Summary
The immune system undergoes age‐associated changes known as immunosenescence, resulting in increased susceptibility to infections, cancers and autoimmunity in the aged. The basis of our understanding of immunosenescence has been derived primarily from studies examining intrinsic defects within many of the cells of the immune system. While these studies have provided insight into the mechanisms of immunosenescence, a picture is now emerging that the stromal microenvironment within lymphoid organs also contributes significantly to the age‐associated decline of immune function. These extrinsic defects appear to impact the functional activity of immune cells and may offer a potential target to recover immune activity. Indeed, rejuvenation studies which have targeted the stromal niche have restored immune function in aged successfully, highlighting the impact of the microenvironment towards the aetiology of immunosenescence.
Keywords: aging, cell differentiation, spleen and lymph nodes, stromal cells, thymus
OTHER ARTICLES PUBLISHED IN THIS REVIEW SERIES
Immunosenescence: the importance of considering age in health and disease. Clinical and Experimental Immunology 2017, 187: 1–3.
The convergence of senescence and nutrient sensing during lymphocyte ageing. Clinical and Experimental Immunology 2017, 187: 4–5.
Innate immune responses in the ageing lung. Clinical and Experimental Immunology 2017, 187: 16–25.
Age‐related alterations in immune responses to West Nile virus infection. Clinical and Experimental Immunology 2017, 187: 26–34.
Intracellular signalling pathways: targets to reverse immunosenescence. Clinical and Experimental Immunology 2017, 187: 35–43.
Ageing and inflammation in patients with HIV infection. Clinical and Experimental Immunology 2017, 187: 44–52.
Considerations for successful cancer immunotherapy in aged hosts. Clinical and Experimental Immunology 2017, 187: 53–63.
Ageing and obesity similarly impair antibody responses. Clinical and Experimental Immunology 2017, 187: 64–70.
The life cycle of a T cell after vaccination – where does immune ageing strike? Clinical and Experimental Immunology 2017, 187: 71–81.
Herpes zoster and the search for an effective vaccine. Clinical and Experimental Immunology 2017, 187: 82–92.
Adult vaccination against tetanus and diphtheria: the European perspective. Clinical and Experimental Immunology 2017, 187: 93–99.
Introduction
The microenvironment of primary and secondary lymphoid organs (e.g. bone marrow, thymus) plays a critical role in the development and activation of immune cells by regulating cellular differentiation and proliferation 1, 2, 3. These highly specialized environments consist of various cell types (fibroblast, endothelial cells, epithelial cells), extracellular matrix molecules and adhesion molecules, which regulate the processes of cellular differentiation and proliferation through the production of soluble factors and cell‐to‐cell interactions 1, 2, 3. Such interactions are essential, as defects within the stromal niche severely hamper the function of primary and secondary lymphoid organs.
It is now evident that, with increasing age, there is a decline in immunological competence, which is displayed by a reduced response to vaccination and infections, together with an increase in the incidence of cancers and autoimmune disorders 4, 5, 6, 7. Our understanding of the mechanisms underlying immunosenescence is based primarily on the identification of intrinsic changes in cells of the immune system 8, 9, 10 and while these studies have provided some insight, what is often overlooked is the potential role of extrinsic factors (see Fig. 1). Moreover, an increasing number of studies have identified the aged microenvironment as a contributing factor to the clinical manifestations of immunosenescence 11. In this review we describe the impact of the stromal niche in the age‐associated decline of immune function.
Figure 1.
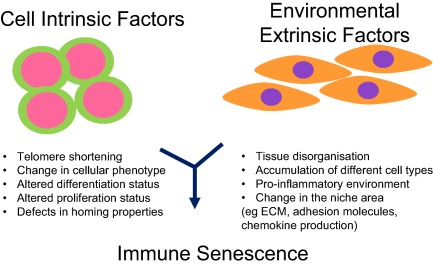
Examples of intrinsic and extrinsic factors that can contribute towards immune senescence.
The aged bone marrow niche
Documented changes in aged haematopoietic stem cells (HSC) include reduced repopulation activity, homing and self‐renewing capacity together with a skewed differentiation along the myeloid lineage 12. Aged HSC show altered gene expression in comparison to young HSC, in particular up‐regulation of the senescent marker p16, which is associated with reduced differentiation and proliferative potential 13 and increased DNA double‐strand breaks 14. These observations imply strongly that the mechanisms involved in HSC ageing are a consequence of cell‐intrinsic changes 12. However, other studies have suggested that perhaps some of these alterations may be due, in part, to the influence of the aged microenvironment 15. For instance, young HSC differentiated preferentially towards the myeloid lineage when transplanted into aged recipients 16, 17. In contrast, transplantation of aged HSC into the young niche produces fewer myeloid cells 18. This could be attributed to the increased inflammatory status within the aged bone marrow (BM) niche, as studies have shown elevated levels of proinflammatory cytokines 18, 19, 20. Indeed, it has been demonstrated that the chemokine RANTES (regulated upon activation normal T cell expressed and secreted), which is elevated in the aged BM, is able to stimulate myeloid‐biased HSC differentiation 18. Furthermore, aged mesenchymal stromal cells (MSC; also known as mesenchymal stem cells 21) show a reduced osteogenic activity while, conversely, preferring to differentiate into adipocytes, correlating with the reduced osteogenesis that is seen in elderly people together with the age‐related increase in yellow BM 22, 23. Kfoury and Scadden recently proposed the term mesenchymal stromal cells 21, not to dispute the presence of stem cells within this population of cells, but they noted that the majority of publications using these cells have not necessary examined their precursor activity. This altered differentiation of aged MSC may be due to the age‐associated reduction in the expression of the CXCR4 receptor 24, as MSC deficient in this receptor exhibit impaired osteogenesis 24, 25. Additionally, aged MSC show a preference to differentiate into adipocytes which might be related to the age‐related increase in yellow marrow and, interestingly, adipocytes appear to exhibit reduced HSC differentiation in human and mice 26, 27.
Such alterations in the aged BM niche have led to the speculation that these changes might also play a role in the development of haematological malignancies 15, which are often age‐related; this is supported by the observation that a pre‐leukaemic cell line showed preferential growth in aged BM 28. In another study by the same authors, they showed that transplantation of transformed HSC clones developed preferentially in the aged BM microenvironment in comparison to the young BM niche 29. Moreover, studies using MSC from multiple myeloma patients (a B cell malignancy) have shown that they exhibit a senescent profile 30, altered phenotype, differentiation and proliferative capacity 30, 31 and produce higher levels of proinflammatory cytokines 30, 32, giving rise to the suggestion that the BM microenvironment may be a key component in the pathogenesis of this disease 33.
