Abstract
The blood-brain barrier (BBB) limits drug delivery to the central nervous system. When combined with microbubbles, ultrasound can transiently permeate blood vessels in the brain. This approach, which can be referred to as sonoporation or sonopermeabilization, holds significant promise for shuttling large therapeutic molecules, such as antibodies, growth factors and nanomedicine formulations, across the BBB. We here describe the basic principles of BBB permeation using ultrasound and microbubbles, and we summarize several (pre-) clinical studies showing the potential of BBB opening for improving the treatment of cancer and neurodegenerative disorders.
Introduction
Efficient delivery of therapeutic agents across the blood-brain barrier (BBB) for the treatment of central nervous system (CNS) disorders remains to be one of the biggest challenges in pharmaceutical research. The BBB consists of endothelial cells linked together by tight junctions, a thick basement membrane and a layer of astrocyte end-feet, together only allowing for the uptake of small lipophilic drugs, while preventing the vast majority of other drug molecules from entering the CNS [1–3].
Numerous (physico-) chemical and pharmacological strategies have been investigated to overcome the BBB and to enable (more) efficient drug delivery to the brain parenchyma. These e.g. include the modification of small water-soluble molecules into small lipid-soluble ones; the use of solute carrier proteins for drug transportation; the targeting of drugs and drug delivery systems to receptors like the transferrin receptor, which are overexpressed on brain endothelial cells and which enable transcytosis; the intra-arterial injection of hyperosmotic solutions like mannitol; the transcranial injection via catheters; and the direct intracerebral injection or implantation of drugs and drug delivery systems [3–8].
In recent years, in addition to the abovementioned strategies, the combination of ultrasound (US) and microbubbles (MB) has attracted a lot of attention for opening up the BBB and for improving drug delivery to the brain [9–11]. In the present manuscript, we describe the mechanisms and factors involved in the opening of the BBB upon the combined use of US and MB, we highlight several preclinical proof-of-principle studies, and we discuss currently ongoing efforts towards clinical translation.
Ultrasound as a diagnostic and therapeutic tool
US imaging is the most ubiquitous imaging modality in the clinic after x-ray radiography, which can be explained by its non-invasive and bedside capable nature, the option of real-time diagnosis of various pathological conditions, the lack of radiation and its low cost [12]. US imaging is used as a standard practice in clinics for routine examinations of fetuses during prenatal development, breast, abdomen, neck, microcirculatory flow and other soft tissue pathologies [13]. The implementation of MB as contrast agents has extended diagnostic US imaging. During ultrasonography, intravenously (i.v.) administered MB are known to cause an acoustic backscatter due to differences in the acoustic impedance of the gas in the MB core and the surrounding tissues [14]. This characteristic of MB very strongly increases their US reflection, and it explains their usefulness as (blood pool) contrast agents [15]. The high echogenicity of circulating MB has improved the US detection and characterization of malignant liver lesions, cardiac pathologies (such as left ventricular opacification and endocardial border delineation), cerebral vessel stenosis and vesico-ureteric reflux, and it is also useful to monitor tumor perfusion in oncological studies [16].
Apart from diagnostic applications, US has also emerged as a powerful tool for therapeutic purposes. For example, magnetic resonance image guided high-intensity focused ultrasound (MR-HIFU) has been employed for the thermal ablation of deep-seated tumors and uterine fibroids [17,18]. In such setups, the US waves and energies are focused deep into the pathological target tissues, which causes a local temperature rise of up to 60°C, resulting in thermal ablation of the lesion. Hybrid imaging together with MRI provides anatomical reference for guidance of focused US therapy, and it at the same time enables real-time monitoring of local temperature increases via MR thermometry [19]. MR-guided US has also been used to induce mild hyperthermia (40-45°C), which can be employed to trigger drug release from temperature-sensitive nanocarriers, such as liposomes [20,21]. Another interesting therapeutic application of US relates to its ability to induce clot lysis, e.g. in patients with stroke. In case of the latter, it has been shown that combining US with MB results in significant improvements in therapeutic outcome [22].
