Summary
Primary infection with varicella zoster virus (VZV), an exclusively human neurotrophic alphaherpsesvirus, results in varicella, known more commonly as chickenpox. Like other alphaherpesviruses, VZV establishes latency in the sensory ganglia and can reactivate to cause herpes zoster (also known as shingles), a painful and debilitating disease, especially in elderly and immunocompromised individuals. The overall incidence of herpes zoster in Europe and the United States is three per 1000 people, but increases sharply after 60 years of age to 10 per 1000 people. Zostavax® is a vaccine approved by the Federal Drug Administration for the prevention of herpes zoster. Unfortunately, this vaccine reduces the incidence of disease by only 51% and the incidence of post‐herpetic neuralgia by 66·5% when administered to those aged 60 and older. Moreover, it is contraindicated for individuals who are immunocompromised or receiving immunosuppressant treatments, although they are at higher risk for herpes zoster compared to immune‐competent older individuals. This paper reviews VZV pathogenesis, host responses and current vaccines available to prevent herpes zoster.
Keywords: herpesvirus, reactivation, shingles, vaccine, Zostavax®
OTHER ARTICLES PUBLISHED IN THIS REVIEW SERIES
Immunosenescence: the importance of considering age in health and disease. Clinical and Experimental Immunology 2017, 187: 1–3.
The convergence of senescence and nutrient sensing during lymphocyte ageing. Clinical and Experimental Immunology 2017, 187: 4–5.
Immune senescence: significance of the stromal microenvironment. Clinical and Experimental Immunology 2017, 187: 6–15.
Innate immune responses in the ageing lung. Clinical and Experimental Immunology 2017, 187: 16–25.
Age‐related alterations in immune responses to West Nile virus infection. Clinical and Experimental Immunology 2017, 187: 26–34.
Intracellular signalling pathways: targets to reverse immunosenescence. Clinical and Experimental Immunology 2017, 187: 35–43.
Ageing and inflammation in patients with HIV infection. Clinical and Experimental Immunology 2017, 187: 44–52.
Considerations for successful cancer immunotherapy in aged hosts. Clinical and Experimental Immunology 2017, 187: 53–63.
Ageing and obesity similarly impair antibody responses. Clinical and Experimental Immunology 2017, 187: 64–70.
The life cycle of a T cell after vaccination – where does immune ageing strike? Clinical and Experimental Immunology 2017, 187: 71–81.
Adult vaccination against tetanus and diphtheria: the European perspective. Clinical and Experimental Immunology 2017, 187: 93–99.
Varicella zoster virus
Varicella zoster virus (VZV) is one of eight human herpesviruses that belongs to the alphaherpesvirus family, together with HSV1 and HSV2. It has a linear double‐stranded DNA genome that is 124 885 base pairs long and encodes 71 unique open reading frames (ORFs) 1. The VZV genome has two isomeric forms consisting of one long and one short covalently linked segments with unique sequences bounded by inverted terminal repeats (ORF69 and ORF70). VZV shares approximately 40 conserved genes with other human herpesviruses that are essential for: viral replication (ORF18 and ORF19), DNA packaging (ORFs 25, 26, 30, 34, 42/45, 43 and 54) tegument proteins (ORF9‐ORF12, 22, 38, 44, 46, 53, 57, 64 and 69), capsid assembly (ORFs 20, 21, 23, 33, 40 and 41) and glycoproteins [gB(ORF31), gC(ORF14), gE(ORF68), gH(ORF37), gI (ORF67), gK(ORF5), gL(ORF60) and gN (ORF9a)] 1, 2, 3. Like other herpesviruses, the VZV virion contains an icosahedral‐shaped nucleocapsid that encloses the viral DNA genome and a lipid envelope containing glycoproteins that facilitate viral entry 1.
VZV entry into cells is not well understood, but is believed to occur through direct fusion with the plasma membrane or endocytosis 4. After viral entry, the virions are uncoated and nucleocapsids attach to nuclear pores and inject their genomic DNA into the nucleus, where it circularizes. Gene expression occurs in a temporal manner with transcription of immediate early, early and then late genes 5. Immediate early and early genes encode for proteins involved in the regulation of gene expression and viral replication, while late genes encode for structural proteins such as nucleocapsids and glycoproteins 6. Viral mRNAs are transported to the cytoplasm, where they undergo translation into proteins that are transported back into the nucleus and used for the viral replication and gene expression to generate viral progeny. Nucleocapsids are then assembled in the nuclei of infected cells. Tegument proteins and glycoproteins are added in the cisternae of the trans‐Golgi network 7. The virus is then released into the cytosol where it fuses with the plasma membrane to bud off. The replication cycle followed by the release of viral progeny only takes 9–12 h in human fibroblasts 5.
Acute VZV infection
Clinical manifestation of varicella
VZV is a highly contagious virus that is spread through the inhalation of saliva droplets containing viral particles or by direct contact with infectious fluid from either varicella or herpes zoster (HZ) vesicles 8, 9. It has an incubation period of 10–21 days, but shorter incubation periods have been observed in immunocompromised people 8, 10. It is believed that VZV replicates initially in the upper respiratory tract and tonsillar lymph nodes before dissemination to the skin. Previous studies proposed a dual viraemia model, where the virus first undergoes amplification in organs such as the liver and spleen followed by a secondary viraemia during which the virus is transported to the skin 11. However, subsequent studies have shown that viral amplification in the spleen and liver is not necessary for viral dissemination to the skin. Studies using fetal human skin xenografts in the severe combined immunodeficiency (SCID) mouse model showed that VZV can disseminate to the skin by infected tonsillar CD4+ T cells that express skin‐homing markers but not by infected skin fibroblasts 12, 13, 14. Other studies suggest that dendritic cells infected at the respiratory mucosa transport VZV to the draining lymph nodes, where they infect T cells that can acquire memory and homing markers and travel to the skin 13, 15. Indeed, more recent studies using time‐of‐flight mass cytometry show that in‐vitro VZV infection VZV remodels tonsillar T cells into activated skin‐homing cells 16, 17. Once infected T cells reach the skin, they are believed to transfer VZV to keratinocytes and skin epidermal cells, resulting in a widespread vesicular rash together with fatigue, fever and itching.
Primary infection of VZV in immune‐competent individuals usually resolves with no complications. However, in individuals who are immunocompromised varicella can be severe and, in some cases, fatal 18, 19. Bacterial infection of skin lesions and pneumonia are the most common complications in both immune‐competent and immunocompromised children 18. Neurological complications are very rare, and occur in one to three per 10 000 cases during acute infection 20. The most serious varicella‐associated neurological complication is acute cerebellar ataxia, which can occur in one in 4000–1 : 100 000 varicella cases in children (depending upon the age of the population studied) 21, 22, 23. Primary infection of varicella has also been associated with increased susceptibility to stroke in immune‐competent children 24 due to VZV infecting endothelial cells lining the cerebral arteries causing inflammation 25. Primary VZV infection in seronegative women during the first 8–20 weeks of gestation could result in fetal varicella syndrome, characterized by cutaneous scars, ocular malformations and limb and central nervous system defects 26. This syndrome occurs in only 1–2% of births to mothers who contract varicella during pregnancy 27. In addition, acute VZV infection during the last 2 weeks of gestation can lead to congenital or neonatal varicella 26. Because of this risk, women of childbearing age are screened for VZV‐specific antibodies, and vaccination is recommended if titres are below detection. People who are at increased risk of severe varicella are often administered anti‐virals such as acyclovir or VZV‐specific immunoglobulins (VariZIG) as prophylaxis following suspected exposure 28.
