Abstract
The attraction of natural enemies towards herbivore‐induced plant volatiles is a well‐documented phenomenon. However, the majority of published studies are carried under optimal water and nutrient regimes and with just one herbivore. But what happens when additional levels of ecological complexity are added? Does the presence of a second herbivore, microorganisms, and abiotic stress interfere with plant–natural enemy communication? or is communication stable enough to withstand disruption by additional biotic and abiotic factors?
Investigating the effects of these additional levels of ecological complexity is key to understanding the stability of tritrophic interactions in natural ecosystems and may aid to forecast the impact of environmental disturbances on these, especially in climate change scenarios, which are often associated with modifications in plant and arthropod species distribution and increased levels of abiotic stress.
This review explores the literature on natural enemy attraction to herbivore‐induced volatiles when, besides herbivory, plants are challenged by additional biotic and abiotic factors.
The aim of this review was to establish the impact of different biotic and abiotic factors on plant–natural enemy communication and to highlight critical aspects to guide future research efforts.
Keywords: climate change, herbivore‐induced plant volatiles, multitrophic interactions, natural enemies, parasitoids, predators
1. Introduction
Volatile compounds serve multiple protective functions for the plants emitting them and are one of the principal currencies mediating plant communication with conspecifics and other trophic levels (Holopainen, 2004). The emission of herbivore‐induced volatiles (HIPVs) has been linked to the attraction of natural enemies of the herbivores in over a hundred tritrophic systems (Hilker & Meiners, 2006; Clavijo McCormick, Unsicker, & Gershenzon, 2012; Mumm & Dicke, 2010). Over the years, considerable progress has been made in elucidating the biosynthetic routes, leading to the formation of volatile compounds and the molecular mechanisms underlying this process, for example, signaling transduction pathways and transcriptome changes in response to herbivory (Arimura, Matsui, & Takabayashi, 2009; Dudareva, Picherski, & Gershenzon, 2004; Stam et al., 2014). We have also advanced in understanding how natural enemies make use of these volatile cues, and the role of learning in their responses to plant volatiles (Allison & Hare, 2009; de Boer & Dicke, 2006; Dicke, 1999; Hoedjes et al., 2011; Clavijo McCormick et al., 2012; Takabayashi, Sabelis, Janssen, Shiojiri, & van Wijk, 2006).
The majority of studies on tritrophic interactions have been performed using monoclonal, herbaceous cultivated species under controlled conditions, which, while useful from a logistical standpoint, poorly reflect natural ecosystems where plants exist as mixed‐genotype populations in heterogeneous landscapes, and usually interact with multiple biotic players under variable abiotic conditions (Bezemer & van Dam, 2005; Dicke & van Loon, 2000; Hunter, 2002; Takabayashi, Dicke, & Posthumus, 1994). An increasing number of field studies demonstrate that attraction of natural enemies to HIPVs is widespread under natural conditions, suggesting that volatile cues are sufficiently robust to withstand certain levels of environmental variation (Birkett et al., 2000; De Moraes, Lewis, Pare, Alborn, & Tumlinson, 1998; Kessler & Baldwin, 2001; Clavijo McCormick, Irmisch, et al. 2014; Thaler, 1999). However, the extent of the impact of interacting biotic and abiotic factors remains poorly documented.
During the last decade, attention has been paid on the potential effects of climate change on multitrophic interactions. However, as a recent meta‐analysis reveals, of over 2000 selected publications on climate change and trophic interactions, the majority dealt with only two trophic levels, and only 15% evaluated the effects of one or more abiotic factors on the outcome of multitrophic interactions (Rosenblatt & Schmitz, 2014). This meta‐analysis suggests that many climate change studies are overlooking ecological complexity, and a question emerges about how can we truly understand the consequences of climate change on these interactions if we do not yet grasp the range of variation occurring under “normal” natural conditions. Hence, one of the main challenges in the study of multitrophic interactions is progressing from evaluating linear systems under controlled settings, into more complex scenarios incorporating additional biotic and abiotic conditions (Dicke, van Loon, & Soler, 2009; Mumm & Dicke, 2010). As volatile compounds are a primary currency mediating plant communication, their study under complex scenarios is vital to understand the community dynamics and how biotic and abiotic factors shape these.
This review explores the available literature on natural enemy attraction to HIPVs in scenarios of multiple herbivores attacking, herbivory in the presence of microorganisms, and herbivory under abiotic stress factors. The aim is to address some relevant questions such as (1) Is plant–natural enemy communication stable enough to withstand disruption by biotic and abiotic factors? (2) Which biotic and abiotic factors disrupt communication between plants and natural enemies? and (3) Are there common patterns allowing us to make predictions about the outcome of these tritrophic interactions under biotic and abiotic stress scenarios?
2. Multiple Variables Affect Plant Volatile Emissions and Natural Enemy Responses
The first attempts to understand and predict the outcome of tritrophic interactions under complex ecological settings come from the knowledge that different types of herbivore damage can elicit different defense signaling pathways. In general, phloem feeders (whiteflies and aphids) activate the salicylic acid (SA)‐dependent shikimic acid pathway, while chewing insects (beetles and caterpillars) and cell‐content feeders (mites and thrips) induce the jasmonic acid (JA)‐dependent octadecanoic pathway. Each of these pathways regulates the expression of different sets of downstream genes associated with indirect plant defenses (i.e., those defenses promoting the efficiency of natural enemies to control herbivores (Gols, 2014), leading to the emission of distinct volatile blends (Erb, Meldau, & Howe, 2012; Heil & Ton, 2008; Walling, 2000).
Initial evidence that the JA and SA pathways act antagonistically led to the hypothesis that induced plant volatile phenotypes and the outcomes of volatile‐mediated interactions may be predictable based on the knowledge of the attacker (Erb et al., 2012; Heil & Ton, 2008; Walling, 2000). For instance, a JA‐inducing herbivore would be expected to disrupt the attraction of natural enemies of a SA‐inducing herbivore under simultaneous attack and vice versa. Although this outcome is possible (Zarate, Kempema, & Walling, 2007), it is now apparent that knowledge of herbivore damage type is insufficient to predict plant volatile phenotypes. For example, recent studies suggest that interactions between the JA and SA pathways do not always result in one pathway disrupting the other, but may involve more back‐and‐forth communication or “cross talk.” Besides, other phytohormones, such as ethylene and abscisic acid, play a significant role in defense signaling cascades acting synergistically or antagonistically with both JA and SA (Bostock, 2005; Dicke et al., 2009; Koornneef & Pieterse, 2008; Pieterse, Leon‐Reyes, Van der Ent, & Van Wees, 2009; Stam et al., 2014).
Changes in volatile phenotypes can also occur as a result of within‐species variation as is the case when different life stages of a given herbivore inflict different patterns (Clavijo McCormick, Boeckler, Köllner, Gershenzon, & Unsicker, 2014; Takabayashi, Takahashi, Dicke, & Posthumus, 1995; Yoneya, Kugimiya, & Takabayashi, 2009) and amounts (Geervliet, Posthumus, Vet, & Dicke, 1997; Maeda & Takabayashi, 2001) of feeding damage (Figure 1). For example, early instar Lymantria dispar caterpillars produce relatively small lesions and attack a larger number of leaves compared to late instars. These differences result in strikingly different patterns of HIPV emission from poplar trees, which may be exploited by parasitoids to obtain information about the suitable developmental stage of their prey (Clavijo McCormick, Boeckler, et al., 2014). Furthermore, different insect‐derived elicitors, for example, those emitted by oviposition vs. salivary compounds, can induce distinct volatile profiles (Alborn et al., 2007; Hilker, Stein, Schroder, Varama, & Mumm, 2005; Louis, Peiffer, Ray, Luthe, & Felton, 2013; Schmelz, Engelberth, Alborn, Tumlinson, & Teal, 2009). Some herbivore species are even able to manipulate the plant defense signaling network to their advantage (Kahl et al., 2000; Musser et al., 2002; Sarmento et al., 2011) (Figure 1). For example, the spider mite Tetranychus evansi blocks the induction of the SA and JA signaling routes, leading to a suppression of direct defenses (i.e., those traits that act upon the herbivore directly (Gols, 2014) and volatile emissions (Sarmento et al., 2011).
Figure 1.
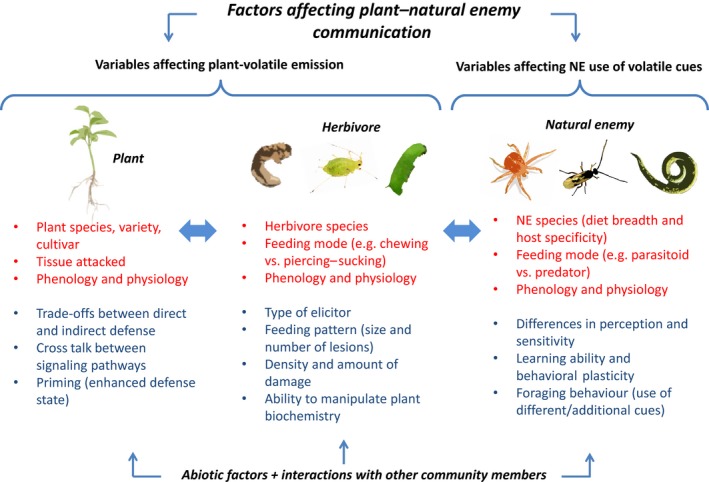
Multiple variables affect plant volatile emissions and natural enemy (NE) responses. The bullet points highlighted in red are critical for the occurrence of a particular plant–herbivore–natural enemy interaction under natural conditions. The points in blue correspond to additional factors having an impact on volatile emission and the use of volatile cues by natural enemies
Volatile profiles also differ in systematic ways among plant species, cultivars, varieties, and genotypes, and even between tissues within the same plant (Jonsson, Lindkvist, & Anderson, 2005; Kappers, Hoogerbrugge, Bouwmeester, & Dicke, 2011; Krips et al., 2001). These responses may be further modified by exposure to the HIPVs of damaged plant parts or nearby attacked neighbors, which “prime” undamaged plants or plant parts to respond more efficiently, and to a higher degree, to subsequent herbivore damage (Engelberth, Alborn, Schmelz, & Tumlinson, 2004; Heil & Kost, 2006; Heil & Silva Bueno, 2007; Ruther & Furstenau, 2005) (Figure 1). As an example of this phenomenon, corn seedlings exposed to green leaf volatiles (GLVs) from neighboring plants produced significantly more JA and volatile sesquiterpenes after mechanical damage in combination with caterpillar regurgitant than seedlings not exposed to GLVs, leading authors to hypothesize that priming may affect plant–plant and plant–insect interactions (Engelberth et al., 2004). Last but not least, trade‐offs between direct and indirect defenses in combination with specific ecological settings can also result in unique “plant defense syndromes” involving differences in HIPV emission (Agrawal & Fishbein, 2006).