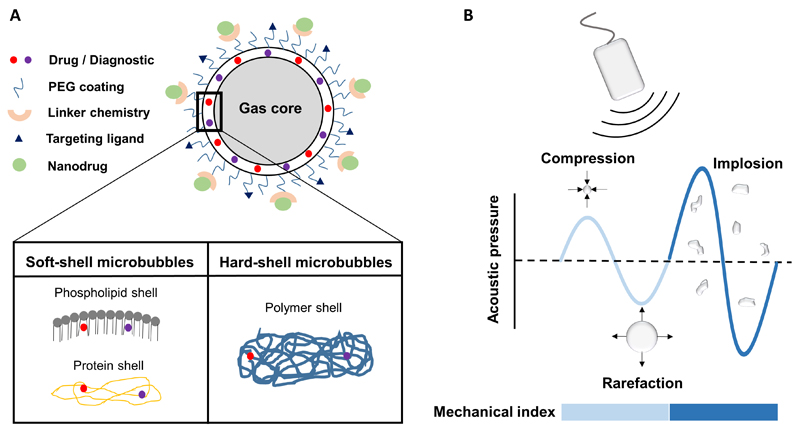
In the last couple of years, the combination of US with MB has also attracted attention for delivering drugs, genes and nanomedicine formulations across biological barriers, including the cellular membrane and the BBB [10,23,24]. In this context, MB can either be used for direct drug delivery (upon loading therapeutic agents into their interior or shell) or for indirect delivery (upon employing stable and inertial cavitation effects to open up biological barriers). MB are 1-5 μm-sized gas-filled vesicles stabilized by phospholipids, proteins or polymers, and their shell can be functionalized with various different moieties, including drugs, imaging agents, PEG and targeting ligands (Figure 1A) [25,26]. Due to the compressible nature of MB, they can either oscillate (i.e. stable cavitation) or implode (i.e. inertial cavitation) depending on the magnitude of mechanical index, which is a measure of cavitation intensity governed by pressure amplitude and US frequency (Figure 1B). Stable and inertial MB cavitation can generate a plethora of biophysical effects that can temporarily open up cell membranes and blood vessels (Figure 2) [25,27]. The former is generally referred to as sonoporation, the latter could alternatively be referred to as sonopermeabilization. For the sake of simplicity, however, we decided to stick to the term sonoporation for describing both cell membrane and blood vessel permeabilization.
Figure 1. Microbubble properties.
A: Schematic depiction of a (multifunctional) microbubble, which can be loaded with drugs or imaging agents, and which can be surface-functionalized with PEG and with targeting ligands. B: When exposed to ultrasound, microbubbles can undergo stable or inertial cavitation, depending on the magnitude of the mechanical index. Light and dark blue regions correspond to low and high mechanical index, respectively.
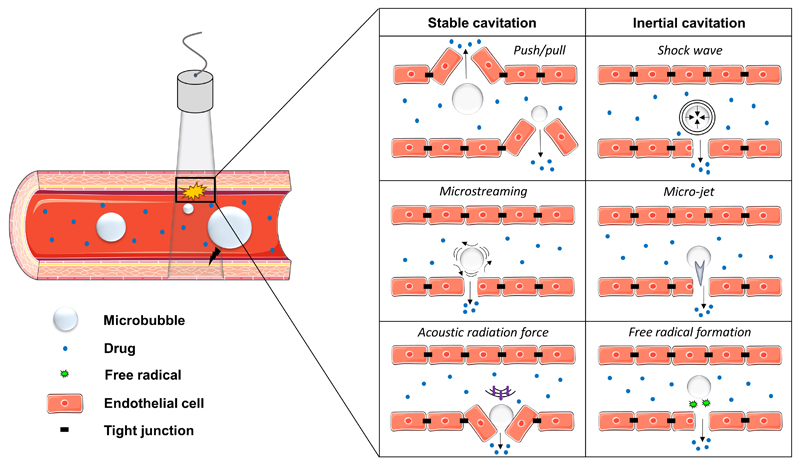
Figure 2. Sonoporation mechanisms.
Schematic overview of the physical mechanisms, based on stable and inertial microbubble cavitation, which contribute to blood vessel (and BBB) permeabilization.
Mechanisms involved in vessel permeabilization
Multiple physical phenomena play a role in sonoporation and vessel permeabilization [27]. At low mechanical index, MB oscillate in a symmetrical manner. The repeated expansion and compression of MB upon exposure to low acoustic pressures is referred to as stable or non-inertial cavitation. The expansion of MB near the vessel wall during the gas inflow phase can push the endothelial or cellular lining apart, while MB shrinkage during the compression phase causes invaginations in the vascular lining, potentially resulting in the opening of tight junctions via push-pull mechanisms (Figure 2). In addition, the rapid expansion and contraction features of MB in an ultrasonic field are known to create fluid flow patterns, known as micro-streams, which can induce high shear stresses of up to several thousand of Pascals, and which can disrupt the integrity of the endothelial lining. A third mechanism which can help to improve drug delivery across the endothelial lining relies on absorption of US energies, which result in pressure gradients and in acoustic radiation forces displacing MB in the direction of US waves. This phenomenon can force MB to strongly push against (and massage) the endothelium, thereby inducing vascular permeability. If mechanical indices exceed a particular threshold, MB violently collapse during the compression phase. This can lead to the fragmentation of the MB, generating extremely high temperatures and pressures within close proximity. This effect is referred to as inertial cavitation and it is generally accompanied by shock waves and by micro-jet formation (Figure 2). Both of these biophysical effects are known to perforate cell membranes and to induce vascular permeability. Other effects, such as the formation of free radicals, have also been proposed to contribute to endothelial permeabilization, e.g. lipid peroxidation mechanisms [28].