Immune response during primary infection
Both the innate and adaptive immune responses play a critical role in controlling viral replication during acute infection. The first mechanism of defence is mediated through natural killer (NK) cells and type 1 interferons (IFNs). Indeed, patients who are deficient in NK cells or lack activated NK cells are at increased risk of severe or fatal varicella 29, 30, 31, 32. NK cells can kill VZV‐infected cells by secreting the anti‐viral factor granulysin, which induces apoptosis in infected cells 33. Type 1 and type 2 IFNs have also been shown to inhibit VZV replication in human skin xenografts 14. Moreover, IFN‐α treatment reduces the number of new varicella lesions in cancer paediatric patients when administered within 72 h after the appearance of the rash 34.
Complete resolution of acute infection requires adaptive immune responses 35. Subjects with T cell deficiencies such as those with lymphoma, undergoing chemotherapy or infected with human immunodeficiency virus (HIV) experience severe varicella 36, 37. VZV‐specific T cells can be detected in the blood 3–7 days after the appearance of rash and peak 1–2 weeks later followed by a gradual decline 35, 36, 37, 38. T cell immunity to VZV is primarily a T helper type 1 (Th1) response, with interleukin (IL)‐2, IL‐12, tumour necrosis factor (TNF)‐α and IFN‐γ being the primary cytokines produced 39. IFN‐γ has been shown to induce the clonal expansion of VZV‐specific T cells 40. Although a comprehensive analysis of the specificity of the anti‐VZV T cell response has yet to be conducted, CD8 T cell responses to VZV immediate early genes ORF4, ORF62 and ORF63, tegument protein ORF10, single‐stranded DNA binding protein ORF29 and glycoproteins ORF67 (gI) and ORF68 (gE) have been described 1, 41, 42, 43, 44.
Humoral immunity can be measured within 3 days after the appearance of the rash with the production of immunoglobulin (Ig)M, IgG and IgA antibodies 45. The specificity of the antibody responses was determined using a protein microarray that contained 69 distinct VZV proteins and sera from subjects ranging from 2 to 70 years of age with no current symptoms of varicella or HZ. This analysis showed that antibodies are directed primarily against VZV glycoproteins (ORF5, ORF14, ORF31, ORF37 and ORF68), capsid proteins (ORF20, ORF23, ORF40), tegument proteins (ORF53, ORF9, ORF11) genes involved in replication and virion assembly (ORF25, ORF26, ORF28), immediate early transactivators ORF12, ORF62 and ORF63 and membrane proteins ORF2 and ORF24 46. ORF2, ORF12 and ORF62 were shown to induce the highest antibody responses 46.
Although both T and B cell responses are generated during acute varicella, early production of VZV‐specific T cells, but not antibodies, correlates with reduced severity of clinical symptoms 35. In line with this observation, patients with agammaglobulinaemia have uncomplicated varicella and are equally protected against a second episode of varicella as individuals with normal B cell responses 47. Moreover, treatment with varicella zoster immunoglobin (VariZIG) is most effective when given within 96 h of exposure and recommended to be administered within 10 days of exposure 48.
VZV latency
Like herpes simplex 1 (HSV‐1) and HSV‐2, VZV establishes latency in sensory ganglia, and co‐infection of the same neurone by multiple herpesviruses has been described 49, 50. There are two (non‐mutually exclusive) theories on how VZV reaches the ganglia: (1) VZV enters into the nerve terminals from the vesicular rash hijacking the retrograde transport mechanism to the ganglia 51, 52; and (2) VZV accesses the distal neurones through the haematogenous route, carried by infected T cells that gain access to the ganglia 53.
During latency, VZV DNA is found in both satellite and neurone cells in sensory ganglia 54 as a circular episomal genome with limited viral gene transcription 55, 56. The most frequently expressed genes found during latency include ORF4, ORF21, ORF29, ORF62, ORF63 and ORF66 57, 58, 59. ORF4 and ORF63 were found to be required for the establishment of latency in a rat model where VZV‐infected melanoma cells were injected directly into the spine 60, 61. Additional studies using rat neurones have shown that ORF63 may also play a role in preventing cell apoptosis 62. Interestingly, during latency, transcripts associated with these ORFs are found in the cytoplasm of neurones, whereas during acute replication in fibroblasts they are found in the nucleus of the cell 63, 64.
VZV reactivation
VZV reactivation results in HZ, a painful and often debilitating disease that affects 1 million individuals per year in the United States alone 65. During VZV reactivation, the virus travels ante‐retrograde from the sensory ganglia to the skin nerve terminals, where it infects and replicates in keratinocytes, and epithelial cells, causing polykaryocytes 53 (Fig. 1). The first symptom of HZ is usually severe prodromal pain and burning which precedes the rash 66. Unlike varicella, the HZ rash is restricted to the dermatome innervated by the ganglia from which the virus reactivated 67. VZV can also reactivate without resulting in a rash. When accompanied by pain this is referred to as zoster sine herpete 68, which is difficult to diagnose and can be confirmed only by measuring VZV DNA in the cerebrospinal fluid 69. Asymptomatic reactivations can also occur during episodes of mild stress or immune suppression. For instance, infectious VZV DNA has been recovered from the saliva of astronauts during and after spaceflight in the absence of disease 70.
Figure 1.
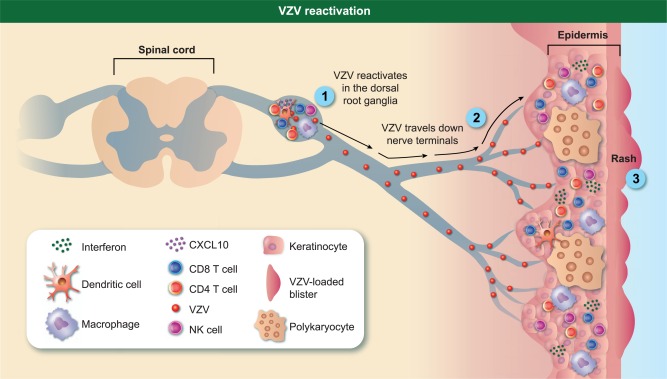
Varicella zoster virus (VZV) immune response during reactivation. When immune responses weaken, VZV reactivates by travelling anterograde towards nerve endings, replicates in keratinocytes and epithelial cells causing the formation of polykaryocytes, leading ultimately to a dermatomal rash. The local immune response in the ganglia is characterized by the infiltration of CD8, CD4, natural killer (NK) cells, macrophages and B cells. The immune response in the skin is characterized by CD4, CD8, NK cells and macrophages along with increased expression of interferon (IFN)‐γ, tumour necrosis factor (TNF)‐α and interleukin (IL)‐6.