The development of B cells is critically dependent upon the stromal environment within the BM 34, and although maturation of B cells resides within a different compartment, which is associated with HSC differentiation, given the age‐related changes within the BM niche 15, the defects of B cell function in the aged could nevertheless be attributed, in part, to the aged microenvironment. Previous studies have shown that primary culture of stromal cells from old and not young fail to support B cell development, due possibly to a defect in the secretion of interleukin (IL)‐7, a key cytokine for B cell maturation, from ageing stromal cells 35. Adoptive transfer experiments suggest that the aged BM stroma may reduce the recombinase activity in B cell progenitors which leads to the inability to undergo gene rearrangement, resulting in a decrease in B cell differentiation 36. Furthermore, adipocytes, which increase in the aged BM, appear to inhibit B cell lymphopoiesis 37. The impact of the aged BM is not confined only to B cell differentiation, as recent transplantation studies involving mixed BM chimeras in mice have shown that the age‐associated decline of natural killer (NK) cell function might also be due to the BM stroma 38, 39, 40. NK cells develop in the BM and their numbers, together with their activity (such as cytotoxicity), decline with age 10; these recent studies 38, 39, 40 have suggested that this may be attributed to the failure of the aged BM stroma to provide the necessary developmental cues.
Aged thymic stromal microenvironment contributes to immunosenescence
The thymus is a central T lymphoid organ responsible for both the production of functional naive T cells and the generation of immune tolerance. It is able to carry out this function due to the presence of cortical and medullary thymic epithelial cells (TEC), which represent a crucial component of the thymic niche 1. Age‐associated thymic involution represents one of the most acknowledged changes in the ageing immune system and appears to occur in all vertebrates, implying that it is an evolutionary conserved event 41. This involution results in the reduced output of naive T cells 42, 43, leading to the oligoclonal expansion of memory T cells. Consequently, the T cell receptor repertoire is diminished 44, 45 together with a decline in T cell functional activity, resulting in immune senescence 5, 9. Furthermore, age‐associated thymic involution also induces defects in the establishment of immune tolerance, thereby resulting in enhanced propensity for autoimmune responses 46.
Several studies have demonstrated that the thymic microenvironment undergoes age‐associated changes, including alterations of TEC cortical and medullary markers 47, 48, changes in TEC gene expression profile 49, 50, which includes a decline in the production of the thymopoietic cytokine IL‐7 51, together with a disruption of the structural organization and integrity of the thymic niche 52, 53. Given that such changes can affect the thymopoietic activity of the thymus 1, it is not unreasonable to propose that the thymic stromal microenvironment contributes towards the process of age‐associated thymic involution 11, 48.
Indeed, this is the conclusion reached from several transplantation studies, which demonstrated that the thymopoietic activity of early thymic precursor (ETP) from young and old mice appear similar and that the defects in age‐associated thymic involution seem to reside in the aged thymic stroma 17, 54, 55, 56. In particular, Zhu and colleagues observed that transplanted fetal thymi were repopulated with equal efficiency in young and old mice, whereas intrathymic injection of ETP from young mice fail to develop in the thymus of old mice 56. However, it should be noted that there are studies showing aged ETP exhibiting reduced proliferative and differentiation potential 57.
The thymus establishes immune tolerance through thymocyte negative selection and the generation of thymus‐derived regulatory T cells (tTregs) 58, mainly by presenting self‐reactive peptide/major histocompatibility complexes (MHC) on medullary TEC (mTEC) 59 to induce either negative selection 60 or the differentiation of tTregs 61, 62, 63. The mechanism of self‐antigen presentation is controlled at least partially by the autoimmune regulatory gene (AIRE) 64, 65, and there is evidence to suggest the aged thymus contains a reduced number of Aire+ mTEC 46, 66, leading possibly to impairment of negative selection which may reflect the increased prevalence of autoimmunity in elderly people 4. However, it is still unclear whether tTreg selection is also disrupted in the involuted thymus, with studies identifying a decrease in the generation of tTreg in the aged thymus 67, while others reveal no reduction in tTregs 46.
It is often stated that thymic involution is initiated at the start of puberty 68, although some have argued that this process may occur earlier in life 69, 70, 71; nevertheless, the thymic stroma has been identified as the target of androgen‐induced regression 72. Furthermore, gene expression analysis comparing young and old thymi revealed that the majority of changes occur within the cortical TEC compartment 50. An extension of this study from the same group revealed that TEC are deficient in the anti‐oxidant enzyme catalase and, by elevating levels of catalase through transgenesis or using anti‐oxidants in the diet, they observed that thymic atrophy was diminished 73. Interestingly, the authors propose that these findings may offer a rationale as to why the thymus begins to ‘age’ much earlier than other organs 74.
Overall, these studies highlight that TEC homeostasis represents an important element in the aetiology of age‐associated thymic involution and factors linked with TEC maintenance and integrity could represent key triggers in involution. One potential trigger appears to be the transcription factor forkhead box nude N1 (FoxN1), which is crucial for TEC development 75. Studies have revealed that the intrathymic expression of FoxN1 shows an age‐associated decrease 49, 76, with a recent study showing that the most dramatic decline of FoxN1 occurs at the onset of thymic involution 77. Moreover, the generation of transgenic mice that have a reduced expression of FoxN1 in the postnatal thymus mimic thymic involution 78, 79, 80. In contrast, over‐expression or induction of expression of FoxN1 in the postnatal thymus can delay thymic involution 80, 81, 82. Similarly, mice deficient in the intrathymic production of retinoblastoma show an enlarged thymus due to the up‐regulation of expression of FoxN1 83.
