Summary
Ageing is a very complex process, the result of the dysregulation of multiple systems interacting in many ways. A prominent change occurring with ageing is related to the architecture and functioning of the immune system, viewed commonly as detrimental and termed ‘immunosenescence’. However, age‐associated changes may also lead to increased function in certain respects, which can be viewed as adaptive. None the less, on balance it is well‐recognized that immunosenescence is accompanied by the low‐grade inflammation observed commonly in elderly people, which has been dubbed ‘inflamm‐ageing’. The exact cause and significance of all these changes is not clear, but there is a consensus that they are related to the occurrence of chronic non‐infectious age‐associated disease, as well as increased susceptibility to infections. Alterations to immune cell signalling may be a prominent cause of malfunctioning immunity. Emerging attempts to reverse immunosenescence have recently targeted the signalling pathways in various different cell types of the immune system. Here, we review and discuss alterations in the signalling pathways of immune cells with ageing and consider current targets and means to modulate altered functions. We discuss the potential dangers as well as the benefits of these interventions, and consider future approaches to this problem.
Keywords: adaptive immunity, aging, immunosenescence, innate immunity, lipid rafts, signalling pathways
OTHER ARTICLES PUBLISHED IN THIS REVIEW SERIES
Immunosenescence: the importance of considering age in health and disease. Clinical and Experimental Immunology 2017, 187: 1–3.
The convergence of senescence and nutrient sensing during lymphocyte ageing. Clinical and Experimental Immunology 2017, 187: 4–5.
Immune senescence: significance of the stromal microenvironment. Clinical and Experimental Immunology 2017, 187: 6–15.
Innate immune responses in the ageing lung. Clinical and Experimental Immunology 2017, 187: 16–25.
Age‐related alterations in immune responses to West Nile virus infection. Clinical and Experimental Immunology 2017, 187: 26–34.
Ageing and inflammation in patients with HIV infection. Clinical and Experimental Immunology 2017, 187: 44–52.
Considerations for successful cancer immunotherapy in aged hosts. Clinical and Experimental Immunology 2017, 187: 53–63.
Ageing and obesity similarly impair antibody responses. Clinical and Experimental Immunology 2017, 187: 64–70.
The life cycle of a T cell after vaccination – where does immune ageing strike? Clinical and Experimental Immunology 2017, 187: 71–81.
Herpes zoster and the search for an effective vaccine. Clinical and Experimental Immunology 2017, 187: 82–92.
Adult vaccination against tetanus and diphtheria: the European perspective. Clinical and Experimental Immunology 2017, 187: 93–99.
Introduction
Ageing is a very complex process involving most of the physiological systems of the body. It may be considered as a dysregulation of these physiological systems interconnected through various regulatory mechanisms 1. One of the most important physiological/regulatory systems is the immune system. It is well established that many aspects of the immune response are decreased with ageing, whereas others are increased, resulting in dysregulation. The result has been designated ‘immunosenescence’ 2. Other prominent age‐associated changes include the presence of a low‐grade inflammation, sometimes referred to as ‘inflamm‐ageing’ 3, 4. An important question is whether the multitude of reported differences between younger and older individuals actually represent changes over time and, if so, whether they are decreases due to ageing of the different systems or an ongoing adaptation/remodelling resulting primarily from lifelong pathogen exposures 5, 6. Thus, in this context, immunosenescence as an adaptation to ageing‐associated deterioration of other bodily systems requires more, or a different type, of protection against internal and external challenges, not only pathogens; therefore, the changes in immune cell signalling that we observe and will discuss during ageing would (or could) also be adaptive in nature, even if leading eventually to functional impairment of the immune system (overall insufficient protection against different challenges). In any event, immunosenescence is viewed as being related to the occurrence of age‐associated diseases, such as cardiovascular, neurodegenerative and endocrine disease. A major aim of research into ageing is not only to understand the systems altered over the lifespan, but also to identify targets for interventions to revert or slow down the age‐associated erosion of the different systems, which is often driving elderly people to disease. However, if we consider the immune changes with ageing as adaptive the question arises as to whether they should be reversed or whether this would be harmful long‐term. We will consider these aspects when discussing potential interventions here.
Following thymic involution, a developmentally programmed event occurring around puberty, phenotypical changes at the single cell level, as well as in terms of cell subset distribution, are observed in the adaptive immune system, both resulting in functional changes; in addition, innate immunity is also affected 2. One of the most important changes underlying the functional alterations is the dysregulation of signalling at the cellular level 7, 8, 9. This is accompanied by hallmarks of senescence, which include the deregulation of mitochondrial function, telomere regulation, nutrient‐sensing pathways, proteostasis and others [10]. Additional to the stimuli and stressors encountered by most cells (DNA damage, free radical damage, etc.), immune cells react upon encountering their specific ligands (pathogen antigens, cancer antigens, etc.) via cell surface receptors, which must process signals and transmit the appropriate information to the nucleus via signalling pathways 10, 11, 12. The communication of external signals to the nucleus through the cytosol is complex, and results ultimately in a response such as cytokine secretion, chemotaxis, phagocytosis, proliferation, acquisition of cytotoxicity or apoptosis 7, 13. We will review here the key alterations during ageing with a focus on the associated signalling pathways as well as ways to modulate these to potentially reverse adaptive and innate immunosenescence.
The innate immune response is different in elderly and young people
Many aspects of innate immunity are impacted by age 14, 15. These functional changes are caused partly by different cell subset distribution, as reflected at the phenotypical level, but cannot be explained fully by this mechanism 16. The changes occurring in the innate compartment may be loss or gain of functions 14, 15. Here, we assume that differences between old and young people actually reflect changes in the individual over time, although this has been demonstrated only rarely in humans because most studies are cross‐sectional.