The incidence of HZ increases dramatically after the age of 50 years from an average of three cases per 1000 adults aged 40–50 years to 10 cases of HZ per 1000 adults aged 80 years or above 71. HZ also occurs frequently in individuals with autoimmune diseases, cancer and organ transplant recipients receiving immunosuppressive drugs 31. Increased incidence of HZ has also been linked to physical trauma, inflammatory bowel disease and diabetes 72, 73. Interestingly, African Americans are significantly less susceptible to HZ compared to Caucasians 74. In contrast, numerous studies in the United States and Europe have demonstrated that the incidence of HZ is significantly higher in women than men 75, 76, 77, 78, 79.
As described for acute infection, VZV‐specific T cell immunity plays a more critical role in the prevention of VZV reactivation than antibodies 38. VZV antibody responses have been shown to be extremely stable, with half‐lives of approximately 50 years, whereas the frequency of VZV‐specific T cells declines dramatically with age 80, 81. Overall, the frequency of VZV‐specific CD4 T cells declines more dramatically with age compared to VZV‐specific CD8 T cells 65, 82. Moreover, CD4 T cell responses in women are smaller than those observed in men, which may account for the increased incidence of HZ in this segment of the population 83. Whether all T cell responses are lost equally or if there is preferential loss of some clonotypes is not currently known, but limited investigations have shown that CD8 T cell responses against the immediate early transactivators ORF62 and ORF63 are reduced in older individuals 42 and patients with malignancies 84, respectively.
VZV reactivation results in a local immune response in the ganglia (Fig. 1). A study that examined ganglia collected from people who suffered from zoster 1–4·5 months before they died from other causes reported the presence of T cells (75% of which were non‐cytolytic CD8 T cells), B cells, macrophages and NK cells, but no dendritic cells in the ganglia 85. The recruitment of T and NK cells to the ganglia could, potentially, be due to VZV‐induced expression of chemokine CXCL10 from neurones, which binds to CXCR3 to induce migration of memory T cells and NK cells 86. Up‐regulation of major histocompatibility complex (MHC)‐I and MHC‐II molecules during reactivation has also been observed, suggesting a second mechanism for T cell retention and activation in the sensory ganglia 85. Skin biopsies collected during reactivation show the presence of CD4, CD8, NK cells and macrophages, along with increased expression of IFN‐γ, TNF‐α and IL‐6, compared to skin biopsies from healthy controls 87 (Fig. 1).
As with primary infection, HZ generally resolves with no complications; however, 26% of patients experience additional complications that are, on rare occasions, fatal 88. The most common complication is post‐herpetic neuralgia (PHN, 19% of HZ cases), defined as chronic pain in the affected dermatomes lasting many months after resolution of the rash due presumably to damaged nerve ending 88. Another serious complication is herpes zoster ophthalmicus (1–10% of HZ cases), where reactivation from the first division of the trigeminal nerve leads to chronic ocular inflammation that could lead ultimately to blindness 88, 89. Additional rare complications include vasculopathy (which can occur with or without rash) where the virus infects the cerebral arteries and causes ischaemic infarctions in the brain or spinal cord, leading to stroke, aneurysm and cerebral haemorrhaging 90. In immunocompromised individuals, VZV reactivation may also result in myelopathy, where the virus infects the spinal cord or spinal arteries 91.
Treatment for most of these complications include anti‐viral therapy; however, the efficacy of anti‐viral drugs initiated later than 72 h after the appearance of the rash is uncertain 92. Corticosteroids have also been shown to reduce morbidity, although their efficacy is short‐lived and does not reduce the risk of PHN 93. Other treatments for PHN include anti‐depressants and anti‐epileptic drugs such as gabapentin 94, 95, opiate analgesic drugs and topical anaesthetic drugs such as lidocaine and capsaicin 96. VZV vasculopathy and myelitis are treated with intravenous acyclovir 90. Unfortunately, a large proportion of patients do not respond to these treatments, or have only moderate relief of pain or adverse side effects to the drugs 97.
Vaccines against herpes zoster
Live attenuated Zostavax®
The Food and Drug Administration (FDA) approved Zostavax® for the prevention in zoster in people aged 60 years and older in May 2006 98. This vaccine contains 19 400 plaque‐forming units (PFU)/dose compared to the varicella vaccine Varivax®, which contains ∼1350 PFU/dose of live attenuated virus 71. Zostavax® was approved after the completion of the Shingles Prevention Study (SPS), a double‐blind, placebo‐controlled study that involved 38 546 people over the age of 60 years 99. Results from the SPS showed that Zostavax® reduced the incidence of disease by 51% and lowered the incidence of PHN and associated pain by 66·5% in subjects aged at least 60 years 99. Efficacy of this vaccine against HZ decreases with increasing age, with only 18% efficacy in individuals aged more than 80 years 99. The FDA lowered the age requirement to 50 years in 2011 due to the increased efficacy in adults aged 50–59 (70%) 100, 101. Long‐term efficacy of this vaccine was shown to drop to 21·1% for HZ and 35·4% for the PHN during the course of 7–10 years 102. A booster dose of Zostavax® 10 years after the first dose has been shown to enhance protection against HZ in people over the age of 70 years 81. Therefore, like Varivax®, boosters may be recommended for this population.
Zostavax® vaccination induces a significant increase in VZV cell‐mediated responses compared to placebo recipients 6 weeks after vaccination; however, as described for efficacy, vaccine‐induced increases in T cell responses correlates negatively with the age of the recipient 103. Moreover, vaccine‐induced cell‐mediated immunity declined dramatically 1 year post‐vaccination, and at the end of a 3‐year follow up T cell immunity had returned to almost pre‐vaccination levels 99. A recent study showed that Zostavax® vaccination increases CD4 T cell responses to ORFs 40, 67, 9, 59, 12, 62 and 18 1 month after vaccination. However, after 6 months only CD4 T cells responses to ORFs 40, 59, 63 and 67 remained higher than pre‐vaccination levels 104. Interestingly, T cell responses to VZV Oka vaccine strain cross‐recognize HSV‐1 and HSV‐2 antigens, which may indicate that the Zostavax® vaccine also provides some degree of protection against HSV 105. Zostavax® vaccination increases a humoral immune response to VZV, albeit to a lesser extent compared to levels achieved after VZV reactivation in the absence of vaccination 65. Zostavax® can boost the antibody titre of individuals who had previously had HZ, and the magnitude of this boost is correlated negatively with time since HZ 106.