From the perspective of natural enemies, there are also several biological and ecological factors playing a role in determining their ability to exploit HIPVs, for instance, their diet breadth and degree of host specificity (Cortesero, De Moraes, Stapel, Tumlinson, & Lewis, 1997; Holt & Lawton, 1994; Shiojiri, Takabayashi, Yano, & Takafuji, 2000a; Steidle & van Loon, 2003; Tamo, Ricard, Held, Davison, & Turlings, 2006), learning capacity and behavioral plasticity (de Boer & Dicke, 2006; Glinwood, Ahmed, Qvarfordt, & Ninkovic, 2011; Hoedjes et al., 2011), and possibly differences in the sensitivity and mechanisms of perception of plant volatiles (Clavijo McCormick et al., 2012) among others.
Nevertheless, a critical factor determining the relative importance of HIPVs, and hence the tolerance to cue disruption, is the foraging behavior of the natural enemy. The foraging behavior is a complex process product of the co‐evolution of prey and predator and is largely determined by the prey's behavior and defense mechanisms, as well as by the community characteristics such as diversity and complexity (Malcom, 2009; de Rijk, Dicke, & Poelman, 2013). In the case of herbivore's natural enemies, the foraging behavior will determine to which extent parasitoids and predators rely on other nonchemical cues (e.g., visual, acoustic, and vibrational signals) and on other sorts of chemical cues rather than HIPVs (e.g., habitat related cues, host‐derived odors, and odors of conspecifics) to find their prey (Steidle & van Loon, 2003; Wäschke, Meiners & Rostas, 2013).
A recent theoretical study (Yoneya & Miki, 2015) suggests that co‐evolution of foraging behavior in herbivores and natural enemies allows both groups of organisms to use HIPVs as multifunctional signals depending on the intensity of the attack. For example, a recent study shows that HIPVs emitted after short‐term (up to 6 hr) damage are attractive to experienced (fed on poplar) L. dispar larvae, whereas volatiles from long‐term damage (24–30 hr) were avoided (Clavijo McCormick, Reinecke, Gershenzon, & Unsicker, 2016). In this case, the first set of volatiles (up to 6 hr) indicated food availability and low competition, whereas the second (24–30 hr) probably signaled high competition and enhanced plant defense. In a similar manner, natural enemies are expected to use different patterns of volatile emission to make foraging decisions.
For all actors involved (plant, herbivore, and natural enemy), physiological and phenological aspects such as the age, previous experience, nutritional state, and “health” conditions are likely to have further effects on the outcome of the interaction (e.g., Anderson & Anton, 2014; Fatouros, van Loon, Hordijk, Smid, & Dicke, 2005; Jonsson et al., 2005; Steinberg, Dicke, Vet, & Wanningen, 1992). All of these factors are influenced by abiotic factors and the interactions with other community members (Figure 1). Due to the complex networks that may arise from the combination of these variables, it seems quite difficult, if not impossible, to generalize or predict the outcome of a tritrophic interaction based only on the study of one individual element (herbivore, plant, or natural enemy).
3. Effects of Biotic and Abiotic Factors on Plant–Natural Enemy Communication
3.1. Multiple herbivory
In nature, most plants are exposed to numerous attackers, acting simultaneously or sequentially (Dicke et al., 2009). Early studies on the effect of multiple herbivory on indirect defense focused on aboveground interactions, but recent work has brought to our attention that simultaneous above‐ and belowground attack can also have profound impacts on natural enemy recruitment (Bezemer & van Dam, 2005; Erb, Ton, Degenhardt, & Turlings, 2008; Van der Putten, Vet, Harvey, & Wäckers, 2001), establishing the role of microbes in this equation is a challenging aspect for further research (Soler, Pozo, Rasmann, & Turlings, 2015).
Available data (Table 1) show that multiple aboveground herbivory can lead to diverse outcomes, including either increased natural enemy attraction, reduced attraction, or no effect, independently of the type of damage and defense pathway elicited by the attackers. For generalist natural enemies, increased attraction often occurs in combination with significant increases in total volatile emission (de Boer, Hordijk, Posthumus, & Dicke, 2008; Moayeri, Ashouri, Poll, & Enkegaard, 2007; Rodriguez‐Saona, Chalmers, Raj, & Thaler, 2005; Shiojiri et al. 2000a; Shiojiri, Takabayashi, Yano, & Takafuji, 2000b; Shiojiri, Takabayashi, Yano, & Takafuji, 2001, 2002), whereas disruption is linked to significant reductions in volatile emission (Shiojiri et al. 2000a,b; Shiojiri et al., 2001, 2002; Zhang et al., 2009). Meanwhile, no effects were observed in situations where there were no measurable differences in volatile emission between single and multiple attackers (Erb, Foresti, & Turlings, 2010; Vos, Berrocal, Karamaouna, Hemerik, & Vet, 2001).
Table 1.
Examples of the effects of multiple herbivory on plant‐volatile emission and plant–natural enemy communication
| Plant species | Outcome | Natural enemy and host specificity | Species and feeding guild of the herbivores | Impact of multiple herbivory on HIPV emission | References |
|---|---|---|---|---|---|
| Aboveground interactions | |||||
| Phaseolus lunatus and Cucumis sativus | Increased attraction | Phytoseiulus persimilis (Generalist predatory mite) | Host: Tetranychus urticae (CH) Nonhost: Spodoptera exigua (CH) |
Significant increase in total HIPV emission in P. lunatus
No significant difference in total HIPV emission in C. sativus |
de Boer et al. (2008) |
| Lycopersicon esculentum | Increased attraction | Cotesia marginiventris (Generalist parasitoid) | Preferred prey: S. exigua (CH) Least preferred prey: Myzus euphorbiae (PF) |
HIPV not quantified | Rodriguez‐Saona et al. (2005) |
| Capsicum annuum | Increased attraction | Macrolophus caliginosus (Generalist predatory bug) | Host: T. urticae (CH) Nonhost: Myzus persicae (PF) |
Significant increase in total HIPV emission | Moayeri et al. (2007) |
| Brassica oleracea | No effect | Cotesia glomerata (Generalist parasitoid) | Host: Pieris rapae (CH) Nonhost: Plutella xylostella (CH) |
HIPV not quantified | Vos et al. (2001) |
| Zea mays | No effect | C. marginiventris (Generalist parasitoid) | Host: S. littoralis (CH) Nonhost: Euscelidius variegatus (PF) |
No significant differences in HIPV emission | Erb et al. (2010) |
| P. lunatus | Disruption | P. persimilis (Generalist predatory mite) | Host: T. urticae (CH) Nonhost: Bemisia tabaci (PF) |
Significant reduction in (E)‐ β‐ocimene emission | Zhang et al. (2009) |
| P. lunatus | Disruption for specialist NE/Increased attraction for generalist NE |
Cotesia plutellae (Specialist parasitoid) C. glomerata (Generalist parasitoid) |
Host to generalist: P. rapae (Lepidoptera) – CH Host to specialist and generalist: P. xylostella (Lepidoptera) – CH |
Variable effects on HIPV emission with some compounds decreasing, for example, DMNT and some increasing, for example, (Z)‐3‐hexenyl acetate and dimethyl sulfide | Shiojiri et al. (2000a,b), Shiojiri et al. (2001, 2002) |
| Aboveground–belowground interactions | |||||
| Brassica nigra | Disruption | C. glomerata (Generalist parasitoid) | Aboveground herbivore: Pieris brassicae (CH) Belowground herbivore: Delia radicum (CH) |
Increased emission o toxic sulfur‐containing compounds and reduced emission of terpenoids | Soler et al. (2005), Soler et al. (2007) |
| Brassica rapa | Disruption | Trybliographa rapae (Specialist parasitoid) | Aboveground herbivore: P. brassicae (CH) Belowground herbivore: D. radicum (CH) |
HIPV not measured | Pierre et al. (2011) |
| Z. mays | Disruption above and belowground |
C. marginiventris (Generalist parasitoid) Heterorhabditis megidis (Generalist entomopathogenic nematode) |
Aboveground herbivore: Spodoptera frugiperda (CH) Belowground herbivore: Diabrotica virgifera virgifera (CH) |
Reduced emission of root HIPV, but no significant differences in the emission of foliar HIPV | Rasmann & Turlings (2007) |
| Vicia faba | Disruption | Trissolcus basalis (Generalist egg parasitoid) | Aboveground herbivore: Nezara viridula (CH) Belowground herbivore: Sitona lineatus (CH) |
Significant changes in total HIPV emission (not specified whether increase or decrease) | Moujahed et al. (2014) |
CH, chewing herbivore; PF, phloem feeder; HIPV, herbivore‐induced plant volatile; GLV, green leaf volatile; DMNT, (E)‐4,8‐dimethyl‐1,3,7‐nonatriene; NE, natural enemy.
In the case of specialists, the only available study reports disruption due to multiple attackers, yet how this relates to changes in HIPV emissions and whether disruption is common for other specialists remain unclear. An exhaustive study of 140 research papers on natural enemy attraction to infochemicals showed that there is no significant difference between specialist and generalist natural enemies in the proportion species that use volatiles during foraging; however, the ability to learn and display plastic responses to these compounds seems to be more common in generalist species (Steidle & van Loon, 2003). Additional studies suggest that generalists and specialists may differ in their use of volatile cues, with generalists relying on widespread damage‐related compounds such as GLVs, while specialists utilize more precise volatile signatures associated with their preferred prey (Cortesero et al., 1997; Ngumbi, Chen, & Fadamiro, 2009, 2010). However, whether differences in feeding specialization render one of these two groups more susceptible to signal disruption than the other remains to be investigated.