Other significant changes that have been identified within the aged thymic microenvironment includes an accumulation of adipose tissue 84, fibroblasts 85 and senescent cells 85, and evidence suggests that such cells, in particular adipocytes and fibroblasts, are derived from TEC 86. Furthermore, the presence of these cell types appears to inhibit thymopoiesis and may therefore contribute to thymic involution 87. Indeed, thymi from caloric‐restricted mice, which exhibit an increased lifespan, show a delayed involution due primarily to a reduction in thymic adipogenesis 84. This reduction may be mediated by fibroblast growth factor 21 (FGF21), which is expressed within the thymic stroma and is up‐regulated in caloric‐restricted mice 88. Moreover, over‐expression of FGF21 inhibited the accumulation of fat within the thymus and abrogated thymic involution 88. The presence of senescent cells within the thymus may correlate with the age‐associated increase in proinflammatory cytokine expression which is seen in the human thymus 89. Such cells, which produce a variety of molecules, are termed senescence‐associated secretory phenotype (SASP) 90 and have been suggested to cause alteration in tissue function and structure 90, 91. Indeed, administration of IL‐6 is known to cause thymic atrophy in mice 89. Further evidence suggesting that TEC are regulators of thymic involution comes from several studies that have rejuvenated the ageing thymus successfully by targeting the thymic stroma 11. These include the administration of IL‐7 92, keratinocyte growth factor 93, IL‐22 94 and ghrelin 95, and in most instances thymic function and structure were restored. In contrast, intrinsic interventions have proved so far to be less efficacious in comparison to targeting the stromal niche.
Secondary lymphoid stromal cells: an underlying contributor to immunosenescence?
Stromal cells in secondary lymphoid organs (lymph nodes, spleen) were once considered purely structural in nature. During the past decade this simplistic view has been overturned by an insurgence of research revealing the integral role of stroma in maintaining and controlling immune cell function. While much of ageing immunology research has been focused heavily upon determining cell intrinsic defects in adaptive and innate immune cells, the contribution of secondary lymphoid stromal cells to age‐related defects in immunity are just beginning to become unravelled 96, 97, 98.
Age‐related alterations of lymph node stromal cells
Lymph nodes are highly organized structures important for the development of adaptive and innate immune responses. In lymph nodes, B cells are segregated to peripheral follicles and T cells remain the central T cell zone, also known as paracortex. The medullary sinus is a site where activated T cells exit the lymph node through lymphatic vessels (Fig. 2a). The majority of secondary lymphoid organ stromal cell research has focused upon the lymph node stromal cells. Recent reviews by Fletcher et al. 99 and Change et al. 100 describe in detail the biology of the various lymph node stromal cell niches. Simplistically, lymph node stroma can be divided into four subsets; lymphatic endothelial cells (LECs), blood endothelial cells (BECs), fibroblastic reticular cells (FRCs) and cells negative for the markers of these subsets, called double‐negative cells (DNCs) 99 (Fig. 2b).
Figure 2.
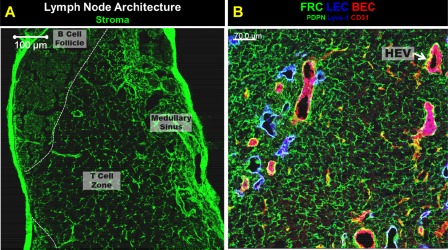
Lymph node architecture. (a) The main architectural components of a lymph node are B cell follicles (dashed white line), T cell zone and lymphatic rich medullary sinus. Stroma are show in green with ER‐TR7 staining. The T cell zone contains a net‐like fibroblastic reticular cell (FRC) network magnified in (b). (b) Image of the stromal cell subsets in the lymph node T cell zone. The FRC network is shown in green as podoplanin (PDPN)+. Lymphatic endothelial cells (LEC) shown in blue with Lyve‐1. Blood endothelial cells (BECs) are shown in red using CD31. High endothelial venules (HEVs), a subset of BECs, are the cuboidal shaped CD31+ areas, one of which is denoted by an arrow. Image was acquired using confocal microscopy. (a) scale bar = 100 μm, (b) scale bar = 70 μm. Images are of mediastinal lymph nodes from C57BL/6 mice, acquired by A. R. M.
Lymphatic endothelial cells compose the lymphatic vessels in lymph nodes 101. Lymphatic vessels are conduits that transport lymph, soluble antigens and immune cells from tissues to draining lymph nodes 102. Aged lymphatic collectors have increased leakiness and a decreased ability to support active lymph flow 103, 104, 105, which results in decreased capacity to transport bacteria 105. Functional attrition of aged lymphatics is due in part to increased oxidative stress and protein carbonylation 105. Defects in cellular and antigenic transport caused by age‐related changes in lymphatic collectors may be a contributing factor behind the delayed initiation to immune responses found in elderly people.
Blood endothelial cells in the lymph node can be separated into capillaries and cuboidal‐shaped high endothelial venules (HEVs) 101. BECs facilitate entry of naive T and B cells into the lymph node 101, but how ageing impacts HEVs or capillaries in the lymph node is still unclear. One recent study by Richner et al. shows that aged naive CD4+ T cells transferred into young mice have delayed entry into the lymph node, and it was observed that aged CD4+ T cells have altered migration through HEVs compared to young T cells 106, suggesting T cell intrinsic defects. However, this study did not examine directly the changes occurring in aged HEVs, which may contribute to the delayed entry of young cells into aged lymph nodes. Ageing of the vascular system is a well‐studied phenomenon characterized by mechanical and structural changes to the vascular including arteriolar stiffening 107. It is likely that lymph node blood vessels would experience age‐related changes which could result in delayed immune responses found with increasing age.
Double‐negative cells are a poorly defined subset of lymph node stromal cells, and they are believed to be contractile FRC‐like pericytes 108. As little is known about these cells, it is not surprising that how ageing changes their function or numbers is unknown.
Fibroblastic reticular cells are a diverse subset of stromal cells in the lymph node. Follicular dendritic cells (FDC) are one type of FRC. The impact of ageing on FDCs is discussed separately below. The most thoroughly studied is lymph node T cell zone FRC biology. T cell zone FRCs maintain the architectural organization of the T cell zone and B cell follicles 109. FRC‐produced chemokines CCL19 and CCL21 interact with their receptor, CCR7, on T cells and dendritic cells controlling the localization of these immune cells to the T cell zone of the lymph node 110. One recent study showed that at steady state, CCL21 concentration was similar in young and aged popliteal lymph nodes, but after infection with West Nile virus aged lymph nodes had lower CCL21 concentrations when compared to young lymph nodes 106. Disruption of B cell follicles in aged lymph nodes 106, 111 may suggest alterations in T cell zone FRCs, but this has not been studied thoroughly. It is unclear whether there are fewer T cell zone FRCs in the aged lymph node or if the aged FRCs are functionally impaired. Future studies are required to understand fully how ageing changes lymph node FRC function. Functional changes in FRCs with age may have a major impact on the initiation and control of adaptive immune responses in aged individuals. Decreased CCL21 concentrations may decrease the recruitment and localization of activated dendritic cells and naive T cells into draining lymph nodes, which could diminish dramatically the immune response magnitude. FRCs are also a major source of IL‐7 and CCL19, both of which are important for survival of naive T cells 112, 113. Ageing reduces the number of naive T cells dramatically, due in part to thymic involution, and results in changes to T cell homeostasis 48. FRCs may contribute to T cell homeostatic difficulties if IL‐7 and CCL19 production is altered.