Adverse reactions to Zostavax® include mainly pain and inflammation at the site of infection 107. Concomitant vaccine administration of zoster and influenza vaccine or pneumococcal vaccines does not affect their immunogenicities adversely 108, 109. Although Zostavax® vaccine has been proved to be safe for HIV‐infected people with 15% CD4 T cells or a CD4 T cell count of 200 cells/μl, it is contraindicated for people who are: (1) taking steroids (40 mg per day for more than 7 days or 20 mg per day for more than 14 days), (2) receiving biologicals such as anti‐TNF (in the past 12 months) or (3) currently undergoing or underwent radiation or chemotherapy in the past 6 months 110, 111, 112. The Center for Disease Control (CDC) also recommends that newly vaccinated people avoid contact with individuals at high risk for varicella complications such immune‐compromised patients. Infectious viral DNA has been found in saliva from Zostavax® vaccinated individuals for up to 4 weeks post‐vaccination 113.
Inactivated adjuvanted subunit vaccine
Another potential strategy to develop a more efficacious vaccine is to generate a subunit vaccine that expresses immunogenic VZV proteins. Subunit vaccines can provoke a strong immune response while being safe for individuals for whom live attenuated vaccines are contraindicated. Two doses of a subunit vaccine (HZ/su) developed by GlaxoSmithKline using adjuvanted recombinant glycoprotein E (ORF68) has shown great immunogenicity and efficacy in older individuals regardless of their age. A Phase III study with HZ/su was completed recently, with a total of 15 411 participants in which 8926 participants received the vaccine and 4466 served as the placebo group. In a 3·2‐year follow up study, the vaccine showed overall 97·2% efficacy rate among all three age groups tested (50–59, 60–69 and ≥ 70 years) 114. However, 81·5% of HZ/su recipients in the Phase III study experienced pain at the site of injection and 66% of recipients had mild to moderate systemic reactions (grade 3 severity in 11·4% of the subjects) such as myalgia (most common), fatigue and headache 114. Glycoprotein E was selected for this subunit vaccine because it is the most abundant viral glycoprotein, and also elicits specific CD4 T cell responses 115, 116. The adjuvant being used is ASO1, which is a liposome‐based adjuvant system containing 3‐O‐desacyl‐4′‐monophosphoryl and saponin QS‐21, which activates the Toll‐like receptor (TLR)‐4 pathway and stimulates both antibody and T helper type 1 (Th1) responses 117. Two doses of HZ/su elicited a stronger anti‐VZV gE, anti‐VZV lysate antibody and anti‐VZV lysate CD4 T cell response than two doses of Zostavax® in adults aged 50–70 years during a 12‐month follow‐up period 118. CD4‐specific T cells and antibody levels to HZ/su dropped by almost half after 42 months 118. Three doses of the HZ/su vaccine were shown to be safe and immunogenic in haematopoietic cell transplants patients for up to 1 year and HIV patients for up to 18 months 119, 120.
Heat‐inactivated vaccines
A heat‐inactivated Varivax® (V212) vaccine may be beneficial to bone marrow transplant recipients and recipients of haematopoietic‐cell transplants 121, 122. Randomized, double‐blind, placebo‐controlled Phase III studies are being conducted currently to test the safety and efficacy of V212 in participants with solid tumours and haematological malignancies during a 5‐year period. Study completion date is expected to the end of February 2017. The pros and cons for the Zostervax®, HZ/su and V212 vaccines are described in Table 1.
Table 1.
Pros and cons for Zostervax®, herpes zoster/subunit vaccine (HZ/su) and V212 vaccines
| Vaccine | Pros | Cons |
|---|---|---|
|
Live, attenuated vaccine Zostavax® |
• Boosts both cellular and humoral immune responses |
• Contraindicated for people with weakened immune systems • Potential for shedding and therefore transmission to people with weakened immune system • Vaccine needs to stay frozen in order to remain potent • Immunity and efficacy wane with increasing age and with time since vaccination |
|
Subunit vaccine HZ/su |
• Only contains one viral protein, making it safe for immune‐deficient people and pregnant women |
• Requires 2 doses • High incidence of adverse events due to the potency of the adjuvant |
|
Heat‐inactivated V212 |
• Safe for immune‐deficient people and pregnant women • Does not require refrigeration |
• Stimulates a weaker immune response than live vaccines • Requires 4 doses, making compliance an issue |
Animal models of VZV studies
Our understanding of VZV pathogenesis remains incomplete. Gaps in our knowledge include: how VZV traffics to the skin and ganglia; when VZV establishes latency; viral transcription profile in the ganglia during acute infection and latency; and host and viral factors that play a role in reactivation. These questions could potentially be addressed by having a robust animal model of VZV infection. However, experimental inoculations of several animal models with VZV have failed to recapitulate all the essential features of VZV infection due to the strict human specificity of VZV. Specifically, infection of rodent models and non‐human primates leads to the establishment of latency without viraemia or rash 60, 61. Similarly, infection of non‐human primates results in abortive infection 123.
Due to the limitations of rodent models, an alternative model has been developed using the non‐human primate homologue of VZV, simian varicella virus (SVV). The structure and size of the SVV genome is related closely to that of VZV 124, 125. Immunization of monkeys with VZV can protect against SVV challenge indicative of antigenic similarities 126. Intrabronchial infection of rhesus macaques with SVV reproduces the cardinal features of VZV infection in humans, including viraemia, replication in lungs, development of cellular and humoral immunity and the establishment of latency in the sensory ganglia with limited transcriptional profile 64, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136.
Using this model, we showed that CD4 T cells play a critical role in controlling acute infection 135 as well as the establishment of latency 136. We also characterized the specificity of the T cell response to SVV during acute infection and latency using IFN‐γ enzyme‐linked immunospot (ELISPOT) following stimulation with overlapping peptide libraries that covered the entire SVV genome 137. Our data show a robust and broad T cell response during acute infection with CD8 T cell responses directed mainly against immediate early and early viral proteins, while CD4 T cell responses were directed against SVV late genes. During latent infection, T cell responses were reduced significantly in magnitude and breadth compared to those observed during acute infection 137. Interestingly, T cell responses against ORF4, ORF11, ORF19, ORF31 and ORF 37 were maintained into latency, albeit at lower levels, whereas T cell responses to ORF10, ORF20, ORF29, ORF31, ORF62, ORF63 and ORF68 showed a significant decrease of about 83% between primary and latent infection 137. These observations may explain the success of the subunit vaccine HZ/su, which contains adjuvanted ORF68 protein and can potentially aid in the development of a multivalent subunit vaccine.
Studies using this model have also shed light on VZV trafficking to the ganglia. African green macaques (AGMs) infected with SVV expressing enhanced green fluorescent protein show that SVV primarily infects memory T cells and demonstrated the presence of SVV infected memory T cells in the ganglia, supporting a role for T cells in transporting SVV into the ganglia 138. This study also showed the infiltration of memory CD8 T cells (most abundant), CD4 T cells, as well as dendritic cells and macrophages into the ganglia during acute SVV infection of AGMs 139. However, given the severity of SVV infection in AGMs and the differences between AGMs and human immune systems, these studies should be validated further in the rhesus macaque model. As described for VZV, SVV can be reactive in macaques that undergo radiation combined with immune suppressive treatments 134. As described for VZV in humans, CD4 and CD8 T cells infiltrate the ganglia during SVV reactivation, which correlates with CXCL10 expression 140.
