Abstract
Introduction
Joint haemorrhage is the principal clinical manifestation of haemophilia frequently leading to advanced arthropathy and arthrofibrosis, resulting in severe disability. The degree and prevalence of arthrofibrosis in hemophilic arthropathy is more severe than in other forms of arthropathy. Expression of connective tissue growth factor (CTGF) has been linked to many fibrotic diseases, but has not been studied in the context of haemophilic arthropathy.
Aim
We aim to compare synovial tissues histologically from haemophilia and osteoarthritis patients with advanced arthropathy in order to compare expression of proteins that are possibly aetiologic in the development of arthrofibrosis.
Methods
Human synovial tissues were obtained from 10 haemophilia and 10 osteoarthritis patients undergoing joint surgery and processed for histology and immunohistochemistry.
Results
All samples from haemophilia patients had synovitis with hypertrophy and hyperplasia of synovial villi. Histologically, synovial tissues contained hyperplastic villi with increased cellularity and abundant haemosiderin-and ferritin-pigmented macrophage-like cells (HMCs), with a perivascular localization in the sub-surface layer. CTGF staining was observed in the surface layer and sub-surface layer in all haemophilia patients, exclusively co-localizing with HMCs. Quantification showed that the extent of CTGF-positive areas was correlated with the degree of detection of HMCs. CTGF was not observed in any of the samples from osteoarthritis patients.
Conclusion
Using histological analysis, we showed that CTGF expression is elevated in haemophilia patients with arthrofibrosis and absent in patients with osteoarthritis. Additionally, we found that CTGF is always associated with haemosiderin-pigmented macrophage-like cells, which suggests that CTGF is produced by synovial A cells following the uptake of blood breakdown products.
Keywords: arthrofibrosis, CTGF, haemophilic arthropathy, haemosiderin, macrophages, synovium
Introduction
Arthrofibrosis involves the formation of excessive fibrous tissue within a joint; it is a well-known complication of trauma and orthopaedic surgery [1]. Progressive fibrosis leads to contracture and atrophy that further restricts motion and impairs function. Haemophilic arthropathy (HA), resulting in joint destruction and severe arthrofibrosis, is the most disabling complication, affecting 50–90% of persons with haemophilia (PWH) [2]. Even with administration of clotting factor, HA persists as a severe problem [3].
Haemophilic arthropathy is characterized by chronic haemorrhagic synovitis, which results in destruction of articular cartilage by direct invasion and enzymatic degradation [4,5]. The morphology of haemophilic synovitis is well established. The blood breakdown products haemosiderin and ferritin are taken up by phagocytic synovial type A cells and returned to the circulation. The number of synovial A cells in haemophilic synovitis is increased compared to that seen in other inflammatory synovitis conditions, such as rheumatoid arthritis [6]. In trauma resulting in a single haemarthrosis, this synovial cell uptake mechanism works well, and returns blood breakdown products to the central circulation. However, following recurrent haemarthrosis in PWH, hypertrophy and hyperplasia of the synovial membrane result in dense intracellular deposition of blood breakdown products [4].
Arthrofibrosis is generally treated with physical therapy, corticosteroid injections, anti-inflammatory drugs and in severe cases, surgery. Surgical intervention in non-haemophilia patients has a higher success rate than in PWH, but recalcitrant cases exist in the non-haemophilia patient population as well. For PWH, arthrofibrosis routinely occurs rapidly following joint replacement as compared to other patients [7]. This arthrofibrosis recurs despite complete cessation of haemarthrosis and debridement of arthrofibrotic tissue [5,8,9]. In PWH, arthrofibrosis is often the most severe and disabling component of their arthropathy and the most refractory to treatment.
The aetiology of arthrofibrosis in haemophilia is not well understood, as there have been few studies addressing this important issue. In non-haemophilia patients, acute intra-articular injuries result in transient formation of reparative fibrotic tissue that eventually is replaced by more normal tissue, allowing return of normal or near-normal motion. In PWH and other patients with recalcitrant arthrofibrosis, the fibrotic tissues do not undergo resolution and replacement. We have hypothesized that in PWH this failure to resolve fibrosis is the result of recurrent exposure to blood breakdown products giving rise to a recalcitrant form of arthrofibrosis. This hypothesis would explain the significant prevalence of arthrofibrosis in PWH compared to other arthritis patients. The goal of our study is to investigate this hypothesis through examination of synovial and other joint tissues from PWH with severe arthrofibrosis in comparison to similar tissues from non-haemophilia patients who underwent total joint replacement or debridement. We anticipate that this information will provide some insights into the pathophysiology of arthrofibrosis in general.
Connective tissue growth factor (CTGF) is a member of the CCN (CYR61-CTGF-NOV) family of matricellular proteins, which includes six members in vertebrates [10–12]. Like other members of the CCN family, CTGF/CCN2 has five distinct domains in order: secretory signal peptide, insulin-like growth factor-binding protein, von Willebrand factor type C repeat, thrombospondin type-1 repeat and C-terminal cystine-knot-containing domains [11–13]. CTGF has been documented as a secreted protein that is required for the development of fibrotic tissues in a variety of organs [10–12]. CTGF has also been shown to be up-regulated in the joint capsule during joint contracture [14,15]. Here, we hypothesize that accumulated blood breakdown products in the synovial tissues in haemophilia patients result in increased pro-fibrotic signalling as compared to synovium in non-haemophilia patients undergoing total joint arthroplasty or debridement.
Methods
Patient samples
Human synovial tissues were obtained from patients undergoing surgery with institutional IRB approval (UCLA, IRB#12-001827). We obtained 10 PWH samples from patients 21 to 59 years of age. Six of the samples were from the knee, two each from the ankle and elbow. Procedures included: knee replacement, radial head excision, and ankle fusion. All PWH had synovectomy at the time of surgery. Ten samples were obtained from non-haemophilia patients with osteoarthritis. This group included four males and six females with ages ranging from 40 to 91 years. All osteoarthritis samples were from patients undergoing hip (3) or knee (7) replacement. All of the PWH had arthrofibrosis preoperative. None of the osteoarthritis patients had arthrofibrosis pre-or postoperative.
Histology and immunohistochemistry
All specimens were fixed with 4% paraformaldehyde overnight and embedded in paraffin and sectioned (5 lm). General histology and immunohistochemistry (IHC) were performed using standard protocols. Antibodies against CTGF (Santa Cruz Biotechnology, Dallas, TX, USA), CD68 (Thermo Fisher, Fremont, CA, USA), vimentin (Millipore, Temecula, CA, USA), transforming growth factor-beta1 (TGF-β1; Thermo Fisher), proliferating cell nuclear antigen (PCNA; Cell Signaling, Boston, MA, USA) and a-smooth muscle actin (αSMA; Dako, Carpinteria, CA, USA) were used for the study.