Age‐related alterations of splenic stromal cells
The spleen is a secondary lymphoid organ located in the upper right quadrant of the abdomen that filters blood, and is a critical component in the defence against blood‐borne pathogens such as encapsulated bacteria 114. The spleen is compartmentalized into red and white pulp, as shown in Fig. 3. The white pulp consists of B cell follicles which surround a T cell periarteriolar sheath. The marginal zone surrounds the B cell follicle. This is where the central arteriole empties and immune cells enter the spleen 114. Bridging channels are FRC‐lined conduits that allow for entry of immune cells into the splenic T cell zone from the marginal sinus 115. A frequently overlooked component of immune system efficacy is locality 116. Immune responses are dependent upon the interaction of rare cells with one another, and the intricate organization of secondary lymphoid organs is designed to increase the probability of these interactions occurring 116. With age, there is considerable attrition of splenic white pulp organization. The splenic marginal zone (B cells 98 and macrophages 96, 117) and follicular dendritic cells 118 show significant disruption with age, and there is also a merging of the B cell follicles and the T cell areas 96, 97, 98. Stromal cells are a non‐haematopoietic component of secondary lymphoid tissues. In the spleen, the markers podoplanin (PDPN) and CD31 can be used to identify three stromal subsets: fibroblastic reticular cells (PDPN+CD31–), blood endothelial cells (CD31+PDPN–) and double‐negative cells (PDPN–CD31–), which are mainly red pulp fibroblasts 119, 120. Unlike the lymph node, spleens do not contain lymphatic endothelial cells.
Figure 3.
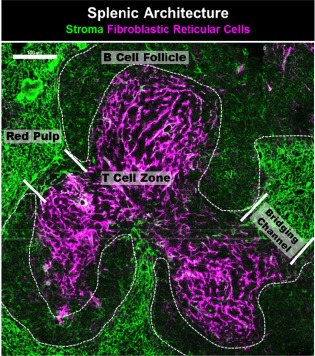
Splenic architecture. The T cell zone of the splenic white pulp is supported by podoplanin (magenta)‐positive fibroblastic reticular cells (FRCs). B cell follicles surround the T cell zone and are devoid of FRCs, but contain follicular dendritic cells. The red pulp consists of ER‐TR7+ red pulp fibroblasts (green). Bridging channels, lined with FRCs, connect the T cell zone of the white pulp to the marginal zone and red pulp of the spleen. Image was acquired using confocal microscopy. Scale bar = 100 μm. Imaged is a C57BL/6 mouse spleen, acquired by A. R. M.
Splenic FRCs (gp38+CD31–ERTR7+) play a variety of roles in the immune response, including providing a conduit for lymphocytes, dendritic cells 115, 121 and antigen 122 trafficking, production of homeostatic chemokines important for T and dendritic cell localization to the T cell area (CCL19, CCL21) 110, production of IL‐7 113, maintenance of the B cell homeostasis and follicular organization 110. A recent report by Aw et al. used microscopy to examine how ageing altered splenic FRC morphology 96. In this study, aged mice had an increased area of splenic FRCs which correlated with the merging of the T cell zones and B cell follicles 96. Splenic FRC production of homeostatic chemokines CCL19 and CCL21 have been shown to decrease in aged mice after antigenic challenge 97, which contributes to improper migration of T cells into the T cell zone 97. One report suggests that aged splenic stroma in vitro have increased production of IL‐6, but this study used a relatively crude stromal cell isolation technique and needs to be repeated 123. Studies of human spleens have noted increased collagen composition in spleens of elderly people 124 and attrition of elastic fibres in splenic capsules 125. Further studies are required to determine how ageing alters red pulp fibroblasts, and splenic arteries. Senescence may also have a profound impact upon age‐related splenic stromal cell dysfunction, but this has yet to be determined. Wang et al. quantified senescent cells in the spleens of aged mice using γH2Ax staining and found that senescence increases with age 126. Further analysis of other senescence markers and careful identification of which splenic cells are senescent needs to be performed. We are just beginning to understand how ageing impacts splenic stromal cells.
Age‐related changes in follicular dendritic cells
Follicular dendritic cells (FDCs) are a subset of FRCs that defines the structure of B cell follicles in secondary lymphoid organs 127. Functionally, FDCs facilitate B cell‐mediated responses by maintaining the germinal centre and facilitating the production of high‐affinity antibodies 128. Ageing is associated with a decline in antibody‐mediated responses which can, in part, be attributed to B cell intrinsic defects 129 and functional attrition of T follicular helper cell responses 97, 106, 130. Age‐related changes in FDC function may also contribute to the decline of humoral response. One way that FDCs maintain the organization of the B cell follicle is through production of the chemokine CXCL13 131. Conflicting reports exist about how ageing changes CXCL13 production. Splenic production of CXCL13 in aged BALB/c mice was shown to be increased compared to young mice at steady state 98, whereas 18 h after antigenic challenge it was determined that CXCL13 localization in the spleen was diffuse and spread into the T cell areas of aged C57BL/6 mice 97. Quantification of CXCL13 in young and aged lymph nodes at steady state showed no significant difference, but after infection with West Nile virus aged mice had lower CXCL13 levels 106. Aged FDCs also have defects in their ability to trap and present immune complexes to B cells 132. Decreased expression of FCγRII, CD21L and FDC‐M2 on FDCs after antigenic challenge may contribute to these defects 118, 132, 133. Defects in aged FDCs may be a major contributor to age‐related defects in the humoral response.