Conclusions
The need for a more effective vaccine against VZV is imperative, due to the increased frequency of older individuals. In fact, the number of individuals over the age of 65 is projected to double or triple by 2050 in most of the developed and developing world. Given the important role of T cell immunity in preventing reactivation, we need to develop novel vaccine strategies that specifically boost T cell immunity. Unfortunately, although several studies have demonstrated T cell responses to specific ORFs, no study to date has examined the immunogenicity of the entire VZV proteome and how it changes with age. Consequently, we do not yet have a complete understanding of which T cell responses decline most significantly with age and should be boosted. We also do not know which T cell responses are protective or how many VZV‐specific T cells are required for protection against reactivation. We should be cautiously optimistic about the early success of the HZ/su vaccine. A similar strategy was employed to develop a vaccine against HSV‐1 and HSV‐2 using gD, which is also the most abundant protein in HSV. However, despite great success in animal models, this subunit vaccine failed in clinical trials 141, 142. Therefore, using subunit vaccines that contain more than just one gene may be more successful at inducing a longer and more protective immune response. Studies have shown that only 6·7% of individuals over the age of 60 have received the current vaccine available for HZ in the United States 143. Therefore, it is especially important to educate people and talk to physicians about this disease and the importance of being vaccinated.
Disclosure
Authors have no competing interests to disclose.
References
- 1. Cohen JI. The varicella‐zoster virus genome. Curr Top Microbiol Immunol 2010; 342:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang Z, Selariu A, Warden C et al Genome‐wide mutagenesis reveals that ORF7 is a novel VZV skin‐tropic factor. PLoS Pathog 2010; 6:e1000971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Visalli MA, House BL, Selariu A, Zhu H, Visalli RJ. The varicella‐zoster virus portal protein is essential for cleavage and packaging of viral DNA. J Virol 2014; 88:7973–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Campadelli‐Fiume G, Menotti L. Entry of alphaherpesviruses into the cell In: Arvin A, Campadelli‐Fiume G, Mocarski E. et al eds. Human herpesviruses: biology, therapy, and immunoprophylaxis. Cambridge: Cambridge University Press; 2007. [PubMed] [Google Scholar]
- 5. Reichelt M, Brady J, Arvin AM. The replication cycle of varicella‐zoster virus: analysis of the kinetics of viral protein expression, genome synthesis, and virion assembly at the single‐cell level. J Virol 2009; 83:3904–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmader K. Herpes zoster in older adults. Clin Infect Dis 2001; 32:1481–6. [DOI] [PubMed] [Google Scholar]
- 7. Zerboni L, Sen N, Oliver SL, Arvin AM. Molecular mechanisms of varicella zoster virus pathogenesis. Nat Rev 2014; 12:197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arvin AM. Varicella‐zoster virus: molecular virology and virus–host interactions. Curr Opin Microbiol 2001; 4:442–9. [DOI] [PubMed] [Google Scholar]
- 9. Grose C. Immunization of inbred guinea pigs with varicella‐zoster virus grown in a syngeneic transformed embryo cell line. J Clin Microbiol 1981; 14:229–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cohen JI. Mutagenesis of the varicella‐zoster virus genome: lessons learned. Arch Virol Suppl 2001; 91–7. [DOI] [PubMed] [Google Scholar]
- 11. Grose C. Variation on a theme by Fenner: the pathogenesis of chickenpox. Pediatrics 1981; 68:735–7. [PubMed] [Google Scholar]
- 12. Moffat JF, Zerboni L, Sommer MH et al The ORF47 and ORF66 putative protein kinases of varicella‐zoster virus determine tropism for human T cells and skin in the SCID‐hu mouse. Proc Natl Acad Sci USA 1998; 95:11969–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ku CC, Padilla JA, Grose C, Butcher EC, Arvin AM. Tropism of varicella‐zoster virus for human tonsillar CD4(+) T lymphocytes that express activation, memory, and skin homing markers. J Virol 2002; 76:11425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ku CC, Zerboni L, Ito H, Graham BS, Wallace M, Arvin AM. Varicella‐zoster virus transfer to skin by T cells and modulation of viral replication by epidermal cell interferon‐alpha. J Exp Med 2004; 200:917–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abendroth A, Morrow G, Cunningham AL, Slobedman B. Varicella‐zoster virus infection of human dendritic cells and transmission to T cells: implications for virus dissemination in the host. J Virol 2001; 75:6183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sen N, Mukherjee G, Sen A et al Single‐cell mass cytometry analysis of human tonsil T cell remodeling by varicella zoster virus. Cell Rep 2014; 8:633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sen N, Arvin AM. Dissecting the molecular mechanisms of the tropism of varicella‐zoster virus for human T cells. J Virol 2016; 90:3284–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gnann JW Jr. Varicella‐zoster virus: atypical presentations and unusual complications. J Infect Dis 2002; 186: S91–8. [DOI] [PubMed] [Google Scholar]
- 19. Wiegering V, Schick J, Beer M et al Varicella‐zoster virus infections in immunocompromised patients – a single centre 6‐years analysis. BMC Pediatr 2011; 11:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Paul R, Singhania P, Hashmi M, Bandyopadhyay R, Banerjee AK. Post chicken pox neurological sequelae: three distinct presentations. J Neurosci Rural Pract 2010; 1:92–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guess HA, Broughton DD, Melton LJ, Kurland LT III. Population‐based studies of varicella complications. Pediatrics 1986; 78:723–7. [PubMed] [Google Scholar]
- 22. Bozzola E, Bozzola M, Tozzi AE et al Acute cerebellitis in varicella: a ten year case series and systematic review of the literature. Ital J Pediatr 2014; 40:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van der Maas NA, Bondt PE, de Melker H, Kemmeren JM. Acute cerebellar ataxia in the Netherlands: a study on the association with vaccinations and varicella zoster infection. Vaccine 2009; 27:1970–3. [DOI] [PubMed] [Google Scholar]
- 24. Askalan R, Laughlin S, Mayank S et al Chickenpox and stroke in childhood: a study of frequency and causation. Stroke 2001; 32:1257–62. [DOI] [PubMed] [Google Scholar]
- 25. Gershon AA. Strokes and infection with varicella zoster virus. Clin Infect Dis 2014; 58:69–71. [DOI] [PubMed] [Google Scholar]
- 26. Ramachandra S, Metta AK, Haneef NS, Kodali S. Fetal varicella syndrome. Indian J Dermatol Venereol Leprol 2010; 76:724. [DOI] [PubMed] [Google Scholar]
- 27. Bruder E, Ersch J, Hebisch G, Ehrbar T, Klimkait T, Stallmach T. Fetal varicella syndrome: disruption of neural development and persistent inflammation of non‐neural tissues. Virchows Arch 2000; 437:440–4. [DOI] [PubMed] [Google Scholar]
- 28. Cohen J, Breuer J. Chickenpox: treatment. BMJ Clin Evid 2015: 2015: 0912. [PMC free article] [PubMed] [Google Scholar]
- 29. Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med 1989; 320:1731–5. [DOI] [PubMed] [Google Scholar]
- 30. Etzioni A, Eidenschenk C, Katz R, Beck R, Casanova JL, Pollack S. Fatal varicella associated with selective natural killer cell deficiency. J Pediatr 2005; 146:423–5. [DOI] [PubMed] [Google Scholar]