Fluorescence detection for dual immunostaining was performed using AlexaFluor-594 and -488-conjugated secondary antibodies (Thermo Fisher). Slides were mounted with Vectashield DAPI-mounting medium (Vector Laboratories, Burlingame, CA) for visualization of the nuclei. Some fluorescent images were overlaid with portions of their corresponding bright-field (BF) images. Specifically, red-brown coloured pixels from BF images were selected, given false colour and subsequently overlaid with the corresponding fluorescent image.
Image quantification and statistics
Three separate images (10×) were taken from random regions of the sample. The total area of the tissue, CTGF-and HMC-positive regions were determined and the area quantified. The reported data for each sample are the total of the three images. The image quantification was reported as mean ± standard deviation. Differences between groups were examined using unpaired t-test. Statistical significance was considered to be achieved if P < 0.05.
Results
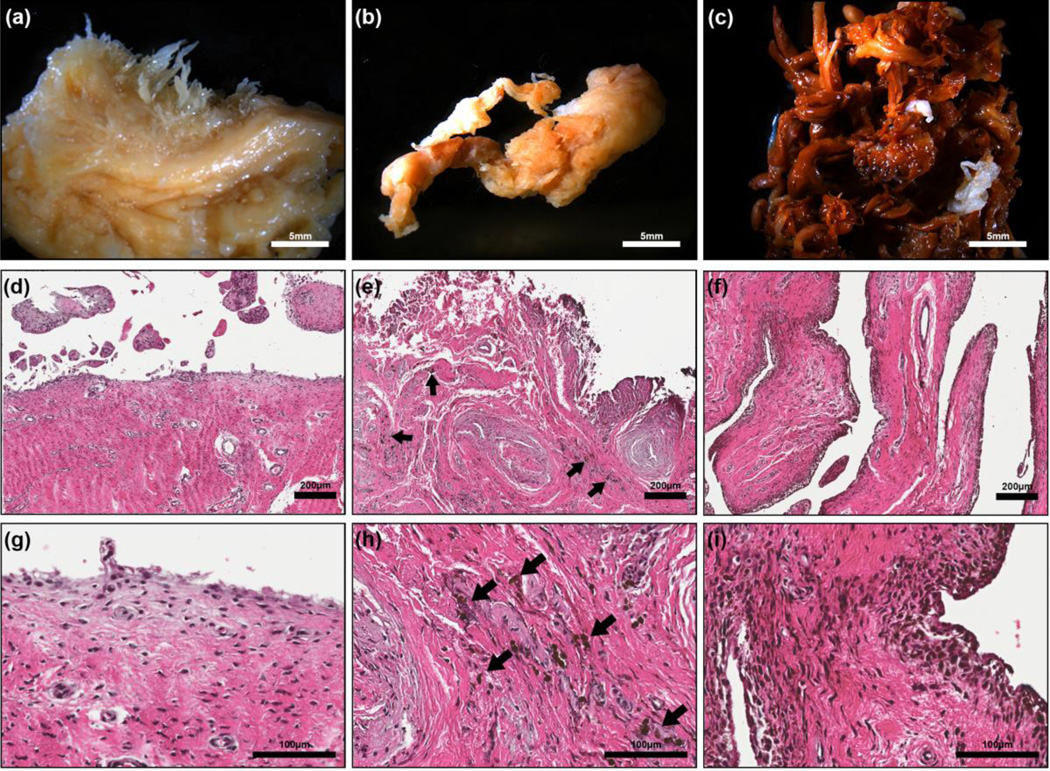
Observation of PWH specimens revealed hyperplastic synovitis with an increased number of synovial villi and characteristic brown haemosiderin hyperpigmentation, indicative of recurrent bleeding into the joint. The degree of hyperplastic synovitis varied from active to quiescent fibrotic synovitis, which is typical of end-stage arthropathy. Gross inspection of synovial tissues from the non-haemophilia patients (Fig. 1a) did not reveal any visible brown-pigmented areas. Gross inspection of samples from patients with active haemophilic synovitis revealed synovial hyperplasia with uniform hyper-pigmentation (Fig. 1c). Tissues from patients with quiescent, fibrotic haemophilic synovitis (Fig. 1b) exhibited less hyperplasia and smaller patches of hyper-pigmented areas within the synovial tissue compared to patients with active synovitis (Fig. 1c).
Figure 1.
Representative images of the synovial tissue from osteoarthritis (a, d & g), PWH with quiescent fibrotic haemophilic synovitis (b, e & h) and PWH with active, hyperplastic synovitis (c, f & i). Gross images of synovial tissue (a, b & c) collected during surgery from osteoarthritis patients (a), haemophilia patient with quiescent fibrotic haemophilic synovitis showing small areas of dense pigment deposition (b) and active synovitis showing enlarged reddish brown synovial villi (c). H&E images of the cross-section of synovia tissue (d, e, f, g, h & i). Note the cells with dense pigment deposition (arrow) in e, h, f & i which are typical of haemophilia patients.
More synovial hyperplasia was observed in PWH as compared to osteoarthritis patients (Fig. 1d–f). Higher magnification images (Fig. 1g–i) demonstrated the presence of numerous HMCs in the surface and sub-surface layers. The number of hypertrophic villi and pigmented HMCs correlated with the macroscopic degree of haemophilic synovial hyperplasia. Synovial tissues from patients with end-stage haemophilic arthropathy (Fig. 1b,e,h) showed less hyperplasia and smaller amounts of HMCs in both the surface and sub-surface layers adjacent to more mature fibrous tissue compared to tissues from patients with active synovitis (Fig. 1c,f,i).
One indication of an ongoing fibrotic process in arthrofibrosis is the presence of αSMA positive myofibroblasts [16]. As expected, perivascular smooth muscle cells were αSMA positive. However, we did not find appreciable numbers of αSMA positive cells elsewhere in the synovial tissues of any patients (Fig. 2). The hyperplastic areas of synovium seen in Fig. 1 are not associated with the presence of differentiated myofibroblasts.
