pilleri P, et al. A&A. 2015;575:A82. [Google Scholar]
- Draine BT. ApJS. 1978;36:595. [Google Scholar]
- Draine BT. ARA&A. 2003;41:241. [Google Scholar]
- Garrod RT, Widicus Weaver SL, Herbst E. ApJ. 2008;682:283. [Google Scholar]
- Gerin M, Pety J, Fuente A, et al. A&A. 2015;577:L2. [Google Scholar]
- Goicoechea JR, Le Bourlot J. A&A. 2007;467:1. [Google Scholar]
- Goicoechea JR, Pety J, Cuadrado S, et al. Nature. 2016;537:207. doi: 10.1038/nature18957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith PF, Langer WD. ApJ. 1999;517:209. [Google Scholar]
- Guzmán VV, Pety J, Gratier P, et al. Faraday Discussions. 2014;168:103. doi: 10.1039/c3fd00114h. [DOI] [PubMed] [Google Scholar]
- He H-Y, Fang W-H. Journal of the American Chemical Society. 2003;125:16139. doi: 10.1021/ja0363157. [DOI] [PubMed] [Google Scholar]
- Hocking WH. Zeitschrift Naturforschung Teil A. 1976;31:1113. [Google Scholar]
- Hollenbach DJ, Tielens AGGM. Reviews of Modern Physics. 1999;71:173. [Google Scholar]
- Ioannoni F, Moule DC, Clouthier DJ. The Journal of Physical Chemistry. 1990;94:2290. [Google Scholar]
- Joblin C, Leger A, Martin P. ApJ. 1992;393:L79. [Google Scholar]
- Jørgensen JK, Harvey PM, Evans NJ, II, et al. ApJ. 2006;645:1246. [Google Scholar]
- Keane JV, Tielens AGGM, Boogert ACA, Schutte WA, Whittet DCB. A&A. 2001;376:254. [Google Scholar]
- Kuze H, Kuga T, Shimizu T. Journal of Molecular Spectroscopy. 1982;93:248. [Google Scholar]
- Le Petit F, Nehmé C, Le Bourlot J, Roueff E. ApJS. 2006;164:506. [Google Scholar]
- Li A, Draine BT. ApJ. 2001;554:778. [Google Scholar]
- Liu S-Y, Mehringer DM, Snyder LE. ApJ. 2001;552:654. [Google Scholar]
- Maçôas EMS, Khriachtchev L, Pettersson M, Fausto R, Räsänen M. J Chem Phys. 2004;121:1331. doi: 10.1063/1.1760733. [DOI] [PubMed] [Google Scholar]
- Maeda S, Taketsugu T, Morokuma K. The Journal of Physical Chemistry Letters. 2012;3:1900. doi: 10.1021/jz300728q. [DOI] [PubMed] [Google Scholar]
- Maeda S, Taketsugu T, Ohno K, Morokuma K. Journal of the American Chemical Soiety. 2015;137:3433. doi: 10.1021/ja512394y. [DOI] [PubMed] [Google Scholar]
- Marcelino N. Universidad de Granada, Spain: 2007. PhD thesis. [Google Scholar]
- Öberg KI, Bottinelli S, Jørgensen JK, van Dishoeck EF. ApJ. 2010;716:825. [Google Scholar]
- Olbert-Majkut A, Ahokas J, Lundell J, Pettersson M. J Chem Phys. 2008;129:041101. doi: 10.1063/1.2955985. [DOI] [PubMed] [Google Scholar]
- Ryan WL, Levy DH. Journal of the American Chemical Society. 2001;123:961. doi: 10.1021/ja003261h. [DOI] [PubMed] [Google Scholar]
- Su H, He Y, Kong F, Fang W, Liu R. J Chem Phys. 2000;113:1891. [Google Scholar]
- Sugarman B. Proceedings of the Physical Society. 1943;55:429. [Google Scholar]
- Tercero B, Cernicharo J, Pardo JR, Goicoechea JR. A&A. 2010;517:A96. [Google Scholar]
- van Dishoeck EF, Black JH. ApJ. 1982;258:533. [Google Scholar]
- Walmsley CM, Natta A, Oliva E, Testi L. A&A. 2000;364:301. [Google Scholar]
- Werner H-J, Knowles PJ, Knizia G, Manby FR, Schtz M. Wiley Interdisciplinary Reviews: Computational Molecular Science. 2012;2:242. [Google Scholar]
- Winnewisser M, Winnewisser BP, Stein M, et al. Journal of Molecular Spectroscopy. 2002;216:259. [Google Scholar]
- Zuckerman B, Ball JA, Gottlieb CA. ApJ. 1971;163:L41. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.