Summary
We have reported previously that T cells from patients with multi‐drug‐resistant tuberculosis (MDR‐TB) express high levels of interleukin (IL)‐17 in response to the MDR strain M (Haarlem family) of Mycobacterium tuberculosis (M. tuberculosis). Herein, we explore the pathways involved in the induction of Th17 cells in MDR‐TB patients and healthy tuberculin reactors [purified protein derivative healthy donors (PPD+ HD)] by the M strain and the laboratory strain H37Rv. Our results show that IL‐1β and IL‐6 are crucial for the H37Rv and M‐induced expansion of IL‐17+interferon (IFN)‐γ– and IL‐17+IFN‐γ+ in CD4+ T cells from MDR‐TB and PPD+ HD. IL‐23 plays an ambiguous role in T helper type 1 (Th1) and Th17 profiles: alone, IL‐23 is responsible for M. tuberculosis‐induced IL‐17 and IFN‐γ expression in CD4+ T cells from PPD+ HD whereas, together with transforming growth factor (TGF‐β), it promotes IL‐17+IFN‐γ– expansion in MDR‐TB. In fact, spontaneous and M. tuberculosis‐induced TGF‐β secretion is increased in cells from MDR‐TB, the M strain being the highest inducer. Interestingly, Toll‐like receptor (TLR)‐2 signalling mediates the expansion of IL‐17+IFN‐γ– cells and the enhancement of latency‐associated protein (LAP) expression in CD14+ and CD4+ T cells from MDR‐TB, which suggests that the M strain promotes IL‐17+IFN‐γ– T cells through a strong TLR‐2‐dependent TGF‐β production by antigen‐presenting cells and CD4+ T cells. Finally, CD4+ T cells from MDR‐TB patients infected with MDR Haarlem strains show higher IL‐17+IFN‐γ– and lower IL‐17+IFN‐γ+ levels than LAM‐infected patients. The present findings deepen our understanding of the role of IL‐17 in MDR‐TB and highlight the influence of the genetic background of the infecting M. tuberculosis strain on the ex‐vivo Th17 response.
Keywords: cytokines, multi‐drug‐resistance, Mycobacterium tuberculosis, pattern recognition receptors, Th17 cells
Introduction
Interleukin‐17 (IL‐17) is a proinflammatory cytokine secreted by haematopoietic cells, including representatives of the adaptive (e.g. CD4+ and CD8+ αβ T cells) and the innate [e.g. natural killer (NK), NK T and γδT cells] immune responses 1, 2. IL‐17 plays an important role in chronic inflammatory disorders as well as in host immune responses against extra‐ and intracellular pathogens 2, 3, 4. T helper type 17 (Th17) cells were described to be protective by accelerating the recruitment of Th1 cells to the site of infection in Mycobacterium tuberculosis‐vaccinated mice 5, contributing to the formation of the granuloma, and reducing the bacterial burden in bacilli Calmette–Guérin (BCG)‐infected mice 6. Nevertheless, excessive production of IL‐17 in response to repeated exposure to mycobacterial antigens has been associated with extensive lung pathology 7.
Human IL‐17‐secreting T cells can produce not only the hallmark interleukins IL‐17A and IL‐17F, but also Tumor necrosis factor (TNF)‐α, IL‐22, IL‐21, IL‐4 and IL‐10, according to the cytokine environment 8. Human Th17 cells produce IFN‐γ in IL‐12‐enriched micro‐environments. IL‐17/IFN‐γ double‐positive CD4+ T cells, named Th17–Th1 cells, were found to be expanded in human inflamed tissues 9, peripheral blood T cells and T cell clones expanded from healthy donors (HD) 10, 11. Conversely, in patients with chronic bronchial asthma and other allergic disorders, cells derived from memory Th17 cells express IL‐4 in response to an IL‐4 enriched environment 12. Also, Th17 cells can produce IL‐10 in response to Staphylococcus aureus 13, supporting the concept of Th17 heterogeneity and plasticity under different microenvironments. Regarding M. tuberculosis‐induced Th17 cells, an increase in IL‐17+IFN‐γ+CD4+ memory T cells was observed in response to the H37Rv strain in non‐BCG‐vaccinated healthy donors (HD) 14. Besides, patients with active pulmonary TB were shown to promote a high Th17 response to cell lysates of H37Rv strain, which was mainly dependent upon IFN‐γ+IL‐17+ CD4+ T lymphocytes 15.
In the early 1990s, two major multi‐drug‐resistant tuberculosis (MDR‐TB) outbreaks were detected in two over‐populated urban areas of Argentina (Buenos Aires and Rosario cities). Epidemiological, bacteriological and genotyping data allowed the identification of the respective outbreak strains named M and Ra, which belong to the Haarlem (H2) and the Latin American–Mediterranean (LAM3) families of M. tuberculosis, respectively. Although these strains have been isolated from human immunodeficiency virus (HIV)‐infected patients 16, they soon disseminated to immunocompetent individuals and managed to perpetuate in the community. In a systematic countrywide survey performed in 2003–09, the M and Ra strains still accounted for 29 and 11% of all MDR‐TB cases in the period 17.
We have demonstrated previously that the M strain induced an altered Th1/Th2 profile in T cells from healthy tuberculin reactors (PPD+ HD) and from TB patients, the M strain being a weaker inducer of IFN‐γ and CD8‐dependent cytotoxic activity 18. In contrast, an expansion of CD4+ and CD8+ IL‐17‐producing T cells was observed in MDR‐TB patients in response to M. tuberculosis strains. This increase was associated with a differential expansion of IL‐17+IFN‐γ– within the CD4+ T cell subset, and this effect was more evident when the M strain was used as an antigen 19. In the present work we explore the underlying mechanisms involved in IL‐17+IFN‐γ– and IL‐17+IFN‐γ+ memory T cell expansion, taking into account the genotype of the infecting strains.
Methods
Ethics statement
This work was carried out in accordance with the revised version of the Declaration of Helsinki (2013) of the World Medical Association, and was reviewed and approved by the following bioethics committees: Academia Nacional de Medicina (Decision Number 23‐03‐2010), Hospital Muñiz (DN 131‐07, Project Number 145) and the Teaching and Research Committee of the Buenos Aires City government (DN 1217 2010).
Patients
Blood samples were obtained from MDR‐TB patients hospitalized at the Phthisiopneumonology Institute University of Buenos Aires in the F. J. Muñiz Hospital, Buenos Aires, Argentina. Patient informed consent was obtained according to the guidelines of the ethics committee of the F. J. Muñiz Hospital. All patients were diagnosed by the presence of recent clinical respiratory symptoms, abnormal chest radiography, a sputum smear test positive for acid‐fast bacilli (AFB) and the identification of M. tuberculosis in culture. Exclusion criteria included a positive test for HIV and the presence of concurrent infectious diseases or non‐infectious conditions (cancer, diabetes or steroid therapy). Sputum smear examination and mycobacterial culture were performed in agreement with standard procedures. Susceptibility to isoniazid, rifampicin, ethambutol and streptomycin was determined according to World Health Organization standards. Susceptibility to kanamycin, p‐aminosalicylic acid and cycloserine was tested following the Canetti, Rist and Grosset method, whereas the pyrazinamidase test was used to infer pyrazinamide susceptibility 20. Available MDR M. tuberculosis isolates were genotyped by IS6110 DNA fingerprinting and spoligotyping, using standardized protocols 21, 22. A total of 31 MDR‐TB patients were included [17 men and 14 women; median age (25th–75th percentiles) 32 (23–55) years]. Percentages of different M. tuberculosis lineages among MDR‐TB patients in this study were as follows: LAM, 43%; Haarlem, 50% (80% of whom were infected with M strain); T, 4%; and 3% other. All MDR‐TB patients showed a radiologically advanced pulmonary disease and were under specific treatment according to the infecting strain's drug susceptibility at the time of the study (Supporting information, Table S1). Twenty‐one patients were sputum smear‐positive; the median number of AFB/field (25th–75th percentiles) for patients infected with Haarlem strains: 3 (0·4–10), and for those infected with LAM strains: 2·5 (0·3–10). Sixteen BGC‐vaccinated healthy volunteer donors were recruited as controls from laboratory personnel of the Academia Nacional de Medicina upon written informed consent, as approved by the research ethics board of the institution. They were classified according to their reactivity to purified protein derivative (PPD) and interferon (IFN)‐γ release assays (QuantiFeron‐TB Gold In‐Tube assay; Qiagen, Valencia, CA, USA) in latently TB‐infected [PPD+HD, PPD+IGRA+, four men and four women, median age (25th–75th percentiles) 30 (27–56) years] and in healthy donors [PPD–HD, PPD–IGRA–, four men and four women, median age 32 (25–55) years].
Peripheral blood mononuclear cells (PBMC)
PBMC from heparinized blood were isolated by Ficoll‐Triyosom gradient centrifugation and suspended in RPMI‐1640 (HyClone; Thermo Scientific, Waltham, MA, USA) containing 100 U/ml penicillin, 100 μg/ml streptomycin and 10% heat‐inactivated fetal calf serum (Gibco®, Thermo Scientific), identified hereafter as complete medium.
Antigens
Clinical isolates representative of the MDR outbreak M. tuberculosis M strain and the laboratory M. tuberculosis strain H37Rv were grown in Middlebrook 7H9 broth (Difco Laboratories, Detroit, MI, USA) at 37°C in 5% CO2. Mycobacteria were harvested in the log phase, washed three times and suspended in pyrogen‐free phosphate‐buffered saline (PBS). Bacteria were inactivated by gamma‐irradiation, suspended in PBS at a 600 nm optical density of 1·0 (≈108 bacteria/ml) and stored at −20°C until use.