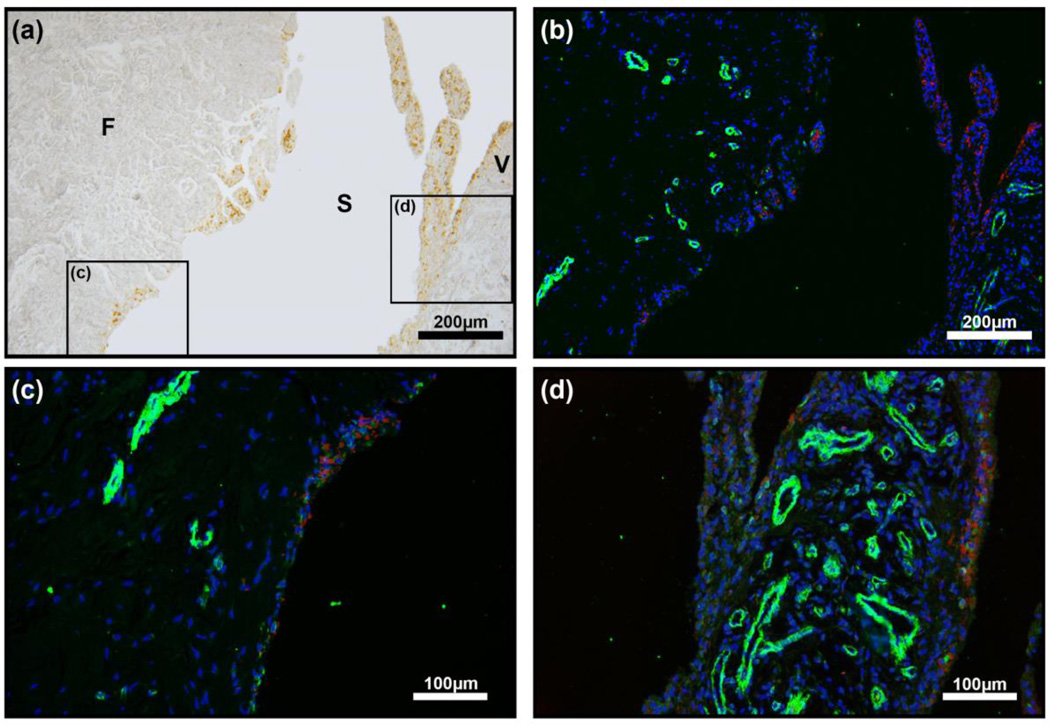
Figure 2.
a-Smooth muscle actin (a-SMA) IHC of synovial tissue in haemophilia patient. (a) Bright field image of synovial tissue showing brown HMCs in the surface layer of synovial membrane. (b–d) a-SMA (green) which is a marker of active myofibroblasts was not readily seen in the synovial tissue of haemophilia patients except surrounding blood vessels in both the fibrous sub-surface layer (F) and synovial villi (V). a-SMA: green; DAPI:blue; HMCs: red.
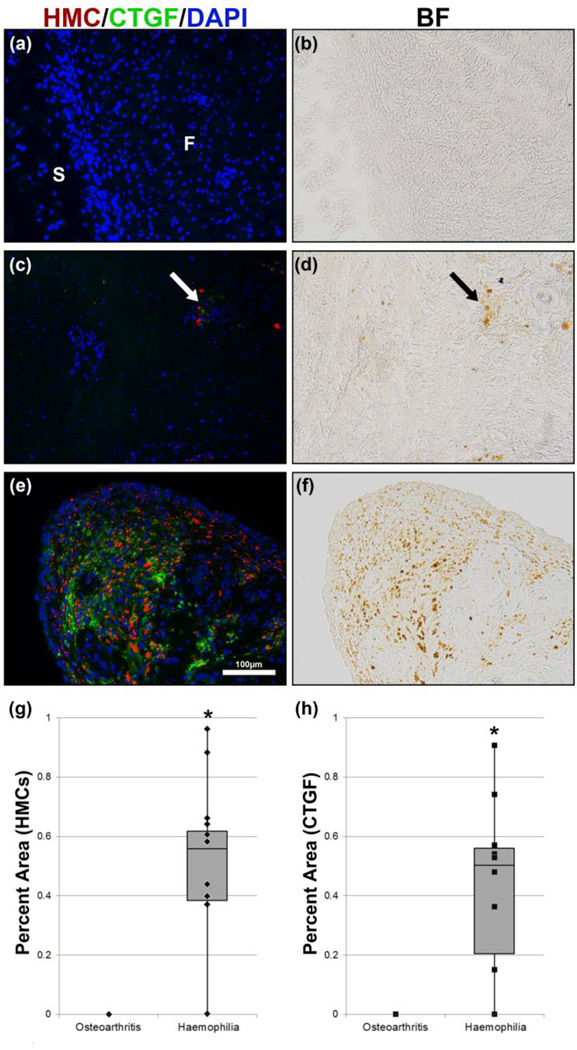
Connective tissue growth factor is expressed in the initial phases of fibrosis, and is required for the excess extracellular matrix accumulation seen in all fibrotic conditions described. Given the established role of CTGF as a mediator of fibrosis [10–12,17], we hypothesized that CTGF would be expressed in collagen-dense regions. In PWH, CTGF staining was seen both in the surface and sub-surface layers, co-localizing with HMCs (Fig. 3). In the fibrous sub-surface layer, we observed CTGF staining only in areas directly associated with the presence of HMCs (Fig. 3a). Using image quantification there was 98% direct correlation between CTGF-positive areas and HMCs (Table 1) in all PWH samples. The per cent area of HMCs (Fig. 3g) and CTGF (Fig. 3f) quantified showed varying degree of HMC and CTGF present in PWH samples (Fig. 3g,h), while no HMCs or CTGF-positive areas were observed in osteoarthritis patients (Table 1).
Figure 3.
CTGF immunostain of synovial tissue in osteoarthritis patient (a) and haemophilia patients (c & e). Bright-field images (b, d & f) showing the presence of HMCs that correspond to CTGF IHC (a, c & e) respectively. CTGF (green) was mainly observed at or adjacent to the brown HMCs (arrow) in both the fibrous sub-surface (c) and surface layer (e) of synovial membrane in haemophilia patients only. Quantification of the per cent area of HMCs (g) and CTGF-positive (h) staining showed a significant increase in PWH (n = 10) compared to osteoarthritis patients (n = 10), *P < 0.001. BF: bright field; F: fibrous sub-surface layer; S: synovium.
Table 1.