Factors affecting vessel permeabilization
The magnitude of blood vessel (and BBB) permeabilization depends on both US parameters and MB properties. US parameters which play an important role include transducer type, pressure amplitude, sonication time, US frequency, burst length and pulse repetition frequency. In case of the BBB, selecting an US transducer with an optimal geometrical design is paramount for efficient propagation of US waves through the skull, which otherwise gets distorted due to skull’s irregular shape and its high acoustic impedance. Particularly, a phased array transducer has been proposed in order to minimize skull heating and attenuation of US waves, thereby improving US propagation into the brain and potentially enhancing sonoporation efficiency [29,30]. Increasing the pressure amplitude and the sonication time increases the magnitude of BBB permeation, however medical complications such as hemorrhages, erythrocyte extravasation and edema formation are observed at higher pressure amplitudes and upon prolonged sonication [31–33]. It was demonstrated that the threshold for BBB permeation linearly increased with US frequency and that less vascular damage was observed at lower frequencies [34]. Similarly, studies have also reported an increase in the extent of BBB opening upon increasing the US burst length or pulse repetition frequency [35–37]. Importantly, however, the magnitude of BBB opening has been shown to be saturable, and at a certain point, no further enhancements in permeation are obtained upon further increasing pressure amplitude, burst length and/or pulse repetition frequency [31–33,35–37].
Apart from US parameters, also the nature and the properties of MB play an important role in determining the extent (and duration) of BBB opening. Some studies have reported that the degree of BBB permeability can be increased by using higher concentrations of MB, while other reports have not observed significant differences in the extent of BBB opening upon using higher amounts of MB [35,36,38]. The permeability of the BBB can also be enhanced by using MB with larger average sizes. In this regard, Konofagou and colleagues demonstrated that the BBB opening volume could be increased by more than a tenfold by increasing the MB size from 1-2 μm to 6-8 μm (at the same acoustic pressure; 0.45 MPa) [39]. The composition of the MB shell, which can be based on lipids, proteins and polymers, likely also plays a crucial role in optimizing BBB opening. In this regard, Hynynen and colleagues showed that Optison® MB, which contain a shell based on denatured albumin, induced much greater BBB disruption (and higher erythrocyte extravasation) than lipid-shelled Definity® MB. However, a definite conclusion cannot yet be drawn, due to differences in the diameter and the dosing of both MB formulations in the study [40]. A more extensive and more detailed analysis would be required to assess the impact of shell properties on the efficiency of BBB opening.
Other factors, such as the mode of MB administration (intravenous bolus injection vs. intravenous infusion), the time delay between MB administration and the initiation of US sonication, and the time of administering drugs, i.e. pre or post sonication, may also influence the effect of BBB opening on drug delivery to the brain. Bearing the above insights and efforts in mind, it becomes clear that there are still quite some factors that can be further refined to enable safe and efficient BBB opening [41].
Preclinical progress
In the 1950's, Barnard and colleagues demonstrated that US can be employed to disrupt the BBB [42]. In the decades that followed, multiple studies confirmed these findings, sometimes at the cost of severe side effects, sometimes without complications (such as hemorrhages and significant tissue damage) [43,44]. To attenuate side effects, Hynynen and co-workers in the early 2000’s employed MRI-guided US in combination with MB to open up the BBB. In comparison to US treatment alone, the administration of MB reduced the energy required for BBB disruption and it minimized the thermal damage done to the brain. At the same time, MRI enabled non-invasive monitoring of BBB opening, together paving the way for more efficient and less harmful strategies for US-mediated drug delivery to the brain [45]. The same group of authors subsequently showed that even large therapeutic agents, such as the monoclonal antibody Herceptin (~15 nm), which is used to treat breast cancer (and breast cancer brain metastases) can be efficiently delivered to the brain upon the co-administration of US and MB. The amount of Herceptin delivered to the brain correlated with the accumulation of Magnevist, i.e. a low-molecular-weight gadolinium-based MRI contrast agent (<1 nm), [46]. McDannold and colleagues extended these efforts by demonstrating, again under MRI and Magnevist guidance, that also liposomal doxorubicin (i.e. Doxil; ~100 nm) accumulates in the brain upon multiple sessions of transcranial sonoporation [47].