The first cells to arrive at the site of aggression are neutrophils. Their adhesive capacity and their phagocytic activity are not different in older or younger people 14, 15, 17. In contrast, chemotaxis and free radical and cytokine production decreases with age 14, 18. Similar changes are also encountered in monocytes/macrophages, although data from humans are scarce 19, 20, 21. Natural killer (NK) cells are also altered, with decreased cytotoxic function at the single cell level, but compensated in most cases by an increase in their number to retain or even increase overall functionality 16. Similar changes were observed in plasmocytoid and myeloid dendritic cells, resulting in impaired antigen presentation and CD4+ T cell activation, which is not compensated by increased cell numbers 22. It is of note that these innate immune cells are able to secrete a significant amount of proinflammatory cytokines especially in the quiescent state, suggesting that there is a higher level of basal activation and activity of these cells in elderly people 2, 23. It is likely that because of the constant immune challenges over the lifetime, cells of the innate immune system are already in a higher basal activation state 18, 24. Most cellular functions are triggered through different receptors, such as Toll‐like receptors (TLRs,) receptors for Fc and C3b. 23, 25, the numbers of most of which do not change 14. Thus, the higher basal activation state must be explained by some mechanism other than unchanged numbers of receptors.
The adaptive immune response is different in elderly and young people
Several important phenotypical changes occur with ageing in the adaptive immune system, mainly in the CD8+ T cell compartment, although qualitatively similar observations in CD4+ T cells have been reported 26. The decrease in number and frequency of naive CD8+ T cells and the increase in the number of memory (CD28–CD8+) and potentially terminally differentiated effector T cells (CD45RA+CD28–CD8+) are explained mainly by the continuous antigenic exposures throughout life 27, 28, 29, 30, 31, 32. Chronic antigenic stimulation may originate either from pathogen sources or from intrinsic stresses such as inflammation, oxidative stress or tissue damage which can modify self‐antigens 33. The most common antigenic stimulation in this context is due to persistent infection with latent cytomegalovirus (CMV) 27, 28, 29. As an opportunistic herpesvirus, CMV has the tendency to reactivate when immune surveillance decreases, which is the case of immunocompromised individuals such as HIV patients but also very likely in healthy elderly people 26. The main issue with CMV is the induction of a bystander effect on other immune cells, as shown by the impressive accumulation of late‐stage, potentially dysfunctional, CD8+ memory T cells and in some cases to a decrease of the CD4/CD8 ratio below unity, where these expansions are especially large. These observations lead to the definition of an immune risk phenotype (IRP) based on results from the Swedish Octo and Nona studies. This IRP was linked to higher mortality during the follow‐up period 34, 35.
Inflamm‐ageing
These changes in the immune response with ageing are often paralleled by inflamm‐ageing 3, a state associated with increased levels of proinflammatory mediators which develops gradually due to continuous antigenic stimulation and cellular deterioration in aged subjects. This stress can be contributed to by pathogens such as CMV, herpes simplex virus‐1 or by cellular and molecular debris arising from damage caused by reactive oxygen species (ROS), by the Maillard reaction (i.e. advanced glycation end‐products), by nitrosylation and even cancer 33. These stressors are often present at the same time, and very probably the ability to cope with each and all together will determine the overall capacity to control inflamm‐ageing. Recently, this concept was complemented by the identification of the senescence‐associated secretory phenotype (SASP) 36, 37, 38. The SASP concept suggests that cells reaching (replicative) senescence display a secretory profile that may generate and/or sustain the low grade inflammatory response in ageing, but may also play a (beneficial) role in other physiological processes such as tissue repair or remodelling. Cells with such a profile seem to accumulate during the ageing process and secrete proinflammatory cytokines. Eventually they may resist elimination by the immune system 39 so, despite being a tumour suppressor mechanism, dysregulated cellular senescence may contribute to the phenomenon of inflamm‐ageing. Recently, we drew attention to the notion that levels of proinflammatory molecules alone cannot explain inflamm‐ageing, as this is a very complex process with various interactions with anti‐inflammatory molecules and the innate immune system 1. Very recently the molecular mechanism behind this sustained inflammatory state was suggested to be ‘trained’ innate immunity, representing a sort of innate memory 40. The trained status of innate immune cells via epigenetic memory presents a persisting proinflammatory phenotype maintained by the age‐related constant challenges resulting in the maintenance of the differential functioning of the immune system, suggesting its contribution to the onset of various age‐related, chronic inflammatory diseases 33, 41, 42.
Most of the above‐mentioned changes in phenotype and alterations of functionality with ageing are still not explained at the molecular level. For instance, expression or loss of markers at the surface is used to define T cell populations but the signals and mechanisms involved are poorly understood. The same applies to dysfunctional immune cells. We believe that intracellular signalling is a key element in this process, as outlined below.
Signal‐aging
Innate immune cells
Receptor signalling is the way a cell communicates the external ligand challenge translated through transcription factors into specific gene expression which ultimately drives the immune response. In innate immune cells (neutrophils, monocyte/macrophages, NK cells) the central signalling events resulting in immune functions are the mitogen‐activated protein kinase (MAPK), the phosphatidylinositol‐4,5‐bisphosphate 3 kinase (PI3K) and the Janus kinase/signal transduction and activator of transcription (JAK/STAT) pathways 24. These signalling pathways are initiated by ligation of the Toll‐like receptors (TLRs), Fcγ, C3b, formyl peptide receptor 1 (FLMP‐R1) or cytokine receptors. Following stimulation of the appropriate receptors there is a lower activation (phosphorylation on tyrosine or threonine) of extracellular‐regulated kinase (ERK)1/2, protein kinase B (Akt) and JAK2/3 molecules 2, 23. These changes occur differentially depending on the receptor studied. More specifically, for example, during TLR‐2/4 stimulation there is a decreased activation of myeloid differentiation primary response gene 88 (MyD88) and interleukin‐1 receptor‐associated kinase 1 (IRAK1) 23, 43; during stimulation via TLR‐3/7 there is an alteration in the regulation of the interferon regulatory factor (IFR) element 44.