PBMC cultures
PBMC (2 × 106 cells/ml) were cultured during 48 h in polystyrene tubes (Falcon; BD Bioscience, Franklin Lakes, NJ, USA) at 37°C in a humidified 5% CO2 atmosphere in complete medium alone or in the presence of gamma‐irradiated bacilli of M or H37Rv strains at a 2 : 1 M. tuberculosis to PBMC ratio. For blocking experiments, monoclonal antibodies directed against (a) cytokines: IL‐23p19 [10 µg/ml, affinity‐purified polyclonal antibody; immunogen: Esherichia coli‐derived recombinant human (rh)IL‐23p19, goat immunoglobulin (Ig)G; R&D Systems, Minneapolis, MN, USA], IL‐1β (10 µg/ml, clone AS10, IgG1, κ, mouse IgG1) and IL‐6 (10 µg/ml, clone MQ2‐13A5, rat IgG1, κ) (both from BD Bioscience) or TGF‐β (10 µg/ml, clone 500‐M66, mouse IgG1, κ, Peprotech Inc., Rocky Hill, NJ, USA), (b) pattern recognition receptors: TLR‐2 (10 µg/ml, clone TL2.1, mouse IgG2a, κκ), TLR‐4 (10 µg/ml, clone HTA, mouse IgG2a, κ) and mannose receptor (10 µg/ml, clone 15‐2, mouse IgG1, κ) (all from Biolegend Inc., San Diego, CA, USA), dectin‐1 (10 µg/ml, clone 259931, mouse IgG2B; R&D Systems) and CD14 (10 µg/ml, clone M5E2, mouse IgG2a, κ; Biolegend) or (c) the corresponding isotype‐matched antibodies (10 μg/ml) were added at the onset of the PBMC cultures in order to determine the role of each cytokine in M. tuberculosis‐induced Th17 immune responses. In some assays, PBMC from PPD+ HD were cultured for 48 h alone or with M. tuberculosis strains in the presence or not of recombinant IL‐23 (1 ng/ml; Biolegend), recombinant TGF‐β (0·5–5 ng/ml; Preprotech) or IL‐23 plus TGF‐β, while PBMC from MDR‐TB patients were cultured for 48 h with the strains in the presence or not of anti‐IL‐23p19, anti‐TGF‐β or anti‐IL‐23 plus anti‐TGF‐β neutralizing antibodies (10 µg/ml for both antibodies). Afterwards, cells were tested for active caspase‐3, IFN‐γ and IL‐17 expression by flow cytometry and supernatants were collected and stored at −70ºC for subsequent IL‐17 and TGF‐β detection.
Immunofluorescence analysis
Surface expression
Surface CD25 and latency‐associated protein (LAP) expression was determined on 48‐h cultured PBMC by staining cells with fluorescein isothiocyanate (FITC) or allophycocyanin (APC)‐conjugated anti‐CD4 and phycoerythrin (PE) or peridinin chlorophyll/cyanin 5 (PerCP/Cy5) anti‐CD25 (all from BD Bioscience) and PerCP/Cy5 anti‐LAP (TGF‐β1) monoclonal antibodies (Biolegend Inc.).
Intracellular cytokine and active caspase‐3 expression
Intracellular IL‐17, IL‐1β, IL‐23 and IFN‐γ expression was determined in PBMC cultures stimulated with M. tuberculosis strains after 24 or in 48 h cultured PBMC (for monocytes and lymphocyte staining, respectively). Briefly, brefeldin A (5 μg/ml; Sigma Chemical Co., St Louis, MO, USA) was added 4 h before finishing the culture to block cytokine secretion, and cells were surface‐stained with the following anti‐human antibodies: PE‐Cy5 or FITC anti‐CD4 (BD Bioscience) or PerCP/Cy5.5 anti‐human CD14 (Biolegend Inc.), then fixed with 0·5% paraformaldehyde and permeabilized with fluorescence‐activated cell sorter permeabilizing solution 2 (BD Bioscience) before PE anti‐IL‐17 (R&D Systems), PE anti‐IL‐1β (eBioscience Inc., San Diego, CA, USA), PE anti‐IL‐23 p19 (R&D Systems), FITC or APC anti‐IFN‐γ (Biolegend Inc.), FITC anti‐active caspase‐3 (BD Bioscience) or the corresponding isotype control antibody was added. In other experiments, surface‐stained CD4+CD25+LAP+ cells were tested for IL‐17 and forkhead box protein 3 (FoxP3) expression by employing a FITC anti‐human FoxP3 staining set (eBioscience Inc.), according to the manufacturer's instructions. Stained cells were analyzed by flow cytometry. Eighty thousand events were acquired for each cell preparation, using a fluorescence‐activated cell sorter (FACS)scan flow cytometer (BD Bioscience) with CellQuest software acquisition. FCS Express software (De Novo Software, Los Angeles, CA, USA) was used for analysis. Lymphocyte or monocyte/macrophage gates were set according to forward‐ and side‐scatter parameters, excluding cell debris and apoptotic ones. The percentage of positive cells in lymphocytes and macrophages gates was determined and then the number of positive cells within 1 × 106 PBMC (n) was calculated for each individual on the basis of the percentage and absolute number of CD4+ and CD14+ cells. As shown previously 18, MDR‐TB patients showed lower levels of CD4+ cells than PPD+ HD.
Results were expressed as (a) number of positive cells/1 × 106 PBMC (n) or (b) percentage of caspase‐3 cells within the IL‐17+IFN‐γ–, IL‐17+IFN‐γ+ and IL‐17–IFN‐γ+ CD4+ cell subsets.
Cytokine assays
IL‐17 and TGF‐β production in 48‐h PBMC culture supernatants was assessed using commercial enzyme‐linked immunosorbent assay (ELISA) kits, according to the manufacturer's instructions: IL‐17 (sensitivity 4 pg/ml, range 4–500 pg/ml) and TGF‐β (sensitivity 8 pg/ml, range 8–1000 pg/ml) (both from eBioscience Inc.).
Statistical analysis
Data in the tables are expressed as medians and 25th–75th percentiles, which are depicted graphically as boxes representing median values (line) and 25th–to 75th percentiles and error bars indicating maximum and minimum values. Data analysis was performed using the following one‐way analysis of variance (anova) tests: (a) non‐parametric Kruskal–Wallis test followed by Dunn's multiple comparison test to compare MDR‐TB patients and PPD+ HD responses; and (b) non‐parametric Friedman test followed by Dunn's multiple comparison test to compare responses to different treatments within each group. All statistical analyses were two‐sided, and the significance level adopted for P‐values was <0·05. Analysis was performed using the statistical software spss version 15.0 for Windows (SPSS Inc., Chicago, IL, USA) and Graphpad Prism version 5.0 (Graphpad Software Inc., San Diego, CA, USA).
Results
IL‐23, TGF‐β, IL‐6 and IL‐1β participate in M. tuberculosis‐induced Th17 response in MDR‐TB patients
It has been shown that IL‐23, IL‐1β and IL‐6 are essential for human Th17 differentiation, while the role of TGF‐β remains controversial 8. Thus, we first evaluated whether these cytokines could modulate the Th17 response by adding specific neutralizing antibodies at the onset of PBMC culture. Cells from MDR‐TB were stimulated with both H37Rv and M strains, while in PPD+ HD and PPD– HD the neutralization assays were performed only in M‐stimulated PBMC cultures, due to the low IL‐17 levels induced by H37Rv 19. As observed in Table 1, M‐ and H37Rv‐induced IL‐17 production by PBMC from MDR‐TB and PPD+ HD was reduced markedly due to IL‐1β, IL‐6 and IL‐23p19 neutralization, while anti‐TGF‐β reduced IL‐17 amounts only in MDR‐TB. In the same line, relative (Supporting information, Fig. S1a) and absolute numbers of CD4+IL‐17+ cells were reduced upon neutralization of IL‐1β, IL‐6 and IL‐23p19 in both MDR‐TB (Supporting information, Fig. S1b) and PPD+ HD (Supporting information, Fig. S1c), while anti‐TGF‐β reduced their numbers only in MDR‐TB. The same trend was observed in PPD– HD but the number of CD4+IL‐17+ cells was negligible (data not shown), and thereafter cells from PPD– HD were withdrawn from further analysis. Our results suggest that IL‐1β, IL‐23 IL‐6 and TGF‐β participate in M. tuberculosis‐specific Th17 responses in MDR‐TB patients.
Table 1.
Interleukin (IL)‐23, transforming growth factor (TGF)‐β, IL‐1‐β and IL‐6 are involved in IL‐17 secretion by multi‐drug‐resistant tuberculosis (MDR‐TB) patients’ peripheral blood mononuclear cells (PBMC)
| No antibodies | a‐IL‐23p19 | a‐TGF‐β | a‐IL‐1β | a‐IL6 | |||
|---|---|---|---|---|---|---|---|
| IL‐17 (pg/ml) | MDR‐TB | C | 87 (41–117) | 64 (60–104) | 91 (95–106) | 76 (70–96) | 65 (59–70) |
| H37Rv | 165 (94–171) | 90 (48–125)* | 92 (53–133)* | 78 (39–101)* | 99 (53–131)* | ||
| M | 207 (82–242) | 83 (47–120)* | 97 (81–116)* | 76 (27–114)* | 73 (29–98)* | ||
| PPD+ HD | C | 5 (4·0–6·0) | < 4 | 6 (4–10) | < 4 | < 4 | |
| M | 17 (10–25) | 7 (4–22)* | 18 (10–29) | 5 (4–15)* | 7 (4–18)* |
PBMC from 10 MDR‐TB patients and eight purified protein derivative (PPD)+ healthy donors (HD) were stimulated for 48 h alone or with the strains H37Rv and M, in the presence or not of monoclonal antibodies against IL‐23p19, TGF‐β, IL‐1β and IL‐6. Then, IL‐17 amounts (pg/ml) were determined in PBMC supernatants by enzyme‐linked immunosorbent assay (ELISA). Results are expressed as median and 25–75 percentiles; statistical differences: *P < 0·05 for antibody‐treated versus non‐treated PBMC (Friedman test followed by Dunn's test).