| % HMCs | % CTGF | % CTGF adjacent to HMCs | |
|---|---|---|---|
| Haemophilia Patients (n=10) | 52.2% ± 24.5% * | 44.2% ± 26.6% * | 98.8% ± 1.4% |
| Osteoarthritis Patients (n=10) | 0 % ± 0% | 0% ± 0% | N/A |
| Others (n=2) | 13.5% ± 8.0% | 12.1% ± 10.0% | 97.7% ± 1.0% |
Significantly higher than osteoarthritis patients, n=10, p<0.001
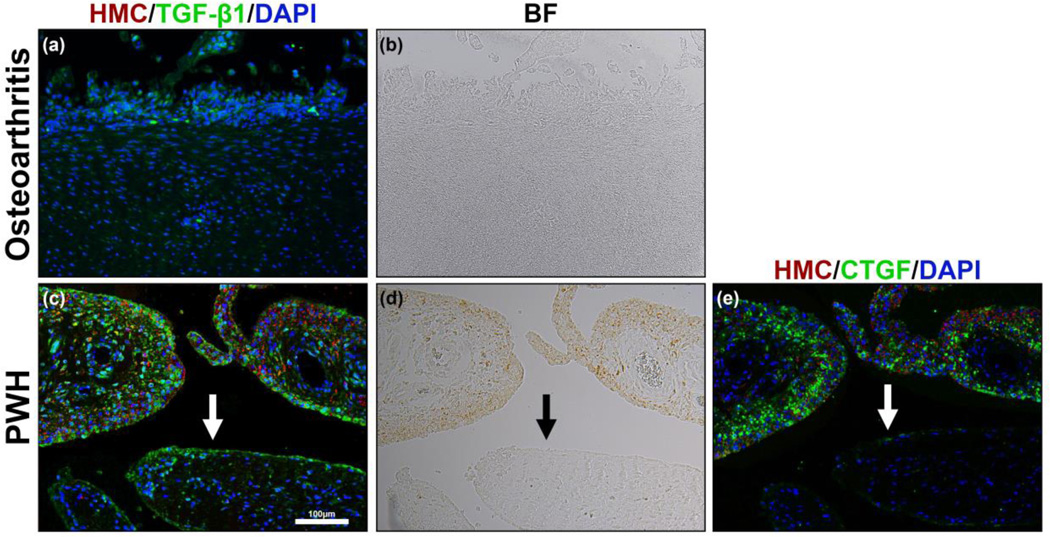
Because transforming growth factor-β (TGF-β) is a direct inducer of CTGF and is thought to trigger expression of CTGF in fibrotic conditions, we also examined the presence of TGF-β1 in our tissue samples using immunofluorescence. The degree of TGF-β staining was variable but overall, not significant in osteoarthritis patients (Fig. 4a). There were relatively high levels of TGF-β1 staining in PWH (Fig. 4c). TGF-β1 localization was observed adjacent to HMCs (Fig. 4c,d). However, we also observed TGF-β1in areas devoid of HMCs (Fig. 4c, bottom arrow). Thus, both CTGF and TGF-β1 are co-localized with HMCs in tissues from PWH but are not co-localized in osteoarthritis patients. These findings are suggestive of an active fibrotic process in PWH.
Figure 4.
TGF-β1 immunostain of synovial tissue in osteoarthritis patient (a) and haemophilia patients (c). No HMCs are seen in the osteoarthritis patients (b). The corresponding immunostain (a) shows minute amounts of TGF-β1. Bright field image (d) shows the presence of HMCs that correspond to TGF-β1 immunostaining (c) in PWH. TGF-β1 was mainly observed in haemophilia patients in both the surface and subsurface layer. Unlike CTGF (e), TGF-b1 was observed both near the brown colored HMCs as well as areas without HMCs (c, arrow). BF: bright field.
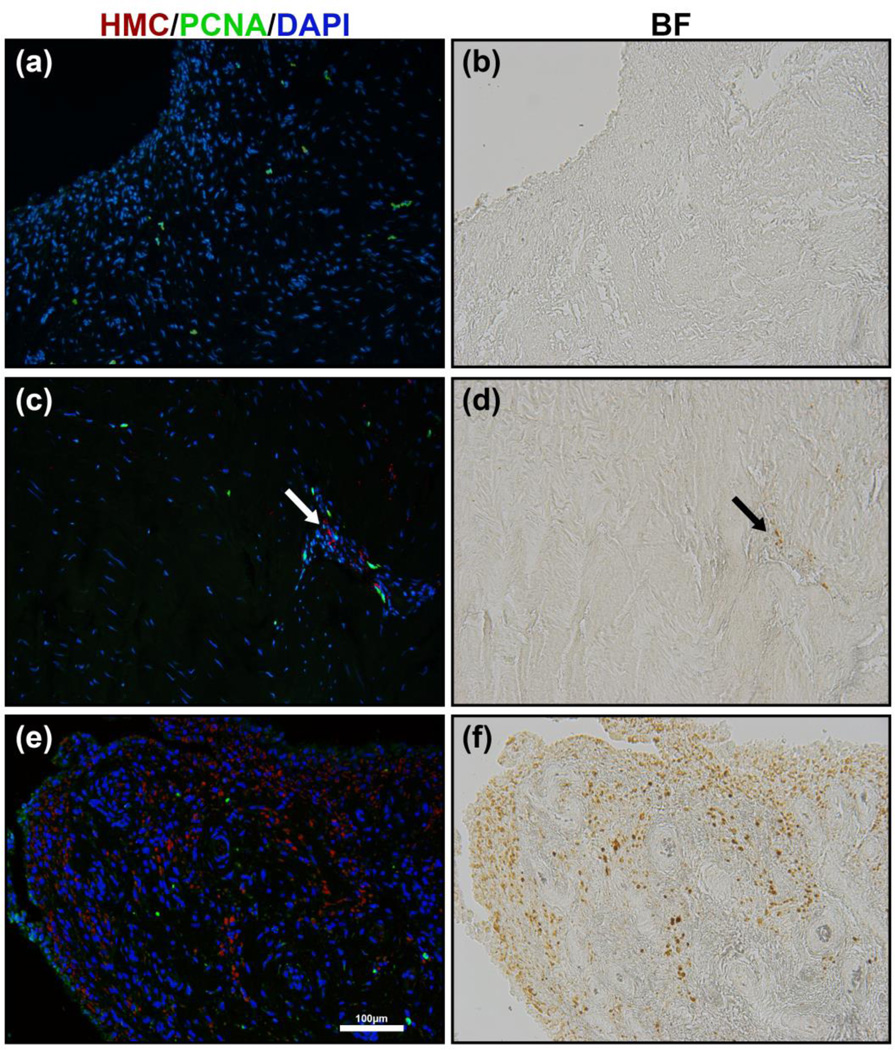
Fibroblast proliferation is an early event in fibrosis. We examined the extent of cell proliferation by immunostaining for PCNA. The PCNA immunostaining did not reveal proliferating cells in the surface layer of the synovial membrane of PWH where there are abundant HMCs. However, we did observe some proliferating cells in the sub-surface layer of tissue from PWH, in areas containing HMCs. The areas with HMCs exhibited increased cellularity compared to adjacent sub-surface areas. It could not be determined whether the increased proliferation observed in these areas is a cause or a consequence of increased cell density. Overall there were few proliferating cells in all samples (Fig. 5).
Figure 5.
Proliferating cell nuclear antigen (PCNA) immunostain for cell proliferation of synovial tissue in non-haemophilia patient (a) and haemophilia patient (c & e). Bright-field images (b, d & f) showing the presence of HMCs that correspond to PCNA immunostain (a, c & e) respectively. Proliferating cells were only observed in the fibrous subsurface layer. In haemophilia patients a slight increase in proliferation was observed near haemosiderin-laden macrophage-like cells in the fibrous sub-surface layer (c, arrow). No significant amount of PCNA staining was observed in the surface layer and synovial villi of haemophilia patients (e).
