Abstract
Antifreeze (glyco)proteins (AF(G)Ps) have potent ice recrystallisation inhibition (IRI) activity – a desirable phenomenon in applications such as cryopreservation, frozen food and more. In Nature AF(G)P activity is regulated by protein expression levels in response to an environmental stimulus; temperature. However, this level of regulation is not possible in synthetic systems. Here, a synthetic macromolecular mimic is introduced, using supramolecular assembly to regulate activity. Catechol-terminated poly(vinyl alcohol) was synthesised by RAFT polymerization. Upon addition of Fe3+, larger supramolecular star polymers form by assembly with two or three catechols. This increase in molecular weight effectively ‘switches on’ the IRI activity and is the first example of external control over the function of AFP mimetics. This provides a simple but elegant solution to the challenge of external control of AFP-mimetic function.
Antifreeze (glyco) proteins (AF(G)Ps) have evolved to modulate ice formation/growth, enabling life to survive in extreme cold environments.1,2 AF(G)Ps have 3 key macroscopic effects: (i) Thermal hysteresis (non-colligative freezing point depression) (TH); (ii) dynamic ice shaping (DIS) and (iii) ice recrystallisation inhibition (IRI). TH and DIS are linked to the AFP’s ability to bind to specific ice crystal faces and require precise 3D arrangements to promote such interactions. It is therefore challenging to reproduce these properties with synthetic materials,3 and particularly with AFGPs.4 However, it is now possible to generate IRI activity without requiring the structural features of AF(G)Ps, with a mode of action that is mechanistically distinct from TH/DIS.5,6 For example, Ben et al. have developed glycopeptides7 and alkylated glycans which exhibit IRI activity, despite few structural similarities to native AFGPs.8 Gibson and co-workers have demonstrated IRI activity using synthetic polymers,9,10 namely poly(vinyl alcohol) (PVA) and a Dr M. I. Gibson poly(ampholytes)11,12 (polymers with mixed cationic and anionic charges). Zirconium acetate has also been found to have some IRI activity, possibly via the formation of polymeric structures.13,14 The design and synthesis of IRI active materials could have major applications, especially in the cryopreservation of donor cells and tissue where ice recrystallisation has been indicated as a major cause of damage. Gibson et al. have demonstrated that the addition of PVA15 or poly(ampholytes)16 to red blood cells significantly enhanced their solvent-free cryopreservation. Similar work using small molecule IRIs with glycerol mediated cryopreservation has also been reported.17
Nature can control the antifreeze activity of AFPs by regulation of protein expression in response to the temperature.18 However, this method of control is not possible with synthetic materials, meaning alternative strategies are required to afford external control over their function. Synthetic polymers can be designed to respond to an external stimulus19 with heat,20 light,21 redox gradients22 and metal binding23 just some of the possible triggers. However, there are no reports of AFP-associated activity being controlled in such a fashion. Previously, we have shown that a significant enhancement in the IRI activity of PVA can be achieved as the degree of polymerisation increases from 10 to 20 units.24 By extension, this observation presents a unique opportunity to modulate AFP activity by triggered supramolecular self-assembly into larger polymers. Herein, this manuscript reports a method to selectively control the IRI activity of PVA using catechol end-groups to increase the effective polymer molecular weight. This is achieved by the addition of Fe3+ which can efficiently co-ordinate 2/3 catechol groups.25
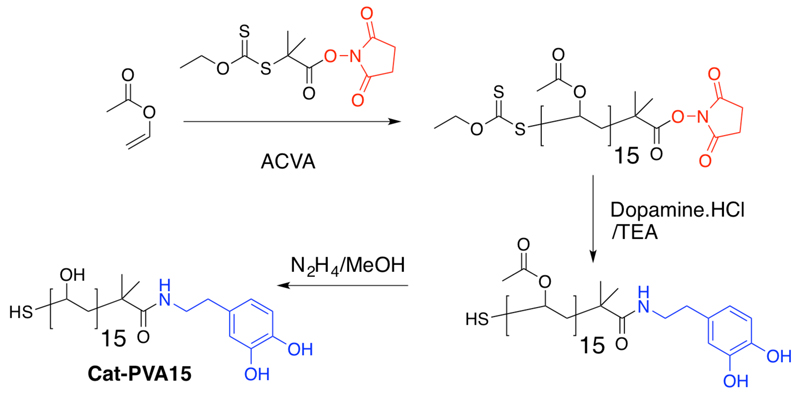
To begin, a well-defined PVA with DP~10 was synthesised bearing a catechol end-group. This polymer can co-ordinate with Fe3+ to give an increase in molecular weight (and hence trigger activity). RAFT/MADIX polymerisation was used to generate well defined polymers with high end-group fidelity, Scheme 1.26 An N-hydroxy succinimide-functional xanthate was synthesised and characterized by 1H and 13C NMR spectroscopy and mass spectrometry (ESI) and used to polymerise vinyl acetate (VAc) in bulk.27 The resulting polymer had an Mn = 1500 g.mol-1 (Ð = 1.25), corresponding to DP = 15.