TGF‐β promotes expansion of IL‐17+IFN‐γ– cells in MDR‐TB patients
We have demonstrated previously that the strong Th17 response observed in MDR patients was the consequence of an increased proportion of CD4+IL‐17+IFN‐γ– cells 19. Herein, we evaluated whether IL‐1β, IL‐6, IL‐23p19 and TGF‐β modified the proportion of IL‐17+IFN‐γ– or IL‐17+IFN‐γ+ cells within the CD4+ cells. As observed in Fig. 1, neutralization of IL‐1β, IL‐6 and IL‐23p19 reduced strongly the number of IL‐17+IFN‐γ– and IL‐17+IFN‐γ+ cells in H37Rv‐ and M‐stimulated PBMC from MDR‐TB. Interestingly, the neutralization of TGF‐β decreased the number of IL‐17+IFN‐γ– cells induced by both strains, while it only increased the number of IL‐17+IFN‐γ+ induced by the M strain in cultures from MDR‐TB patients. In PPD+ HD, neutralization of IL‐1β and IL‐6 reduced the numbers of both Th17 cell subsets, while anti‐IL‐23p19 and anti‐TGF‐β had no effect. These results indicate that IL‐1β, IL‐6 and IL‐23 are crucial for the expansion of both IL‐17+ T cells subsets independently of the strain, whereas TGF‐β shifts Th17 cells towards an IL‐17+IFN‐γ– phenotype in M‐stimulated CD4+ T cells from MDR‐TB patients.
Figure 1.
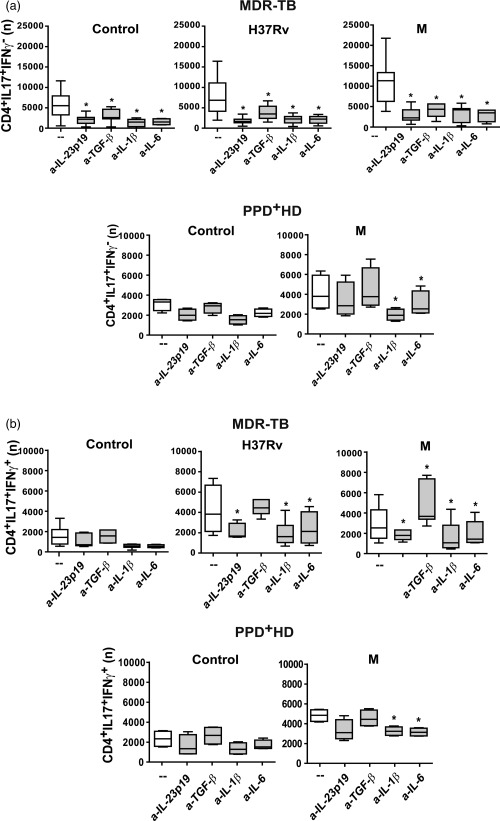
Interleukin (IL)‐23, transforming growth factor (TGF)‐β, IL‐6 and IL‐1β participate differentially in Mycobacterium tuberculosis‐induced T helper type 17 (Th17) response. Peripheral blood mononuclear cells (PBMC) from 31 multi‐drug‐resistant tuberculosis (MDR‐TB) and eight purified protein derivative (PPD)+ healthy donors (HD) were stimulated for 48 h alone (control) or with H37Rv and M strains (2 : 1 bacteria to PBMC ratio), in the absence (–) or presence of antibodies against IL‐23p19, TGF‐β, IL‐1β and IL‐6 (a‐IL‐23p19, a‐TGF‐β, a‐IL‐1β and a‐IL‐6, respectively). IL‐17 and interferon (IFN)‐γ expression was determined in CD4+ T cells by flow cytometry [fluorescence‐activated cell sorter (FACS)] and the number (n) of (a) CD4+IL‐17+IFN‐γ– and (b) CD4+IL‐17+IFN‐γ+ cells present in 1×106 cultured PBMC was calculated for each individual. Results are expressed as median and 25th–75th percentiles (boxes) with maximum and minimum values (error bars). Statistical differences: *P < 0·05 for treated versus non‐treated PBMC (Friedman test followed by Dunn's test).
IL‐23 and TGF‐β promote the expansion of CD4+IL‐17+IFN‐γ– cells by inducing IFN‐γ+ cell death in MDR‐TB
To confirm the modulatory role of IL‐23 and TGF‐β on the M. tuberculosis‐induced expansion of Th17 memory cells observed in MDR‐TB, PBMC from six PPD+ HD were cultured for 48 h alone or with the M strain with or without the addition of IL‐23 and/or TGF‐β and then IL‐17 and IFN‐γ expression was tested within CD4+ T cells. As shown in Fig. 2a, IL‐23 alone enlarged the number of IL‐17+ cell subsets in M‐stimulated cells and TGF‐β alone inhibited IL‐17 and IFN‐γ expression, while co‐addition of IL‐23 and TGF‐β enhanced IL‐17+ T cell numbers through the expansion of IL‐17+IFN‐γ– cells.
Figure 2.
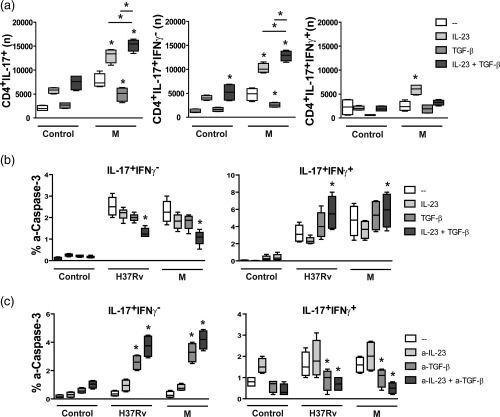
Interleukin (IL)‐23 and transforming growth factor (TGF)‐β promoted the expansion of IL‐17+interferon (IFN)‐γ– cells and modulated IL‐17+IFN‐γ+ cell death. (a,b) Peripheral blood mononuclear cells (PBMC) from six PPD+ healthy donors (HD) were cultured for 48 h alone (control) or with H37Rv and M strains in the presence or absence of IL‐23 (1 ng/ml) and/or TGF‐β (5 µg/ml), and then IL‐17, IFN‐γ and active‐caspase‐3 (a‐caspase‐3) expression was tested within CD4+ T cells by fluorescence‐activated cell sorter (FACS). Results are expressed as number (n) of CD4+IL‐17+ cells, CD4+IL‐17+IFN‐γ– and CD4+IL‐17+IFN‐γ+ cells in 1 × 106 cultured PBMC (a) and percentage of a‐caspase‐3+ cells within IL‐17+IFN‐γ– and IL‐17+IFN‐γ+ CD4+ cells (% a‐caspase‐3) (b). (c) PBMC from six MDR‐TB patients were cultured for 48 h alone (control) or with the strains H37Rv and M in the presence or absence of anti‐IL‐23p19 (a‐IL‐23, 10 µg/ml) and/or anti‐TGF‐β (a‐TGF‐β, 10 µg/ml) monoclonal antibodies. Then the percentage of a‐caspase‐3+ cells was determined within CD4+IL‐17+IFN‐γ– and CD4+IL‐17+IFN‐γ+ cells by FACS. Box‐plots indicating medians and 25th–75th percentiles with maximum and minimum values are shown. Statistical differences: *P < 0·05 for treated versus non‐treated PBMC (Friedman test followed by Dunn's test).
Considering that human Th17 clones are less susceptible to TGF‐β‐induced cell death than Th1 and Th2 clones 23, we evaluated whether the M. tuberculosis‐induced expansion of IL‐17+IFN‐γ– cells observed in MDR‐TB was due to TGF‐β‐induced cell death of IFN‐γ+ cells. For this purpose, the expression of active caspase‐3 (a‐caspase‐3), which is associated with the cell death signalling pathway 24, was evaluated in (a) PBMC from PPD+ HD cultured for 48 h in the presence or not of IL‐23 and/or TGF‐β and (b) PBMC from MDR‐TB patients cultured with or without anti‐TGF‐β and/or anti‐IL‐23p19. As shown in Fig. 2b, H37Rv and M strains enhanced the expression of a‐caspase‐3 in IL‐17+ cells from PPD+ HD and co‐addition of IL‐23 and TGF‐β increased the percentages of a‐caspase‐3+ cells in IL‐17+IFN‐γ+ subsets but reduced their percentage markedly in CD4+IL‐17+IFN‐γ– cells (Fig. 2b). Interestingly, a low proportion of a‐caspase‐3+ cells was observed within the IL‐17+IFN‐γ– subset from MDR‐TB patients, and this proportion increased when IL‐23 and TGF‐β were neutralized simultaneously (Fig. 2c). In contrast, IL‐17+IFN‐γ+ cells showed a high percentage of a‐caspase‐3+ cells that were diminished by IL‐23 and TGF‐β neutralization. A similar tendency was observed in IL‐17–IFN‐γ+ cells from MDR‐TB patients (data not shown). These results suggest that IL‐23 and TGF‐β could act in concert to promote the expansion of Th17 cells with an IFN‐γ– phenotype in response to M. tuberculosis strains by inducing cell death in IFN‐γ+ cells from MDR‐TB patients.