In simultaneous above‐ and belowground herbivory scenarios, the most common outcome is decreased natural enemy attraction (both above‐ and belowground), independently of the feeding guild of the natural enemy or the changes in total volatile emissions (Table 1). There are two nonexclusive hypotheses that may explain why disruption occurs: simultaneous above‐ and belowground herbivory may cause a systemic response, leading to an increased production of defense‐related compounds (including volatiles), which may deter natural enemies (van Dam et al., 2003). Alternatively, due to the importance of roots as nutrient providers for the plant, belowground damage could cause severe constraints on resource allocation. Lack of nutrients and water would affect both primary and secondary metabolism, and the signaling pathways leading to volatile emission, causing a decrease in the overall volatile emission or a significant reduction (or no emission) of particular compounds used as cues by natural enemies (Bezemer & van Dam, 2005; Soler et al., 2007).
Root herbivory is likely to be a major factor disrupting plant–natural enemy communication in nature, due to its significant negative impact on plant and herbivore communities (Blossey & Hunt‐Joshi, 2003). The available studies evidence that disruption of natural enemy attraction due to the presence of belowground herbivores is a common outcome. However, it remains unclear whether the disruption is due to a complete inhibition or reduced emission of volatile cues, or because natural enemies (both specialists and generalists) can obtain information about the quality of the herbivores as hosts based on differing plant volatile profiles, and avoid those feeding on highly defended or low‐quality plants.
There is abundant evidence of specificity in the use of volatile cues by predators and parasitoids to support the second argument (Clavijo McCormick et al., 2012; and references therein). Nevertheless, a major challenge in the resolution of this issue is that we still ignore what part of the complex volatile blend emitted by the plant comprises the actual cue (i.e., individual compounds, a subset of compounds in specific ratios, or whole blends). Most research investigating the role of volatiles on tritrophic interactions has focused on changes in the emission of abundant compounds (terpenoids and GLVs). Yet minor compounds, and those belonging to other chemical classes, for example, sulfur‐ and nitrogen‐containing compounds, are known to play important roles in plant–natural enemy interactions and be more resistant to environmental degradation than terpenoids and GLVs and therefore should not be overlooked (D'Alessandro, Brunner, von Merey, & Turlings, 2009; Clavijo McCormick, Gershenzon & Unsicker, 2014; Pinto, Nerg, & Holopainen, 2007; Pinto, Blande, et al. 2007).
3.2. Presence of microorganisms
Plants are not only challenged by multiple herbivores but by beneficial microorganisms and pathogens, which can also elicit distinct signaling pathways. For example, biotrophic pathogens (those growing and feeding within the living cells of their hosts) typically elicit SA‐mediated induced defenses. Necrotrophic pathogens (those killing its host cells and then feeding on the dead matter) often induce JA/ethylene‐mediated defenses (Glazebrook, 2005; Thomma, Penninckx, Broekaert, & Cammue, 2001), and interactions with beneficial microorganisms are generally mediated by the JA signaling pathway (Glazebrook, 2005).
In addition to the attacker‐specific responses, microorganisms can elicit other lines of defense. Pathogens that establish as local infections can elicit systemic acquired resistance (SAR) via a SA‐dependent signaling cascade. As a result, the entire plant is primed to resist or tolerate subsequent attack (Conrath, 2006; Durrant & Dong, 2004). A similar priming of defense occurs when plants associate with beneficial bacteria, eliciting induced systemic resistance (ISR), which is commonly JA‐mediated, and leads to a broad spectrum of long‐lasting resistance traits, such as cell wall changes, production of pathogenesis‐related proteins and phytoalexins (Heil & Bostock, 2002; Pieterse et al., 2014; Van der Ent, Van Wees, & Pieterse, 2009).
Although much is known about the molecular basis of plant–pathogen interactions, few studies have explored the effect of herbivore attack in combination with microorganisms on plant volatile emission and its effects on natural enemy recruitment (Ponzio, Gols, Pieterse, & Dicke, 2013). Available studies involving beneficial and nonpathogenic microorganisms report multiple outcomes (Table 2). As one study involving three different species of arbuscular mycorrhizae points out, the outcome of the interaction may be strongly driven by the species of microorganism and the phenotypic changes (morphological or chemical) it induces on the plant. These changes may have either a negative or positive impact on herbivore quality as prey, or on the access of natural enemies to the herbivores (Gange, Brown, & Aplin, 2003).
Table 2.
Examples of effects of microorganisms on plant‐volatile emission and plant–natural enemy communication
| Plant species | Microorganism | Natural enemy and host specificity | Species and feeding guild of the herbivore | Outcome and effect on HIPV emission and suggested explanation | References |
|---|---|---|---|---|---|
| Beneficial and nonpathogenic microorganisms | |||||
| Lycopersicon esculentum | Arbuscular mycorrhizae (AM) | Aphidius ervi (Generalist parasitoid) | Host: Macrosiphum euphorbiae (PF) | The NE is unable to distinguish plants infested by its host from those only colonized by AM HIPV not measured Symbiosis induces deceptive volatile signals attracting NE to plants bearing no herbivores |
Guerrieri et al. (2004) |
| Leucanthemum vulgare | Three different species of AM | Diglyphus isaea (Generalist parasitoid) | Host: Chromatomyia syngenesiae (Leaf miner) | Some fungal combinations increased parasitism, some decreased it, while others had no effect HIPV not measured The outcome of the interaction depends on the species of AM and the phenotypic changes they induce on the plants and herbivores |
Gange et al. (2003) |
| Arabidopsis | Nonpathogenic Pseudomonas fluorescens associated with ISR |
Cotesia rubecula (Specialist parasitoid) | Host: Pieris rapae (CH) | No effect on the attraction of NE when control and P. fluorescence challenged plants were offered in combination with herbivore damage HIPV not measured ISR‐associated pseudomonas do not have a negative impact on herbivore growth and development |
Van Oosten et al. (2008) |
| Pathogenic microorganisms | |||||
| Arachis hypogaea | Sclerotium rolfsii (White mold fungus) | Cotesia marginiventris (Generalist parasitoid) | Host: Spodoptera exigua (CH) | Increased attraction of the NE toward mold‐infested plants plus herbivores Increased HIPV emission, the blend has unique compounds associated with fungal attack, for example, methyl salicylate and 3‐octanone. Changes in plant quality lead to increased preference and performance of the herbivore, which correlates with NE choice |
Cardoza et al. (2002), Cardoza et al. (2003) |
| Quercus robur | Erysiphe alphitoides (oak powdery mildew) | An assembly of naturally occurring parasitoids | Host: Tischeria ekebladella (Leaf miner) | Increased parasitism rates on mildew infested leaves HIPV not measured Negative effects on larval phenology facilitate parasitoid attack (e.g., slower developmental rates) |
Tack et al. (2012) |
| Citrus trees Citrus spp. |
Candidatus Liberibacter asiaticus | Tamarixia radiate (Specialist parasitoid) | Host: Diaphorina citri (PF and vector of the pathogen) | Increased NE attraction toward pathogen‐infested plants Increased methyl salicylate emission The pathogen manipulates VOC emission of the plants to attract its vector, and this in turn attracts more NE |
Martini et al. (2014) |
| Zea mays | Setosphaeria turcica (Northern corn leaf blight) |
C. marginiventris (Generalist parasitoid) Microplitis rufiventris (Specialist parasitoid) |
Host: Spodoptera littoralis (CH) | No effect on NE attraction Reduced HIPV emissions but similar blend composition Herbivore performance is not affected by the presence of the pathogen |
Rostás et al. (2006) |
CH, chewing herbivore; PF, phloem feeder; HIPV, herbivore‐induced plant volatile; NE, natural enemy; AM, arbuscular mycorrhizae.
Contrastingly, the few studies on pathogenic microorganisms show an increased attraction of natural enemies toward pathogen‐infested plants (Table 2), indicating that tritrophic interactions can withstand pathogen disruption. The authors of these studies hypothesize that pathogens have a strong effect on plant nutrients and defense compounds affecting plant quality for herbivores, making them either better quality hosts or lower quality, but more apt preys (Cardoza, Teal, & Tumlinson, 2003; Tack, Gripenberg, & Roslin, 2012). For example, infestation by white mold fungus (Sclerotium rolfsii) on peanut plants causes an increase in levels of soluble sugars and decreases soluble phenolics (defense compounds). These changes in nutrient and defense compounds had a significant positive effect on preference and performance of the herbivore, which correlated with natural enemy preference, suggesting once more that predators and parasitoids can infer host quality based on volatile cues (Cardoza et al., 2003).
An exception is the case of cultivated corn Zea mays, where Spodoptera littoralis preference and performance were not affected by northern corn leaf blight infection. The composition of the volatile blend remained quite stable (albeit reduced), and there were no significant effects on the attraction of a generalist or a specialist parasitoid (Rostás, Ton, Mauch‐Mani, & Turlings, 2006). This plant species emits a fairly constant volatile blend, not only in the presence of pathogens but also in the presence of multiple aboveground (Erb et al., 2010) and aboveground–belowground herbivores (Rasmann & Turlings, 2007), suggesting high stress tolerance regarding HIPV emissions. However, it is likely that cultivated plant species have reduced responses to biotic and abiotic stress. In these plants, selection pressures leading to maintaining defense traits have been alleviated by moving them to geographical ranges where they escape their native herbivores, are artificially protected them from herbivores, or selectively bred giving priority to other traits (Kempel, Schädler, Chrobock, Fischer, & van Kleunen, 2011). Further studies involving wild and cultivated plant varieties are required to investigate the impact of cultivation and breeding practices on plant responses to herbivory and their repercussion at the community level.
Another interesting study case shows that infection by a vector‐borne pathogen increases natural enemy attraction (Martini, Pelz‐Stelinski, & Stelinski, 2014). There is evidence that vector‐borne plant pathogens (e.g., viruses and phytoplasmas) can manipulate HIPV emission of plants to attract arthropod vectors (Martini et al., 2014; Mauck, De Moraes, & Mescher, 2010), so further studies are required to explore the consequences of this manipulation on natural enemy recruitment.