Scheme 1.
Synthesis of catechol-functionalised PVA (full synthetic details in the ESI.)
To install the desired catechol functionality, dopamine hydrochloride was reacted with the terminal N-hydroxy succinimide group on PVAc. Functionalisation was confirmed by 1H and 13C NMR spectroscopic analysis, where the unique aromatic peaks were observed. Finally, the acetate groups were quantitatively removed using hydrazine hydrate solution, as confirmed by IR and 1H NMR spectroscopy, without affecting the dopamine amide-linkage. Full details and spectra are in the ESI.
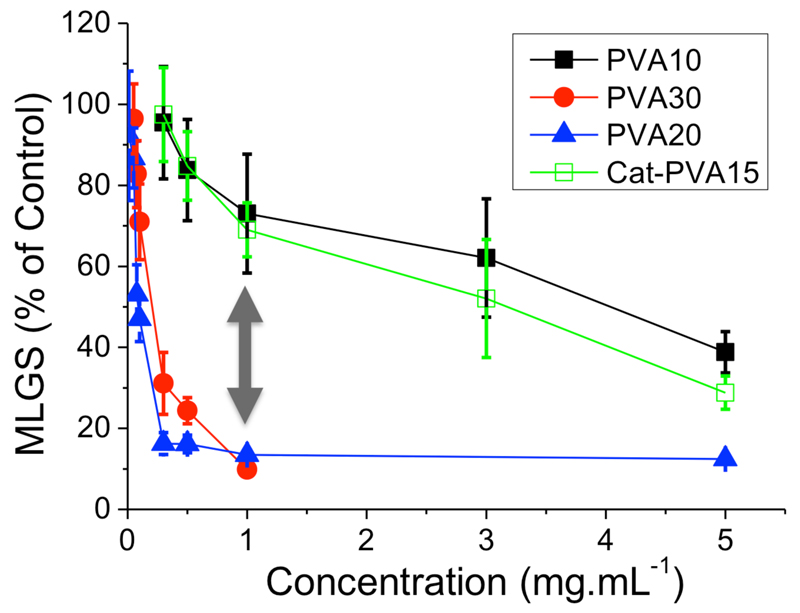
This polymer was then tested for ice recrystallisation inhibition (IRI) activity using a modified splat assay (see ESI). This involves measuring the growth of a polynucleated ice wafer where smaller values indicate less ice growth and hence greater activity. All values reported are calculated from the largest four crystals in each of three micrographs to give a mean largest grain size (MLGS), relative to a PBS control. Figure 1 shows that Cat-PVA15 had similar activity to the non-catechol PVA10, and less than PVA20/PVA30. This activity gap is essential for the molecular-weight triggered activation of IRI (vide infra).
Figure 1.
IRI activity by splat assay. PVA10 – 30 have ester end-groups and their activity has been reported previously.24 Arrow guides the eye to the molecular-weight dependence. MLGS is reported relative to a PBS control. Error bars are ± standard deviation from n ≥3.
With these polymers to hand, the supra-molecular control of IRI activity was evaluated. Addition of Fe3+ to catechol-terminated polymers promotes the formation of 2- and 3-arm star polymers (based upon octahedral assembly around the metal ion) with an ideal Fe3+:catechol ratio of 0.33:1, and has been confirmed by DOSY NMR spectroscopic analysis.23 Figure 2 shows the results of IRI testing of the various polymers with a variety metal ions.