In neutrophils several receptors mediate cellular effector functions. Historically, N‐formylmethionyl‐leucyl‐phenylalanine (fMLP), Fcγ and the C3b receptors have been studied extensively. Functions mediated by these receptors are altered with ageing, explained by changes in the associated signalling pathways, mainly MAPK, PI3K and Akt 24. The reported changes in these signalling pathways are not related to changes in the number of receptors triggered, but rather to impaired signalosome formation that contributed largely to age‐related impairment of neutrophil functions 45. Neutrophils of aged individuals present alterations in TLR signalling due to modified MyD88 and IRAKs activation 23. Whereas the number of these receptors is not affected significantly with ageing, there is a significant alteration in the trafficking of the pathway‐associated signalling molecules in the plasma membrane 46. Membrane microdomains called lipid rafts support early intracellular signalling events by enabling membrane‐bound receptors and their adaptor proteins to coalesce at the site of stimulation 47. This signalling platform is critical for the downstream signalling cascade. Studies have reinforced the role of the membrane in driving the signalling events leading to optimal responses 48, 49. We and others have shown that disruption of the tightly regulated composition of these microdomains leads to faulty signalling with direct consequences for cellular function 23, 50. Not only is the location of the signalling molecules in these lipid rafts important, but the biochemical composition is also crucial. The cholesterol‐rich microdomains have a very specific lipid composition that enables them to coalescence. Many studies suggest that dysregulation of cellular lipid turnover could be an important driver of cell dysfunction 48, 51.
Adaptive immune cells: focus on T cell signalling
T cell activation requires recognition of antigenic epitopes presented by professional antigen‐presenting cells (APC) within the context of major histocompatibility complex (MHC) class I or class II molecules (signal 1) at the immunological synapse (IS), where assembly of the T cell signalling machinery occurs within lipid rafts and involves ad hoc formation of multi‐molecular complexes called signalosomes 52, 53, 54. One of the first events following TCR ligation is the up‐regulation of lymphocyte‐specific protein tyrosine kinase (Lck) activity that targets immunoreceptor tyrosine‐based activation motif (ITAM) motifs of the CD3 complex and initiates recruitment and activation of zeta‐chain‐associated protein kinase 70/linker for activation of T cells/SRC homology 2 domain‐containing leucocyte phosphoprotein of 76 kDa (ZAP70/LAT/SLP76) 55, 56. At every step of the signalling cascade following TCR and co‐stimulatory receptor (e.g. CD28) ligation, age‐associated alterations have been reported which can lead to altered nuclear factor kappa B (NF‐kB) and nuclear translocation of nuclear factor of activated T cells (NFAT) translocation 7. The most notable alterations are at the very early phases of the signalling pathway with the Src tyrosine kinases, Lck notably being the most affected. The activation of Lck, which transduces signals via the phosphorylation of Zap70 to the whole machinery, is altered with ageing 57. All were shown to have differential lipid raft association during ageing 8. For instance, high levels of phosphorylated p38 have been recorded in CD4+ T cells displaying a CD27–CD45RA+ phenotype 58. Signals from the TCR/CD3 complex, co‐stimulatory receptors and cytokine receptors converge leading to p38 phosphorylation which, in turn, leads to interleukin (IL)‐1β, tumour necrosis factor (TNF)‐α and IL‐6 cytokine production. It was demonstrated that anti‐TNF treatment in rheumatoid arthritis patients depletes CD8+ effector memory RA (EMRA) T cells 59 while leaving other T cell subpopulations unaffected, suggesting a role for TNF‐α in the signalling events leading to the generation of CD8+ EMRA T cells in vivo. The relationship between cytokine secretion switch and p38 signalling‐mediated T cell senescence remains to be defined more clearly, but it is likely that dysregulation of TCR signalling cascades will also influence differentiation, as suggested previously 58, 60, 61. Not only is the forward signalling compromised, leading to activation through tyrosine phosphorylation to the tyrosine kinase activation, but also the feedback control.
The phosphatases are part of the feedback control of the signalling pathways in both the innate and the adaptive immune responses. There is evidence that these pathways are also affected by ageing at least at two check‐points in neutrophils and lymphocytes. Src homology region 2 domain‐containing phosphatase‐1 (SHP)‐1 activity could not be modulated in neutrophils and lymphocytes of elderly subjects when these cells were stimulated through specific receptors, in contrast to younger individuals. In neutrophils, SHP‐1 exerts negative control on Lyn tyrosine kinase but cannot function properly because of lipid raft alterations 51. Similarly, in T lymphocytes SHP‐1 activity was not decreased to permit the activation of Lck to transmit the signal adequately for clonal expansion and IL‐2 secretion 57. In fact, SHP‐1 activity was higher in healthy elderly subjects than in young individuals, an observation consistent with the decreased T cell response. In addition, there were significant differences in active (pY394) and inactive (pY505) forms of Lck in response to T cell activation with ageing. In T cells it was also shown that altered ERK activation was due to altered activities of dual specificity phosphatase 4 (DUSP4) and DUSP6 via mRNA‐181 62. Thus, the dysregulation of the negative regulation of immune cell activation could be an important driver of immune dysfunction in ageing.