3.3. Abiotic factors
Abiotic stress is expected to have a large impact on tritrophic interactions as it affects plant nutritional quality, phenology, and architecture, as well as the production of secondary metabolites (both volatile and nonvolatile) (Boullis, Francis, & Verheggen, 2015; Chen, Olson, & Ruberson, 2010; Gershenzon, 1984; Ramakrishna & Ravishankar, 2011). However, several volatile compounds such as isoprene and monoterpenes are known to protect the plants from drought, radiation, thermal and oxidative stress and could play an important role in stabilizing volatile‐mediated tritrophic interactions in scenarios of abiotic stress (Holopainen, 2004; Lavoir et al., 2011; Peñuelas J., & Llusià J. 2003; Sharkey, Wiberley, & Donohue, 2008; Way, Schnitzler, Monson, & Jackson, 2011).
Despite the expected negative effects, the available reports (Table 3) indicate that plant–natural enemy communication can withstand several abiotic stresses, with a couple of exceptions in the case of drought and changes in CO2 concentration. Disruption due to alterations in CO2 levels and drought is comprehensible as carbon dioxide and water are crucial for primary metabolism, which in turn is the main energy provider for plant growth and development, as well as for the production of secondary metabolites involved in plant defense (Bolton, 2009; Lawlor & Cornic, 2002). However, as shown in the case of CO2, different plant genotypes (Sun, Feng, Gao, & Ge, 2011) and natural enemy species react differently when tested under similar conditions (Fonseca, Santos, & Auad, 2014; Vuorinen, Nerg, Ibrahim, Reddy, & Holopainen, 2004), indicating there may be variability in the tolerance to abiotic stress factors at both ends of the scale (plant and natural enemy).
Table 3.
Effects of abiotic factors on plant‐volatile emission and plant–natural enemy communication
| Plant species | Abiotic factor | Natural enemy and host specificity | Species and feeding guild of the herbivore | Outcome and effect on HIPV emission | References |
|---|---|---|---|---|---|
| Gossypium hirsutum | Drought | Microplitis croceipes (Specialist parasitoid) | Spodoptera exigua (CH) | Disruption HIPV not quantified |
Olson et al. (2009) |
| Brassica oleracea | Drought | Microplitis mediator (Generalist parasitoid) | Mamestra brassicae (CH) | No disruption Enhanced emission of green leaf volatile, nitriles and DMNT in drought stressed plant samples with herbivory |
Weldegergis et al. (2015) |
| Two cultivars of B. oleracea | Elevated CO2 |
Cotesia plutellae (Specialist parasitoid) Podisus maculiventris (Generalist predator) |
Plutella xylostella (CH) | Disruption for C. plutellae Disruption for P. maculiventris on one cultivar No significant effect on HIPV emissions, albeit minor reductions in the emission of some terpenoids |
Vuorinen et al. (2004) |
| Pennisetum purpureum | Elevated CO2 |
Cycloneda sanguinea (Generalist predator) Diomus seminulus (Generalist predator) |
Sipha flava (PF) | Disruption for D. seminulus
No disruption for C. sanguinea HIPV not measured |
Fonseca et al. (2014) |
| Brassica napus | Elevated CO2 | Cotesia vestalis (Specialist parasitoid) | P. xylostella (CH) | No disruption Increased terpenoid emissions |
Himanen et al. (2009) |
| B. oleracea | Elevated O3 | C. plutellae (Specialist parasitoid) | P. xylostella (CH) | No disruption Degradation of most herbivore‐induced terpenes and green leaf volatiles |
Pinto, Blande et al. (2007), Pinto, Nerg et al. (2007), Pinto et al. (2008) |
| Phaseolus lunatus | Elevated O3 | Phytoseiulus persimilis (Oligophagous predator) | Tetranychus urticae (PH) | No disruption Degradation of most herbivore‐induced terpenes and green leaf volatiles |
Pinto, Blande et al. (2007), Pinto, Nerg et al. (2007), Pinto et al. (2008) |
| G. hirsutum | Excess and Lack N2 | M. croceipes (Specialist parasitoid) | Spodoptera exigua (CH) | No disruption Lower volatile emissions due to excess or lack of N2 |
Olson et al. (2009) |
| Glycine max | Low N2 | Cotesia marginiventris (Generalist parasitoid) | Spodoptera frugiperda (CH) | No disruption No significant differences in HIPV emission |
Winter & Rostás (2010) |
| G. max | Reduction of UV radiation | C. marginiventris (Generalist parasitoid) | S. frugiperda (CH) | No disruption No significant differences in HIPV emission |
Winter & Rostás (2008) |
| B. oleracea | Increased UV‐B radiation | C. plutellae (Specialist parasitoid) | P. xylostella (CH) | Increased attraction HIPV not measured |
Foggo et al. (2007) |
| Different plant species | Increased temperature | Aphidius matricariae (Generalist parasitoid) | Myzus persicae (PF) | Increased attraction HIPV not measured |
Bezemer et al. (1998) |
CH, chewing herbivore; PF, phloem feeder; HIPV, herbivore‐induced plant volatiles; DMNT, (E)‐4,8‐dimethyl‐1,3,7‐nonatriene.
Abiotic stress has been reported to have negative bottom‐up effects on natural enemy fitness and performance in correlation with poor‐quality hosts (Calatayud, Polania, Seligmann, & Bellotti, 2002; Chen et al., 2010; Klaiber, Najar‐Rodriguez, Dialer, & Dorn, 2013; Winter & Rostás, 2010). However, this is not always the case (Bezemer, Jones, & Knight, 1998; Stacey & Fellowes, 2002; Sun et al., 2011). For example, a study on the long‐term effects of temperature on populations of the aphid Myzus persicae and its parasitoid Aphidius matricariae reported that elevated temperature decreased plant biomass while increasing leaf nitrogen concentrations, which in turn enhanced herbivore abundance and increased parasitism rates (Bezemer et al., 1998). Such studies evidence that bottom‐up effects of abiotic stress are not always negative.
Another interesting aspect is that under controlled settings, plant–natural enemy communication can withstand disruption due to abiotic stress, yet when offered a choice, natural enemies would prefer “healthy” herbivore‐induced plants to those under stress conditions (Olson, Cortesero, Rains, Potter, & Lewis, 2009). The main question is how this translates into field scenarios, as plants growing under similar conditions are likely to experience similar levels of abiotic stress. What happens when there is no choice? Up to which extent can plant–natural enemy communication withstand abiotic stress?
It is possible that effects of abiotic factors on natural enemy recruitment vary depending on the magnitude of the stress and its impacts on the plant metabolism, with severe stress having stronger effects due to constraints in resource availability and allocation affecting HIPV production and release. For example, existing studies show that mild drought increases HIPV emissions or has no effect, whereas severe drought decreases emissions (Becker et al., 2015; Lavoir et al., 2009; Peñuelas & Staudt, 2010). Moreover, responses may vary for individual plant species, as some plants have evolved unique adaptations to stress, and the presence or absence of stress‐tolerance traits will determine the threshold levels for a particular species (Bray, 1997; Pareek, Sopory, Bohnert, & Govindjee, 2010; Wang, Vinocur, & Altman, 2003).
It is evident that individual abiotic factors affect HIPV emission, but there is much potential for interaction among them, leading to different outcomes from those caused by a single stress or those expected by additive effects (Becker et al., 2015; Bezemer et al., 1998; Peñuelas & Staudt, 2010). Studying these interactions among abiotic factors is necessary, especially in scenarios of global warming where multiple abiotic stress factors are likely to occur simultaneously.
The predicted impacts of climate change on natural enemies are severe and include, but are no restricted to: loss of fitness due to poor prey quality, lower susceptibility of herbivores to parasitism or predation due to changes in plant phenology and altered timing of herbivore life cycles, permanent loss of prey due to prey extinction or changes in plant and herbivore distribution, and increased competition with new natural enemies, due to changes in distribution ranges (Boullis et al., 2015; Hance, Van Baaren, Vernon, & Boivin, 2006; Thomson, Macfadyen, & Hoffmann, 2010). In agricultural systems, a number of additional effects may appear as a result of adaptive management strategies adopted by farmers to cope with climate change (Thomson et al., 2010). Whether disruption in plant–natural enemy communication needs to be incorporated to the list remains to be investigated.
3.4. Combining biotic and abiotic factors: a new approach
Recently, two pioneer studies have brilliantly incorporated the effects of abiotic factors with above‐ and belowground organisms and their effects on the attraction of natural enemies (Johnson, Staley, McLeod, & Hartley, 2011; Tariq, Wright, Bruce, & Staley, 2013). The first study evaluated the effects of summer drought on plant community containing Hordeum vulgare (barley), Capsella bursa‐pastoris (shepherd's purse), and Senecio vulgaris (common groundsel), in the presence of the earthworm Aporrectodea caliginosa, the aphid Rhopalosiphum padi and its parasitoid, Aphidius ervi (Johnson et al., 2011). Johnson and co‐authors found that summer drought alone had a negative impact on plant shoot and root biomass, but the addition of earthworms significantly reduced root biomass loss. Drought also led to a significant decrease in aphid abundance, which was moderated by the presence of earthworms, and these effects reflected on parasitism rates. Interestingly, the effect of earthworms was much higher in one‐plant species plots than in multiple species plots, suggesting that other community members can also have an impact on the outcome of tritrophic interactions.
The second study evaluated the effect of drought in a system comprising Brassica oleracea, the root herbivore Delia radicum, the aphids Myzus persicae and Brevicoryne brassicae, and the parasitoids Aphidius colemani and Diaeretiella rapae (Tariq et al., 2013). Their results showed that drought conditions and root herbivory separately had negative effects on parasitism rates. However, there was a significant interaction between drought and root herbivory, in which drought stress partially reversed the negative effect of root herbivory on parasitism rates.
These rare examples demonstrate that multiple biotic and abiotic factors interact, having a strong impact on plant–natural enemy communication. It is hoped that we will be seeing more such studies in the future, which are closer to the natural situation of plants under both cultivated and natural conditions. Similar studies could be useful to investigate plant–natural enemy communication in climate change scenarios.