Concluding remarks
Much of our understanding of the underlying mechanisms of immunosenescence has come from examining intrinsic defects of immune cells, which has provided valuable insight. However, the impact of the stromal microenvironment to the process of immunosenescence is often overlooked, despite the importance of the stromal niche in the development, maintenance and proliferation of immune cells. There are now a number of studies identifying the significance of the stromal niche in immunosenescence, and moreover it is tempting to postulate that perhaps some of the intrinsic defects are derived through interaction with the aged microenvironment. The stromal environment‐induced immunosenescence is largely unknown and worth being determined, as it appears to offer a potential target to rejuvenate the ageing immune system.
Disclosures
The authors have no financial or commercial conflicts of interest.
Acknowledgements
The authors would like to acknowledge the following support for our research: L. H. is funded by National Institute of Aging Program grant no. AG021600 and D. B. P. is funded by Research into Ageing (UK) and BBSRC.
References
- 1. Anderson G, Jenkinson EJ. Lymphostromal interactions in thymic development and function. Nat Rev Immunol 2001; 1:31–40. [DOI] [PubMed] [Google Scholar]
- 2. Junt T, Scandella E, Ludewig B. Form follows function: lymphoid tissue microarchitecture in antimicrobial immune defence. Nat Rev Immunol 2008; 8:764–75. [DOI] [PubMed] [Google Scholar]
- 3. Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med 2014; 20:833–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology 2007; 120:435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haynes L, Swain SL. Why aging T cells fail: implications for vaccination. Immunity 2006; 24:663–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pawelec G, Akbar A, Caruso C et al Human immunosenescence: is it infectious? Immunol Rev 2005; 205:257–68. [DOI] [PubMed] [Google Scholar]
- 7. Chen WH, Kozlovsky BF, Effros RB et al Vaccination in the elderly: an immunological perspective. Trends Immunol 2009; 30:351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cancro MP, Hao Y, Scholz JL et al B cells and aging: molecules and mechanisms. Trends Immunol 2009; 30:313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Akbar AN, Henson SM. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat Rev Immunol 2011; 11:289–95. [DOI] [PubMed] [Google Scholar]
- 10. Hazeldine J, Lord JM. Innate immunosenescence: underlying mechanisms and clinical relevance. Biogerontology 2015; 16:187–201. [DOI] [PubMed] [Google Scholar]
- 11. Su DM, Aw D, Palmer DB. Immunosenescence: a product of the environment? Curr Opin Immunol 2013; 25:498–503. [DOI] [PubMed] [Google Scholar]
- 12. Geiger H, Denkinger M, Schirmbeck R. Hematopoietic stem cell aging. Curr Opin Immunol 2014; 29:86–92. [DOI] [PubMed] [Google Scholar]
- 13. Janzen V, Forkert R, Fleming HE et al Stem‐cell ageing modified by the cyclin‐dependent kinase inhibitor p16INK4a. Nature 2006; 443:421–6. [DOI] [PubMed] [Google Scholar]
- 14. Rossi DJ, Bryder D, Seita J et al Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature 2007; 447:725–9. [DOI] [PubMed] [Google Scholar]
- 15. Nakamura‐Ishizu A, Suda T. Aging of the hematopoietic stem cells niche. Int J Hematol 2014; 100:317–25. [DOI] [PubMed] [Google Scholar]
- 16. Liang Y, Van Zant G, Szilvassy SJ. Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood 2005; 106:1479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sun L, Brown R, Chen S et al Aging induced decline in T‐lymphopoiesis is primarily dependent on status of progenitor niches in the bone marrow and thymus. Aging (Albany NY) 2012; 4:606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ergen AV, Boles NC, Goodell MA. Rantes/Ccl5 influences hematopoietic stem cell subtypes and causes myeloid skewing. Blood 2012; 119:2500–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Henry CJ, Casas‐Selves M, Kim J et al Aging‐associated inflammation promotes selection for adaptive oncogenic events in B cell progenitors. J Clin Invest 2015; 125:4666–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cheleuitte D, Mizuno S, Glowacki J. In vitro secretion of cytokines by human bone marrow: effects of age and estrogen status. J Clin Endocrinol Metab 1998; 83:2043–51. [DOI] [PubMed] [Google Scholar]
- 21. Kfoury Y, Scadden DT. Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell 2015; 16:239–53. [DOI] [PubMed] [Google Scholar]
- 22. Bellantuono I, Aldahmash A, Kassem M. Aging of marrow stromal (skeletal) stem cells and their contribution to age‐related bone loss. Biochim Biophys Acta 2009; 1792:364–70. [DOI] [PubMed] [Google Scholar]
- 23. Baker N, Boyette LB, Tuan RS. Characterization of bone marrow‐derived mesenchymal stem cells in aging. Bone 2015; 70:37–47. [DOI] [PubMed] [Google Scholar]
- 24. Guang LG, Boskey AL, Zhu W. Age‐related CXC chemokine receptor‐4‐deficiency impairs osteogenic differentiation potency of mouse bone marrow mesenchymal stromal stem cells. Int J Biochem Cell Biol 2013; 45:1813–20. [DOI] [PubMed] [Google Scholar]
- 25. Zhu W, Liang G, Huang Z et al Conditional inactivation of the CXCR4 receptor in osteoprecursors reduces postnatal bone formation due to impaired osteoblast development. J Biol Chem 2011; 286:26794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tuljapurkar SR, McGuire TR, Brusnahan SK et al Changes in human bone marrow fat content associated with changes in hematopoietic stem cell numbers and cytokine levels with aging. J Anat 2011; 219:574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Naveiras O, Nardi V, Wenzel PL et al Bone‐marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 2009; 460:259–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vas V, Wandhoff C, Dorr K et al Contribution of an aged microenvironment to aging‐associated myeloproliferative disease. PLoS One 2012; 7:e31523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vas V, Senger K, Dorr K et al Aging of the microenvironment influences clonality in hematopoiesis. PLOS ONE 2012; 7:e42080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Andre T, Meuleman N, Stamatopoulos B et al Evidences of early senescence in multiple myeloma bone marrow mesenchymal stromal cells. PLOS ONE 2013; 8:e59756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reagan MR, Ghobrial IM. Multiple myeloma mesenchymal stem cells: characterization, origin, and tumor‐promoting effects. Clin Cancer Res 2012; 18:342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zdzisinska B, Bojarska‐Junak A, Dmoszynska A et al Abnormal cytokine production by bone marrow stromal cells of multiple myeloma patients in response to RPMI8226 myeloma cells. Arch Immunol Ther Exp (Warsz) 2008; 56:207–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Garcia‐Gomez A, Sanchez‐Guijo F, Del Canizo MC et al Multiple myeloma mesenchymal stromal cells: contribution to myeloma bone disease and therapeutics. World J Stem Cells 2014; 6:322–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Melchers F. Checkpoints that control B cell development. J Clin Invest 2015; 125:2203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stephan RP, Reilly CR, Witte PL. Impaired ability of bone marrow stromal cells to support B‐lymphopoiesis with age. Blood 1998; 91:75–88.] [PubMed] [Google Scholar]