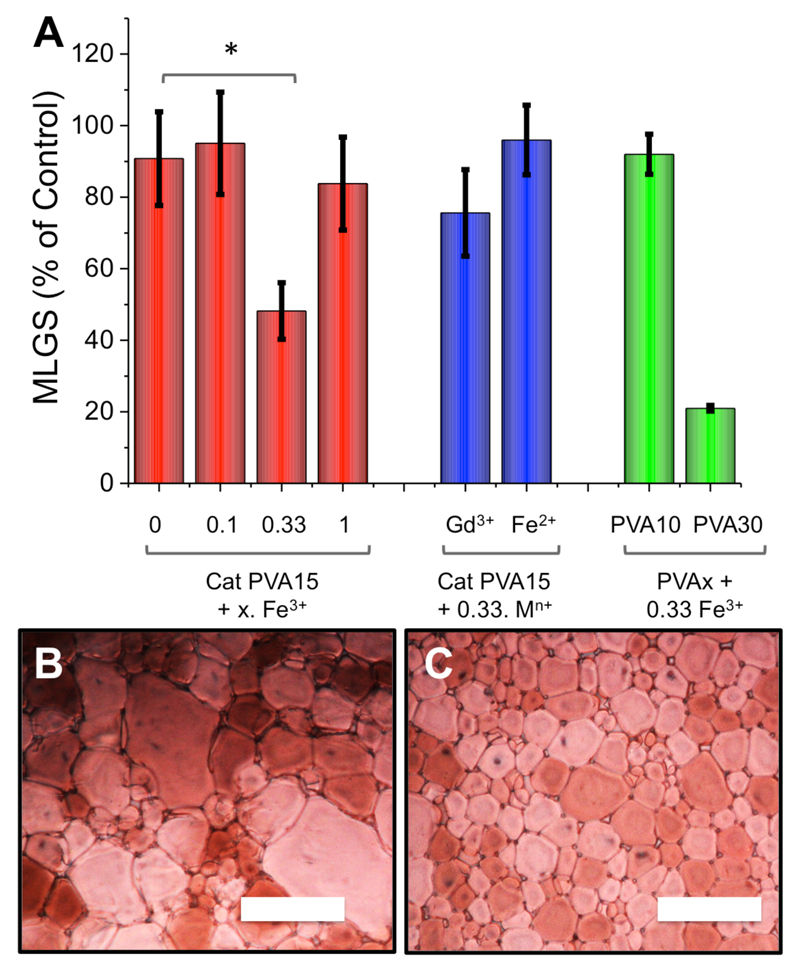
Figure 2.
IRI activity modulation by addition of Fe3+ (FeCl3) as a supramolecular trigger. A) Quantitative evaluation of IRI activity; Cryomicrographs of Cat-PVA15 (B) and with 0.33 equivalents of Fe3+ (C). Error bars are ± standard deviation from n ≥3. * indicates significant differences (p < 0.005). Scale bar = 100 μM. Red colour due to cross-polarisers.
A saline solution of Cat-PVA15 gave MLGS = 90 % indicating no activity at this concentration. The addition of 0.1 molar equivalents of Fe3+ did not cause any significant changes in the observed activity, presumably due to the dynamic nature of the catechol-Fe3+ interaction: A low concentration of Fe3+ will not promote the formation of many high molecular weight species. Increasing [Fe3+] to 0.33 molar equivalents resulted in a dramatic ‘switching on’ of the IRI activity, with the MLGS decreasing to ~ 50 %. This corresponds to the formation of polymers with higher molecular weights driven by catechol binding. The observed magnitude of the activity is less than DP30 PVA suggesting a mixture of 2/3 arms stars forming. Example micrographs showing the difference in crystal size upon activation by Fe3+ are shown in Figure 2B/C. The addition of 1 molar equivalent of Fe3+ removed all IRI activity as at this concentration, all the catechols would be saturated preventing supramolecular assembly. These changes are shown schematically in Figure 3. A series of control experiments were also conducted to assess the specificity of the response. The addition of Fe2+, which does not bind with catechols, failed to significantly change the MLGS. Moreover, the addition of another hexavalent metal, Gd3+, resulted in a small, but not statistically significant, decrease in the MLGS in line with previous reports of catechol binding behavior.28 PVA10 and PVA30 which have ester end-groups (rather than catechols) showed no change in activity upon the addition of 0.33 molar equivalents of Fe3+, confirming the catechol was responsible for this activity. Fe3+-doped saline solution gave identical results to saline alone.