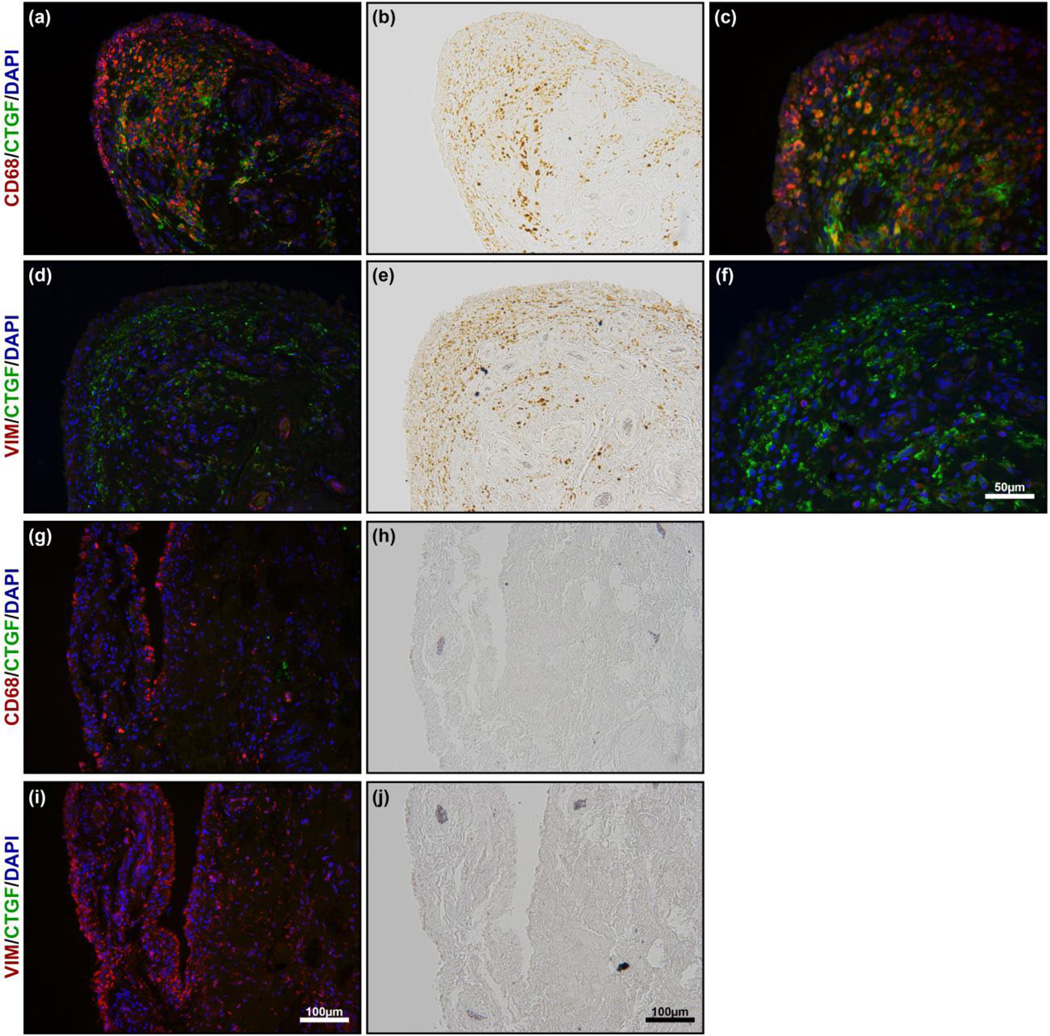
In terms of arthrofibrosis, the defining characteristic of synovial tissue from PWH was the co-localization of HMCs with CTGF. We performed immunostaining to investigate the identity of the HMCs using CD68, a macrophage marker, or vimentin (VIM), a fibroblast marker. As seen in Fig. 6, the HMCs are indeed macrophages that are positive for CD68 and negative for vimentin. This is consistent with previous literature where it has been shown that macrophage-like synovial type A cells are CD68-positive [18] and are responsible for the uptake of both haemosiderin and ferritin when there is bleeding into the joint [6].
Figure 6.
Double immunostain of haemophilia patients (a, c, d & f) and osteoarthritis patients (g & i). No HMCs are seen in osteoarthritis patients (h & i). The corresponding immunostain (g & i) shows the location of CD68 positive macrophages and VIM positive fibroblasts. Bright-field images (b & e) showing the presence of HMCs in PWH. The corresponding to immunostain (a, d) shows CD68 positive macrophage (a & c) and VIM positive fibroblasts (d & f) to identify the HMCs that are associated with the CTGF staining (a, c, d & f; green). The majority of the HMCs is positive for CD68, while negative for VIM and was co-localized with CTGF.
Discussion
Gross observations and general histological analysis showed increased synovial villi and pigmented HMCs in tissues of PWH, as is typical of chronic haemophilic synovitis [8]. Our study focused on synovial tissue to help determine its role in PWH, and showed that CTGF was almost exclusively co-localized with HMCs.
Connective tissue growth factor has been implicated in many fibrotic diseases [10–12,17] and has been shown in experimental animals to be up-regulated specifically in the joint capsule during joint contracture [14,15]. Our study focused on synovial tissue, as much less is known about this region and its role in arthrofibrosis in PWH, and demonstrated that CTGF was almost exclusively co-localized with HMCs (Fig. 3). The correlation between CTGF expression and the presence of HMCs was observed in all PWH and none of the osteoarthritis patients. CTGF is known to be an inflammatory modulator and can modulate macrophage adhesion [19] and migration [20]. CTGF has been shown to co-localize with macrophages in non-haemophilia patients [21–24] and some have postulated that macrophages ingest CTGF coming from a source in the surrounding environment [21,22]. However, other studies indicate that macro-phages themselves can produce CTGF [23,24]. In our samples, we identified the HMCs as macrophages that are positive for CD68. This is consistent with previous studies showing that the majority of the haemosiderin-and ferritin-pigmented cells in haemophilic synovitis are CD68-positive type A synovial macrophage-like cells [6,18]. Looking for possible CTGF involvement, we examined two patients outside our study parameters. One was a patient with sickle cell anemia with osteonecrosis of the knee and the other a patient with an infected total knee replacement (TKR) who had a history of 17 prior operations and severe arthrofibrosis. In these two patients we detected small areas with HMCs (13.5 ± 8.0%) and a limited region of CTGF staining (12.1 ± 10.0%). In these two patients, we also observed a 97.7 ± 1.0% correlation between the presence of HMCs and the areas that were positive for CTGF. Based on these findings, the presence of HMCs, characteristic of haemophilic synovitis and other disorders where haemarthrosis occurs are highly correlated with the expression of the pro-fibrotic protein CTGF. We thus postulate that the CTGF, in association with HMCs, is directly produced by these macrophages in response to haemarthrosis. We plan to confirm this hypothesis with future experiments. Additionally, our data indicate this is not an isolated process that only occurs in PWH, as we showed a similar correlation between CTGF and HMCs in a patient with sickle cell anaemia and one with chronic joint infection with arthrofibrosis. To further explore the aetiology of fibrosis that develops in the non-haemophilia patient, we plan to examine tissues from patients with arthrofibrosis after total joint replacement as well as patients with arthrofibrosis following haemarthrosis such as following ACL rupture.