- 36. Labrie JE 3rd, Sah AP, Allman DM et al Bone marrow microenvironmental changes underlie reduced RAG‐mediated recombination and B cell generation in aged mice. J Exp Med 2004; 200:411–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bilwani FA, Knight KL. Adipocyte‐derived soluble factor(s) inhibits early stages of B lymphopoiesis. J Immunol 2012; 189:4379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chiu BC, Martin BE, Stolberg VR et al The host environment is responsible for aging‐related functional NK cell deficiency. J Immunol 2013; 191:4688–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nair S, Fang M, Sigal LJ. The natural killer cell dysfunction of aged mice is due to the bone marrow stroma and is not restored by IL‐15/IL‐15Ralpha treatment. Aging Cell 2015; 14:180–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shehata HM, Hoebe K, Chougnet CA. The aged nonhematopoietic environment impairs natural killer cell maturation and function. Aging Cell 2015; 14:191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shanley DP, Aw D, Manley NR et al An evolutionary perspective on the mechanisms of immunosenescence. Trends Immunol 2009; 30:374–81. [DOI] [PubMed] [Google Scholar]
- 42. Swain S, Clise‐Dwyer K, Haynes L. Homeostasis and the age‐associated defect of CD4 T cells. Semin Immunol 2005; 17:370–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hale JS, Boursalian TE, Turk GL et al Thymic output in aged mice. Proc Natl Acad Sci USA 2006; 103:8447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Goronzy JJ, Lee WW, Weyand CM. Aging and T‐cell diversity. Exp Gerontol 2007; 42:400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yager EJ, Ahmed M, Lanzer K et al Age‐associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J Exp Med 2008; 205:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Coder B, Su DM. Thymic involution beyond T‐cell insufficiency. Oncotarget 2015; 6:21777–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chinn IK, Blackburn CC, Manley NR et al Changes in primary lymphoid organs with aging. Semin Immunol 2012; 24:309–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Palmer DB. The effect of age on thymic function. Front Immunol 2013; 4:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ortman CL, Dittmar KA, Witte PL et al Molecular characterization of the mouse involuted thymus: aberrations in expression of transcription regulators in thymocyte and epithelial compartments. Int Immunol 2002; 14:813–22. [DOI] [PubMed] [Google Scholar]
- 50. Griffith AV, Fallahi M, Venables T et al Persistent degenerative changes in thymic organ function revealed by an inducible model of organ regrowth. Aging Cell 2012; 11:169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Andrew D, Aspinall R. Age‐associated thymic atrophy is linked to a decline in IL‐7 production. Exp Gerontol 2002; 37:455–63. [DOI] [PubMed] [Google Scholar]
- 52. Aw D, Taylor‐Brown F, Cooper K et al Phenotypical and morphological changes in the thymic microenvironment from ageing mice. Biogerontology 2009; 10:311–22. [DOI] [PubMed] [Google Scholar]
- 53. Flores KG, Li J, Sempowski GD et al Analysis of the human thymic perivascular space during aging. J Clin Invest 1999; 104:1031–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gui J, Zhu X, Dohkan J et al The aged thymus shows normal recruitment of lymphohematopoietic progenitors but has defects in thymic epithelial cells. Int Immunol 2007; 19:1201–11. [DOI] [PubMed] [Google Scholar]
- 55. Mackall CL, Punt JA, Morgan P et al Thymic function in young/old chimeras: substantial thymic T cell regenerative capacity despite irreversible age‐associated thymic involution. Eur J Immunol 1998; 28:1886–93. [DOI] [PubMed] [Google Scholar]
- 56. Zhu X, Gui J, Dohkan J et al Lymphohematopoietic progenitors do not have a synchronized defect with age‐related thymic involution. Aging Cell 2007; 6:663–72. [DOI] [PubMed] [Google Scholar]
- 57. Min H, Montecino‐Rodriguez E, Dorshkind K. Reduction in the developmental potential of intrathymic T cell progenitors with age. J Immunol 2004; 173:245–50. [DOI] [PubMed] [Google Scholar]
- 58. Abbas AK, Benoist C, Bluestone JA et al Regulatory T cells: recommendations to simplify the nomenclature. Nat Immunol 2013; 14:307–8. [DOI] [PubMed] [Google Scholar]
- 59. Irla M, Hollander G, Reith W. Control of central self‐tolerance induction by autoreactive CD4+ thymocytes. Trends Immunol 2010; 31:71–9. [DOI] [PubMed] [Google Scholar]
- 60. Gascoigne NR, Palmer E. Signaling in thymic selection. Curr Opin Immunol 2011; 23:207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kronenberg M, Rudensky A. Regulation of immunity by self‐reactive T cells. Nature 2005; 435:598–604. [DOI] [PubMed] [Google Scholar]
- 62. Sakaguchi S, Ono M, Setoguchi R et al Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self‐tolerance and autoimmune disease. Immunol Rev 2006; 212:8–27. [DOI] [PubMed] [Google Scholar]
- 63. Wirnsberger G, Hinterberger M, Klein L. Regulatory T‐cell differentiation versus clonal deletion of autoreactive thymocytes. Immunol Cell Biol 2011; 89:45–53. [DOI] [PubMed] [Google Scholar]
- 64. Anderson MS, Venanzi ES, Chen Z et al The cellular mechanism of Aire control of T cell tolerance. Immunity 2005; 23:227–39. [DOI] [PubMed] [Google Scholar]
- 65. Anderson MS, Venanzi ES, Klein L et al Projection of an immunological self shadow within the thymus by the aire protein. Science 2002; 298:1395–401. [DOI] [PubMed] [Google Scholar]
- 66. Xia J, Wang H, Guo J et al Age‐related disruption of steady‐state thymic medulla provokes autoimmune phenotype via perturbing negative selection. Aging Dis 2012; 3:248–59. [PMC free article] [PubMed] [Google Scholar]