4. Conclusions and Outlook
To wrap up this review, I will answer the questions proposed in the introduction in light of the available literature.
-
1
Is plant–natural enemy communication stable enough to withstand disruption by biotic and abiotic factors?
The existing literature shows that many volatile‐mediated plant–natural enemy interactions can withstand disruption due to multiple biotic and abiotic factors. However, there are exceptions in all cases, and with so few studies available, the risk of hasty generalization is high. The overall stability of the interaction is likely to depend on the individual variability at both ends of the scale (e.g., the levels of plant tolerance to stress or foraging behavior of the natural enemy), and on the bottom‐up effects of biotic and abiotic stress factors.
-
Which biotic and abiotic factors disrupt communication between plants and natural enemies?
Due to the limited amount of available of literature, it is difficult to predict accurately which factors disrupt plant–natural enemy communication. Each system is unique and needs to be explored in the ecological context in which it occurs, including the interactions between multiple biotic and abiotic factors. However, the literature reviewed here suggests that belowground herbivory consistently disrupts natural enemy attraction, presumably due to the strong effects of root herbivory on nutrient uptake and plant metabolism that impact plant signaling and herbivore quality as a prey. More studies are required to support or reject this hypothesis.
-
Are there common patterns allowing us to make predictions about the outcome of these tritrophic interactions under biotic and abiotic stress scenarios?
Although it may be tempting trying to predict the outcome of plant–natural enemy interactions by investigating only one the actors involved, this is often insufficient and pays no heed to ecological complexity. A more systemic approach is needed to understand the stability and direction of these interactions in nature, and under biotic and abiotic stress. There is a common thread in the existing reports, suggesting that natural enemies can infer host quality based on volatile cues. Hence, the bottom‐up effects (both positive and negative) of biotic and abiotic factors on plant quality for the herbivore, and of this as host for the natural enemies, are likely to play an important role determining the outcome of the interaction. Therefore, investigating these bottom‐up effects is crucial for further studies aiming to understand the impact of biotic and abiotic factors on plant–natural enemy interactions.
Research on multitrophic interactions has slowly progressed from evaluating linear plant–herbivore–natural enemy systems under controlled conditions into more complex models incorporating multiple attackers and abiotic conditions. However, even at this level, there is a high risk of oversimplification, as both biotic and abiotic factors are likely to interact in complex ways, rather than just having additive effects.
Critical aspects for future research to understand the stability of plant–natural enemy interactions in nature include the effects of biotic and abiotic stress on natural enemy foraging behavior, the impact of the stress intensity on volatile emission and natural enemy recruitment, and the complex role of microorganisms on plant–natural enemy interactions. The ultimate goal is to establish the impact of multiple co‐occurring biotic and abiotic factors that recreate natural and climate change scenarios, and the identification and exploration of newly emerged and threatened interactions as a result of climate change.
Conflict of Interest
None declared.
Acknowledgments
I would like to thank the members of the group Biocommunication and Entomology at ETH Zurich, who have critically read this manuscript and made valuable suggestions to its earlier versions, especially Dr. Kerry Mauck, Dr. Sean Halloran, Dr. Mark Mescher, and Prof. Consuelo De Moraes. I am also grateful to Prof. Jonathan Gershenzon and Prof. Mary Morgan‐Richards for their comments and support and to anonymous reviewers for the valuable suggestions.
Clavijo McCormick, A. (2016), Can plant–natural enemy communication withstand disruption by biotic and abiotic factors? Ecology and Evolution, 6: 8569–8582. doi: 10.1002/ece3.2567
References
- Agrawal, A. A. , & Fishbein, M. (2006). Plant defense syndromes. Ecology, 87, S132–S149. [DOI] [PubMed] [Google Scholar]
- Alborn, H. T. , Hansen, T. V. , Jones, T. H. , Bennett, D. C. , Tumlinson, J. H. , Schmelz, E. A. , & Teal, P. E. A. (2007). Disulfooxy fatty acids from the American bird grasshopper Schistocerca americana, elicitors of plant volatiles. Proceedings of the National Academy of Sciences of the United States of America, 104, 12976–12981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison, J. D. , & Hare, D. J. (2009). Learned and naive natural enemy responses and the interpretation of volatile organic compounds as cues or signals. New Phytologist, 184, 768–782. [DOI] [PubMed] [Google Scholar]
- Anderson, P. , & Anton, S. (2014). Experience‐based modulation of behavioural responses to plant volatiles and other sensory cues in insect herbivores. Plant, Cell and Environment, 37, 1826–1835. [DOI] [PubMed] [Google Scholar]
- Arimura, G. , Matsui, K. , & Takabayashi, J. (2009). Chemical and molecular ecology of herbivore‐induced plant volatiles: Proximate factors and their ultimate functions. Plant and Cell Physiology, 50, 911–923. [DOI] [PubMed] [Google Scholar]
- Becker, C. , Desneux, N. , Monticelli, L. , Fernandez, X. , Michel, T. , & Lavoir, A.‐V. (2015). Effects of abiotic factors on HIPV‐mediated interactions between plants and parasitoids. BioMed Research International, 2015, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezemer, T. M. , Jones, T. H. , & Knight, K. J. (1998). Long‐term effects of elevated CO2 and temperature on populations of the peach potato aphid Myzus persicae and its parasitoid Aphidius matricariae . Oecologia, 116, 128–135. [DOI] [PubMed] [Google Scholar]
- Bezemer, T. M. , & van Dam, N. M. (2005). Linking aboveground and belowground interactions via induced plant defenses. Trends in Ecology & Evolution, 20, 617–624. [DOI] [PubMed] [Google Scholar]
- Birkett, M. A. , Campbell, C. A. , Chamberlain, K. , Guerrieri, E. , Hick, A. J. , Martin, J. L. , ··· Woodcock, C. M. (2000). New roles for cis‐jasmone as an insect semiochemical and in plant defense. Proceedings of the National Academy of Sciences of the United States of America, 97, 9329–9334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blossey, B. , & Hunt‐Joshi, T. R. (2003). Belowground herbivory by insects: Influence on plants and aboveground herbivores. Annual Review of Entomology, 48, 521–547. [DOI] [PubMed] [Google Scholar]
- de Boer, J. , & Dicke, M. (2006). Olfactory learning by predatory arthropods. Animal Biology, 56, 143–155. [Google Scholar]
- de Boer, J. , Hordijk, C. , Posthumus, M. , & Dicke, M. (2008). Prey and non‐prey arthropods sharing a host plant: Effects on induced volatile emission and predator attraction. Journal of Chemical Ecology, 34, 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton, M. D. (2009). Primary metabolism and plant defense‐fuel for the fire. Molecular Plant‐Microbe Interactions, 22, 487–497. [DOI] [PubMed] [Google Scholar]
- Bostock, R. M. (2005). Signal crosstalk and induced resistance: Straddling the line between cost and benefit. Annual review of Phytopathology, 43, 545–580. Annual Reviews, Palo Alto. [DOI] [PubMed] [Google Scholar]
- Boullis, A. , Francis, F. , & Verheggen, F. J. (2015). Climate change and tritrophic interactions: Will modifications to greenhouse gas emissions increase the vulnerability of herbivorous insects to natural enemies? Environmental Entomology, 44, 277–286. [DOI] [PubMed] [Google Scholar]
- Bray, E. A. (1997). Plant responses to water deficit. Trends in Plant Science, 2, 48–54. [Google Scholar]
- Calatayud, P.‐A. , Polania, M. , Seligmann, C. , & Bellotti, A. C. (2002). Influence of water‐stressed cassava on Phenacoccus herreni and three associated parasitoids. Entomologia Experimentalis et Applicata, 102, 163–175. [Google Scholar]
- Cardoza, Y. J. , Alborn, H. T. , & Tumlinson, J. H. (2002). In vivo volatile emissions from peanut plants induced by simultaneous fungal infection and insect damage. Journal of Chemical Ecology, 28, 161–174. [DOI] [PubMed] [Google Scholar]
- Cardoza, Y. J. , Teal, P. E. A. , & Tumlinson, J. H. (2003). Effect of peanut plant fungal infection on oviposition preference by Spodoptera exigua and on host‐searching behaviour by Cotesia marginiventris . Environmental Entomology, 32, 970–976. [Google Scholar]
- Chen, Y. G. , Olson, D. M. , & Ruberson, J. R. (2010). Effects of nitrogen fertilization on tritrophic interactions. Arthropod‐Plant Interactions, 4, 81–94. [Google Scholar]
- Clavijo McCormick, A. , Boeckler, A. G. , Köllner, T. G. , Gershenzon, J. , & Unsicker, S. B. (2014). The timing of herbivore‐induced volatile emission in black poplar (Populus nigra) and the influence of herbivore age and identity affect the value of individual volatiles as cues for herbivore enemies. BMC Plant Biology, 14, 304. doi:10.1186/s12870‐014‐0304‐5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavijo McCormick, A. , Gershenzon, J. , & Unsicker, S. (2014). Little peaks with big effects: Establishing the role of minor plant volatiles in plant‐insect interactions. Plant, Cell and Environment, 37, 1836–1844. [DOI] [PubMed] [Google Scholar]