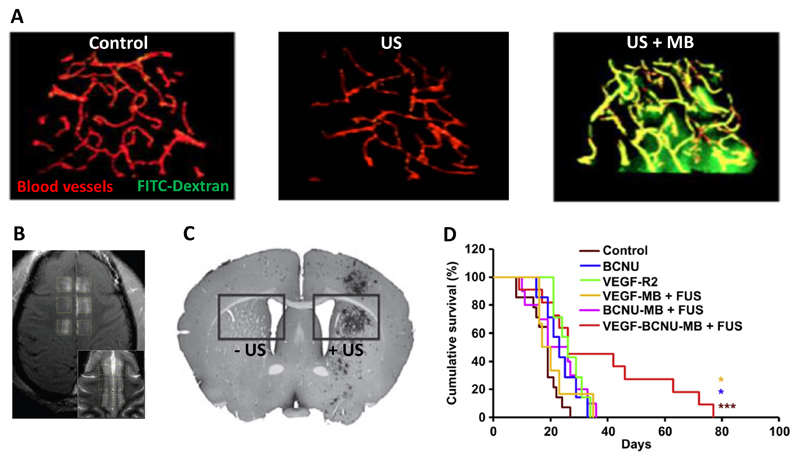
Keeping the abovementioned efforts in mind, an interesting step forward would be the development of MB which can mediate and monitor BBB permeabilization at the same time. We therefore recently developed polymeric MB based on poly(butyl cyanoacrylate) (PBCA) which contain ultrasmall superparamagnetic iron oxide (USPIO) nanoparticles within their ~50 nm-sized shell [9]. These USPIO-loaded MB were co-administered together with FITC-dextran (70 kDa; ~10 nm), and upon exposure to transcranial US pulses, they were employed to facilitate and image the delivery of this relatively large macromolecular model drug to the brain. As shown in Figure 3A, we observed significant extravasation and accumulation of FITC-dextran in the brain upon combining US and USPIO-MB. The accumulation and penetration of FITC-dextran, as determined by means of 2D and 3D microscopy, correlated well with the findings obtained using MRI [9]. Such theranostic materials and methods might be useful to fine-tune BBB opening, and to enable safe and efficient drug delivery to the brain.
Figure 3. Preclinical proof-of-concept for US- and MB-mediated drug delivery to the brain.
A: Two-photon microscopy imaging showing extravasation of 70 kDa FITC-Dextran across the BBB upon combining US with MB. B: Contrast enhanced T1-weighted MRI showing delivery of gadolinium-DPTA to six distinct regions within the cingulate cortex upon the implementation of focused US. The inset shows the corresponding T2-weighted imaging. C: Immunohistochemistry exemplifying efficient gene delivery to the right striatum upon combining MB with US. D: When combined with focused US, BCNU-loaded and VEGFR2-targeted MB were able to substantially prolong survival in a rat glioma model. Images adapted and reproduced, with permission, from [9,26,48,49].
Progress in the development of soft- and hardware tools to accurately focus US waves to specific regions within the brain, such as the putamen, thalamus, cortex, striatum and hippocampus, is also considered to be instrumental to enable safe and efficient drug delivery to the brain. In this context, McDannold and co-workers provided important proof-of-principle, showing that transcranial MRI-guided focused US can be employed to deliver gadolinium-based MRI contrast agents to specific regions in the cingulate cortex in rhesus macaques by electronically steering the beam to distinct volumetric regions in the brain without physically moving the US transducer (Figure 3B) [48]. In line with this, viral vectors could be locally accumulated and activated in distinct brain regions upon the application of focused US (Figure 3C) [49].
Besides at the technological level, significant progress has also been made at the therapeutic level. Preclinically, the combined use of US and MB has already been employed to relieve disease symptoms in pathologies such as brain cancer, Alzheimer’s disease and Huntington’s disease [26,50,51]. In the case of cancer, both indirect drug delivery approaches, based on the co-administration of drugs and MB, as well as direct drug delivery strategies, based on the use of drug-loaded MB, have been employed. And interesting example of the latter has been published by Yeh and colleagues, who encapsulated BCNU (carmustine) in the shell of VEGFR2-targeted MB, and who showed that upon combining these drug-containing MB with transcranial US, significant improvements could be achieved in the survival of rats suffering from orthotopic gliomas (Figure 3D) [26]. Similarly, in mice suffering from Alzheimer’s disease, Hynynen and colleagues showed that indirect drug delivery strategies, based on the co-administration of MB and US together with antibodies directed against toxic amyloid-beta peptides, efficiently suppressed disease symptoms, as exemplified by reductions in the number, size and total surface area of Aβ plaques in the brains of these mice [50].
Besides in rodents, brain sonopermeabilization studies have also been performed in large animals. Xie and colleagues for instance showed that the permeability of the BBB can be safely and efficiently increased in pigs by combining US with MB [52]. Similarly, also in non-human primates, sonopermeabilization was able to open up the BBB in a non-invasive, efficient and safe manner. The cavitation effects resulting from transcranial US exposure were investigated by Konofagou and colleagues, and were found to correlate with US pressure and MB size [53]. In line with this, McDannold and co-workers demonstrated efficient (and repeated) disruption of the BBB in rhesus macaques, by employing a transcranial MRI-guided focused US system (TcMRgFUS), which is designed for treating patients [48]. In none of these studies, significant tissue damage or functional deficits were observed, although it needs to be stressed that treatment protocols were optimized to minimize the occurrence of side effects.