One other potentially important signalling pathway with implications for the erosion of immunity with age is in the mammalian target of rapamycin (mTOR) pathway. This critical pathway regulates many processes but has been linked mainly to glucose metabolism and longevity. Recently, it became evident that mTOR, as a serine threonine kinase, may also play an important role in T cell activation and differentiation especially of naive CD4+ T cells in their differentiation towards the T helper type 1 (Th1) or Th17 phenotype 10. The mTOR signalling pathway activation is under the control of TCR/CD28 stimulation 63, 64. Target of rapamycin complex 1 (TORC1) is activated through Akt phosphorylation via the 3‐phosphoinositide dependent kinase‐1 (PDK1) pathway. TORC2 regulates naive CD4+ T cell differentiation towards the Th2 phenotype. There are few studies on T cell mTOR alterations with ageing and most are in mice. Thus, Perkey et al. 65 showed recently that TORC2 signalling is increased in murine CD4+ T cells in ageing and its enforced over‐expression in CD4+ T cells of young mice reproduced age‐related CD4+ T cell functional changes. Our own data suggest differential phosphorylation status [cAMP response element‐binding protein (CREB, Akt, S6, eukaryotic translation initiation factor 4E‐binding protein (ElF4E), 4EBP1] of memory compared to naive T cells. Very recently, Arnold et al. 66 demonstrated that TCR stimulation induced autophagy in CD8+CD28+ T cells, while in the CD8+CD28– subset autophagy was decreased, which largely seems to compromise their survival under specific antigen stimulation. These emerging data underline the importance of mTOR‐related metabolic control interwined with the TCR feed‐forward and negative signalling pathways to induce efficient T cell activation leading to appropriate functioning. Therefore, further investigation of this signalling pathway is fundamental for the understanding of the functional changes in T cells with human ageing. An oppositely directed (stimulating) regulator of the autophagy pathway is the metabolic sensor 5'‐adenosine monophosphate‐activated protein kinase (AMPK), already implicated in the regulation of inflammatory processes 66. It was demonstrated recently that AMPK activates p38 which leads to T cell immunosenescence, which can be prevented by blocking the AMPK 60.
Thus, with ageing there are many signalling alterations contributing to the changes in immune cell functions leading to the well‐known modification of immune reactivity 9, 13, 14. Consequently, the question arises as to whether the various modulations of these signalling alterations may result in changes in functions which would offer an opportunity to revert to immunosenescence. There are some experimental data that suggest that modulation of signalling pathways may lead to beneficial changes in the altered functions of immune cells. However, it remains questionable whether the changes in immune cell functions represent a reversal per se of immunosenescence or only changes in individual functions from the context of the immune regulation occurring with ageing.
Signalling as a target for reversing immunosenescence
From the perspective that we consider the changes occurring in the immune system with ageing as detrimental, it seems that altered signalling represents a good candidate for trials to reverse immunosenescence. This notion is strengthened by the present tendency towards personalized medicine. These molecules are perfectly specific targets to be considered when we wish to change specific functions, such as phagocytosis, proliferation and others. There are still some drawbacks, as we are currently not able to target individual and specific cell populations specifically in a human‐orientated approach. In the following section, we will summarize trials aimed at modulating signalling and their effects on immune cells with ageing. We discuss the innate and the adaptive immune response separately, because despite many similarities they display significant differences in signalling pathways.
Modulation of signalling in innate immune cells with ageing
There are a few studies aiming at modulating innate immune cell functions through signalling 51, 67. All these studies have been performed in polymorphonuclear neutrophils (PMN). Our study targeted SHP‐1 in neutrophils to increase some of their crucial functions. Granulocyte–macrophage colony‐stimulating factor (GM‐CSF) is a well‐known modulator of PMN functions, which were found previously to be altered with ageing 2, 45. We found strong tyrosine phosphorylation of Lyn in lipid rafts of PMN from young subjects following GM‐CSF stimulation compared with the almost non‐phosphorylated basal status. In contrast, there is no phosphorylation of Lyn with GM‐CSF stimulation in PMN of elderly donors compared with the higher phosphorylated basal status. A similar situation has been demonstrated already for MAPKs in PMN of elderly 24. The use of protein tyrosine phosphatase (PTP) inhibitors has a strong influence on Lyn phosphorylation and recruitment to lipid rafts. The inhibition of phosphatase activity, including SHP‐1, revealed that it is necessary to maintain phospho‐Lyn in rafts to achieve optimal cellular activation. Additionally, incubation of PMN with a PTP inhibitor cocktail, followed by GM‐CSF stimulation, resulted in a significant increase in ROS production and chemotaxis. It is of note that the PTP inhibitor cocktail induced a significant increase in ROS production in PMN of elderly people compared with GM‐CSF alone (and not observed for chemotaxis). This suggests that modulation of signalling could lead to effective modulation of PMN functions. This was also true for essential cellular processes such as susceptibility to apoptosis. In PMN of young subjects, the PTP inhibitor alone or PTP inhibitor and GM‐CSF treatment blunted the GM‐CSF apoptosis‐rescuing effect. In contrast, preincubation of PMN from elderly people with the PTP inhibitor before the 18‐h culture with GM‐CSF resulted in recovery of the lost GM‐CSF‐induced rescue from apoptosis. Together, these results suggest that by modulating phosphatase activity, such as SHP‐1, PMN functions can be improved with ageing.