- Clavijo McCormick, A. , Irmisch, S. , Reinecke, A. , Boeckler, G. A. , Veit, D. , Reichelt, M. , ··· Unsicker, S. B. (2014). Herbivore‐induced volatile emission in black poplar: Regulation and role in attracting herbivore enemies. Plant, Cell and Environment, 37, 1909–1923. [DOI] [PubMed] [Google Scholar]
- Clavijo McCormick, A. , Reinecke, A. , Gershenzon, J. , & Unsicker, S. B. (2016). Feeding experience affects the behavioral response of polyphagous gypsy moth caterpillars to herbivore‐induced poplar volatiles. Journal of Chemical Ecology, 42, 382. doi:10.1007/s10886‐016‐0698‐7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavijo McCormick, A. , Unsicker, S. B. , & Gershenzon, J. (2012). The specificity of herbivore‐induced plant volatiles in attracting herbivore enemies. Trends in Plant Science, 17, 303–310. [DOI] [PubMed] [Google Scholar]
- Conrath, U. (2006). Systemic acquired resistance. Plant Signalling & Behaviour, 1, 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortesero, A. M. , De Moraes, C. M. , Stapel, J. O. , Tumlinson, J. H. , & Lewis, W. J. (1997). Comparisons and contrasts in host‐foraging strategies of two larval parasitoids with different degrees of host specificity. Journal of Chemical Ecology, 23, 1589–1606. [Google Scholar]
- D'Alessandro, M. , Brunner, V. , von Merey, G. , & Turlings, T. C. J. (2009). Strong attraction of the parasitoid Cotesia marginiventris towards minor volatile compounds of maize. Journal of Chemical Ecology, 35, 999–1008. [DOI] [PubMed] [Google Scholar]
- van Dam, N. M. , Harvey, J. A. , Wäckers, F. L. , Bezemer, T. M. , van der Putten, W. H. , & Vet, L. E. M. (2003). Interactions between aboveground and belowground induced responses against phytophages. Basic and Applied Ecology, 4, 63–77. [Google Scholar]
- De Moraes, C. M. , Lewis, W. J. , Pare, P. W. , Alborn, H. T. , & Tumlinson, J. H. (1998). Herbivore‐infested plants selectively attract parasitoids. Nature, 393, 570–573. [Google Scholar]
- Dicke, M. (1999). Are herbivore‐induced plant volatiles reliable indicators of herbivore identity to foraging carnivorous arthropods? Entomologia Experimentalis et Applicata, 91, 131–142. [Google Scholar]
- Dicke, M. , & van Loon, J. J. A. (2000). Multitrophic effects of herbivore‐induced plant volatiles in an evolutionary context. Entomologia Experimentalis et Applicata, 97, 237–249. [Google Scholar]
- Dicke, M. , van Loon, J. J. A. , & Soler, R. (2009). Chemical complexity of volatiles from plants induced by multiple attack. Nature Chemical Biology, 5, 317–324. [DOI] [PubMed] [Google Scholar]
- Dudareva, N. , Picherski, E. , & Gershenzon, J. (2004). Biochemistry of plant volatiles. Plant Physiology, 135, 1893–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant, W. E. , & Dong, X. (2004). Systemic acquired resistance. Annual review of Phytopathology, 42, 185–209. [DOI] [PubMed] [Google Scholar]
- Engelberth, J. , Alborn, H. T. , Schmelz, E. A. , & Tumlinson, J. H. (2004). Airborne signals prime plants against insect herbivore attack. Proceedings of the National Academy of Sciences of the United States of America, 101, 1781–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb, M. , Foresti, N. , & Turlings, T. (2010). A tritrophic signal that attracts parasitoids to host‐damaged plants withstands disruption by non‐host herbivores. BMC Plant Biology, 1, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb, M. , Meldau, S. , & Howe, G. A. (2012). Role of phytohormones in insect‐specific plant reactions. Trends in Plant Science, 17, 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb, M. , Ton, J. , Degenhardt, J. , & Turlings, T. C. J. (2008). Interactions between arthropod‐induced aboveground and belowground defenses in plants. Plant Physiology, 146, 867–874. doi:10.1104/pp.107.112169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatouros, N. E. , van Loon, J. J. , Hordijk, K. A. , Smid, H. M. , & Dicke, M. (2005). Herbivore‐induced plant volatiles mediate in‐flight host discrimination by parasitoids. Journal of Chemical Ecology, 31, 2033–2047. [DOI] [PubMed] [Google Scholar]
- Foggo, A. , Higgins, S. , Wargent, J. J. , & Coleman, R. A. (2007). Tri‐trophic consequences of UV‐B exposure: Plants, herbivores and parasitoids. Oecologia, 154, 505–512. [DOI] [PubMed] [Google Scholar]
- Fonseca, M. G. , Santos, D. R. , & Auad, A. M. (2014). Impact of different carbon dioxide concentrations in the olfactory response of Sipha flava (Hemiptera: Aphididae) and its Predators. Journal of Insect Behavior, 27, 722–728. [Google Scholar]
- Gange, A. C. , Brown, V. K. , & Aplin, D. M. (2003). Multitrophic links between arbuscular mycorrhizal fungi and insect parasitoids. Ecology Letters, 6, 1051–1055. [Google Scholar]
- Geervliet, J. B. F. , Posthumus, M. A. , Vet, L. E. M. , & Dicke, M. (1997). Comparative analysis of headspace volatiles from different caterpillar‐infested or uninfested food plants of Pieris species. Journal of Chemical Ecology, 23, 2935–2954. [Google Scholar]
- Gershenzon, J. (1984). Changes in the levels of plant secondary metabolites under water and nutrient stress In Timmermann B. N., Steelink C. & Loewus F. A. (Eds.), Phytochemical adaptations to stress (pp. 273–320). Boston, MA, USA: Springer. [Google Scholar]
- Glazebrook, J. (2005). Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annual review of Phytopathology, 43, 205–227. [DOI] [PubMed] [Google Scholar]
- Glinwood, R. , Ahmed, E. , Qvarfordt, E. , & Ninkovic, V. (2011). Olfactory learning of plant genotypes by a polyphagous insect predator. Oecologia, 166, 637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gols, R. (2014). Direct and indirect chemical defences against insects in a multitrophic framework. Plant, Cell and Environment, 37, 1741–1752. [DOI] [PubMed] [Google Scholar]
- Guerrieri, E. , Lingua, G. , Digilio, M. C. , Massa, N. , & Berta, G. (2004). Do interactions between plant roots and the rhizosphere affect parasitoid behaviour? Ecological Entomology, 29, 753–756. [Google Scholar]
- Hance, T. , Van Baaren, J. , Vernon, P. , & Boivin, G. (2006). Impact of extreme temperatures on parasitoids in a climate change perspective. Annual Review of Entomology, 52, 107. [DOI] [PubMed] [Google Scholar]
- Heil, M. , & Bostock, R. M. (2002). Induced systemic resistance (ISR) against pathogens in the context of induced plant defences. Annals of Botany, 89, 503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil, M. , & Kost, C. (2006). Priming of indirect defences. Ecology Letters, 9, 813–817. [DOI] [PubMed] [Google Scholar]
- Heil, M. , & Silva Bueno, J. C. (2007). Within‐plant signalling by volatiles leads to induction and priming of an indirect plant defense in nature. Proceedings of the National Academy of Sciences of the United States of America, 104, 5467–5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil, M. , & Ton, J. (2008). Long‐distance signalling in plant defence. Trends in Plant Science, 13, 264–272. [DOI] [PubMed] [Google Scholar]
- Hilker, M. , & Meiners, T. (2006). Early herbivore alert: Insect eggs induce plant defense. Journal of Chemical Ecology, 32, 1379–1397. [DOI] [PubMed] [Google Scholar]
- Hilker, M. , Stein, C. , Schroder, R. , Varama, M. , & Mumm, R. (2005). Insect egg deposition induces defence responses in Pinus sylvestris: Characterisation of the elicitor. Journal of Experimental Biology, 208, 1849–1854. [DOI] [PubMed] [Google Scholar]
- Himanen, S. J. , Nerg, A. M. , Nissinen, A. , Pinto, D. M. , Stewart, C. N. , Poppy, G. M. , & Holopainen, J. K. (2009). Effects of elevated carbon dioxide and ozone on volatile terpenoid emissions and multitrophic communication of transgenic insecticidal oilseed rape (Brassica napus). New Phytologist, 181, 174–186. [DOI] [PubMed] [Google Scholar]
- Hoedjes, K. M. , Kruidhof, H. M. , Huigens, M. E. , Dicke, M. , Vet, L. E. M. , & Smid, H. M. (2011). Natural variation in learning rate and memory dynamics in parasitoid wasps: Opportunities for converging ecology and neuroscience. Proceedings of the Royal Society. B: Biological sciences, 22, 889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holopainen, J. K. (2004). Multiple functions of inducible plant volatiles. Trends in Plant Science, 9, 529–533. [DOI] [PubMed] [Google Scholar]
- Holt, R. D. , & Lawton, J. H. (1994). The ecological consequences of shared natural enemies. Annual Review of Ecology and Systematics, 25, 495–520. [Google Scholar]
- Hunter, M. D. (2002). A breath of fresh air: Beyond laboratory studies of plant volatile–natural enemy interactions. Agricultural and Forest Entomology, 4, 81–86. [Google Scholar]
- Johnson, S. N. , Staley, J. T. , McLeod, F. A. , & Hartley, S. E. (2011). Plant‐mediated effects of soil invertebrates and summer drought on above‐ground multitrophic interactions. Journal of Ecology, 99, 57–65. [Google Scholar]
- Jonsson, M. , Lindkvist, A. , & Anderson, P. (2005). Behavioural responses in three ichneumonid pollen beetle parasitoids to volatiles emitted from different phenological stages of oilseed rape. Entomologia Experimentalis et Applicata, 115, 363–369. [Google Scholar]
- Kahl, J. , Siemens, D. H. , Aerts, R. J. , Gäbler, R. , Kühnemann, F. , Preston, C. A. , & Baldwin, I. T. (2000). Herbivore‐induced ethylene suppresses a direct defense but not a putative indirect defense against an adapted herbivore. Planta, 210, 336–342. [DOI] [PubMed] [Google Scholar]