Clinical translation
Extending the above efforts, US-mediated drug delivery has recently made notable advancements in the clinic. For instance, Kotopoulis and colleagues initiated a clinical trial in Norway in which patients suffering from advanced pancreatic cancer were given gemcitabine in conjunction with SonoVue® MB and US [54]. Care was taken to maximize the safety of the intervention, and therefore stable cavitation was employed, rather than inertial cavitation. The patients tolerated the treatment very well, and a higher number of treatment cycles could be given as compared to patients on gemcitabine treatment alone. In multiple patients, tumor size temporally decreased, while in other patients, an inhibition of tumor growth was observed. As compared to a historical cohort, which showed an overall survival time of 8-9 months, which is typical for pancreatic cancer, sonoporation more than doubled the efficacy of the intervention, with survival times of up to 19 months. Although the number of patients was low, and although no direct comparator arm was available, these findings suggest that sonopermeabilization might hold significant potential for improving the treatment of high medical need malignancies, such as pancreatic cancer.
Along the same line of thought, Hynynen and colleagues at Sunnybrook Health Sciences Center in Toronto recently initiated the first clinical BBB sonopermeabilization study in patients suffering from glioblastoma multiforme (GBM) [55]. As pancreatic cancer, GBM is known to be among the most deadly forms of cancer. In this study, which was started at the end of 2015, GBM patients are treated with doxorubicin, and US and MB are employed to locally open up the BBB to enable efficient drug delivery to the tumor. MRI guidance is employed to tailor the sonopermeabilization protocol. In the first patient, in which two different GBM regions were exposed to focused US, efficient BBB opening could be confirmed by contrast-enhanced T1-weighted MRI. Additionally, after the intervention, a small portion of the tumor was surgically excised to validate the accumulation of doxorubicin at the target site. This study will recruit up to ten patients, and even though its primary goal only is to demonstrate the safety of this novel type of externally triggered and targeted therapy, its (efficacy) results are eagerly awaited.
Conclusions and future outlook
The BBB remains to be one of the most insurmountable barriers in the drug delivery field. When combined with MB, focused US may assist in overcoming this barrier, by opening the BBB in a temporally and spatially controlled manner. Multiple physical principles, such as push-pull-mechanisms, microstreaming, micro-jets and shock waves, may contribute to US- and MB-mediated BBB opening, and non-invasive (MR) imaging may help to tailor efficient and safe sonopermeabilization. Significant progress has already been made at the preclinical level, both in rodents and in large animal models. At the clinical level, several pioneering studies have recently been initiated in patients, suffering from pancreatic cancer and from glioblastoma multiforme. Although it may be too early to draw any definite conclusions, the results obtained thus far are very promising. If the initial findings obtained in cancer patients can be confirmed in larger (and case-controlled) clinical trials, sonopermeabilization may also become relevant for the treatment of neurodegenerative disorders, such as Parkinson’s, Alzheimer’s and Huntington’s disease. To more efficiently and more rapidly move the field forward, concerted actions between several different basic and applied scientific disciplines are necessary, bridging biology, medicine, chemistry, physics and engineering, and involving input from both academia and industry, as well as from funding agencies, governmental bodies and health policy makers.
Acknowledgements
This work is supported by the European Research Council (ERC Starting Grant 309495: NeoNaNo), by the German Research Foundation (DFG: LA2937/1-2), by the China Scholarship Council (CSC: 201506910070), and by the European Union (EU 7th Framework Programme: ITN-642028-NABBA).
Footnotes
Conflict of interest: The authors have no conflict of interest to declare.
References
- [1].Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR. Pericytes regulate the blood-brain barrier. Nature. 2010;468(7323):557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- [2].Abbott NJ, Romero IA. Transporting therapeutics across the blood-brain barrier. Molecular medicine today. 1996;2(3):106–113. doi: 10.1016/1357-4310(96)88720-x. [DOI] [PubMed] [Google Scholar]