As mentioned above, phosphorylation of signalling molecules in resting cells from elderly individuals is dysregulated. This higher basal phosphorylation level suggests that PMN are already primed for action and is characteristic for the PMN of elderly people. This is the consequence of a low‐grade, chronic inflammation that we referred to earlier as inflamm‐ageing. Many signalling molecules, such as Lyn, ERK1/2 and PI3K, were shown to exhibit this higher activation state in neutrophils with ageing. It is of interest that increased phosphorylation of PI3K was demonstrated in neutrophils, with its consequent decreased further activation during specific receptor stimulation 67. This increased basal activation was linked mainly to altered chemotaxis, but also phagocytosis and free radical production 67. Sapey et al. 67 have shown that inhibiting specifically PI3Kγ and PI3Kδ at the basal state in neutrophils of elderly people increases PI3K activity under stimulation which will lead to increased chemotaxis. The authors concluded that ‘targeting PI3K signalling may therefore offer new strategies in improving neutrophil functions during infections and reduce inappropriate inflammation in older patients’. Despite the attractiveness of this approach, it remains to be determined whether this is a meaningful approach in the whole ageing organism, resulting ultimately in a better defence against infections or in the decrease of low‐grade inflammation. It is not to be excluded that the higher basal phosphorylation status of signalling molecules, besides altering the threshold for cellular activation, also increases the susceptibility of these cells to develop the secretory phenotype (SASP) described earlier, which would further suggest the appropriateness of such interventions but perhaps at an earlier period of life. This area needs further research, and eventually clinical studies to confirm the global effects of these changes in the evolutionary perspective of immune changes as part of the collection of other adaptations that happen during ageing.
Modulation of signalling in adaptive immune cells with ageing
There are many more studies targeting signalling molecules specifically to reverse functional changes in T cells. Historically, changes in the T cell compartment with ageing were considered more important than in the innate compartment. Studies are beginning to reveal that this may not be true, especially in light of new knowledge that the majority of immune cells resides in tissues and that most approaches to study immunosenescence are restricted to the peripheral blood. Nevertheless, alterations of T cell signalling pathway are better characterized.
We have shown that pharmacological inhibition of SHP‐1 results in recovery of TCR/CD28‐dependent lymphocyte proliferation and IL‐2 production to levels similar to those of young adults 57. These studies provide a lead for a strategy aimed at modulation of the negative feedback loop of T cell activation by targeting SHP‐1 and PTPases in general. The inhibition of SHP‐1 activity resulted in recovered Lck activity modulation in T cells of elderly subjects. This approach is supported further by recent data from other laboratories where higher DUSP4 and DUSP6 phosphatase activities were inhibited by various means, such as siRNA, a specific allosteric inhibitor, or miR‐181a, and resulted in increased T cell signalling and associated functions 64. This modulation targeted the activity of ERK1/2, which improved significantly in T cells, especially in CD4+ T cells of elderly subjects. Thus, these phosphatases are potential targets to restore T cell functions in elderly subjects, with the aim of improving response to vaccination or control persistent infections such as CMV more effectively. However, based on an extensive literature search we can say that such an approach has not yet been tested in vivo, due possibly to limited specificity and other problems with the in‐vivo use of the available PTP inhibitors, such as possible autoimmunity 68.
As mentioned above, ageing is characterized by the increase of putatively terminal effector memory CD8+ T cells (TTE), at least some of which are very likely to be senescent, and as such to have lost their proliferative capacity. Thus, there is great interest to reverse this functionally semi‐inert state. Furthermore, the modulation of inhibitory receptors such as programmed death 1 (PD‐1) (also acting through phosphatases such as SHP‐1) increased the functions of old CD8+ T cells successfully 72. Moreover, the inhibition of p38 MAPK resulted in inhibition of TNF‐α secretion. It is of note that simultaneous PD‐1 inhibition counteracted this decreased TNF‐α secretion. Thus, simultaneous inhibition of the PD‐1 and p38 MAPK signalling pathways may result in unblocking terminal‐effector CD8+ T cell proliferation capacity. Together with their sustained cytokine production capabilities, this may result in reverting the status from TTE to ‘TEM‐like’. There are risks associated with this modulation, as long‐term blockade of p38 may result in cell escape from senescence to malignant transformation. However, combined short‐term inhibition could reduce the extent of immune ageing in populations at risk (e.g. frail category or those with intense immunological history) in a specific situation where the functionality of the immune response may be critical for morbidity.
There have also been attempts to modulate immune cell functions through global changes in the signalling pathway network. One of these methods consists of using nutrition or physical activity as a modulator of T cell function. In particular, it was shown that high‐density lipoprotein (HDL) may influence various signalling pathways affecting T cell proliferation 69. However, the exact mechanism is not yet understood. Of interest, elderly individuals under physical exercise treatment showed increased circulating levels of killer‐cell lectin like receptor G1 (KLRG‐1)+ T cells 70. This may simply reflect mobilization of cells from the tissue to the periphery. More studies are required to identify whether exercise is a way to regulate the organ‐specific immune senescence burden or whether this is simply a response to stress. Together, mimicking ageing of immune cells has shown value for understanding the role of specific alterations on cellular functions. For instance, increasing cholesterol levels in T cell membranes (and lipid rafts) has resulted in similar functional defects as in T cells from elderly individuals. Approaches are still needed to rejuvenate immune cell functions by restoring the right biochemical properties of membranes.
Very recently it was shown that mTOR inhibition can improve immune function in elderly people. This is the first demonstration in vivo that the modulation of a signalling pathway could result in significant functional changes leading to a clinically significant effect; namely, improving the response to influenza vaccination 71. Everolimus (RAD001) administered to elderly subjects enhanced the antibody response to influenza vaccination by 20%. This intervention was relatively well tolerated. The mechanism seemed to be via a decrease of the PD‐1 receptor on CD4+ and CD8+ T cells, as this receptor inhibits T cell signalling and is expressed more highly with ageing. However, it is not known whether this increased response was translated to a better protection against influenza in these subjects. None the less, this first human study shows the feasibility of some interventions (Fig. 1).
Figure 1.