- Kappers, I. F. , Hoogerbrugge, H. , Bouwmeester, H. J. , & Dicke, M. (2011). Variation in herbivory‐induced volatiles among cucumber (Cucumis sativus L.) varieties has consequences for the attraction of carnivorous natural enemies. Journal of Chemical Ecology, 37, 150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempel, A. , Schädler, M. , Chrobock, T. , Fischer, M. , & van Kleunen, M. (2011). Tradeoffs associated with constitutive and induced plant resistance against herbivory. Proceedings of the National Academy of Sciences of the United States of America, 108, 5685–5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler, A. , & Baldwin, I. T. (2001). Defensive function of herbivore‐induced plant volatile emissions in nature. Science, 291, 2141–2144. [DOI] [PubMed] [Google Scholar]
- Klaiber, J. , Najar‐Rodriguez, A. , Dialer, E. , & Dorn, S. (2013). Elevated carbon dioxide impairs the performance of a specialized parasitoid of an aphid host feeding on Brassica plants. Biological Control, 66, 49–55. [Google Scholar]
- Koornneef, A. , & Pieterse, C. M. J. (2008). Cross talk in defense signalling. Plant Physiology, 146, 839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krips, O. E. , Willems, P. E. L. , Gols, R. , Posthumus, M. A. , Gort, G. , & Dicke, M. (2001). Comparison of cultivars of ornamental crop Gerbera jamesonii on production of spider mite‐induced volatiles, and their attractiveness to the predator Phytoseiulus persimilis . Journal of Chemical Ecology, 27, 1355–1372. [DOI] [PubMed] [Google Scholar]
- Lavoir, A. V. , Duffet, C. , Mouillot, F. , Rambal, S. , Ratte, J. P. , Schnitzler, J. P. , & Staudt, M. (2011). Scaling‐up leaf monoterpene emissions from a water limited Quercus ilex woodland. Atmospheric Environment, 45, 2888–2897. [Google Scholar]
- Lavoir, A. , Staudt, M. , Schnitzler, J. , Landais, D. , Massol, F. , Rocheteau, A. , ··· Rambal, S. (2009). Drought reduced monoterpene emissions from Quercus ilex trees: Results from a throughfall displacement experiment within a forest ecosystem. Biogeosciences Discussions, 6, 863. [Google Scholar]
- Lawlor, D. , & Cornic, G. (2002). Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant, Cell and Environment, 25, 275–294. [DOI] [PubMed] [Google Scholar]
- Louis, J. , Peiffer, M. , Ray, S. , Luthe, D. S. , & Felton, G. W. (2013). Host‐specific salivary elicitor(s) of European corn borer induce defenses in tomato and maize. New Phytologist, 199, 66–73. [DOI] [PubMed] [Google Scholar]
- Maeda, T. , & Takabayashi, J. (2001). Production of herbivore‐induced plant volatiles and their attractiveness to Phytoseius persimilis (Acari: Phytoseiidae) with changes of Tetranychus urticae (Acari: Tetranychidae) density on a plant. Applied Entomology and Zoology, 36, 47–52. [Google Scholar]
- Malcom, S. B. (2009) Prey defense and predator foraging In Crawley M. J. (Ed.), Natural enemies: The population biology of predators, parasites and diseases (pp. 458–475). doi: 10.1002/9781444314076.ch20 USA: Blackwel publishing Ltd. [Google Scholar]
- Martini, X. , Pelz‐Stelinski, K. S. , & Stelinski, L. L. (2014). Plant pathogen‐induced volatiles attract parasitoids to increase parasitism of an insect vector. Frontiers in Ecology and Evolution, 2, doi: 10.3389/fevo.2014.00008. [Google Scholar]
- Mauck, K. E. , De Moraes, C. M. , & Mescher, M. C. (2010). Deceptive chemical signals induced by a plant virus attract insect vectors to inferior hosts. Proceedings of the National Academy of Sciences of the United States of America, 107, 3600–3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moayeri, H. R. S. , Ashouri, A. , Poll, L. , & Enkegaard, A. (2007). Olfactory response of a predatory mirid to herbivore induced plant volatiles: Multiple herbivory vs. single herbivory. Journal of Applied Entomology, 131, 326–332. [Google Scholar]
- Moujahed, R. , Frati, F. , Cusumano, A. , Salerno, G. , Conti, E. , Peri, E. , & Colazza, S. (2014). Egg parasitoid attraction toward induced plant volatiles is disrupted by a non‐host herbivore attacking above or belowground plant organs. Frontiers in Plant Science, 5, doi: 10.3389/fpls.2014.00601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumm, R. , & Dicke, M. (2010). Variation in natural plant products and the attraction of bodyguards involved in indirect plant defense. Canadian Journal of Zoology, 88, 628–667. [Google Scholar]
- Musser, R. O. , Hum‐Musser, S. M. , Eichenseer, H. , Peiffer, M. , Ervin, G. , Murphy, J. B. , & Felton, G. W. (2002). Herbivory: Caterpillar saliva beats plant defences. Nature, 416, 599–600. [DOI] [PubMed] [Google Scholar]
- Ngumbi, E. , Chen, L. , & Fadamiro, H. Y. (2009). Comparative GC‐EAD responses of a specialist (Microplitis croceipes) and a generalist (Cotesia marginiventris) parasitoid to cotton volatiles induced by two caterpillar species. Journal of Chemical Ecology, 35, 1009–1020. [DOI] [PubMed] [Google Scholar]
- Ngumbi, E. , Chen, L. , & Fadamiro, H. (2010). Electroantennogram (EAG) responses of Microplitis croceipes and Cotesia marginiventris and their lepidopteran hosts to a wide array of odor stimuli: Correlation between EAG response and degree of host specificity? Journal of Insect Physiology, 56, 1260–1268. [DOI] [PubMed] [Google Scholar]
- Olson, D. M. , Cortesero, A. M. , Rains, G. C. , Potter, T. , & Lewis, W. J. (2009). Nitrogen and water affect direct and indirect plant systemic induced defense in cotton. Biological Control, 49, 239–244. [Google Scholar]
- Pareek, A. , Sopory, S. , Bohnert, H. , & Govindjee. (2010) Abiotic stress adaptation in plants. Dordrecht, Nederlands: Springer. [Google Scholar]
- Peñuelas, J. , & Llusià, J. (2003). BVOCs: plant defense against climate warming?. Trends Plant Sci, 8, 105–109. [DOI] [PubMed] [Google Scholar]
- Peñuelas, J. , & Staudt, M. (2010). BVOCs and global change. Trends in Plant Science, 15, 133–144. [DOI] [PubMed] [Google Scholar]
- Pierre, P. S. , Dugravot, S. , Ferry, A. , Soler, R. , van Dam, N. M. , & Cortesero, A. M. (2011). Aboveground herbivory affects indirect defences of brassicaceous plants against the root feeder Delia radicum Linnaeus: Laboratory and field evidence. Ecological Entomology, 36, 326–334. [Google Scholar]
- Pieterse, C. M. , Leon‐Reyes, A. , Van der Ent, S. , & Van Wees, S. C. (2009). Networking by small‐molecule hormones in plant immunity. Nature Chemical Biology, 5, 308–316. [DOI] [PubMed] [Google Scholar]
- Pieterse, C. M. J. , Zamioudis, C. , Berendsen, R. L. , Weller, D. M. , Van Wees, S. C. M. , & Bakker, P. A. H. M. (2014). Induced systemic resistance by beneficial microbes. Annual review of Phytopathology, 52, 347–375. [DOI] [PubMed] [Google Scholar]
- Pineda, A. , Soler, R. , Pozo, M. J. , Rasmann, S. , & Turlings, T. C. (2015). Above‐belowground interactions involving plants, microbes and insects. Frontiers in Plant Science, 6, 318. doi:10.3389/fpls.2015.00318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto, D. M. , Blande, J. D. , Nykanen, R. , Dong, W. X. , Nerg, A. M. , & Holopainen, J. K. (2007). Ozone degrades common herbivore‐induced plant volatiles: Does this affect herbivore prey location by predators and parasitoids? Journal of Chemical Ecology, 33, 683–694. [DOI] [PubMed] [Google Scholar]
- Pinto, D. , Himanen, S. , Nissinen, A. , Nerg, A.‐M. , & Holopainen, J. (2008). Host location behaviour of Cotesia plutellae Kurdjumov (Hymenoptera: Braconidae) in ambient and moderately elevated ozone in field conditions. Environmental Pollution, 156, 227–231. [DOI] [PubMed] [Google Scholar]
- Pinto, D. M. , Nerg, A. M. , & Holopainen, J. K. (2007). The role of ozone‐reactive compounds, terpenes, and green leaf volatiles (GLVs), in the orientation of Cotesia plutellae . Journal of Chemical Ecology, 33, 2218–2228. [DOI] [PubMed] [Google Scholar]
- Ponzio, C. , Gols, R. , Pieterse, C. M. , & Dicke, M. (2013). Ecological and phytohormonal aspects of plant volatile emission in response to single and dual infestations with herbivores and phytopathogens. Functional Ecology, 27, 587–598. [Google Scholar]
- Ramakrishna, A. , & Ravishankar, G. A. (2011). Influence of abiotic stress signals on secondary metabolites in plants. Plant Signalling & Behaviour, 6, 1720–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmann, S. , & Turlings, T. C. J. (2007). Simultaneous feeding by aboveground and belowground herbivores attenuates plant‐mediated attraction of their respective natural enemies. Ecology Letters, 10, 926–936. [DOI] [PubMed] [Google Scholar]
- de Rijk, M. , Dicke, M. , & Poelman, E. H. (2013). Foraging behaviour by parasitoids in multiherbivore communities. Animal Behaviour, 85, 1517–1528. [Google Scholar]
- Rodriguez‐Saona, C. , Chalmers, J. , Raj, S. , & Thaler, J. (2005). Induced plant responses to multiple damagers: Differential effects on an herbivore and its parasitoid. Oecologia, 143, 566–577. [DOI] [PubMed] [Google Scholar]
- Rosenblatt, A. E. , & Schmitz, O. J. (2014). Interactive effects of multiple climate change variables on trophic interactions: A meta‐analysis. Climate Change Responses, 1, 1–10. [Google Scholar]
- Rostás, M. , Ton, J. , Mauch‐Mani, B. , & Turlings, T. C. J. (2006). Fungal infection reduces herbivore‐induced plant volatiles of maize but does not affect naive parasitoids. Journal of Chemical Ecology, 32, 1897–1909. [DOI] [PubMed] [Google Scholar]