Figure 3.
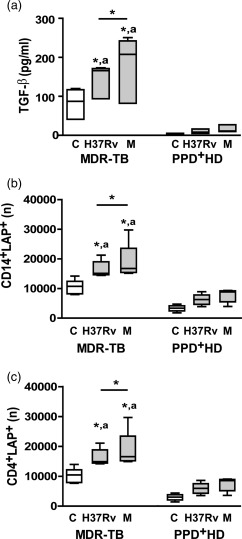
Enhanced transforming growth factor (TGF)‐β secretion and absolute number of latency‐associated protein (LAP)+ cells in peripheral blood mononuclear cells (PBMC) from multi‐drug‐resistant tuberculosis (MDR‐TB) patients. PBMC from 10 MDR‐TB and six purified protein derivative (PPD)+ healthy donors (HD) were cultured for 48 h alone (control, C) or with Mycobacterium tuberculosis strains and then TGF‐β secretion was determined in PBMC supernatants by enzyme‐linked immunosorbent assay (ELISA) as well as LAP expression was determined in CD14+ and CD4+ cells by FACS. (a). TGF‐β secretion. Results are expressed as pg/ml and medians and 25th–75th percentiles are shown. Statistical differences: *P < 0·05 for control versus M. tuberculosis‐stimulated PBMC or differences among strains (Friedman test followed by Dunn's test); (a) P < 0·05 for M‐ or Ra‐stimulated PBMC from MDR‐TB versus the corresponding data from PPD+ HD (Kruskal–Wallis statistics followed by Dunn's test). (b,c). Number (n) of CD14+ LAP+ cells (b) and CD4+LAP+ cells (c) present in 1× 106 cultured PBMC; medians and 25th–75th percentiles with maximum and minimum values are shown. Statistical differences: *P < 0·05 for treated versus non‐treated PBMC or differences between strains (Friedman test followed by Dunn's test); (a) P < 0·05 for MDR‐TB patients versus PPD+ HD (Kruskal–Wallis statistics followed by Dunn's test).
TGF‐β secretion and LAP+ cells are enhanced in CD4+ and CD14+ cells from MDR‐TB
It was shown that spontaneous and M. tuberculosis‐induced TGF‐β production is enhanced in PBMC from patients with active TB 25. Thus, we evaluated the production of TGF‐β in supernatants of cultured PBMC. Spontaneous TGF‐β production was increased markedly in MDR‐TB when compared to PPD+ HD and the strains enhanced TGF‐β levels only in MDR‐TB (Fig. 3), the M strain being the highest inducer.
Mature TGF‐β is associated with a latency‐associated protein (LAP), which provides a disulphide‐linked shell hindering interaction of TGF‐β with its cellular receptors and to a latent TGF‐β‐binding protein, which anchors the complex to the cell surface 26. This latent TGF‐β complex has been detected in the cell surface of macrophages and T cells that exert their inhibitory activity through secretion of the bound latent complex upon activation. Therefore, we determined the numbers of CD14+ and CD4+ cells that express latent TGF‐β by measuring surface membrane LAP expression in 48 h‐cultured PBMC. As observed in Fig. 3, the number of CD14+LAP+ and CD4+LAP+ cells increased in non‐stimulated PBMC from MDR‐TB compared to PPD+ HD. Both strains enhanced LAP+ cell values, M being a higher inducer when comparing with H37Rv in MDR‐TB. Considering that LAP has been found in activated human CD4+FoxP3+ regulatory T cells (Tregs) 27, as well as circulating CD4+FoxP3– T cells with suppressor activity 28, and taking into account that MDR‐TB patients show enhanced proportion of circulating CD4+CD25high (Tregs) 18, we wondered if the enhanced LAP expression in CD4+ T cells was ascribed to this subset. We found that not only Tregs but also conventional activated CD4+CD25low T cells showed enhanced LAP expression and M. tuberculosis strains enhanced its expression in both subsets (Supporting information, Fig. S2a). Furthermore, we observed that 90–95% of IL‐17 expression was detected in M. tuberculosis‐stimulated CD4+FoxP3–LAP– cell subsets (data not shown), suggesting that LAP+ cells participate as regulatory cells and not as direct Th17 effector cells in MDR‐TB. These findings, in combination with the results of blocking experiments, suggest that the high amounts of free TGF‐β and cells binding latent TGF‐β could be involved in the expansion of IL‐17+IFN‐γ– cells via IFN‐γ down‐regulation.
Dectin‐1 and TLR‐2 induce expansion of IL‐17+IFN‐γ– CD4+ T cells in MDR‐TB patients
As the recognition of M. tuberculosis is mediated by a complex network of pattern recognition receptors 29, we also wondered if TLR‐2, TLR‐4, MR and dectin‐1 participated in the Th17 response induced by MDR clinical isolates of M. tuberculosis. As shown in Table 2, blockade of TLR‐2, TLR‐4 and dectin‐1 diminished IL‐17 amounts in PBMC supernatants from MDR‐TB and PPD+ HD, while no differences were observed by blocking MR. We also evaluated if these receptors were involved in the expansion of both Th17 subsets in MDR‐TB and in PPD+ HD. As observed in Fig. 4a,b, blockade of dectin‐1 markedly diminished the number of antigen‐stimulated CD4+ IL‐17+IFN‐γ– and IL‐17+IFN‐γ+ cells from MDR‐TB and PPD+ HD, making no difference among strains. Blockade of TLR‐2 inhibited M. tuberculosis‐induced IL‐17+IFN‐γ– cell numbers, while enhanced numbers of M‐induced IL‐17+IFN‐γ+ cells only in MDR‐TB. Anti‐TLR‐4 also reduced the number of M. tuberculosis‐induced IL‐17+IFN‐γ+ in PPD+ HD. These results propose that while dectin‐1 is essential for a whole Th17 response, TLR‐4 sustains IL‐17+IFN‐γ+ cells in PPD+ HD and TLR‐2 shifts IL‐17+ cells towards an IFN‐γ– phenotype in MDR‐TB.
Table 2.
Dectin‐1, Toll‐like receptor (TLR)‐2 and TLR‐4 are involved in high T helper type 17 (Th17) response in multi‐drug‐resistant tuberculosis (MDR‐TB) patients
| No mAb | α‐TLR‐2 | α‐TLR‐4 | a‐MR | α‐Dectin‐1 | |||
|---|---|---|---|---|---|---|---|
| IL‐17 (pg/ml) | MDR‐TB | C | 94 (66–114) | 66 (59–109) | 90 (62–110) | 103 (94–112) | 87 (71–112) |
| Rv | 171 (144–270) | 127 (55–171)* | 133 (76–190)* | 159 (105–225) | 101 (54–168)* | ||
| M | 254 (177–423) | 120 (38–149)* | 141 (108–220)* | 214 (159–353) | 121 (28–149)* | ||
| PPD+HD | C | 5 (4·2–6·5) | 5 (4·8–5·7) | 4 (4·0–4·5) | 4·5 (4·1–6·5) | 4 (4·1–6·3) | |
| M | 20 (10·1–25·7) | 18 (9–29) | 7 (4·5–22·5)* | 17 (9·2–23·3) | 5 (4·0–15·2)* |
Peripheral blood mononuclear cells (PBMC) from 10 MDR‐TB patients and six purified protein derivative (PPD)+ healthy donor (HD) controls were stimulated for 48 h alone or with Mtb strains, in the presence or not of monoclonal antibodies against TLR‐2, TLR‐4, mannose receptor (MR) and dectin‐1. Then, IL‐17 amounts (pg/ml) were determined in PBMC supernatants by enzyme‐linked immunosorbent assay (ELISA). Results are expressed as median and 25th–75th percentiles; statistical differences: *P < 0·05 for antibody‐treated versus non‐treated PBMC (Friedman test followed by Dunn's test). mAb = monoclonal antibodies.
Figure 4.