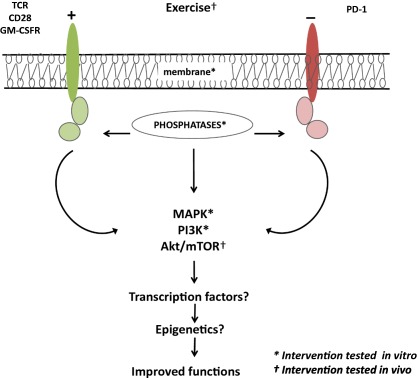
Different signalling pathway targets already used for interventions to reverse immunosenescence. Immune cells use receptor‐mediated signalling pathways to respond to their ligands. With ageing, the changes in the signalling pathways may lead to changes in immune cell functions of the innate and the adaptive immune response. To improve the functioning of the immune cells with ageing, these signalling pathways seem to be good targets. The main targets used in various studies in vitro (*) and in vivo (†) are indicated. ? Suggests that other possible targets not yet explored in the context of immunosenescence.
Conclusion
Together, the data reviewed here suggest that age‐related signalling changes may be targeted for restoring immune function in vitro in immune cells. However, considering the concept of immune adaptation/remodelling of the immune system with ageing we do not know what the physiological consequences of the reverted changes within the immune system could be. Would they completely unbalance the whole system? Would they cause even more damage than good? Before being able to conclude that a beneficial effect of the restoration of immunosenescence is the most likely outcome, these questions should be addressed. However, the study with the mTOR inhibitor RAD001 holds promise, but even in this case the long‐term effects have not yet been investigated. In theory, each signalling molecule/pathway may be targeted 72, 73, but a systems approach is needed to choose the best‐defined hubs to intervene more precisely and without compromising other beneficial pathways.
Disclosure
The authors declare no competing interests.
Acknowledgements
This work was supported partly by grants from the Canadian Institutes of Health Research (CIHR) (No. 106634 and No. 106701), Canada Research Chairs (SCC) the Université de Sherbrooke, and the Research Center on Aging to TF and by the Singapore Immunology Network (SIgN) and A*STAR Joint Council Office DP grant [1434m00115] to AL and by the Polish Ministry of Science and Higher Education statutory grant 02‐0058/07/262 to J. M. W. and by Funding of the European Commission under Grant Agreement FP7 259679, Integrated research on developmental determinants of ageing and longevity, ‘IDEAL’ to G. P.
References
- 1. Morrisette‐Thomas V, Cohen AA, Fülöp T et al Inflamm‐aging does not simply reflect increases in pro‐inflammatory markers. Mech Aging Dev 2014; 139:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pera A, Campos C, López N et al Immunosenescence: implications for response to infection and vaccination in older people. Maturitas 2015; 82:50–5. [DOI] [PubMed] [Google Scholar]
- 3. Franceschi C, Bonafè M, Valensin S et al Inflamm‐aging. An evolutionary perspective on immunosenescence. Ann NY Acad Sci 2000; 908:244–54. [DOI] [PubMed] [Google Scholar]
- 4. Paolisso G, Barbieri M, Bonafè M, Franceschi C. Metabolic age modelling: the lesson from centenarians. Eur J Clin Invest 2000; 30:888–94. [DOI] [PubMed] [Google Scholar]
- 5. Franceschi C, Monti D, Sansoni P, Cossarizza A. The immunology of exceptional individuals: the lesson of centenarians. Immunol Today 1995; 16:12–6. [DOI] [PubMed] [Google Scholar]
- 6. Fulop T, Dupuis G, Baehl S et al From inflamm‐aging to immune‐paralysis – a slippery slope during aging for immune‐adaptation. Biogerontology 2016; 17:147–57. [DOI] [PubMed] [Google Scholar]
- 7. Fulop T, Le Page A, Fortin C, Witkowski JM, Dupuis G, Larbi A. Cellular signaling in the aging immune system. Curr Opin Immunol 2014; 29C:105–11. [DOI] [PubMed] [Google Scholar]
- 8. Larbi A, Dupuis G, Khalil A, Douziech N, Fortin C, Fulop T Jr. Differential role of lipid rafts in the functions of CD4+ and CD8+ human T lymphocytes with aging. Cell Signal 2006; 18:1017–30. [DOI] [PubMed] [Google Scholar]
- 9. Goronzy JJ, Li G, Yu M, Weyand CM. Signaling pathways in aged T cells – a reflection of T cell differentiation, cell senescence and host environment. Semin Immunol 2012; 24:365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chisolm DA, Weinmann AS. TCR‐signaling events in cellular metabolism and specialization. Front Immunol 2015; 6:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nika K, Acuto O. Membrane nanodomains in T‐cell antigen receptor signalling. Essays Biochem 2015; 57:165–75. [DOI] [PubMed] [Google Scholar]
- 12. Yasuda T. MAP kinase cascades in antigen receptor signaling and physiology. Curr Top Microbiol Immunol 2016; 393:211–31. [DOI] [PubMed] [Google Scholar]
- 13. Larbi A, Rymkiewicz P, Vasudez A et al The immune system in the elderly: a fair fight against diseases? Aging Health 2013; 9:35–47. [Google Scholar]
- 14. Solana R, Tarazona R, Inmaculada G, Lesur O, Dupuis G, Fülöp T. Innate immunosenescence: effect of aging on cells and receptors of the innate immune system in humans. Semin Immunol 2012; 24:331–41. [DOI] [PubMed] [Google Scholar]
- 15. Montgomery RR, Shaw AC. Paradoxical changes in innate immunity in aging: recent progress and new directions. J Leukoc Biol 2015; 98:937–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Solana R, Campos C, Pera A, Tarazona R. Shaping of NK cell subsets by aging. Curr Opin Immunol 2014; 29:56–61. [DOI] [PubMed] [Google Scholar]
- 17. Baëhl S, Garneau H, Le Page A et al Altered neutrophil functions in elderly patients during a 6‐month follow‐up period after a hip fracture. Exp Gerontol 2015; 65:58–68. [DOI] [PubMed] [Google Scholar]