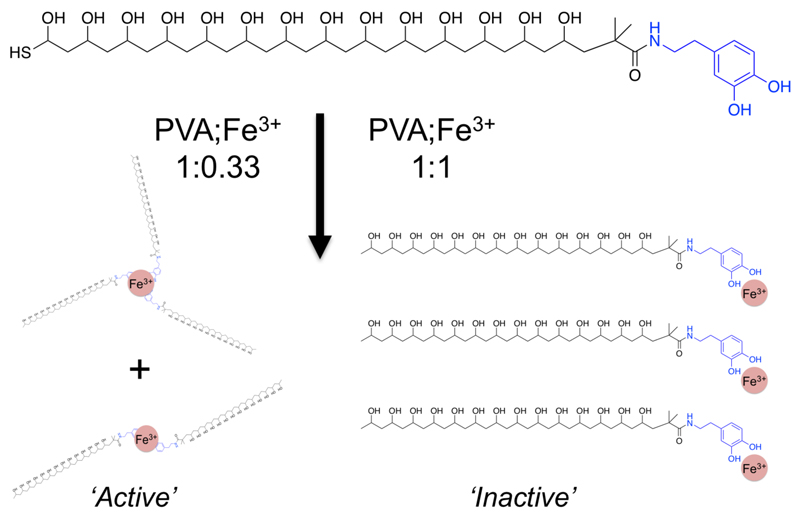
Figure 3.
Schematic explaining the observed effect of Fe3+ concentration on IRI activity: A polymer:Fe3+ ratio of 1:0.33 provides optimum binding and hence largest molecular weight increase.
The above data demonstrates that the incorporation of a supramolecular binding motif enables the AFP mimetic activity of PVA to be triggered upon application of an external stimulus providing an alternative to Nature’s method of controlling AFP function. Such external control could particularly be of use for understanding the mechanism of activity of AFPs, as well as being an example of a new biomimetic
Conclusions
Here we demonstrate the first example of a stimuli-responsive antifreeze protein biomimetic with activity triggered by application of a specific metal ion. A well-defined poly(vinyl alcohol) bearing a catechol end-group was synthesised with the molecular weight tuned such that it was too short to have significant ice recrystallisation inhibition activity. Upon addition of Fe3+, the catechol groups could self-assemble to give 2/3 armed star shaped polymers. This effective increase in molecular weight enhanced the ice recrystallisation inhibition activity enabling it to be activated ordeactivated based upon Fe3+ concentration, but not with other ions. While the Fe3+ trigger itself might not find application, this report demonstrates the first example of external control of AFP mimetic function and the development of ‘smart’ ice controlling materials, and may be a useful tool for understanding the origins fo the IRI activity.
Supplementary Material
Acknowledgements
Equipment used was supported by the Innovative Uses for Advanced Materials in the Modern World (AM2), with support from Advantage West Midlands (AWM) and part funded by the European Regional Development Fund (ERDF. This work was supported by a Research Project Grant from the Royal Society. TRC was funded by Leverhulme Trust research grant RPG-144. DJP acknowledges the University of Warwick for a studentship. MIG acknowledges the ERC for a Starter Grant (CRYOMAT 638661).