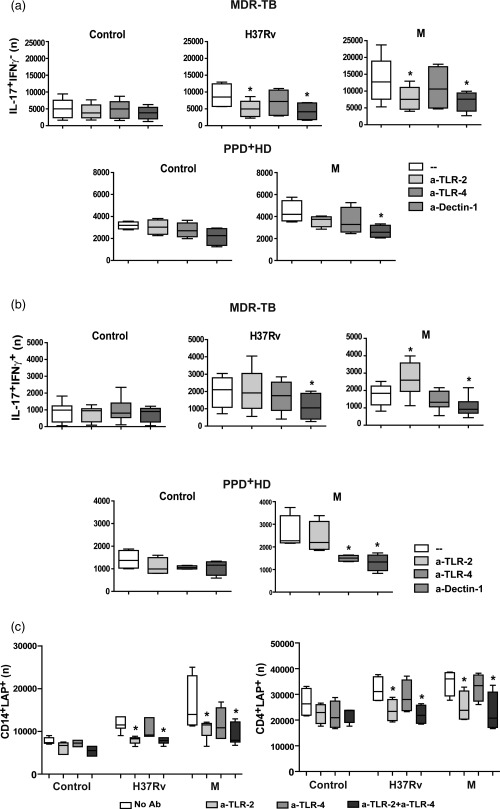
Dectin‐1, Toll‐like receptor (TLR)‐2 and TLR‐4 are involved differentially in high T helper type 17 (Th17) response and latency‐associated protein (LAP)+ cells expansion in multi‐drug‐resistant tuberculosis (MDR‐TB) patients. (a,b). PBMC from 10 MDR‐TB patients and six PPD+ healthy donors (HD) controls were stimulated for 48 h alone (control, C) or with Mtb strains, in the absence (–) or presence of antibodies against TLR‐2, TLR‐4 and dectin‐1 (a‐TLR‐2, a‐TLR‐4 and a‐dectin‐1). Then, the IL‐17 and interferon (IFN)‐γ expression was determined within the CD4+ subset by FACS and the number (n) of CD4+IL‐17+IFN‐γ– and of CD4+IL‐17+IFN‐γ+ cells in 1×106 cultured PBMC was calculated. Box‐plots show median and 25th–75th percentiles with maximum and minimum values; statistical differences: *P < 0·05 for treated versus non‐treated PBMC (Friedman test followed by Dunn's test). (c). PBMC from six MDR‐TB patients were stimulated for 48 h alone or with Mtb strains, in the absence (–) or presence of anti‐TLR‐2 or anti‐TLR‐4 antibodies. Then LAP expression was determined in CD4+ and CD14+ cells by FACS. Results are expressed as number (n) of CD4+LAP+ and CD14+LAP+ cells in 1 × 106 PBMC (median and 25th–75th percentiles with maximum and minimum values). Statistical differences: *P < 0·05 for antibody‐treated versus non‐treated PBMC (Friedman test followed by Dunn's test).
The expansion of LAP+ cells in MDR‐TB patients is dependent upon TLR‐2 signalling
Given that interactions between microbial ligands and TLRs in APCs and/or T cells will shape the T cell profiles evoked by modulating their expansion and functionality 30, we assessed whether dectin‐1, TLR‐2 and TLR‐4 were involved in the expansion of CD4+LAP+ T cells induced by M. tuberculosis strains in MDR‐TB. As observed in Fig. 4c, anti‐TLR‐2 reduced the number of M. tuberculosis‐stimulated CD14+ and CD4+LAP+ cells from MDR‐TB, while anti‐TLR‐4 did not. In line with this, anti‐TLR‐2 decreased the number of Tregs and conventional activated CD4+ T cells in antigen‐stimulated PBMC from MDR‐TB (Supporting information, Fig. S2b). In PPD+ HD the differences were negligible, due to low LAP+ cell levels (data not shown). Altogether, these results suggest that TLR‐2 would be involved in the induction of LAP expression in CD4+ T and CD14+ cells from MDR‐TB.
TGF‐β secretion and LAP+ cells levels correlate with bacillary load
We have shown previously that the strong Th17 response observed in MDR‐TB patients correlates with the presence of acid‐fast bacilli (AFB) in sputum smear 19. Herein, we evaluated whether TGF‐β secretion and the number of LAP+ cells in cultured PBMC was associated with the presence/absence of AFB in sputum using Ziehl–Neelsen‐stained smears and bacillary load (bacilli/field). As shown in Fig. 5, not only CD4+LAP+ cell values but also TGF‐β levels correlated with the bacillary load in M. tuberculosis‐stimulated PBMC of all the AFB+ patients studied.
Figure 5.
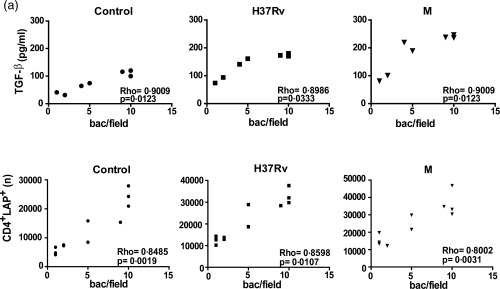
Correlation between transforming growth factor (TGF)‐β levels, percentage of CD4+ latency‐associated protein (LAP)+ cells and bacillary load. Correlation between TGF‐β levels, CD4+LAP+ cells and bacillary load in multi‐drug‐resistant tuberculosis (MDR‐TB): TGF‐β levels (pg/ml) (a) and number (n) of CD4+LAP+ cells (b) were compared with the number of bacilli per field detected in sputum from MDR‐TB. Individual data and Spearman's rho coefficients are shown.
MDR‐TB patients infected with Haarlem strains show a high proportion of CD4+IL‐17+IFN‐γ– cells
We finally evaluated whether MDR‐TB infected with strains belonging to Haarlem or LAM families showed differential in‐vitro expansion of IL‐17+IFN‐γ– and IL‐17+IFN‐γ+ cells. For this purpose, MDR‐TB patients were divided into four groups according to the infecting strain and the presence of AFB in sputum smears at the time of the study. As some patients were infected with MDR Mtb strains belonging to the LAM family, PBMC from all patients were also stimulated with the MDR Ra strain, which belongs to this family. Haarlem‐ and LAM‐infected AFB+ patients showed higher IL‐17+ cell numbers than the AFB– (Fig. 6). However, higher numbers of CD4+IL‐17+ cells were observed in PBMC from Haarlem‐infected patients when compared to LAM‐infected patients, despite the fact that both groups showed similar sputum smear bacillary loads [bacilli/field: Haarlem = 7 (0·6–10); LAM = 7 (0·8–10)]. M‐stimulated CD4+ T cells from AFB+ patients produced higher numbers of IL‐17+IFN‐γ– cells compared with cells from AFB– patients. Also, IL‐17+IFN‐γ– cell numbers were higher in cells from Haarlem‐infected patients than in cells from LAM‐infected patients. In contrast, in AFB+ patients, the numbers of CD4+IL‐17+IFN‐γ+ T cells were lower than in AFB– patients, and AFB+ Haarlem‐infected patients showed the lowest levels. Remarkably, the M strain was the highest IL‐17+IFN‐γ– and the lowest IL‐17+IFN‐γ+ cell inducer.
Figure 6.
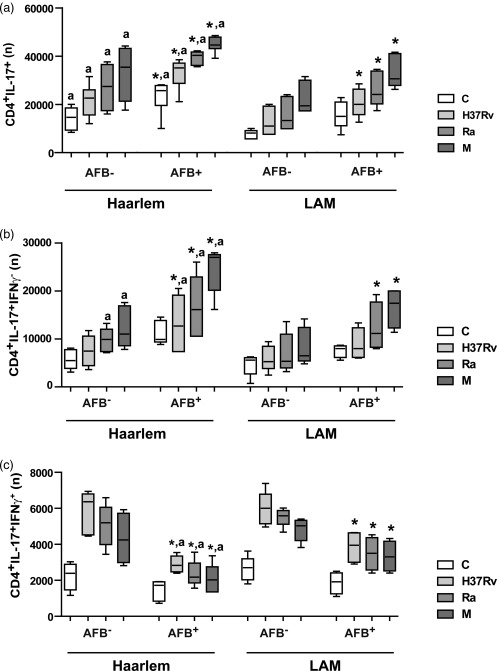
Multi‐drug‐resistant tuberculosis (MDR‐TB) patients infected with the M strain showed a high proportion of CD4+interleukin (IL)‐17+ interferon (IFN)‐γ– T cells. MDR‐TB patients were grouped according to the presence or absence of AFB in the sputum (AFB+ and AFB–) and to the infecting strains (Haarlem family: AFB+ n = 10, AFB– n = 5; Latin American–Mediterranean (LAM) family: AFB+ n = 8, AFB– n = 5). The numbers (n) of CD4+IL‐17+ (a), CD4+IL‐17+IFN‐γ– cells (b) and of CD4+IL‐17+IFN‐γ+ cells (c) present in unstimulated or H37Rv, M and Ra‐stimulated peripheral blood mononuclear cells (PBMC) cultures were calculated for each group (medians and 25th–75th percentiles). Statistical differences: *P < 0·05 for AFB+ versus AFB– individuals; a: Haarlem‐ versus LAM‐infected patients (Kruskal–Wallis statistics followed by Dunn's test).
Discussion
It is well known that triggering naive CD4 αβ T cells with MHC/peptide antigens in the presence of IL‐6 and/or IL‐21, IL‐1β and TGF‐β directs their differentiation to the Th17 lineage, while IL‐23 tends to strengthen and maintain this lineage commitment 8. As we have reported previously that MDR‐TB patients show a high proportion of IL‐17‐producing cells 19, in the present study we explored the involvement of the above‐mentioned cytokines in Th17 responses of MDR‐TB patients and PPD+ HD. Our results showed that IL‐1β and IL‐6 are involved critically in the Th17 response induced by M. tuberculosis strains by promoting the expansion of IL‐17+IFN‐γ– and IL‐17+IFN‐γ+CD4+ T cells in MDR‐TB as well as in PPD+ HD. We also found that the neutralization of both cytokines inhibits the expansion of IL‐17–IFN‐γ+ cells in PPD+ HD (data not shown), suggesting that IL‐1β and IL‐6 are also implicated in the M. tuberculosis‐induced IFN‐γ response, in agreement with Saunders et al. 31.