- 18. Sauce D, Dong Y, Campillo‐Gimenez L et al Reduced oxidative burst by primed neutrophils in the elderly individuals is associated with increased levels of the CD16bright/CD62Ldim immunosuppressive subset. J Gerontol A Biol Sci Med Sci in press 2016. [DOI] [PubMed] [Google Scholar]
- 19. Linton PJ, Thoman ML. Immunosenescence in monocytes, macrophages, and dendritic cells: lessons learned from the lung and heart. Immunol Lett 2014; 162:290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. do Nascimento MP, Pinke KH, Penitenti M, Ikoma MR, Lara VS. Aging does not affect the ability of human monocyte‐derived dendritic cells to phagocytose Candida albicans . Aging Clin Exp Res 2015; 27:785–9. [DOI] [PubMed] [Google Scholar]
- 21. Stervbo U, Meier S, Mälzer JN et al Effects of aging on human leukocytes (part I): immunophenotyping of innate immune cells. Age (Dordr) 2015; 37:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Magrone T, Jirillo E. Disorders of innate immunity in human aging and effects of nutraceutical administration. Endocr Metab Immune Disord Drug Targets 2014; 14:272–82. [DOI] [PubMed] [Google Scholar]
- 23. Fulop T, Larbi A, Douziech N et al Signal transduction and functional changes in neutrophils with aging. Aging Cell 2004; 3:217–26. [DOI] [PubMed] [Google Scholar]
- 24. Larbi A, Douziech N, Fortin C, Linteau A, Dupuis G, Fulop T Jr. The role of the MAPK pathway alterations in GM‐CSF modulated human neutrophil apoptosis with aging. Immun Aging 2005; 2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thomas CJ, Schroder K. Pattern recognition receptor function in neutrophils. Trends Immunol 2013; 34:317–28. [DOI] [PubMed] [Google Scholar]
- 26. Larbi A, Fulop T. From ‘truly naïve’ to ‘exhausted senescent’ T cells: when markers predict functionality. Cytometry A 2014; 85:25–35. [DOI] [PubMed] [Google Scholar]
- 27. Pawelec G, Derhovanessian E, Larbi A, Strindhall J, Wikby A. Cytomegalovirus and human immunosenescence. Rev Med Virol 2009; 19:47–56. [DOI] [PubMed] [Google Scholar]
- 28. Pawelec G. Immunosenenescence: role of cytomegalovirus. Exp Gerontol 2014; 54:1–5. [DOI] [PubMed] [Google Scholar]
- 29. Fülöp T, Larbi A, Pawelec G. Human T cell aging and the impact of persistent viral infections. Front Immunol 2013; 4:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Palmer DB. The effect of age on thymic function. Front Immunol 2013; 4:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Qi Q, Zhang DW, Weyand CM, Goronzy JJ. Mechanisms shaping the naïve T cell repertoire in the elderly ‐ thymic involution or peripheral homeostatic proliferation? Exp Gerontol 2014; 54:71–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Appay V, Sauce D. Naive T cells: the crux of cellular immune aging? Exp Gerontol 2014; 54:90–3. [DOI] [PubMed] [Google Scholar]
- 33. Fulop T, Dupuis G, Witkowski JM, Larbi A. The role of immunosenescence in the development of age‐related diseases. Rev Inves Clin 2016; 68:84–91. [PubMed] [Google Scholar]
- 34. Wikby A, Ferguson F, Forsey R et al An immune risk phenotype, cognitive impairment, and survival in very late life: impact of allostatic load in Swedish octogenarian and nonagenarian humans. J Gerontol A Biol Sci Med Sci 2005; 60:556–65. [DOI] [PubMed] [Google Scholar]
- 35. Wikby A, Johansson B, Olsson J, Löfgren S, Nilsson BO, Ferguson F. Expansions of peripheral blood CD8 T‐lymphocyte subpopulations and an association with cytomegalovirus seropositivity in the elderly: the Swedish NONA immune study. Exp Gerontol 2002; 37:445–53. [DOI] [PubMed] [Google Scholar]
- 36. Burton DGA, Krizhanovsky V. Physiological and pathological consequences of cellular senescence. Cell Mol Life Sci 2014; 71:4373–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Laberge RM, Sun Y, Orjalo AV et al MTOR regulates the pro‐tumorigenic senescence‐associated secretory phenotype by promoting IL1A translation. Nat Cell Biol 2015; 17:1049–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Van Deursen JM. The role of senescent cells in aging. Nature 2014; 509:439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest 2013; 123:966–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Netea MG, Latz E, Mills KH, O'Neill LA. Innate immune memory: a paradigm shift in understanding host defense. Nat Immunol 2015; 16:675–9. [DOI] [PubMed] [Google Scholar]
- 41. Kleinnijenhuis J, Quintin J, Preijers F et al Bacille Calmette–Guerin induces NOD2‐dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci USA 2012; 109:17537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kyburz D, Karouzakis E, Ospelt C. Epigenetic changes: the missing link. Best Pract Res Clin Rheumatol 2014; 28:577–87. [DOI] [PubMed] [Google Scholar]
- 43. Casanova JL, Abel L, Quintana‐Murci L. Human TLRs and IL‐1Rs in host defense: natural insights from evolutionary, epidemiological, and clinical genetics. Annu Rev Immunol 2011; 29:447–91. [DOI] [PubMed] [Google Scholar]
- 44. Shaw AC, Panda A, Joshi SR, Qian F, Allore HG, Montgomery RR. Dysregulation of human Toll‐like receptor function in aging. Aging Res Rev 2011; 10:346–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fortin CF, McDonald PP, Lesur O, Fülöp T Jr. Aging and neutrophils: there is still much to do. Rejuvenation Res 2008; 11:873–82. [DOI] [PubMed] [Google Scholar]
- 46. Shaw AC, Godstein DR, Montgomery RR. Age‐dependent dysregulation of innate immunity. Nat Rev Immunol 2013; 13:875–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fortin C, Fülöp T. Isolation of lipid rafts from human neutrophils by density gradient centrifugation. Methods Mol Biol 2015; 1343:1–7. [DOI] [PubMed] [Google Scholar]