- [3].Pardridge WM. Blood–brain barrier delivery. Drug discovery today. 2007;12(1):54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- [4].Zhang EY, Knipp GT, Ekins S, Swaan PW. Structural biology and function of solute transporters: implications for identifying and designing substrates. Drug metabolism reviews. 2002;34(4):709–750. doi: 10.1081/dmr-120015692. [DOI] [PubMed] [Google Scholar]
- [5].Gan CW, Feng SS. Transferrin-conjugated nanoparticles of poly (lactide)-D-α-tocopheryl polyethylene glycol succinate diblock copolymer for targeted drug delivery across the blood–brain barrier. Biomaterials. 2010;31(30):7748–7757. doi: 10.1016/j.biomaterials.2010.06.053. [DOI] [PubMed] [Google Scholar]
- [6].Kroll RA, Neuwelt EA. Outwitting the blood-brain barrier for therapeutic purposes: osmotic opening and other means. Neurosurgery. 1998;42(5):1083–99. doi: 10.1097/00006123-199805000-00082. [DOI] [PubMed] [Google Scholar]
- [7].Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proceedings of the National Academy of Sciences. 1994;91(6):2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Brem H, Gabikian P. Biodegradable polymer implants to treat brain tumors. Journal of controlled release. 2001;74(1):63–67. doi: 10.1016/s0168-3659(01)00311-x. [DOI] [PubMed] [Google Scholar]
- [9].Lammers T, Koczera P, Fokong S, Gremse F, Ehling J, Vogt M, Pich A, Storm G, van Zandvoort M, Kiessling F. Theranostic USPIO-Loaded Microbubbles for Mediating and Monitoring Blood- Brain Barrier Permeation. Advanced functional materials. 2015;25(1):36–43. doi: 10.1002/adfm.201401199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ting CY, Fan CH, Liu HL, Huang CY, Hsieh HY, Yen TC, Wei KC, Yeh CK. Concurrent blood–brain barrier opening and local drug delivery using drug-carrying microbubbles and focused ultrasound for brain glioma treatment. Biomaterials. 2012;33(2):704–712. doi: 10.1016/j.biomaterials.2011.09.096. [DOI] [PubMed] [Google Scholar]
- [11].Raymond SB, Treat LH, Dewey JD, McDannold NJ, Hynynen K, Bacskai BJ. Ultrasound enhanced delivery of molecular imaging and therapeutic agents in Alzheimer's disease mouse models. PloS one. 2008;3(5):e2175. doi: 10.1371/journal.pone.0002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shung KK. Diagnostic ultrasound: Past, present, and future. J Med Biol Eng. 2011;31(6):371–374. [Google Scholar]
- [13].Kiessling F, Fokong S, Koczera P, Lederle W, Lammers T. Ultrasound microbubbles for molecular diagnosis, therapy, and theranostics. Journal of nuclear medicine. 2012;53(3):345–348. doi: 10.2967/jnumed.111.099754. [DOI] [PubMed] [Google Scholar]
- [14].Dayton PA, Ferrara KW. Targeted imaging using ultrasound. Journal of magnetic resonance imaging. 2002;16(4):362–377. doi: 10.1002/jmri.10173. [DOI] [PubMed] [Google Scholar]
- [15].Foster FS, Burns PN, Simpson DH, Wilson SR, Christopher DA, Goertz DE. Ultrasound for the visualization and quantification of tumor microcirculation. Cancer and Metastasis reviews. 2000;19(1-2):131–138. doi: 10.1023/a:1026541510549. [DOI] [PubMed] [Google Scholar]
- [16].Kiessling F, Fokong S, Bzyl J, Lederle W, Palmowski M, Lammers T. Recent advances in molecular, multimodal and theranostic ultrasound imaging. Advanced drug delivery reviews. 2014;72:15–27. doi: 10.1016/j.addr.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Al-Bataineh O, Jenne J, Huber P. Clinical and future applications of high intensity focused ultrasound in cancer. Cancer treatment reviews. 2012;38(5):346–353. doi: 10.1016/j.ctrv.2011.08.004. [DOI] [PubMed] [Google Scholar]
- [18].Voogt MJ, Trillaud H, Kim YS, Mali WTM, Barkhausen J, Bartels LW, Deckers R, Frulio N, Rhim H, Lim HK, Eckey T. Volumetric feedback ablation of uterine fibroids using magnetic resonance-guided high intensity focused ultrasound therapy. European radiology. 2012;22(2):411–417. doi: 10.1007/s00330-011-2262-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Quesson B, de Zwart JA, Moonen CT. Magnetic resonance temperature imaging for guidance of thermotherapy. Journal of Magnetic Resonance Imaging. 2000;12(4):525–533. doi: 10.1002/1522-2586(200010)12:4<525::aid-jmri3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- [20].Weinstein JN, Magin RL, Yatvin MB, Zaharko DS. Liposomes and local hyperthermia: selective delivery of methotrexate to heated tumors. Science. 1979;204(4389):188–191. doi: 10.1126/science.432641. [DOI] [PubMed] [Google Scholar]
- [21].Maruyama K, Unezaki S, Takahashi N, Iwatsuru M. Enhanced delivery of doxorubicin to tumor by long-circulating thermosensitive liposomes and local hyperthermia. Biochimica et Biophysica Acta (BBA)-Biomembranes. 1993;1149(2):209–216. doi: 10.1016/0005-2736(93)90203-c. [DOI] [PubMed] [Google Scholar]