Notes and references
- 1.Harding MM, Anderberg PI, Haymet ADJ. Eur J Biochem. 2003;270:1381–1392. doi: 10.1046/j.1432-1033.2003.03488.x. [DOI] [PubMed] [Google Scholar]
- 2.Harding MM, Ward LG. Eur J Biochem. 1999;264:653–665. doi: 10.1046/j.1432-1327.1999.00617.x. [DOI] [PubMed] [Google Scholar]
- 3.Tachibana Y, Fletcher GL, Fujitani N, Tsuda S, Monde K, Nishimura S-I. Angew Chem Int Ed. 2004;116:874–880. doi: 10.1002/anie.200353110. [DOI] [PubMed] [Google Scholar]
- 4.Wilkinson BL, Stone RS, Capicciotti CJ, Thaysen-Andersen M, Matthews JM, Packer NH, Ben RN, Payne RJ. Angew Chem Int Ed. 2012;51:3606–3610. doi: 10.1002/anie.201108682. [DOI] [PubMed] [Google Scholar]
- 5.Yu SO, Brown A, Middleton AJ, Tomczak MM, Walker VK, Davies PL. Cryobiology. 61:327–334. doi: 10.1016/j.cryobiol.2010.10.158. [DOI] [PubMed] [Google Scholar]
- 6.Eniade A, Purushotham M, Ben R, Wang JB, Horwath K. Cell Biochem Biophys. 2003;38:115–124. doi: 10.1385/CBB:38:2:115. [DOI] [PubMed] [Google Scholar]
- 7.Liu S, Ben RN. Org Lett. 2005;7:2385–2388. doi: 10.1021/ol050677x. [DOI] [PubMed] [Google Scholar]
- 8.Capicciotti CJ, Leclère M, Perras FA, Bryce DL. Chem Sci. 2012;3:1408. [Google Scholar]
- 9.Gibson MI, Barker CA, Spain SG, Albertin L, Cameron NR. Biomacromolecules. 2009;10:328–333. doi: 10.1021/bm801069x. [DOI] [PubMed] [Google Scholar]
- 10.Gibson MI. Polym Chem. 2010;1:1141–1152. [Google Scholar]
- 11.Mitchell DE, Lilliman M, Spain SG, Gibson MI. Biomater Sci. 2014;2:1787–1795. doi: 10.1039/c4bm00153b. [DOI] [PubMed] [Google Scholar]
- 12.Matsumura K, Bae JY, Hyon S-H. Cell Transplant. 2010;19:691–699. doi: 10.3727/096368910X508780. [DOI] [PubMed] [Google Scholar]
- 13.Deville S, Viazzi C, Guizard C. Langmuir. 2012;28:14892–14898. doi: 10.1021/la302275d. [DOI] [PubMed] [Google Scholar]
- 14.Mizrahy O, Bar-Dolev M, Guy S, Braslavsky I. PLoS ONE. 2013;8:e59540. doi: 10.1371/journal.pone.0059540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deller RC, Deller RC, Vatish M, Mitchell DA, Gibson MI. Nat Commun. 2014;5 doi: 10.1038/ncomms4244. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell D, Cameron NR, Gibson MI. Chem Commun. 2015 doi: 10.1039/c5cc04647e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capicciotti CJ, Kurach JDR, Turner TR, Mancini RS, Acker JP, Ben RN. Sci Rep. 2015;5 doi: 10.1038/srep09692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duncker BP, Koops MD, Walker VK, Davies PL. FEBS Lett. 1995;377:185–188. doi: 10.1016/0014-5793(95)01340-7. [DOI] [PubMed] [Google Scholar]
- 19.Stuart MAC, Huck WTS, Genzer J, Muller M, Ober C, Stamm M, Sukhorukov GB, Szleifer I, Tsukruk VV, Urban M, Winnik F, et al. Nat Mater. 2010;9:101–113. doi: 10.1038/nmat2614. [DOI] [PubMed] [Google Scholar]
- 20.Phillips DJ, Gibson MI. Biomacromolecules. 2012;13:3200–3208. doi: 10.1021/bm300989s. [DOI] [PubMed] [Google Scholar]
- 21.Jochum FD, Theato P. Chem Soc Rev. 2013;42:7468–7483. doi: 10.1039/c2cs35191a. [DOI] [PubMed] [Google Scholar]
- 22.Phillips DJ, Gibson MI. Chem Commun. 2012;48:1054–1056. doi: 10.1039/c1cc16323j. [DOI] [PubMed] [Google Scholar]
- 23.Phillips DJ, Prokes I, Davies GL, Gibson MI. ACS Macro Lett. 2014;3:1225–1229. doi: 10.1021/mz500686w. [DOI] [PubMed] [Google Scholar]
- 24.Congdon T, Notman R, Gibson MI. Biomacromolecules. 2013;14:1578–1586. doi: 10.1021/bm400217j. [DOI] [PubMed] [Google Scholar]
- 25.Hider RC, Kong X. Nat Prod Rep. 2010;27:637–657. doi: 10.1039/b906679a. [DOI] [PubMed] [Google Scholar]
- 26.Moad G, Rizzardo E, Thang SH. Aust J Chem. 2005;58:379–410. [Google Scholar]
- 27.Stenzel MH, Cummins L, Roberts GE, Davis TP, Vana P, Barner-Kowollik C. Macromol Chem Phys. 2003;204:1160–1168. [Google Scholar]
- 28.Phillips DJ, Davies G-L, Gibson MI. J Mater Chem B. 2015;3:270–275. doi: 10.1039/c4tb01501k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.