- Ruther, J. , & Furstenau, B. (2005). Emission of herbivore‐induced volatiles in absence of a herbivore‐response of Zea mays to green leaf volatiles and terpenoids. Zeitschrift für Naturforschung C, 60, 743–756. [DOI] [PubMed] [Google Scholar]
- Sarmento, R. A. , Lemos, F. , Bleeker, P. M. , Schuurink, R. C. , Pallini, A. , Oliveira, M. G. A. , ··· Janssen, A. (2011). A herbivore that manipulates plant defence. Ecology Letters, 14, 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz, E. A. , Engelberth, J. , Alborn, H. T. , Tumlinson, J. H. , & Teal, P. E. A. (2009). Phytohormone‐based activity mapping of insect herbivore‐produced elicitors. Proceedings of the National Academy of Sciences of the United States of America, 106, 653–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey, T. D. , Wiberley, A. E. , & Donohue, A. R. (2008). Isoprene emission from plants: Why and how. Annals of Botany, 101, 5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiojiri, K. , Takabayashi, J. , Yano, S. , & Takafuji, A. (2000a). Flight response of parasitoids toward plant‐herbivore complexes: A comparative study of two parasitoid‐herbivore systems on cabbage plants. Applied Entomology and Zoology, 35, 87–92. [Google Scholar]
- Shiojiri, K. , Takabayashi, J. , Yano, S. , & Takafuji, A. (2000b). Herbivore‐species‐specific interactions between crucifer plants and parasitic wasps (Hymenoptera: Braconidae) that are mediated by infochemicals present in areas damaged by herbivores. Applied Entomology and Zoology, 35, 519–524. [Google Scholar]
- Shiojiri, K. , Takabayashi, J. , Yano, S. , & Takafuji, A. (2001). Infochemically mediated tritrophic interaction webs on cabbage plants. Population Ecology, 43, 23–29. [Google Scholar]
- Shiojiri, K. , Takabayashi, J. , Yano, S. , & Takafuji, A. (2002). Oviposition preferences of herbivores are affected by tritrophic interaction webs. Ecology Letters, 5, 186–192. [Google Scholar]
- Soler, R. , Bezemer, T. M. , Van der Putten, W. H. , Vet, L. E. M. , & Harvey, J. A. (2005). Root herbivore effects on above‐ground herbivore, parasitoid and hyperparasitoid performance via changes in plant quality. Journal of Animal Ecology, 74, 1121–1130. [Google Scholar]
- Soler, R. , Harvey, J. A. , Kamp, A. F. D. , Vet, L. E. M. , Van der Putten, W. H. , Van Dam, N. M. , ··· Bezemer, T. M. (2007). Root herbivores influence the behaviour of an aboveground parasitoid through changes in plant‐volatile signals. Oikos, 116, 367–376. [Google Scholar]
- Stacey, D. A. , & Fellowes, M. D. (2002). Influence of elevated CO2 on interspecific interactions at higher trophic levels. Global Change Biology, 8, 668–678. [Google Scholar]
- Stam, J. M. , Kroes, A. , Li, Y. , Gols, R. , van Loon, J. J. , Poelman, E. H. , & Dicke, M. (2014). Plant interactions with multiple insect herbivores: From community to genes. Annual Review of Plant Biology, 65, 689–713. [DOI] [PubMed] [Google Scholar]
- Steidle, J. L. M. , & van Loon, J. J. A. (2003). Dietary specialization and infochemical use in carnivorous arthropods: Testing a concept. Entomologia Experimentalis et Applicata, 108, 133–148. [Google Scholar]
- Steinberg, S. , Dicke, M. , Vet, L. E. M. , & Wanningen, R. (1992). Response of the braconid parasitoid Cotesia (=Apanteles) glomerata to volatile infochemicals: Effects of bioassay set‐up, parasitoid age and experience and barometric flux. Entomologia Experimentalis et Applicata, 63, 163–175. [Google Scholar]
- Sun, Y. C. , Feng, L. , Gao, F. , & Ge, F. (2011). Effects of elevated CO2 and plant genotype on interactions among cotton, aphids and parasitoids. Insect Sci., 18, 451–461. [Google Scholar]
- Tack, A. J. , Gripenberg, S. , & Roslin, T. (2012). Cross‐kingdom interactions matter: Fungal‐mediated interactions structure an insect community on oak. Ecology Letters, 15, 177–185. [DOI] [PubMed] [Google Scholar]
- Takabayashi, J. , Dicke, M. , & Posthumus, M. A. (1994). Volatile herbivore‐induced terpenoids in plant mite interactions – variation caused by biotic and abiotic factors. Journal of Chemical Ecology, 20, 1329–1354. [DOI] [PubMed] [Google Scholar]
- Takabayashi, J. , Sabelis, M. W. , Janssen, A. , Shiojiri, K. , & van Wijk, M. (2006). Can plants betray the presence of multiple herbivore species to predators and parasitoids? The role of learning in phytochemical information networks. Ecological Research, 21, 3–8. [Google Scholar]
- Takabayashi, J. , Takahashi, S. , Dicke, M. , & Posthumus, M. A. (1995). Developmental stage of herbivore Pseudaletia separata affects production of herbivore‐induced synomone by corn plants. Journal of Chemical Ecology, 21, 273–287. [DOI] [PubMed] [Google Scholar]
- Tamo, C. , Ricard, I. , Held, M. , Davison, A. C. , & Turlings, T. C. J. (2006). A comparison of naive and conditioned responses of three generalist endoparasitoids of lepidopteran larvae to host‐induced plant odours. Animal Biology, 56, 205–220. [Google Scholar]
- Tariq, M. , Wright, D. J. , Bruce, T. J. A. , & Staley, J. T. (2013). Drought and root herbivory interact to alter the response of above‐ground parasitoids to aphid infested plants and associated plant volatile signals. PLoS One, 8, e69013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler, J. S. (1999). Jasmonate‐inducible plant defences cause increased parasitism of herbivores. Nature, 399, 686–688. [Google Scholar]
- Thomma, B. P. , Penninckx, I. A. , Broekaert, W. F. , & Cammue, B. P. (2001). The complexity of disease signalling in Arabidopsis . Current Opinion in Immunology, 13, 63–68. [DOI] [PubMed] [Google Scholar]
- Thomson, L. J. , Macfadyen, S. , & Hoffmann, A. A. (2010). Predicting the effects of climate change on natural enemies of agricultural pests. Biological Control, 52, 296–306. [Google Scholar]
- Van der Ent, S. , Van Wees, S. C. , & Pieterse, C. M. (2009). Jasmonate signalling in plant interactions with resistance‐inducing beneficial microbes. Phytochemistry, 70, 1581–1588. [DOI] [PubMed] [Google Scholar]
- Van der Putten, W. H. , Vet, L. E. M. , Harvey, J. A. , & Wäckers, F. L. (2001). Linking above‐ and belowground multitrophic interactions of plants, herbivores, pathogens, and their antagonists. Trends in Ecology & Evolution, 16, 547–554. [Google Scholar]
- Van Oosten, V. R. , Bodenhausen, N. , Reymond, P. , Van Pelt, J. A. , Van Loon, L. C. , Dicke, M. , & Pieterse, C. M. (2008). Differential effectiveness of microbially induced resistance against herbivorous insects in Arabidopsis . Molecular Plant‐Microbe Interactions, 21, 919–930. [DOI] [PubMed] [Google Scholar]
- Vos, M. , Berrocal, S. M. , Karamaouna, F. , Hemerik, L. , & Vet, L. E. M. (2001). Plant‐mediated indirect effects and the persistence of parasitoid–herbivore communities. Ecology Letters, 4, 38–45. [Google Scholar]
- Vuorinen, T. , Nerg, A.‐M. , Ibrahim, M. , Reddy, G. , & Holopainen, J. K. (2004). Emission of Plutella xylostella‐induced compounds from cabbages grown at elevated CO2 and orientation behaviour of the natural enemies. Plant Physiology, 135, 1984–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walling, L. L. (2000). The myriad plant responses to herbivores. Journal of Plant Growth Regulation, 19, 195–216. [DOI] [PubMed] [Google Scholar]
- Wang, W. , Vinocur, B. , & Altman, A. (2003). Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta, 218, 1–14. [DOI] [PubMed] [Google Scholar]
- Wäschke, N. , Meiners, T. & Rostas, M. (2013). Foraging strategies of insect parasitoids in complex chemical environments In: Wajnberg E. & Colazza S. (Eds.), Recent Advances in Chemical Ecology of Insect Parasitoids, Wiley‐Blackwell, pp. 193‐224. [Google Scholar]
- Way, D. A. , Schnitzler, J. P. , Monson, R. K. , & Jackson, R. B. (2011). Enhanced isoprene‐related tolerance of heat‐ and light‐stressed photosynthesis at low, but not high, CO2 concentrations. Oecologia, 166, 273–282. [DOI] [PubMed] [Google Scholar]
- Weldegergis, B. T. , Zhu, F. , Poelman, E. H. , & Dicke, M. (2015). Drought stress affects plant metabolites and herbivore preference but not host location by its parasitoids. Oecologia, 177, 701. doi:10.1007/s00442‐014‐3129‐x [DOI] [PubMed] [Google Scholar]
- Winter, T. R. , & Rostás, M. (2010). Nitrogen deficiency affects bottom‐up cascade without disrupting indirect plant defense. Journal of Chemical Ecology, 36, 642–651. [DOI] [PubMed] [Google Scholar]
- Winter, T. R. , & Rostás, M. (2008). Ambient ultraviolet radiation induces protective responses in soybean but does not attenuate indirect defense. Environmental Pollution, 155, 290–297. [DOI] [PubMed] [Google Scholar]
- Yoneya, K. , Kugimiya, S. , & Takabayashi, J. (2009). Can herbivore‐induced plant volatiles inform predatory insect about the most suitable stage of its prey? Physiological Entomology, 34, 379–386. [Google Scholar]
- Yoneya, K. , & Miki, T. (2015). Co‐evolution of foraging behaviour in herbivores and their natural enemies predicts multifunctionality of herbivore‐induced plant volatiles. Functional Ecology, 29, 451–461. [Google Scholar]
- Zarate, S. I. , Kempema, L. A. , & Walling, L. L. (2007). Silverleaf whitefly induces salicylic acid Defenses and suppresses effectual jasmonic acid defenses. Plant Physiology, 143, 866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, P. , Zheng, S. , van Loon, J. , Boland, W. , David, A. , Mumm, R. , & Dicke, M. (2009). Whiteflies interfere with indirect plant defense against spider mites in Lima bean. Proceedings of the National Academy of Sciences of the United States of America, 106, 21202–21207. [DOI] [PMC free article] [PubMed] [Google Scholar]