- [22].Molina CA, Ribo M, Rubiera M, Montaner J, Santamarina E, Delgado-Mederos R, Arenillas JF, Huertas R, Purroy F, Delgado P, Alvarez-Sabín J. Microbubble administration accelerates clot lysis during continuous 2-MHz ultrasound monitoring in stroke patients treated with intravenous tissue plasminogen activator. Stroke. 2006;37(2):425–429. doi: 10.1161/01.STR.0000199064.94588.39. [DOI] [PubMed] [Google Scholar]
- [23].Mead BP, Mastorakos P, Suk JS, ? AL, Hanes J, Price RJ. Targeted gene transfer to the brain via the delivery of brain-penetrating DNA nanoparticles with focused ultrasound. Journal of Controlled Release. 2016;223:109–117. doi: 10.1016/j.jconrel.2015.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Theek B, Baues M, Ojha T, Möckel D, Veettil SK, Steitz J, van Bloois L, Storm G, Kiessling F, Lammers T. Sonoporation enhances liposome accumulation and penetration in tumors with low EPR. Journal of Controlled Release. 2016;231:77–85. doi: 10.1016/j.jconrel.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sirsi SR, Borden MA. State-of-the-art materials for ultrasound-triggered drug delivery. Advanced drug delivery reviews. 2014;72:3–14. doi: 10.1016/j.addr.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fan CH, Ting CY, Liu HL, Huang CY, Hsieh HY, Yen TC, Wei KC, Yeh CK. Antiangiogenic-targeting drug-loaded microbubbles combined with focused ultrasound for glioma treatment. Biomaterials. 2013;34(8):2142–2155. doi: 10.1016/j.biomaterials.2012.11.048. [DOI] [PubMed] [Google Scholar]
- [27].Lentacker I, De Cock I, Deckers R, De Smedt SC, Moonen CTW. Understanding ultrasound induced sonoporation: definitions and underlying mechanisms. Advanced drug delivery reviews. 2014;72:49–64. doi: 10.1016/j.addr.2013.11.008. [DOI] [PubMed] [Google Scholar]
- [28].Basta G, Venneri L, Lazzerini G, Pasanisi E, Pianelli M, Vesentini N, Del Turco S, Kusmic C, Picano E. In vitro modulation of intracellular oxidative stress of endothelial cells by diagnostic cardiac ultrasound. Cardiovascular research. 2003;58(1):156–161. doi: 10.1016/s0008-6363(02)00665-x. [DOI] [PubMed] [Google Scholar]
- [29].Hynynen K, Jolesz FA. Demonstration of potential noninvasive ultrasound brain therapy through an intact skull. Ultrasound in medicine & biology. 1998;24(2):275–283. doi: 10.1016/s0301-5629(97)00269-x. [DOI] [PubMed] [Google Scholar]
- [30].Hynynen K, McDannold N. MRI guided and monitored focused ultrasound thermal ablation methods: a review of progress. International journal of hyperthermia. 2004;20(7):725–737. doi: 10.1080/02656730410001716597. [DOI] [PubMed] [Google Scholar]
- [31].Hynynen K, McDannold N, Sheikov NA, Jolesz FA, Vykhodtseva N. Local and reversible blood–brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage. 2005;24(1):12–20. doi: 10.1016/j.neuroimage.2004.06.046. [DOI] [PubMed] [Google Scholar]
- [32].Chopra R, Vykhodtseva N, Hynynen K. Influence of exposure time and pressure amplitude on blood– brain-barrier opening using transcranial ultrasound exposures. ACS chemical neuroscience. 2010;1(5):391–398. doi: 10.1021/cn9000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yang FY, Lin YS, Kang KH, Chao TK. Reversible blood–brain barrier disruption by repeated transcranial focused ultrasound allows enhanced extravasation. Journal of controlled release. 2011;150(1):111–116. doi: 10.1016/j.jconrel.2010.10.038. [DOI] [PubMed] [Google Scholar]
- [34].McDannold N, Vykhodtseva N, Hynynen K. Blood-brain barrier disruption induced by focused ultrasound and circulating preformed microbubbles appears to be characterized by the mechanical index. Ultrasound in medicine & biology. 2008;34(5):834–840. doi: 10.1016/j.ultrasmedbio.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].McDannold N, Vykhodtseva N, Hynynen K. Effects of acoustic parameters and ultrasound contrast agent dose on focused-ultrasound induced blood-brain barrier disruption. Ultrasound in medicine & biology. 2008;34(6):930–937. doi: 10.1016/j.ultrasmedbio.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Choi JJ, Selert K, Gao Z, Samiotaki G, Baseri B, Konofagou EE. Noninvasive and localized blood–brain barrier disruption using focused ultrasound can be achieved at short pulse lengths and low pulse repetition frequencies. Journal of Cerebral Blood Flow & Metabolism. 2011;31(2):725–737. doi: 10.1038/jcbfm.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Choi JJ, Selert K, Vlachos F, Wong A, Konofagou EE. Noninvasive and localized neuronal delivery using short ultrasonic pulses and microbubbles. Proceedings of the National Academy of Sciences. 2011;108(40):16539–16544. doi: 10.1073/pnas.1105116108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Treat LH, McDannold N, Vykhodtseva N, Zhang Y, Tam K, Hynynen K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI- guided focused ultrasound. International journal of cancer. 2007;121(4):901–907. doi: 10.1002/ijc.22732. [DOI] [PubMed] [Google Scholar]