- 31. Yawn BP, Wollan PC, Kurland MJ, St Sauver JL, Saddier P. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc 2011; 86:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vossen MT, Biezeveld MH, de Jong MD et al Absence of circulating natural killer and primed CD8+ cells in life‐threatening varicella. J Infect Dis 2005; 191:198–206. [DOI] [PubMed] [Google Scholar]
- 33. Levy O, Orange JS, Hibberd P et al Disseminated varicella infection due to the vaccine strain of varicella‐zoster virus, in a patient with a novel deficiency in natural killer T cells. J Infect Dis 2003; 188:948–53. [DOI] [PubMed] [Google Scholar]
- 34. Arvin AM, Kushner JH, Feldman S, Baehner RL, Hammond D, Merigan TC. Human leukocyte interferon for the treatment of varicella in children with cancer. N Engl J Med 1982; 306:761–5. [DOI] [PubMed] [Google Scholar]
- 35. Arvin AM, Koropchak CM, Williams BR, Grumet FC, Foung SK. Early immune response in healthy and immunocompromised subjects with primary varicella‐zoster virus infection. J Infect Dis 1986; 154:422–9. [DOI] [PubMed] [Google Scholar]
- 36. Buchbinder SP, Katz MH, Hessol NA et al Herpes zoster and human immunodeficiency virus infection. J Infect Dis 1992; 166:1153–6. [DOI] [PubMed] [Google Scholar]
- 37. Jura E, Chadwick EG, Josephs SH et al Varicella‐zoster virus infections in children infected with human immunodeficiency virus. Pediatr Infect Dis J 1989; 8:586–90. [DOI] [PubMed] [Google Scholar]
- 38. Weinberg A, Lazar AA, Zerbe GO et al Influence of age and nature of primary infection on varicella‐zoster virus‐specific cell‐mediated immune responses. J Infect Dis 2010; 201:1024–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Torigoe S, Ihara T, Kamiya HIL. 12, IFN‐gamma and TNF‐alpha released from mononuclear cells inhibit the spread of varicella‐zoster virus at an early stage of varicella. Microbiology and Immunology 2000; 44:1027–31. [DOI] [PubMed] [Google Scholar]
- 40. Wallace MR, Woelfl I, Bowler WA et al Tumor necrosis factor, interleukin‐2, and interferon‐gamma in adult varicella. J Med Virol 1994; 43:69–71. [DOI] [PubMed] [Google Scholar]
- 41. Sadzot‐Delvaux C, Kinchington PR, Debrus S, Rentier B, Arvin AM. Recognition of the latency‐associated immediate early protein IE63 of varicella‐zoster virus by human memory T lymphocytes. J Immunol 1997; 159:2802–6. [PubMed] [Google Scholar]
- 42. Arvin AM, Sharp M, Moir M et al Memory cytotoxic T cell responses to viral tegument and regulatory proteins encoded by open reading frames 4, 10, 29, and 62 of varicella‐zoster virus. Viral Immunol 2002; 15:507–16. [DOI] [PubMed] [Google Scholar]
- 43. Malavige GN, Jones L, Black AP, Ogg GS. Rapid effector function of varicella‐zoster virus glycoprotein I‐specific CD4+ T cells many decades after primary infection. J Infect Dis 2007; 195:660–4. [DOI] [PubMed] [Google Scholar]
- 44. Malavige GN, Jones L, Black AP, Ogg GS. Varicella zoster virus glycoprotein E‐specific CD4+ T cells show evidence of recent activation and effector differentiation, consistent with frequent exposure to replicative cycle antigens in healthy immune donors. Clin Exp Immunol 2008; 152:522–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Arvin AM. Varicella‐zoster virus. Clin Microbiol Rev 1996; 9:361–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ceroni A, Sibani S, Baiker A et al Systematic analysis of the IgG antibody immune response against varicella zoster virus (VZV) using a self‐assembled protein microarray. Mol Biosyst 2010; 6:1604–10. [DOI] [PubMed] [Google Scholar]
- 47. Good RA, Zak SJ. Disturbances in gamma globulin synthesis as experiments of nature. Pediatrics 1956; 18:109–49. [PubMed] [Google Scholar]
- 48. Centers for Disease Control and Prevention. Updated recommendations for use of VariZIG – United States, 2013. Morb Mortal Wkly Rep 2013; 62:574–6. [PMC free article] [PubMed] [Google Scholar]
- 49. Gilden DH, Vafai A, Shtram Y, Becker Y, Devlin M, Wellish M. Varicella‐zoster virus DNA in human sensory ganglia. Nature 1983; 306:478–80. [DOI] [PubMed] [Google Scholar]
- 50. Sloutskin A, Yee MB, Kinchington PR, Goldstein RS. Varicella zoster virus and herpes simplex virus type 1 can infect and replicate in the same neurons whether co‐ or superinfected. J Virol 2014; 88:5079–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bearer EL, Breakefield XO, Schuback D, Reese TS, LaVail JH. Retrograde axonal transport of herpes simplex virus: evidence for a single mechanism and a role for tegument. Proc Natl Acad Sci USA 2000; 97:8146–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Eshleman E, Shahzad A, Cohrs RJ. Varicella zoster virus latency. Future Virol 2011; 6:341–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Arvin AM, Moffat JF, Sommer M et al Varicella‐zoster virus T cell tropism and the pathogenesis of skin infection. Curr Top Microbiol Immunol 2010; 342:189–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lungu O, Annunziato PW, Gershon A et al Reactivated and latent varicella‐zoster virus in human dorsal root ganglia. Proc Natl Acad Sci USA 1995; 92:10980–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Clarke P, Beer T, Cohrs R, Gilden DH. Configuration of latent varicella‐zoster virus DNA. J Virol 1995; 69:8151–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cohrs R, Mahalingam R, Dueland AN, Wolf W, Wellish M, Gilden DH. Restricted transcription of varicella‐zoster virus in latently infected human trigeminal and thoracic ganglia. J Infect Dis 1992; 166:S24–9. [DOI] [PubMed] [Google Scholar]
- 57. Cohrs RJ, Barbour M, Gilden DH. Varicella‐zoster virus (VZV) transcription during latency in human ganglia: detection of transcripts mapping to genes 21, 29, 62, and 63 in a cDNA library enriched for VZV RNA. J Virol 1996; 70:2789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kennedy PG, Grinfeld E, Gow JW. Latent varicella‐zoster virus in human dorsal root ganglia. Virology 1999; 258:451–4. [DOI] [PubMed] [Google Scholar]
- 59. Cohrs RJ, Gilden DH, Kinchington PR, Grinfeld E, Kennedy PG. Varicella‐zoster virus gene 66 transcription and translation in latently infected human Ganglia. J Virol 2003; 77:6660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cohen JI, Cox E, Pesnicak L, Srinivas S, Krogmann T. The varicella‐zoster virus open reading frame 63 latency‐associated protein is critical for establishment of latency. J Virol 2004; 78:11833–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cohen JI, Krogmann T, Ross JP, Pesnicak L, Prikhod'ko EA. Varicella‐zoster virus ORF4 latency‐associated protein is important for establishment of latency. J Virol 2005; 79:6969–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hood C, Cunningham AL, Slobedman B et al Varicella‐zoster virus ORF63 inhibits apoptosis of primary human neurons. J Virol 2006; 80:1025–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lungu O, Panagiotidis CA, Annunziato PW, Gershon AA, Silverstein SJ. Aberrant intracellular localization of varicella‐zoster virus regulatory proteins during latency. Proc Natl Acad Sci USA 1998; 95:7080–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kennedy PG, Grinfeld E, Traina‐Dorge V, Gilden DH, Mahalingam R. Neuronal localization of simian varicella virus DNA in ganglia of naturally infected African green monkeys. Virus Genes 2004; 28:273–6. [DOI] [PubMed] [Google Scholar]