- 67. Thiault N, Darrigues J, Adoue V et al Peripheral regulatory T lymphocytes recirculating to the thymus suppress the development of their precursors. Nat Immunol 2015; 16:628–34. [DOI] [PubMed] [Google Scholar]
- 68. Lynch HE, Goldberg GL, Chidgey A et al Thymic involution and immune reconstitution. Trends Immunol 2009; 30:366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Min H, Montecino‐Rodriguez E, Dorshkind K. Reassessing the role of growth hormone and sex steroids in thymic involution. Clin Immunol 2006; 118:117–23.] [DOI] [PubMed] [Google Scholar]
- 70. Steinmann GG, Klaus B, Muller‐Hermelink HK. The involution of the ageing human thymic epithelium is independent of puberty. A morphometric study. Scand J Immunol 1985; 22:563–75. [DOI] [PubMed] [Google Scholar]
- 71. Aw D, Palmer DB. It's not all equal: a multiphasic theory of thymic involution. Biogerontology 2012; 13:77–81. [DOI] [PubMed] [Google Scholar]
- 72. Olsen NJ, Olson G, Viselli SM et al Androgen receptors in thymic epithelium modulate thymus size and thymocyte development. Endocrinology 2001; 142:1278–83. [DOI] [PubMed] [Google Scholar]
- 73. Griffith AV, Venables T, Shi J et al Metabolic damage and premature thymus aging caused by stromal catalase deficiency. Cell Rep 2015; 12:1071–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Richardson RB, Allan DS, Le Y. Greater organ involution in highly proliferative tissues associated with the early onset and acceleration of ageing in humans. Exp Gerontol 2014; 55:80–91. [DOI] [PubMed] [Google Scholar]
- 75. Nehls M, Pfeifer D, Schorpp M et al New member of the winged‐helix protein family disrupted in mouse and rat nude mutations. Nature 1994; 372:103–7. [DOI] [PubMed] [Google Scholar]
- 76. Reis MD, Csomos K, Dias LP et al Decline of FOXN1 gene expression in human thymus correlates with age: possible epigenetic regulation. Immun Ageing 2015; 12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. O'Neill KE, Bredenkamp N, Tischner C et al Foxn1 Is dynamically regulated in thymic epithelial cells during embryogenesis and at the onset of thymic involution. PLOS ONE 2016; 11:e0151666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chen L, Xiao S, Manley NR. Foxn1 is required to maintain the postnatal thymic microenvironment in a dosage‐sensitive manner. Blood 2009; 113:567–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cheng L, Guo J, Sun L et al Postnatal tissue‐specific disruption of transcription factor FoxN1 triggers acute thymic atrophy. J Biol Chem 2010; 285:5836–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sun L, Guo J, Brown R et al Declining expression of a single epithelial cell‐autonomous gene accelerates age‐related thymic involution. Aging Cell 2010; 9:347–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zook EC, Krishack PA, Zhang S et al Overexpression of Foxn1 attenuates age‐associated thymic involution and prevents the expansion of peripheral CD4 memory T cells. Blood 2011; 118:5723–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bredenkamp N, Nowell CS, Blackburn CC. Regeneration of the aged thymus by a single transcription factor. Development 2014; 141:1627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Garfin PM, Min D, Bryson JL et al Inactivation of the RB family prevents thymus involution and promotes thymic function by direct control of Foxn1 expression. J Exp Med 2013; 210:1087–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yang H, Youm YH, Dixit VD. Inhibition of thymic adipogenesis by caloric restriction is coupled with reduction in age‐related thymic involution. J Immunol 2009; 183:3040–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Aw D, Silva AB, Maddick M et al Architectural changes in the thymus of aging mice. Aging Cell 2008; 7:158–67. [DOI] [PubMed] [Google Scholar]
- 86. Youm YH, Yang H, Sun Y et al Deficient ghrelin receptor‐mediated signaling compromises thymic stromal cell microenvironment by accelerating thymic adiposity. J Biol Chem 2009; 284:7068–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yang H, Youm YH, Vandanmagsar B et al Obesity accelerates thymic aging. Blood 2009; 114:3803–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Youm YH, Horvath TL, Mangelsdorf DJ et al Prolongevity hormone FGF21 protects against immune senescence by delaying age‐related thymic involution. Proc Natl Acad Sci USA 2016; 113:1026–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sempowski GD, Hale LP, Sundy JS et al Leukemia inhibitory factor, oncostatin M, IL‐6, and stem cell factor mRNA expression in human thymus increases with age and is associated with thymic atrophy. J Immunol 2000; 164:2180–7. [DOI] [PubMed] [Google Scholar]
- 90. Campisi J, Andersen JK, Kapahi P et al Cellular senescence: a link between cancer and age‐related degenerative disease? Semin Cancer Biol 2011; 21:354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. van Deursen JM. The role of senescent cells in ageing. Nature 2014; 509:439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Aspinall R, Pido‐Lopez J, Imami N et al Old rhesus macaques treated with interleukin‐7 show increased TREC levels and respond well to influenza vaccination. Rejuvenation Res 2007; 10:5–17. [DOI] [PubMed] [Google Scholar]
- 93. Min D, Panoskaltsis‐Mortari A, Kuro OM, et al Sustained thymopoiesis and improvement in functional immunity induced by exogenous KGF administration in murine models of aging. Blood 2007; 109:2529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Dudakov JA, Hanash AM, Jenq RR et al Interleukin‐22 drives endogenous thymic regeneration in mice. Science 2012; 336:91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Dixit VD, Yang H, Sun Y et al Ghrelin promotes thymopoiesis during aging. J Clin Invest 2007; 117:2778–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Aw D, Hilliard L, Nishikawa Y et al Disorganization of the splenic microanatomy in ageing mice. Immunology 2016; 148:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lefebvre JS, Maue AC, Eaton SM et al The aged microenvironment contributes to the age‐related functional defects of CD4 T cells in mice. Aging Cell 2012; 11:732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wols HA, Johnson KM, Ippolito JA et al Migration of immature and mature B cells in the aged microenvironment. Immunology 2010; 129:278–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Fletcher AL, Acton SE, Knoblich K. Lymph node fibroblastic reticular cells in health and disease. Nat Rev Immunol 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Chang JE, Turley SJ. Stromal infrastructure of the lymph node and coordination of immunity. Trends Immunol 2015; 36:30–9. [DOI] [PubMed] [Google Scholar]