- [39].Vlachos F, Tung YS, Konofagou E. Permeability dependence study of the focused ultrasound- induced blood–brain barrier opening at distinct pressures and microbubble diameters using DCE- MRI. Magnetic resonance in medicine. 2011;66(3):821–830. doi: 10.1002/mrm.22848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].McDannold N, Vykhodtseva N, Hynynen K. Use of ultrasound pulses combined with Definity for targeted blood-brain barrier disruption: a feasibility study. Ultrasound in medicine & biology. 2007;33(4):584–590. doi: 10.1016/j.ultrasmedbio.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Aryal M, Arvanitis CD, Alexander PM, McDannold N. Ultrasound-mediated blood–brain barrier disruption for targeted drug delivery in the central nervous system. Advanced drug delivery reviews. 2014;72:94–109. doi: 10.1016/j.addr.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Barnard JW, Fry WJ, Fry FJ, Krumins RF. Effects of high intensity ultrasound on the central nervous system of the cat. Journal of Comparative Neurology. 1955;103(3):459–484. doi: 10.1002/cne.901030304. [DOI] [PubMed] [Google Scholar]
- [43].Vykhodtseva N, McDannold N, Hynynen K. Progress and problems in the application of focused ultrasound for blood–brain barrier disruption. Ultrasonics. 2008;48(4):279–296. doi: 10.1016/j.ultras.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Mesiwala AH, Farrell L, Wenzel HJ, Silbergeld DL, Crum LA, Winn HR, Mourad PD. High-intensity focused ultrasound selectively disrupts the blood-brain barrier in vivo. Ultrasound in medicine & biology. 2002;28(3):389–400. doi: 10.1016/s0301-5629(01)00521-x. [DOI] [PubMed] [Google Scholar]
- [45].Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR Imaging–guided Focal Opening of the Blood-Brain Barrier in Rabbits 1. Radiology. 2001;220(3):640–646. doi: 10.1148/radiol.2202001804. [DOI] [PubMed] [Google Scholar]
- [46].Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood–brain barrier disruption. Proceedings of the National Academy of Sciences. 2006;103(31):11719–11723. doi: 10.1073/pnas.0604318103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Aryal M, Vykhodtseva N, Zhang YZ, McDannold N. Multiple sessions of liposomal doxorubicin delivery via focused ultrasound mediated blood–brain barrier disruption: A safety study. Journal of Controlled Release. 2015;204:60–69. doi: 10.1016/j.jconrel.2015.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].McDannold N, Arvanitis CD, Vykhodtseva N, Livingstone MS. Temporary disruption of the blood–brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques. Cancer research. 2012;72(14):3652–3663. doi: 10.1158/0008-5472.CAN-12-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Thévenot E, Jordão JF, O'Reilly MA, Markham K, Weng YQ, Foust KD, Kaspar BK, Hynynen K, Aubert I. Targeted delivery of self-complementary adeno-associated virus serotype 9 to the brain, using magnetic resonance imaging-guided focused ultrasound. Human gene therapy. 2012;23(11):1144–1155. doi: 10.1089/hum.2012.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Jordão JF, Ayala-Grosso CA, Markham K, Huang Y, Chopra R, McLaurin J, Hynynen K, Aubert I. Antibodies targeted to the brain with image-guided focused ultrasound reduces amyloid-β plaque load in the TgCRND8 mouse model of Alzheimer's disease. PloS one. 2010;5(5):e10549. doi: 10.1371/journal.pone.0010549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Burgess A, Huang Y, Querbes W, Sah DW, Hynynen K. Focused ultrasound for targeted delivery of siRNA and efficient knockdown of Htt expression. Journal of controlled release. 2012;163(2):125–129. doi: 10.1016/j.jconrel.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Xie F, Boska MD, Lof J, Uberti MG, Tsutsui JM, Porter TR. Effects of transcranial ultrasound and intravenous microbubbles on blood brain barrier permeability in a large animal model. Ultrasound in medicine & biology. 2008;34(12):2028–2034. doi: 10.1016/j.ultrasmedbio.2008.05.004. [DOI] [PubMed] [Google Scholar]
- [53].Tung YS, Marquet F, Teichert T, Ferrera V, Konofagou EE. Feasibility of noninvasive cavitation-guided blood-brain barrier opening using focused ultrasound and microbubbles in nonhuman primates. Applied physics letters. 2011;98(16):163704. doi: 10.1063/1.3580763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kotopoulis S, Dimcevski G, Gilja OH, Hoem D, Postema M. Treatment of human pancreatic cancer using combined ultrasound, microbubbles, and gemcitabine: a clinical case study. Medical physics. 2013;40(7):072902. doi: 10.1118/1.4808149. [DOI] [PubMed] [Google Scholar]
- [55].Radovini NN. World first: blood–brain barrier opened non-invasively to deliver chemotherapy – Sunnybrook Hospital. Sunnybrook Canada: Press Release; 2015. [Google Scholar]