- 65. Weinberg A, Zhang JH, Oxman MN et al Varicella‐zoster virus‐specific immune responses to herpes zoster in elderly participants in a trial of a clinically effective zoster vaccine. J Infect Dis 2009; 200:1068–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wareham DW, Breuer J. Herpes zoster. BMJ 2007; 334:1211–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Oxman MN. Immunization to reduce the frequency and severity of herpes zoster and its complications. Neurology 1995; 45:S41–6. [DOI] [PubMed] [Google Scholar]
- 68. Schwab IR. Herpes zoster sine herpete. A potential cause of iridoplegic granulomatous iridocyclitis. Ophthalmology 1997; 104:1421–5. [DOI] [PubMed] [Google Scholar]
- 69. Furuta Y, Ohtani F, Mesuda Y, Fukuda S, Inuyama Y. Early diagnosis of zoster sine herpete and antiviral therapy for the treatment of facial palsy. Neurology 2000; 55:708–10. [DOI] [PubMed] [Google Scholar]
- 70. Cohrs RJ, Mehta SK, Schmid DS, Gilden DH, Pierson DL. Asymptomatic reactivation and shed of infectious varicella zoster virus in astronauts. J Med Virol 2008; 80:1116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Keating GM. Shingles (herpes zoster) vaccine (zostavax((R))): a review of its use in the prevention of herpes zoster and postherpetic neuralgia in adults aged >/=50 years. Drugs 2013; 73:1227–44. [DOI] [PubMed] [Google Scholar]
- 72. Long MD, Martin C, Sandler RS, Kappelman MD. Increased risk of herpes zoster among 108 604 patients with inflammatory bowel disease. Aliment Pharmacol Ther 2013; 37:420–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhang JX, Joesoef RM, Bialek S, Wang C, Harpaz R. Association of physical trauma with risk of herpes zoster among Medicare beneficiaries in the United States. J Infect Dis 2013; 207:1007–11. [DOI] [PubMed] [Google Scholar]
- 74. Schmader K, George LK, Burchett BM, Hamilton JD, Pieper CF. Race and stress in the incidence of herpes zoster in older adults. J Am Geriatr Soc 1998; 46:973–7. [DOI] [PubMed] [Google Scholar]
- 75. Insinga RP, Itzler RF, Pellissier JM, Saddier P, Nikas AA. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med 2005; 20:748–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fleming DM, Cross KW, Cobb WA, Chapman RS. Gender difference in the incidence of shingles. Epidemiol Infect 2004; 132:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Opstelten W, Van Essen GA, Schellevis F, Verheij TJ, Moons KG. Gender as an independent risk factor for herpes zoster: a population‐based prospective study. Ann Epidemiol 2006; 16:692–5. [DOI] [PubMed] [Google Scholar]
- 78. Ultsch B, Siedler A, Rieck T, Reinhold T, Krause G, Wichmann O. Herpes zoster in Germany: quantifying the burden of disease. BMC Infect Dis 2011; 11:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gialloreti LE, Merito M, Pezzotti P et al Epidemiology and economic burden of herpes zoster and post‐herpetic neuralgia in Italy: a retrospective, population‐based study. BMC Infect Dis 2010; 10:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med 2007; 357:1903–15. [DOI] [PubMed] [Google Scholar]
- 81. Levin MJ, Smith JG, Kaufhold RM et al Decline in varicella‐zoster virus (VZV)‐specific cell‐mediated immunity with increasing age and boosting with a high‐dose VZV vaccine. J Infect Dis 2003; 188:1336–44. [DOI] [PubMed] [Google Scholar]
- 82. Asanuma H, Sharp M, Maecker HT, Maino VC, Arvin AM. Frequencies of memory T cells specific for varicella‐zoster virus, herpes simplex virus, and cytomegalovirus by intracellular detection of cytokine expression. J Infect Dis 2000; 181:859–66. [DOI] [PubMed] [Google Scholar]
- 83. Klein NP, Holmes TH, Sharp MA et al Variability and gender differences in memory T cell immunity to varicella‐zoster virus in healthy adults. Vaccine 2006; 24:5913–8. [DOI] [PubMed] [Google Scholar]
- 84. Malavige GN, Rohanachandra LT, Jones L et al IE63‐specific T‐cell responses associate with control of subclinical varicella zoster virus reactivation in individuals with malignancies. Br J Cancer 2010; 102:727–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Steain M, Sutherland JP, Rodriguez M, Cunningham AL, Slobedman B, Abendroth A. Analysis of T cell responses during active varicella‐zoster virus reactivation in human ganglia. J Virol 2014; 88:2704–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Steain M, Gowrishankar K, Rodriguez M, Slobedman B, Abendroth A. Upregulation of CXCL10 in human dorsal root ganglia during experimental and natural varicella‐zoster virus infection. J Virol 2011; 85:626–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nikkels AF, Sadzot‐Delvaux C, Pierard GE. Absence of intercellular adhesion molecule 1 expression in varicella zoster virus‐infected keratinocytes during herpes zoster: another immune evasion strategy? Am J Dermatopathol 2004; 26:27–32. [DOI] [PubMed] [Google Scholar]
- 88. Volpi A. Severe complications of herpes zoster. Herpes 2007; 14:35–9. [PubMed] [Google Scholar]
- 89. Liesegang TJ. Herpes zoster ophthalmicus natural history, risk factors, clinical presentation, and morbidity. Ophthalmology 2008; 115:S3–12. [DOI] [PubMed] [Google Scholar]
- 90. Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol 2009; 8:731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gilden D, Nagel MA, Ransohoff RM, Cohrs RJ, Mahalingam R, Tanabe JL. Recurrent varicella zoster virus myelopathy. J Neurol Sci 2009; 276:196–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Johnson RW, Wasner G, Saddier P, Baron R. Herpes zoster and postherpetic neuralgia: optimizing management in the elderly patient. Drugs Aging 2008; 25:991–1006. [DOI] [PubMed] [Google Scholar]
- 93. Li Q, Chen N, Yang J et al Antiviral treatment for preventing postherpetic neuralgia. Cochrane Database Syst Rev 2009; CD006866. [DOI] [PubMed] [Google Scholar]
- 94. Mehta N, Bucior I, Bujanover S, Shah R, Gulati A. Relationship between pain relief, reduction in pain‐associated sleep interference, and overall impression of improvement in patients with postherpetic neuralgia treated with extended‐release gabapentin. Health Qual Life Outcomes 2016; 14:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Moore RA, Wiffen PJ, Derry S, Toelle T, Rice AS. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev 2014; 4:CD007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Johnson RW, Rice AS. Clinical practice. Postherpetic neuralgia. N Engl J Med 2014; 371:1526–33. [DOI] [PubMed] [Google Scholar]
- 97. Watson CP, Tyler KL, Bickers DR, Millikan LE, Smith S, Coleman E. A randomized vehicle‐controlled trial of topical capsaicin in the treatment of postherpetic neuralgia. Clin Ther 1993; 15:510–26. [PubMed] [Google Scholar]
- 98. Harpaz R, Ortega‐Sanchez IR, Seward JF, Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb Mort Wkly Rep Recomm Rep 2008; 57:1–30; quiz CE2‐4. [PubMed] [Google Scholar]
- 99. Oxman MN, Levin MJ, Johnson GR et al A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271–84. [DOI] [PubMed] [Google Scholar]
- 100. Sutradhar SC, Wang WW, Schlienger K et al Comparison of the levels of immunogenicity and safety of Zostavax in adults 50 to 59 years old and in adults 60 years old or older. Clin Vaccine Immunol 2009; 16:646–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.US Food and Drug Administration. FDA approves Zostavax vaccine to prevent shingles in individuals 50 to 59 years of age; 2011. Available at: www.fdagovNewsEvents/Newsroom/PressAnnouncements/ucm248390htm.