- 101. Girard JP, Moussion C, Forster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol 2012; 12:762–73. [DOI] [PubMed] [Google Scholar]
- 102. Betterman KL, Harvey NL. The lymphatic vasculature: development and role in shaping immunity. Immunol Rev 2016; 271:276–92. [DOI] [PubMed] [Google Scholar]
- 103. Akl TJ, Nagai T, Cote GL et al Mesenteric lymph flow in adult and aged rats. Am J Physiol Heart Circ Physiol 2011; 301:H1828–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chevalier S, Ferland G, Tuchweber B. Lymphatic absorption of retinol in young, mature, and old rats: influence of dietary restriction. FASEB J 1996; 10:1085–90. [DOI] [PubMed] [Google Scholar]
- 105. Zolla V, Nizamutdinova IT, Scharf B et al Aging‐related anatomical and biochemical changes in lymphatic collectors impair lymph transport, fluid homeostasis, and pathogen clearance. Aging Cell 2015; 14:582–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Richner JM, Gmyrek GB, Govero J et al Age‐dependent cell trafficking defects in draining lymph nodes impair adaptive immunity and control of West Nile virus infection. PLOS Pathog 2015; 11:e1005027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Mendonca GV, Pezarat‐Correia P, Vaz JR et al Impact of aging on endurance and neuromuscular physical performance: the role of vascular senescence. Sports Med 2016; DOI:10.1007/s40279-016-0596-8. [DOI] [PubMed] [Google Scholar]
- 108. Malhotra D, Fletcher AL, Astarita J et al Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nat Immunol 2012; 13:499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Cremasco V, Woodruff MC, Onder L et al B cell homeostasis and follicle confines are governed by fibroblastic reticular cells. Nat Immunol 2014; 15:973–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Luther SA, Tang HL, Hyman PL et al Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci USA 2000; 97:12694–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hadamitzky C, Spohr H, Debertin AS et al Age‐dependent histoarchitectural changes in human lymph nodes: an underestimated process with clinical relevance? J Anat 2010; 216:556–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Jiang Q, Li WQ, Aiello FB et al Cell biology of IL‐7, a key lymphotrophin. Cytokine Growth Factor Rev 2005; 16:513–33. [DOI] [PubMed] [Google Scholar]
- 113. Link A, Vogt TK, Favre S et al Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol 2007; 8:1255–65. [DOI] [PubMed] [Google Scholar]
- 114. Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol 2005; 5:606–16. [DOI] [PubMed] [Google Scholar]
- 115. Bajenoff M, Glaichenhaus N, Germain RN. Fibroblastic reticular cells guide T lymphocyte entry into and migration within the splenic T cell zone. J Immunol 2008; 181:3947–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Qi H, Kastenmuller W, Germain RN. Spatiotemporal basis of innate and adaptive immunity in secondary lymphoid tissue. Annu Rev Cell Dev Biol 2014; 30:141–67. [DOI] [PubMed] [Google Scholar]
- 117. Birjandi SZ, Ippolito JA, Ramadorai AK et al Alterations in marginal zone macrophages and marginal zone B cells in old mice. J Immunol 2011; 186:3441–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Aydar Y, Balogh P, Tew JG et al Follicular dendritic cells in aging, a ‘bottle‐neck’ in the humoral immune response. Ageing Res Rev 2004; 3:15–29. [DOI] [PubMed] [Google Scholar]
- 119. den Haan JM, Mebius RE, Kraal G. Stromal cells of the mouse spleen. Front Immunol 2012; 3:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Mueller SN, Germain RN. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat Rev Immunol 2009; 9:618–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bajenoff M, Egen JG, Koo LY et al Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity 2006; 25:989–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Nolte MA, Belien JA, Schadee‐Eestermans I et al A conduit system distributes chemokines and small blood‐borne molecules through the splenic white pulp. J Exp Med 2003; 198:505–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Park J, Miyakawa T, Shiokawa A et al Splenic stromal cells from aged mice produce higher levels of IL‐6 compared to young mice. Mediat Inflamm 2014; 2014:826987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Al'fonsova EV. Functional morphology of conjunctive tissue stroma of spleen in the age aspect. Adv Gerontol 2012; 25:415–21. [PubMed] [Google Scholar]
- 125. Rodrigues CJ, Sacchetti JC, Rodrigues AJ Jr. Age‐related changes in the elastic fiber network of the human splenic capsule. Lymphology 1999; 32:64–9. [PubMed] [Google Scholar]
- 126. Wang C, Jurk D, Maddick M et al DNA damage response and cellular senescence in tissues of aging mice. Aging Cell 2009; 8:311–23. [DOI] [PubMed] [Google Scholar]
- 127. Wang X, Cho B, Suzuki K et al Follicular dendritic cells help establish follicle identity and promote B cell retention in germinal centers. J Exp Med 2011; 208:2497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Heesters BA, Myers RC, Carroll MC. Follicular dendritic cells: dynamic antigen libraries. Nat Rev Immunol 2014; 14:495–504. [DOI] [PubMed] [Google Scholar]
- 129. Blomberg BB, Frasca D. Quantity, not quality, of antibody response decreased in the elderly. J Clin Invest 2011; 121:2981–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Sage PT, Tan CL, Freeman GJ et al Defective TFH cell function and increased TFR cells contribute to defective antibody production in aging. Cell Rep 2015; 12:163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Ansel KM, Ngo VN, Hyman PL et al A chemokine‐driven positive feedback loop organizes lymphoid follicles. Nature 2000; 406:309–14. [DOI] [PubMed] [Google Scholar]
- 132. Aydar Y, Balogh P, Tew JG et al Age‐related depression of FDC accessory functions and CD21 ligand‐mediated repair of co‐stimulation. Eur J Immunol 2002; 32:2817–26. [DOI] [PubMed] [Google Scholar]
- 133. Aydar Y, Balogh P, Tew JG et al Altered regulation of Fc gamma RII on aged follicular dendritic cells correlates with immunoreceptor tyrosine‐based inhibition motif signaling in B cells and reduced germinal center formation. J Immunol 2003; 171:5975–87. [DOI] [PubMed] [Google Scholar]