Cytokines promoting the differentiation of human naive T cells into IL‐17‐secreting cells are also committed to the regulation of IL‐17 production by memory T cells 32. Similarly, human Th17 cells can be generated from effector memory CD4+ T cells 32, 33, 34 and Treg cells 35. The role of IL‐1β and IL‐6 that we found for the overall Th17 response in MDR‐TB is in agreement with results demonstrating that IL‐1β, alone or in association with IL‐23 and IL‐6, increases IL‐17 production in CCR6+ memory T cells from healthy donors, while IL‐1β and IL‐12 promote the differentiation of memory CCR6+CXCR3+ Th17/Th1 cells specific for pathogenic and commensal microbes 36. Regarding IL‐23, it was found previously to have certain overlapping functions with IL‐12 by inducing IFN‐γ secretion in human CD4+ T cells 37. However, we found an ambiguous role for IL‐23 in M. tuberculosis‐induced IFN‐γ expression in CD4+ cells. On one hand, IL‐23 amplifies IFN‐γ expression in T cells from PPD+ HD and, on the other hand, it strongly enhances the IL‐17+IFN‐γ– profile in MDR‐TB, suggesting a direct role of IL‐23 on the Th1 shift towards IL‐17+IFN‐γ– cells in these patients. This dual behaviour was confirmed in cells from PPD+ HD in which the addition of IL‐23 increases IFN‐γ expression in CD4+ T cells, and the co‐addition of TGF‐β leads to a marked reduction of IFN‐γ expression together with a significant expansion of the IL‐17+IFN‐γ– population, in agreement with Santarlasci et al. 23. The involvement of TGF‐β in Th17 cell differentiation is ambiguous, having positive 38 or negative effects 32, 39, 40 according to its concentration along the T cell differentiation/activation processes. As shown herein, and in accordance with the literature 41, high amounts of TGF‐β are present in control and M. tuberculosis‐stimulated cells from MDR‐TB. In fact, the high TGF‐β secretion and the enhanced percentage of CD14+ and CD4+ T cells binding latent TGF‐β might suppress the Th1 response and, together with IL‐23, increase the proportion of IL‐17+IFN‐γ– cells in these patients, as supported by our TGF‐β neutralization assay results. It has been demonstrated that a‐CD3/a‐CD28‐stimulated Th17 clones are less prone to TGF‐β‐induced apoptosis than Th1 cells, and it has been proposed that TGF‐β favours Th17 expansion by directly inhibiting Th1 cell survival 23. Herein, we show that a‐caspase 3+ cells levels induced by the strains were lower in IL‐17+IFN‐γ– cells and higher in IL‐17+IFN‐γ+ and in IL‐17–IFN‐γ+ cells from MDR‐TB patients than in PPD+ HD. Furthermore, the assays carried out with neutralization of TGF‐β and IL‐23 in MDR‐TB cells and their addition to PPD+ HD cells suggest that both cytokines would sustain the expansion of IL‐17+IFN‐γ+ cells by inducing the subtle cell death of IFN‐γ‐expressing CD4+ T cells.
The balance between Th1 and Th17 responses has been associated with the expression of C‐lectin‐ and Toll‐like receptors on APC within the TB context 14, 33, 34, 42, 43. In our hands, the blockade of dectin‐1 decreased the M. tuberculosis‐induced Th17 response in MDR‐TB and in PPD+ HD by inhibiting the expansion of IL‐17+IFN‐γ– and IL‐17+IFN‐γ+ cells but not of IL‐17–IFN‐γ+ cells, confirming that IL‐17+IFN‐γ+ cells are derived from the Th17 and not the Th1 subsets 10, 44. TLR‐2 participated in the M. tuberculosis‐induced expansion of IL‐17+IFN‐γ+ cells and the inhibition of IL‐17–IFN‐γ+ cells in MDR‐TB patients. However, we did not find participation of TLR‐2 in the production of IL‐17 from PPD+ HD cells, as has been reported in HD, without previous exposure to M. tuberculosis or BCG vaccination 14. We consider that our results could be ascribed to an enhanced TLR‐2‐mediated production of TGF‐β by APCs 45 or cell–cell contact suppression by CD4+CD25+ T cells binding the TGF‐β/LAP complex, which are known to express TLR‐2 46, 47 and up‐regulate TGF‐β expression upon TLR‐2 signalling 48. Indeed, TLR‐2 blockade inhibited the M. tuberculosis‐induced LAP expression in CD14+ cells as well as in Tregs and conventional activated CD4+ T cells from MDR‐TB patients.
Conversely, we showed that TLR‐4 signalling is involved in M. tuberculosis‐induced expansion of IL‐17+IFN‐γ+ cells from MDR‐TB and from PPD+ HD, but it does not affect the Th1 response, suggesting a differential contribution of TLR‐4 and TLR‐2 in the modulation of Th17 subsets and Th1 responses in MDR‐TB and in PPD+ HD, in agreement with Van der Weerdonk 14. Thus, our results indicate that the M strain promotes a high Th17 response in MDR‐TB by the expansion of IL‐17+IFN‐γ– cells as a consequence of a strong TLR‐2‐dependent TGF‐β production by APC and Tregs and conventional activated CD4+ T cells (summarized schematically in Supporting information, Fig. S3).
Interestingly, we found that free TGF‐β and LAP+CD4+ T cell levels correlated with bacillary loads in sputum from MDR‐TB patients at the time of the study, which is in agreement with experimental models showing that TGF‐β suppresses Th1 responses and lung inflammation and enhances lung bacillary load during the chronic phase in the murine model of infection 49. Thus, it is tempting to speculate that the strong TGF‐β elicited by the host in an attempt to diminish the severe inflammatory process could favour the persistence of the high numbers of bacilli in MDR‐TB.
Finally, we demonstrated that patients who are infected with Haarlem strains and have a positive AFB sputum show the highest numbers of IL‐17+IFN‐γ– cells and the lowest numbers of IL‐17+IFN‐γ+ cells, underlining the strong ability of the M strain, which belongs to the Haarlem family, to switch the Th17 profile to an IL‐17+IFN‐γ– phenotype. Thus, our present work extends our previous results 19, and shows that the genetic background of the infecting M. tuberculosis strain and its bacterial burden can modulate in‐vitro Th17 profiles through an expansion of IL‐17+IFN‐γ– cells.
Disclosure
None to declare.
Author contributions
J. B. and S. de la B. conceived and designed the study. J. B., D. K., M. R. and C. S. y G. performed the experiments. J. B., L. B., M. S. and S. de la B. interpreted the data. J. M., B. L. and V. R. provided the clinical isolates and performed the genotype analysis of patients’ infecting strains. A. G., M. V., P. G. M. and D. P. were responsible for patient recruitment and collected the clinical data. M. S., J. B., L B. and S de la B. wrote the paper.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Interleukin (IL)‐23, transforming growth factor (TGF)‐β, IL‐6 and IL‐1β are involved in Mycobacterium tuberculosis‐induced T helper type 17 (Th17) response. Peripheral blood mononuclear cells (PBMC) from 31 multi‐drug‐resistant tuberculosis (MDR‐TB) patients (a,b) and eight purified protein derivative (PPD)+ (C) healthy individuals were cultured for 48 h alone (control) or with H37Rv and M strains in the absence (–) or presence of anti‐IL‐23p19, anti‐TGF‐β, anti‐IL‐1β or anti‐IL‐6 antibodies. Then the percentage of CD4+ cells expressing intracellular IL‐17 was determined by fluorescence‐activated cell sorter (FACS) and number of CD4+IL‐17+ cells present in 1 × 106 cultured PBMC (n) was calculated for each individual. (a) Dot‐plots from one MDR‐TB patient are shown and are representative of 31 patients studied. The numbers in the upper right quadrant represent the percentage of CD4+IL‐17+ cells within live lymphocyte gate. (b,c) Results are expressed as medians and 25th–75th percentiles (boxes) with maximum and minimum values (error bars). *P < 0·05 for treated versus untreated PBMC (Friedman test followed by Dunn's test).
Fig. S2. High proportion of CD25+latency‐associated protein (LAP)+CD4+ T cells in multi‐drug‐resistant tuberculosis (MDR‐TB) patients; role of Toll‐like receptor (TLR)‐2 on their expansion. (a). Peripheral blood mononuclear cells (PBMC) from eight MDR‐TB patients and six purified protein derivative (PPD)+ healthy donors (HD) were cultured for 48 h alone (control) or with H37Rv, Ra or M strains. Then LAP and CD25 expression was determined in CD4+ cells by flow cytometry. CD4+ cells were classified in CD4+CD25high [regulatory T cells (Tregs)] and CD4+CD25low (conventional activated) according to their CD25 fluorescence intensity. Results are expressed as number of CD4+CD25high or CD25low expressing LAP+ cells in 1 × 106 cultured PBMC (n) and are depicted as boxes showing median and 25th–75th percentiles with maximum and minimum error bars. Statistical differences: *P < 0·05 for control versus Mtb‐stimulated cells (Friedman test followed by Dunn's test); (a) = P < 0·05 for MDR‐TB versus PPD+ HD (Kruskal–Wallis statistics followed by Dunn's test). (b). PBMC from six MDR‐TB patients were stimulated for 48 h alone or with Mycobacterium tuberculosis strains, in the presence or not of anti‐TLR‐2 or anti‐TLR‐4 monoclonal antibodies. Then the number of CD4+CD25high/low LAP+ cells was determined. Box‐plots show median and 25th–75th percentiles with maximum and minimum values. Statistical differences: *P < 0·05 for antibody‐treated versus non‐treated PBMC (Friedman test followed by Dunn's test).