We did not observe extravascular myofibroblasts in any of the specimens examined. Additionally, we did not observe robust proliferation in our PWH samples, but we did observe small areas of focal proliferation in the sub-surface layer near HMCs in PWH samples. These observations are consistent with previous studies showing that arthrofibrosis might not follow the standard course seen in other fibrotic processes. Animal models of trauma-induced joint contracture have shown an increase in myofibroblasts in the posterior capsules of knees after 2 weeks [15,25], but at longer time point no changes were seen in the number of myofibroblasts as compared to non-operated control [26,27]. Thus, the phase represented by the presence of myofibroblasts in this model is likely a transient one. A current model of trauma-induced arthrofibrosis proposes that there is an initial inflammation phase, followed in order by a proliferative phase and a regenerative phase, which includes maturation and scar formation. During the proliferative phase of healing, fibroblasts are activated to become myofibroblasts, which then disappear [28]. This model works well for acute joint injuries and also seems to apply to the present study in PWH [26,27]. While myofibroblasts may play a role in the initial healing process, our data indicate that the long-standing contractures seen in our haemophilia patients do not show significant active persistent myofibroblasts. Additionally, the hyperplastic villi seen in PWH showed abundant HMC and CTGF staining, and we did not observe any active proliferation or myofibroblasts in these areas. This is most likely because our haemophilia samples in this study were from patients with end-stage arthropathy undergoing knee replacement. Patients with end-stage arthropathy rarely exhibit bleeds into the joint. The synovium has involuted, and the marked vasodilatation and robust cell proliferation seen in earlier stages have been replaced by mature fibrous tissue mostly in the subsurface synovial layer and capsule. Furthermore, as Fig. 6 shows, the hyperplastic villi are mainly composed of CD68-positive macrophages with very sparse fibroblasts, which could be another reason neither proliferation nor myofibroblasts were observed in this area. It is important to note that the present study included the fibrotic sub-surface layer of the synovium, but did not include joint capsule where myofibroblasts have been detected in animal studies, as the joint capsule is not normally removed during the surgeries performed in our study.
Because arthrofibrosis and joint contracture are fibrotic diseases mediated by progressive formation of fibrous tissue, we believe that in PWH, bleeding into the joint provides a pro-proliferative and pro-fibrotic state. This is evident in our PCNA staining, where we observed an increase in proliferating fibroblast-like cells only adjacent to the relatively small amount of perivascular HMCs in the fibrous sub-surface layer. Fibroblast proliferation could be a direct effect of CTGF, as in other diseases where CTGF has been shown to induce fibroblast proliferation [29–31]. We postulate that the favorable micro-environment produced by blood breakdown products taken up by macrophage synovial A cells stimulates native fibroblasts to proliferate and enhances their function, resulting in fibrous joint adhesions, thickened joint capsule and contractures.
Previous studies of arthrofibrosis in non-haemophilia patients have focused primarily on the role of TGF-β [32–35]. We found an increased expression of TGF-β1 in PWH and most areas that were positive for CTGF and HMCs were also positive for TGF-β1. Studies have shown that both TGF-β and CTGF are required for the onset and persistent fibrotic response [32,36,37]. Our study demonstrates that the co-localization of TGF-β and CTGF, as observed in multiple fibrotic conditions, is also a defining feature of HA. Our data provide a molecular explanation of why arthrofibrosis in PWH is persistent and is unresponsive to treatment [5,7]. How these two molecules interact with each other to mediate persistent fibrosis is still unknown. CTGF is a direct target of TGF-β in some tissues [11,38], but other studies show that CTGF expression can be independent of TGF-β signalling [39]. In arthrofibrosis, some studies demonstrated an up-regulation of both TGF-β and CTGF expression in the joint capsule during joint contracture [25,26]. In another study, injection of TGF-β1 into the joint resulted in transient fibrotic tissue formation, but failed to significantly up-regulate CTGF expression [32]. As shown in the present study, TGF-β was detected in all areas where CTGF was identified; raising the possibility that TGF-β is a key regulator of CTGF expression in haemophilic arthropathy. CTGF appears to be produced by synovial A cell macro-phages, possibly as a response to the presence of haemosiderin and ferritin. How haemosiderin, ferritin and TGF-β interact to regulate CTGF expression in haemophilic arthropathy and the function of CTGF in this process are important issues that will be a topic of future investigation. Most cases of arthrofibrosis in non-haemophilia patients are initiated by joint injury or surgery that causes bleeding into the joint. While this is less blood exposure than the chronic recurrent bleeding in haemophilia patients, it is reasonable to assume the cellular response to the blood breakdown product by the synovial A cells would be similar. Thus, we believe the mechanism shown here could also be applicable to the development of arthrofibrosis in non-haemophilia patients. Arthrofibrosis in PWH is universal and recalcitrant to treatment and continuously active, which makes this population attractive for studying the mechanisms of arthrofibrosis. The identification of the relationship between hemarthrosis and arthrofibrosis in PWH supports the prevention of hemarthrosis through prophylactic clotting factor replacement, joint aspiration, and synovectomy in refractory cases.
Regardless of mechanism, our studies show that CTGF is seen in synovial tissue in all patients with HA. Thus, CTGF might serve as a useful biomarker to assess risk of arthrofibrosis, as there currently is none, potentially allowing intervention before significant loss of motion has occurred. The finding that CTGF can be detected in the urine of patients with diabetic nephropathy using ELISA [40–43], raises the possibility that CTGF could be a useful marker.
Conclusion
Connective tissue growth factor has been implicated in all fibrotic conditions described to date, and has been detected in joint capsule tissue or contracted joints [14,15]. For the first time, this paper demonstrates an association of CTGF with arthrofibrosis in the haemophilia patient population. The increased CTGF is detected in the surface and sub-surface layers of the synovium in haemophilia patients. Moreover, we report that CTGF is exclusively colocalized with CD68-positive HMCs, synovial A cells. We found TGF-β1 is also present in high levels in PWH. The expression of TGF-β1 and CTGF in the same areas raises the possibility that TGF-β1 and CTGF cooperate to mediate persistent arthrofibrosis in PWH. The finding that TGF-β1 and CTGF consistently seen in joint tissues from PWH but not in most non-haemophilia patients provides a potential explanation for why PWH are more likely to experience chronic and progressive joint contractures refractory to current treatment.