- 48. Masoud R, Bizouarn T, Houée‐Levin C. Cholesterol: a modulator of the phagocyte NADPH oxidase activity – a cell‐free study. Redox Biol 2014; 3:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gorgojo J, Lamberti Y, Valdez H, Harvill ET, Rodríguez ME. Bordetella parapertussis survives the innate interaction with human neutrophils by impairing bactericidal trafficking inside the cell through a lipid raft‐dependent mechanism mediated by the lipopolysaccharide O antigen. Infect Immun 2012; 80:4309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Scheel‐Toellner D, Wang K, Assi LK et al Clustering of death receptors in lipid rafts initiates neutrophil spontaneous apoptosis. Biochem Soc Trans 2004; 32:679–81. [DOI] [PubMed] [Google Scholar]
- 51. Fortin CF, Larbi A, Lesur O, Douziech N, Fulop T Jr. Impairment of SHP‐1 down‐regulation in the lipid rafts of human neutrophils under GM‐CSF stimulation contributes to their age‐related, altered functions. J Leukoc Biol 2006; 79:1061–72. [DOI] [PubMed] [Google Scholar]
- 52. Ventimiglia LN, Alonso MA. The role of membrane rafts in Lck transport, regulation and signalling in T cells. Biochem J 2013; 454:169–79. [DOI] [PubMed] [Google Scholar]
- 53. Brownlie RJ, Zamoyska R. T cell receptor signalling networks: branched, diversified and bounded. Nat Rev Immunol 2013; 13:257–69. [DOI] [PubMed] [Google Scholar]
- 54. Alarcón B, Mestre D, Martínez‐Martín N. The immunological synapse: a cause or consequence of T‐cell receptor triggering? Immunology 2011; 133:420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schoenborn JR, Tan YX, Zhang C, Shokat KM, Weiss A. Feedback circuits monitor and adjust basal Lck‐dependent events in T cell receptor signaling. Sci Signal 2011; 4:ra59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Coussens NP, Hayashi R, Brown PH et al Multipoint binding of the SLP‐76 SH2 domain to ADAP is critical for oligomerization of SLP‐76 signaling complexes in stimulated T cells. Mol Cell Biol 2013; 33:4140–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Le Page A, Fortin C, Garneau H et al Downregulation of inhibitory SRC homology 2 domain‐containing phosphatase‐1 (SHP‐1) leads to recovery of T cell responses in elderly. Cell Commun Signal 2014; 12:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Di Mitri D, Azevedo RI, Henson SM et al Reversible senescence in human CD4+CD45RA+CD27– memory T cells. J Immunol 2011; 187:2093–100. [DOI] [PubMed] [Google Scholar]
- 59. Maculay R, Akbar AN, Henson SM. The role of the T cell in age‐related inflammation. Age 2013; 35:563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lanna A, Henson SM, Escors D, Akbar AN. The kinase p38 activated by the metabolic regulator AMPK and scaffold TAB1 drives the senescence of human T cells. Nat Immunol 2014; 15:965–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Henson SM, Lanna A, Riddell NE et al p38 signaling inhibits mTORC1‐independent autophagy in senescent human CD8+ T cells. J Clin Invest 2014; 124:4004–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li G, Yu M, Lee WW et al Decline in miR‐181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nat Med 2012; 18:1518–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pearce EL. Fuelling immunity: insights into metabolism and lymphocyte function. Science 2013; 342:1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Arnold CR, Theresa Pritz T, Brunner S et al T cell receptor‐mediated activation is a potent inducer of macroautophagy in human CD8+CD28+ T cells but not in CD8+CD28− T cells. Exp. Gerontol 2014; 54:75–83. [DOI] [PubMed] [Google Scholar]
- 65. Perkey E, Fingar D, Miller RA, Garcia GG. Increased mammalian target of rapamycin complex 2 signalling promotes age‐related decline in CD4 T cell signaling and function. J Immunol 2013; 191:4648–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Deretic V, Kimura T, Timmins G, Moseley P, Chauhan S, Mandell M. Immunologic manifestations of autophagy. J Clin Invest 2015; 125:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sapey E, Greenwood H, Walton G et al Phosphoinositide 3‐kinase inhibition restores neutrophil accuracy in the elderly: toward targeted treatments for immunosenescence. Blood 2014; 123:239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Blaskovich MA. Drug discovery and protein tyrosine phosphatases. Curr Med Chem 2009; 16:2095–176. [DOI] [PubMed] [Google Scholar]
- 69. Larbi A, Fortin C, Dupuis G, Berrougui H, Khalil A, Fulop T. Immunomodulatory role of high‐density lipoproteins: impact on immunosenescence. Age (Dordr) 2014; 36:9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Spielmann G, Bollard CM, Bigley AB et al The effects of age and latent cytomegalovirus infection on the redeployment of CD8+ T cell subsets in response to acute exercise in humans. Brain Behav Immun 2014; 39:142–51. [DOI] [PubMed] [Google Scholar]
- 71. Mannick JB, Del Giudice G, Lattanzi M et al mTOR inhibition improves immune function in the elderly. Sci Transl Med 2014; 6:268ra179. [DOI] [PubMed] [Google Scholar]
- 72. Lopez‐Otın C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging. Cell 2013; 153:1194–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Henson SM, Macaulay R, Riddell NE, Nunn CJ, Akbar AN. Blockade of PD‐1 or p38 MAP kinase signaling enhances senescent human CD8(+) T‐cell proliferation by distinct pathways. Eur J Immunol 2015; 45:1441–51. [DOI] [PubMed] [Google Scholar]