Fig. S3. Schematic model representing the mechanisms used by M strain to induce high levels of transforming growth factor (TGF)‐β secretion by antigen‐presenting cells (APCs) and CD4+latency‐associated protein (LAP)+ T cells leading to the interleukin (IL)‐17+interferon (IFN)‐γ– cell subset expansion in multi‐drug‐resistant tuberculosis (MDR‐TB) patients. Upper panel: IL‐17 secretion: antigen‐presenting cells (APCs) from MDR‐TB patients and purified protein derivative (PPD)+ healthy donors (HD) recognize Mycobacterium tuberculosis (Mtb) strains (H37Rv and MDR M strain) through dectin‐1, Toll‐like receptor (TLR)‐2 and TLR‐4 pattern recognition receptors. Consequently, APCs secrete IL‐1β, IL‐6, IL‐23 and TGF‐β triggering the production of IL‐17 by CD4+ T cells (upper grey panel). Lower panels: differential expansion of IL‐17+ T cell subsets in MDR‐TB and PPD+ HD: IL‐1β and IL‐6 secreted by APCs are essential for IL‐17+IFN‐γ+ and IL‐17+IFN‐γ–CD4+ T cells expansion in MDR‐TB and PPD+ HD (lower grey panel). Particularly, in PPD+ HD, APCs recognize Mtb strains through TLR‐4 and together with IL‐23 promote IL‐17+IFN‐γ+ cell expansion (green panel). In the case of MDR‐TB patients, APCs recognize M strain via TLR‐2 secreting large amounts of TGF‐β. Additionally, M strain can be recognized further by CD4+CD25highforkhead box protein 3 (FoxP3+) [regulatory T cells (Treg)] and CD4+CD25lowFoxP3– (conventional activated cells) through TLR‐2, inducing up‐regulation of the LAP/TGF‐β complex (LAP) expression and promoting the expansion of both subsets. TGF‐β secreted by APCs and CD4+LAP+ T cells acts jointly with IL‐23 to support the marked expansion of IL‐17+IFNγ–CD4+ T cells (pink panel), which are responsible for the enhanced T helper type 17 (Th17) response observed in MDR‐TB patients.
Table S1. Additional clinical feature of tuberculosis (TB) patients.
Acknowledgements
The authors gratefully acknowledge Dr Nora Galassi, Dr Norma Riera and Miss Marta Felippo for their technical assistance in flow cytometry. This work was supported by grants from the Agencia Nacional de Promoción Científica y Tecnológica (PICT 2011‐0572) and Consejo Nacional de Investigaciones Científicas y Técnicas (PIP 112‐2013‐0100202).
References
- 1. Sallusto F, Zielinski CE, Lanzavecchia A. Human Th17 subsets. Eur J Immunol 2012; 42:2215–20. [DOI] [PubMed] [Google Scholar]
- 2. Annunziato F, Romagnani C, Romagnani S. The 3 major types of innate and adaptive cell‐mediated effector immunity. J Allergy Clin Immunol 2015; 135:626–35. [DOI] [PubMed] [Google Scholar]
- 3. Crome SQ, Wang AY, Levings MK. Translational mini‐review series on Th17 cells: function and regulation of human T helper 17 cells in health and disease. Clin Exp Immunol 2009; 159:109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cosmi L, Santarlasci V, Maggi L, Liotta F, Annunziato F. Th17 plasticity: pathophysiology and treatment of chronic inflammatory disorders. Curr Opin Pharmacol 2014; 17:12–6. [DOI] [PubMed] [Google Scholar]
- 5. Khader SA, Bell GK, Pearl JE et al IL‐23 and IL‐17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol 2007; 8:369–77. [DOI] [PubMed] [Google Scholar]
- 6. Okamoto Yoshida Y, Umemura M, Yahagi A et al Essential role of IL‐17A in the formation of a mycobacterial infection‐induced granuloma in the lung. J Immunol 2010; 184:4414–22. [DOI] [PubMed] [Google Scholar]
- 7. Cruz A, Fraga AG, Fountain JJ et al Pathological role of interleukin 17 in mice subjected to repeated BCG vaccination after infection with Mycobacterium tuberculosis . J Exp Med 2010; 207:1609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. Defining the human T helper 17 cell phenotype. Trends Immunol 2012; 33:505–12. [DOI] [PubMed] [Google Scholar]
- 9. Annunziato F, Cosmi L, Santarlasci V et al Phenotypic and functional features of human Th17 cells. J Exp Med 2007; 204:1849–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brucklacher‐Waldert V, Steinbach K, Lioznov M, Kolster M, Holscher C, Tolosa E. Phenotypical characterization of human Th17 cells unambiguously identified by surface IL‐17A expression. J Immunol 2009; 183:5494–501. [DOI] [PubMed] [Google Scholar]
- 11. Acosta‐Rodriguez EV, Rivino L, Geginat J et al Surface phenotype and antigenic specificity of human interleukin 17‐producing T helper memory cells. Nat Immunol 2007; 8:639–46. [DOI] [PubMed] [Google Scholar]
- 12. Cosmi L, Maggi L, Santarlasci V et al Identification of a novel subset of human circulating memory CD4(+) T cells that produce both IL‐17A and IL‐4. J Allergy Clin Immunol 2010; 125:222–30 e1‐4. [DOI] [PubMed] [Google Scholar]
- 13. Zielinski CE, Mele F, Aschenbrenner D et al Pathogen‐induced human TH17 cells produce IFN‐gamma or IL‐10 and are regulated by IL‐1beta. Nature 2012; 484:514–8. [DOI] [PubMed] [Google Scholar]
- 14. van de Veerdonk FL, Teirlinck AC, Kleinnijenhuis J et al Mycobacterium tuberculosis induces IL‐17A responses through TLR4 and dectin‐1 and is critically dependent on endogenous IL‐1. J Leukoc Biol 2010; 88:227–32. [DOI] [PubMed] [Google Scholar]
- 15. Jurado JO, Pasquinelli V, Alvarez IB et al IL‐17 and IFN‐gamma expression in lymphocytes from patients with active tuberculosis correlates with the severity of the disease. J Leukoc Biol 2012; 91:991–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ritacco V, Di Lonardo M, Reniero A et al Nosocomial spread of human immunodeficiency virus‐related multidrug‐resistant tuberculosis in Buenos Aires. J Infect Dis 1997; 176:637–42. [DOI] [PubMed] [Google Scholar]
- 17. Ritacco V, Lopez B, Ambroggi M et al HIV infection and geographically bound transmission of drug‐resistant tuberculosis, Argentina. Emerg Infect Dis 2012; 18:1802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Geffner L, Yokobori N, Basile J et al Patients with multidrug‐resistant tuberculosis display impaired Th1 responses and enhanced regulatory T‐cell levels in response to an outbreak of multidrug‐resistant Mycobacterium tuberculosis M and Ra strains. Infect Immun 2009; 77:5025–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Basile JI, Geffner LJ, Romero MM et al Outbreaks of mycobacterium tuberculosis MDR strains induce high IL‐17 T‐cell response in patients with MDR tuberculosis that is closely associated with high antigen load. J Infect Dis 2011; 204:1054–64. [DOI] [PubMed] [Google Scholar]
- 20. World Health Organization (WHO) . Global Tuberculosis Programme. Laboratory Services in Tuberculosis ControlWHO/TB/98258. Geneva, Switzerland: WHO, 1998. [Google Scholar]
- 21. Kamerbeek J, Schouls L, Kolk A et al Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol 1997; 35:907–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Embden JD, Cave MD, Crawford JT et al Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol 1993; 31:406–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Santarlasci V, Maggi L, Capone M et al TGF‐beta indirectly favors the development of human Th17 cells by inhibiting Th1 cells. Eur J Immunol 2009; 39:207–15. [DOI] [PubMed] [Google Scholar]
- 24. Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol 1999; 15:269–90. [DOI] [PubMed] [Google Scholar]
- 25. Dlugovitzky D, Bay ML, Rateni L et al In vitro synthesis of interferon‐gamma, interleukin‐4, transforming growth factor‐beta and interleukin‐1 beta by peripheral blood mononuclear cells from tuberculosis patients: relationship with the severity of pulmonary involvement. Scand J Immunol 1999; 49:210–7. [DOI] [PubMed] [Google Scholar]
- 26. Govinden R, Bhoola KD. Genealogy, expression, and cellular function of transforming growth factor‐beta. Pharmacol Ther 2003; 98:257–65. [DOI] [PubMed] [Google Scholar]
- 27. Tran DQ, Andersson J, Hardwick D, Bebris L, Illei GG, Shevach EM. Selective expression of latency‐associated peptide (LAP) and IL‐1 receptor type I/II (CD121a/CD121b) on activated human FOXP3+ regulatory T cells allows for their purification from expansion cultures. Blood 2009; 113:5125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gandhi R, Farez MF, Wang Y, Kozoriz D, Quintana FJ, Weiner HL. Cutting edge: human latency‐associated peptide+ T cells: a novel regulatory T cell subset. J Immunol 2010; 184:4620–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kleinnijenhuis J, Oosting M, Joosten LA, Netea MG, Van Crevel R. Innate immune recognition of Mycobacterium tuberculosis. Clin Dev Immunol 2011; 2011:405310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jin B, Sun T, Yu XH, Yang YX, Yeo AE. The effects of TLR activation on T‐cell development and differentiation. Clin Dev Immunol 2012; 2012:836485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saunders BM, Frank AA, Orme IM, Cooper AM. Interleukin‐6 induces early gamma interferon production in the infected lung but is not required for generation of specific immunity to Mycobacterium tuberculosis infection. Infect Immun 2000; 68:3322–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu H, Rohowsky‐Kochan C. Regulation of IL‐17 in human CCR6+ effector memory T cells. J Immunol 2008; 180:7948–57. [DOI] [PubMed] [Google Scholar]