Acknowledgments
This study was funded in part by the H and H Lee Surgical Research Grant (JJ), the Orthopaedic Institute for Children, the Orthopaedic Haemophilia Treatment Center (JVL), and NIH grant R01 AR056228 (to KML), and the generous support of haemophilia research by Karon and Joe Walvis (JVL) is acknowledged. JJ designed and performed the study and drafted the manuscript. NL assisted in drafting and edited the manuscript. UK edited the manuscript and conducted immunostains. TMP edited the manuscript and conducted immunostains. KML helped design the study and edited the manuscript. JVL proposed, helped design and oversee the study, obtained IRB approval and collected patient samples, authored the clinical section and edited the manuscript. The authors wish to express their appreciation to Gloria Kiel-Gutierrez for her assistance with the IRB application and coordination of the samples.
Footnotes
Disclosures
The authors stated that they had no interests which might be perceived as posing a conflict or bias.
References
- 1.Magit D, Wolff A, Sutton K, Medvecky MJ. Arthrofibrosis of the knee. J Am Acad Orthop Surg. 2007;15:682–694. doi: 10.5435/00124635-200711000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Atkins RM, Henderson NJ, Duthie RB. Joint contractures in the hemophilias. Clin Orthop Relat Res. 1987:97–106. [PubMed] [Google Scholar]
- 3.Löfqvist T, Nilsson IM, Berntorp E, Pettersson H. Haemophilia prophylaxis in young patients--a long-term follow-up. J Intern Med. 1997;241:395–400. doi: 10.1046/j.1365-2796.1997.130135000.x. [DOI] [PubMed] [Google Scholar]
- 4.Silva M, Luck JV, Jr, Llinás A. Chronic hemophilic synovitis: the role of radiosynovectomy. Treatment of Hemophilia World Fed Hemophilia. 2004;33:1–10. [Google Scholar]
- 5.Solimeno L, Goddard N, Pasta G, Mohanty S, Mortazavi S, Pacheco L, et al. Management of arthrofibrosis in haemophilic arthropathy. Haemophilia. 2010;16(Suppl 5):115–120. doi: 10.1111/j.1365-2516.2010.02308.x. [DOI] [PubMed] [Google Scholar]
- 6.Morris CJ, Blake DR, Wainwright AC, Steven MM. Relationship between iron deposits and tissue damage in the synovium: an ultrastructural study. Ann Rheum Dis. 1986;45:21–26. doi: 10.1136/ard.45.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silva M, Luck JV. Long-term results of primary total knee replacement in patients with hemophilia. J Bone Joint Surg Am. 2005;87:85–91. doi: 10.2106/JBJS.C.01609. [DOI] [PubMed] [Google Scholar]
- 8.Luck JV, Silva M, Rodriguez-Merchan EC, Ghalambor N, Zahiri CA, Finn RS. Hemophilic arthropathy. J Am Acad Orthop Surg. 2004;12:234–245. doi: 10.5435/00124635-200407000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Valentino LA. Blood-induced joint disease: the pathophysiology of hemophilic arthropathy. J Thromb Haemost. 2010;8:1895–1902. doi: 10.1111/j.1538-7836.2010.03962.x. [DOI] [PubMed] [Google Scholar]
- 10.Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–4810. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- 11.Jun JI, Lau LF. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov. 2011;10:945–963. doi: 10.1038/nrd3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kubota S, Takigawa M. Cellular and molecular actions of CCN2/CTGF and its role under physiological and pathological conditions. Clin Sci (Lond) 2015;128:181–196. doi: 10.1042/CS20140264. [DOI] [PubMed] [Google Scholar]
- 13.Takigawa M, Nakanishi T, Kubota S, Nishida T. Role of CTGF/HCS24/ecogenin in skeletal growth control. J Cell Physiol. 2003;194:256–266. doi: 10.1002/jcp.10206. [DOI] [PubMed] [Google Scholar]
- 14.Hagiwara Y, Chimoto E, Takahashi I, Ando A, Sasano Y, Itoi E. Expression of transforming growth factor-beta1 and connective tissue growth factor in the capsule in a rat immobilized knee model. Ups J Med Sci. 2008;113:221–234. doi: 10.3109/2000-1967-223. [DOI] [PubMed] [Google Scholar]
- 15.Hildebrand KA, Zhang M, Hart DA. Myofibroblast upregulators are elevated in joint capsules in posttraumatic contractures. Clin Orthop Relat Res. 2007;456:85–91. doi: 10.1097/BLO.0b013e3180312c01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Unterhauser FN, Bosch U, Zeichen J, Weiler A. Alpha-smooth muscle actin containing contractile fibroblastic cells in human knee arthrofibrosis tissue. Winner of the AGA-DonJoy Award 2003. Arch Orthop Trauma Surg. 2004;124:585–591. doi: 10.1007/s00402-004-0742-x. [DOI] [PubMed] [Google Scholar]
- 17.Kular L, Pakradouni J, Kitabgi P, Laurent M, Martinerie C. The CCN family: a new class of inflammation modulators? Biochimie. 2011;93:377–388. doi: 10.1016/j.biochi.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Athanasou NA, Quinn J. Immunocytochemical analysis of human synovial lining cells: phenotypic relation to other marrow derived cells. Ann Rheum Dis. 1991;50:311–315. doi: 10.1136/ard.50.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schober JM, Chen N, Grzeszkiewicz TM, Jovanovic I, Emeson EE, Ugarova TP, et al. Identification of integrin alpha(M)beta(2) as an adhesion receptor on peripheral blood monocytes for Cyr61 (CCN1) and connective tissue growth factor (CCN2): immediate-early gene products expressed in atherosclerotic lesions. Blood. 2002;99:4457–4465. doi: 10.1182/blood.v99.12.4457. [DOI] [PubMed] [Google Scholar]
- 20.Liu SC, Hsu CJ, Fong YC, Chuang SM, Tang CH. CTGF induces monocyte chemoattractant protein-1 expression to enhance monocyte migration in human synovial fibroblasts. Biochim Biophys Acta. 2013;1833:1114–1124. doi: 10.1016/j.bbamcr.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Cicha I, Yilmaz A, Klein M, et al. Connective tissue growth factor is overexpressed in complicated atherosclerotic plaques and induces mononuclear cell chemotaxis in vitro. Arterioscler Thromb Vasc Biol. 2005;25:1008–1013. doi: 10.1161/01.ATV.0000162173.27682.7b. [DOI] [PubMed] [Google Scholar]