- 33. Evans HG, Gullick NJ, Kelly S et al In vivo activated monocytes from the site of inflammation in humans specifically promote Th17 responses. Proc Natl Acad Sci USA 2009; 106:6232–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van Beelen AJ, Zelinkova Z, Taanman‐Kueter EW et al Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin‐17 production in human memory T cells. Immunity 2007; 27:660–9. [DOI] [PubMed] [Google Scholar]
- 35. Koenen HJ, Smeets RL, Vink PM, van Rijssen E, Boots AM, Joosten I. Human CD25highFoxp3pos regulatory T cells differentiate into IL‐17‐producing cells. Blood 2008; 112:2340–52. [DOI] [PubMed] [Google Scholar]
- 36. Duhen T, Campbell DJ. IL‐1beta promotes the differentiation of polyfunctional human CCR6+CXCR3+ Th1/17 cells that are specific for pathogenic and commensal microbes. J Immunol 2014; 193:120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oppmann B, Lesley R, Blom B et al Novel p19 protein engages IL‐12p40 to form a cytokine, IL‐23, with biological activities similar as well as distinct from IL‐12. Immunity 2000; 13:715–25. [DOI] [PubMed] [Google Scholar]
- 38. Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)‐17 cells requires transforming growth factor‐beta and induction of the nuclear receptor RORgammat. Nat Immunol 2008; 9:6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Acosta‐Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor‐beta are essential for the differentiation of interleukin 17‐producing human T helper cells. Nat Immunol 2007; 8:942–9. [DOI] [PubMed] [Google Scholar]
- 40. Wilson NJ, Boniface K, Chan JR et al Development, cytokine profile and function of human interleukin 17‐producing helper T cells. Nat Immunol 2007; 8:950–7. [DOI] [PubMed] [Google Scholar]
- 41. Castro AZ, Diaz‐Bardalez BM, Oliveira EC et al Abnormal production of transforming growth factor beta and interferon gamma by peripheral blood cells of patients with multidrug‐resistant pulmonary tuberculosis in Brazil. J Infect 2005; 51:318–24. [DOI] [PubMed] [Google Scholar]
- 42. Zenaro E, Donini M, Dusi S. Induction of Th1/Th17 immune response by Mycobacterium tuberculosis: role of dectin‐1, mannose receptor, and DC‐SIGN. J Leukoc Biol 2009; 86:1393–401. [DOI] [PubMed] [Google Scholar]
- 43. Dennehy KM, Willment JA, Williams DL, Brown GD. Reciprocal regulation of IL‐23 and IL‐12 following co‐activation of Dectin‐1 and TLR signaling pathways. Eur J Immunol 2009; 39:1379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Boniface K, Blumenschein WM, Brovont‐Porth K et al Human Th17 cells comprise heterogeneous subsets including IFN‐gamma‐producing cells with distinct properties from the Th1 lineage. J Immunol 2010; 185:679–87. [DOI] [PubMed] [Google Scholar]
- 45. Chatterjee S, Dwivedi VP, Singh Y et al Early secreted antigen ESAT‐6 of Mycobacterium tuberculosis promotes protective T helper 17 cell responses in a toll‐like receptor‐2‐dependent manner. PLOS Pathog 2011; 7:e1002378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sutmuller RP, Morgan ME, Netea MG, Grauer O, Adema GJ. Toll‐like receptors on regulatory T cells: expanding immune regulation. Trends Immunol 2006; 27:387–93. [DOI] [PubMed] [Google Scholar]
- 47. Liu H, Komai‐Koma M, Xu D, Liew FY. Toll‐like receptor 2 signaling modulates the functions of CD4+ CD25+ regulatory T cells. Proc Natl Acad Sci USA 2006; 103:7048–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nyirenda MH, Sanvito L, Darlington PJ et al TLR2 stimulation drives human naive and effector regulatory T cells into a Th17‐like phenotype with reduced suppressive function. J Immunol 2011; 187:2278–90. [DOI] [PubMed] [Google Scholar]
- 49. Hernandez‐Pando R, Orozco‐Esteves H, Maldonado HA et al A combination of a transforming growth factor‐beta antagonist and an inhibitor of cyclooxygenase is an effective treatment for murine pulmonary tuberculosis. Clin Exp Immunol 2006; 144:264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Interleukin (IL)‐23, transforming growth factor (TGF)‐β, IL‐6 and IL‐1β are involved in Mycobacterium tuberculosis‐induced T helper type 17 (Th17) response. Peripheral blood mononuclear cells (PBMC) from 31 multi‐drug‐resistant tuberculosis (MDR‐TB) patients (a,b) and eight purified protein derivative (PPD)+ (C) healthy individuals were cultured for 48 h alone (control) or with H37Rv and M strains in the absence (–) or presence of anti‐IL‐23p19, anti‐TGF‐β, anti‐IL‐1β or anti‐IL‐6 antibodies. Then the percentage of CD4+ cells expressing intracellular IL‐17 was determined by fluorescence‐activated cell sorter (FACS) and number of CD4+IL‐17+ cells present in 1 × 106 cultured PBMC (n) was calculated for each individual. (a) Dot‐plots from one MDR‐TB patient are shown and are representative of 31 patients studied. The numbers in the upper right quadrant represent the percentage of CD4+IL‐17+ cells within live lymphocyte gate. (b,c) Results are expressed as medians and 25th–75th percentiles (boxes) with maximum and minimum values (error bars). *P < 0·05 for treated versus untreated PBMC (Friedman test followed by Dunn's test).
Fig. S2. High proportion of CD25+latency‐associated protein (LAP)+CD4+ T cells in multi‐drug‐resistant tuberculosis (MDR‐TB) patients; role of Toll‐like receptor (TLR)‐2 on their expansion. (a). Peripheral blood mononuclear cells (PBMC) from eight MDR‐TB patients and six purified protein derivative (PPD)+ healthy donors (HD) were cultured for 48 h alone (control) or with H37Rv, Ra or M strains. Then LAP and CD25 expression was determined in CD4+ cells by flow cytometry. CD4+ cells were classified in CD4+CD25high [regulatory T cells (Tregs)] and CD4+CD25low (conventional activated) according to their CD25 fluorescence intensity. Results are expressed as number of CD4+CD25high or CD25low expressing LAP+ cells in 1 × 106 cultured PBMC (n) and are depicted as boxes showing median and 25th–75th percentiles with maximum and minimum error bars. Statistical differences: *P < 0·05 for control versus Mtb‐stimulated cells (Friedman test followed by Dunn's test); (a) = P < 0·05 for MDR‐TB versus PPD+ HD (Kruskal–Wallis statistics followed by Dunn's test). (b). PBMC from six MDR‐TB patients were stimulated for 48 h alone or with Mycobacterium tuberculosis strains, in the presence or not of anti‐TLR‐2 or anti‐TLR‐4 monoclonal antibodies. Then the number of CD4+CD25high/low LAP+ cells was determined. Box‐plots show median and 25th–75th percentiles with maximum and minimum values. Statistical differences: *P < 0·05 for antibody‐treated versus non‐treated PBMC (Friedman test followed by Dunn's test).
Fig. S3. Schematic model representing the mechanisms used by M strain to induce high levels of transforming growth factor (TGF)‐β secretion by antigen‐presenting cells (APCs) and CD4+latency‐associated protein (LAP)+ T cells leading to the interleukin (IL)‐17+interferon (IFN)‐γ– cell subset expansion in multi‐drug‐resistant tuberculosis (MDR‐TB) patients. Upper panel: IL‐17 secretion: antigen‐presenting cells (APCs) from MDR‐TB patients and purified protein derivative (PPD)+ healthy donors (HD) recognize Mycobacterium tuberculosis (Mtb) strains (H37Rv and MDR M strain) through dectin‐1, Toll‐like receptor (TLR)‐2 and TLR‐4 pattern recognition receptors. Consequently, APCs secrete IL‐1β, IL‐6, IL‐23 and TGF‐β triggering the production of IL‐17 by CD4+ T cells (upper grey panel). Lower panels: differential expansion of IL‐17+ T cell subsets in MDR‐TB and PPD+ HD: IL‐1β and IL‐6 secreted by APCs are essential for IL‐17+IFN‐γ+ and IL‐17+IFN‐γ–CD4+ T cells expansion in MDR‐TB and PPD+ HD (lower grey panel). Particularly, in PPD+ HD, APCs recognize Mtb strains through TLR‐4 and together with IL‐23 promote IL‐17+IFN‐γ+ cell expansion (green panel). In the case of MDR‐TB patients, APCs recognize M strain via TLR‐2 secreting large amounts of TGF‐β. Additionally, M strain can be recognized further by CD4+CD25highforkhead box protein 3 (FoxP3+) [regulatory T cells (Treg)] and CD4+CD25lowFoxP3– (conventional activated cells) through TLR‐2, inducing up‐regulation of the LAP/TGF‐β complex (LAP) expression and promoting the expansion of both subsets. TGF‐β secreted by APCs and CD4+LAP+ T cells acts jointly with IL‐23 to support the marked expansion of IL‐17+IFNγ–CD4+ T cells (pink panel), which are responsible for the enhanced T helper type 17 (Th17) response observed in MDR‐TB patients.
Table S1. Additional clinical feature of tuberculosis (TB) patients.