- 22.Maybin JA, Barcroft J, Thiruchelvam U, Hirani N, Jabbour HN, Critchley HO. The presence and regulation of connective tissue growth factor in the human endometrium. Hum Reprod. 2012;27:1112–1121. doi: 10.1093/humrep/der476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikezumi Y, Suzuki T, Yamada T, Hasegawa H, Kaneko U, Hara M, et al. Alternatively activated macrophages in the pathogenesis of chronic kidney allograft injury. Pediatr Nephrol. 2015;30:1007–1017. doi: 10.1007/s00467-014-3023-0. [DOI] [PubMed] [Google Scholar]
- 24.Ikezumi Y, Suzuki T, Karasawa T, Hasegawa H, Yamada T, Imai N, et al. Identification of alternatively activated macrophages in new-onset paediatric and adult immunoglobulin A nephropathy: potential role in mesangial matrix expansion. Histopathology. 2011;58:198–210. doi: 10.1111/j.1365-2559.2011.03742.x. [DOI] [PubMed] [Google Scholar]
- 25.Abdel MP, Morrey ME, Barlow JD, Kreofsky CR, An KN, Steinmann SP, et al. Myofibroblast cells are preferentially expressed early in a rabbit model of joint contracture. J Orthop Res. 2012;30:713–719. doi: 10.1002/jor.21588. [DOI] [PubMed] [Google Scholar]
- 26.Hildebrand KA, Sutherland C, Zhang M. Rabbit knee model of post-traumatic joint contractures: the long-term natural history of motion loss and myofibroblasts. J Orthop Res. 2004;22:313–320. doi: 10.1016/j.orthres.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Barlow JD, Hartzler RU, Abdel MP, Morrey ME, An KN, Steinmann SP, et al. Surgical capsular release reduces flexion contracture in a rabbit model of arthrofibrosis. J Orthop Res. 2013;31:1529–1532. doi: 10.1002/jor.22385. [DOI] [PubMed] [Google Scholar]
- 28.Darby IA, Laverdet B, Bonté F, Desmoulière A. Fibroblasts and myofibroblasts in wound healing. Clin Cosmet Investig Dermatol. 2014;7:301–311. doi: 10.2147/CCID.S50046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grotendorst GR, Duncan MR. Individual domains of connective tissue growth factor regulate fibroblast proliferation and myofibroblast differentiation. FASEB J. 2005;19:729–738. doi: 10.1096/fj.04-3217com. [DOI] [PubMed] [Google Scholar]
- 30.Grotendorst GR, Rahmanie H, Duncan MR. Combinatorial signaling pathways determine fibroblast proliferation and myofibroblast differentiation. FASEB J. 2004;18:469–479. doi: 10.1096/fj.03-0699com. [DOI] [PubMed] [Google Scholar]
- 31.Sakai N, Chun J, Duffield JS, Wada T, Luster AD, Tager AM. LPA1-induced cytoskeleton reorganization drives fibrosis through CTGF-dependent fibroblast proliferation. FASEB J. 2013;27:1830–1846. doi: 10.1096/fj.12-219378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watson RS, Gouze E, Levings PP, Bush ML, Kay JD, Jorgensen MS, et al. Gene delivery of TGF-β1 induces arthrofibrosis and chondrometaplasia of synovium in vivo. Lab Invest. 2010;90:1615–1627. doi: 10.1038/labinvest.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hannafin JA, Chiaia TA. Adhesive capsulitis. A treatment approach. Clin Orthop Relat Res. 2000:95–109. [PubMed] [Google Scholar]
- 34.Rodeo SA, Hannafin JA, Tom J, Warren RF, Wickiewicz TL. Immunolocalization of cytokines and their receptors in adhesive capsulitis of the shoulder. J Orthop Res. 1997;15:427–436. doi: 10.1002/jor.1100150316. [DOI] [PubMed] [Google Scholar]
- 35.Ryu JD, Kirpalani PA, Kim JM, Nam KH, Han CW, Han SH. Expression of vascular endothelial growth factor and angiogenesis in the diabetic frozen shoulder. J Shoulder Elbow Surg. 2006;15:679–685. doi: 10.1016/j.jse.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Wang Q, Usinger W, Nichols B, Gray J, Xu L, Seeley TW, et al. Cooperative interaction of CTGF and TGF-β in animal models of fibrotic disease. Fibrogenesis Tissue Repair. 2011;4:4. doi: 10.1186/1755-1536-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mori T, Kawara S, Shinozaki M, Hayashi N, Kakinuma T, Igarashi A, et al. Role and interaction of connective tissue growth factor with transforming growth factor-beta in persistent fibrosis: A mouse fibrosis model. J Cell Physiol. 1999;181:153–159. doi: 10.1002/(SICI)1097-4652(199910)181:1<153::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 38.Holmes A, Abraham DJ, Sa S, Shiwen X, Black CM, Leask A. CTGF and SMADs, maintenance of scleroderma phenotype is independent of SMAD signaling. J Biol Chem. 2001;276:10594–10601. doi: 10.1074/jbc.M010149200. [DOI] [PubMed] [Google Scholar]
- 39.Leask A. Transcriptional profiling of the scleroderma fibroblast reveals a potential role for connective tissue growth factor (CTGF) in pathological fibrosis. Keio J Med. 2004;53:74–77. doi: 10.2302/kjm.53.74. [DOI] [PubMed] [Google Scholar]
- 40.Gilbert RE, Akdeniz A, Weitz S, Usinger WR, Molineaux C, Jones SE, et al. Urinary connective tissue growth factor excretion in patients with type 1 diabetes and nephropathy. Diabetes Care. 2003;26:2632–2636. doi: 10.2337/diacare.26.9.2632. [DOI] [PubMed] [Google Scholar]
- 41.Bao J, Tu Z, Wang J, Ye F, Sun H, Qin M, et al. A novel accurate rapid ELISA for detection of urinary connective tissue growth factor, a biomarker of chronic allograft nephropathy. Transplant Proc. 2008;40:2361–2364. doi: 10.1016/j.transproceed.2008.07.122. [DOI] [PubMed] [Google Scholar]
- 42.Cheng O, Thuillier R, Sampson E, Schultz G, Ruiz P, Zhang X, et al. Connective tissue growth factor is a biomarker and mediator of kidney allograft fibrosis. Am J Transplant. 2006;6:2292–2306. doi: 10.1111/j.1600-6143.2006.01493.x. [DOI] [PubMed] [Google Scholar]
- 43.Yue L, Xia Q, Luo GH, Lu YP. Urinary connective tissue growth factor is a biomarker in a rat model of chronic nephropathy. Transplant Proc. 2010;42:1875–1880. doi: 10.1016/j.transproceed.2009.11.041. [DOI] [PubMed] [Google Scholar]