- 102. Morrison VA, Johnson GR, Schmader KE et al Long‐term persistence of zoster vaccine efficacy. Clin Infect Dis 2015; 60:900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Levin MJ, Oxman MN, Zhang JH et al Varicella‐zoster virus‐specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis 2008; 197:825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Laing KJ, Russell RM, Dong L et al Zoster vaccination increases the breadth of CD4+ T cells responsive to varicella zoster virus. J Infect Dis 2015; 212:1022–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Jing L, Laing KJ, Dong L et al Extensive CD4 and CD8 T cell cross‐reactivity between alpha herpes viruses. J Immunol 2016; 196:2205–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Mills R, Tyring SK, Levin MJ et al Safety, tolerability, and immunogenicity of zoster vaccine in subjects with a history of herpes zoster. Vaccine 2010; 28:4204–9. [DOI] [PubMed] [Google Scholar]
- 107. Schmader KE, Levin MJ, Gnann JW Jr et al Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50‐59 years. Clin Infect Dis 2012; 54:922–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kerzner B, Murray AV, Cheng E et al Safety and immunogenicity profile of the concomitant administration of ZOSTAVAX and inactivated influenza vaccine in adults aged 50 and older. J Am Geriatr Soc 2007; 55:1499–507. [DOI] [PubMed] [Google Scholar]
- 109. Wyman MJ, Stabi KL. Concomitant administration of pneumococcal‐23 and zoster vaccines provides adequate herpes zoster coverage. Ann Pharmacother 2013; 47:1064–8. [DOI] [PubMed] [Google Scholar]
- 110. Levin MJ, Gershon AA, Weinberg A, Song LY, Fentin T, Nowak B. Administration of live varicella vaccine to HIV‐infected children with current or past significant depression of CD4(+) T cells. J Infect Dis 2006; 194:247–55. [DOI] [PubMed] [Google Scholar]
- 111. Sanford M, Keating GM. Zoster vaccine (Zostavax): a review of its use in preventing herpes zoster and postherpetic neuralgia in older adults. Drugs Aging 2010; 27:159–76. [DOI] [PubMed] [Google Scholar]
- 112. R Harpaz, IR Ortega‐Sanchez, JF Seward. Advisory Committee on Immunization Practices Centers for Disease C, Prevention. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2008; 57:1–30; quiz CE2‐4. [PubMed] [Google Scholar]
- 113. Perella D, Fiks AG, Jumaan A et al Validity of reported varicella history as a marker for varicella zoster virus immunity among unvaccinated children, adolescents, and young adults in the post‐vaccine licensure era. Pediatrics 2009; 123:e820–8. [DOI] [PubMed] [Google Scholar]
- 114. Lal H, Cunningham AL, Godeaux O et al Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015; 372:2087–96. [DOI] [PubMed] [Google Scholar]
- 115. Harper DR, Kangro HO, Heath RB. Antibody responses in recipients of varicella vaccine assayed by immunoblotting. J Med Virol 1990; 30:61–7. [DOI] [PubMed] [Google Scholar]
- 116. Arvin AM, Kinney‐Thomas E, Shriver K et al Immunity to varicella‐zoster viral glycoproteins, gp I (gp 90/58) and gp III (gp 118), and to a nonglycosylated protein, p 170. J Immunol 1986; 137:1346–51. [PubMed] [Google Scholar]
- 117. Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity 2010; 33:492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Leroux‐Roels I, Leroux‐Roels G, Clement F et al A phase 1/2 clinical trial evaluating safety and immunogenicity of a varicella zoster glycoprotein e subunit vaccine candidate in young and older adults. J Infect Dis 2012; 206:1280–90. [DOI] [PubMed] [Google Scholar]
- 119. Stadtmauer EA, Sullivan KM, Marty FM et al A phase 1/2 study of an adjuvanted varicella‐zoster virus subunit vaccine in autologous hematopoietic cell transplant recipients. Blood 2014; 124:2921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Berkowitz EM, Moyle G, Stellbrink HJ et al Safety and immunogenicity of an adjuvanted herpes zoster subunit candidate vaccine in HIV‐infected adults: a phase 1/2a randomized, placebo‐controlled study. J Infect Dis 2015; 211:1279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Redman RL, Nader S, Zerboni L et al Early reconstitution of immunity and decreased severity of herpes zoster in bone marrow transplant recipients immunized with inactivated varicella vaccine. J Infect Dis 1997; 176:578–85. [DOI] [PubMed] [Google Scholar]
- 122. Hata A, Asanuma H, Rinki M et al Use of an inactivated varicella vaccine in recipients of hematopoietic‐cell transplants. N Engl J Med 2002; 347:26–34. [DOI] [PubMed] [Google Scholar]
- 123. Meyer C, Engelmann F, Arnold N et al Abortive intrabronchial infection of rhesus macaques with varicella‐zoster virus provides partial protection against simian varicella virus challenge. J Virol 2015; 89:1781–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Gray WL, Pumphrey CY, Ruyechan WT, Fletcher TM. The simian varicella virus and varicella zoster virus genomes are similar in size and structure. Virology 1992; 186:562–72. [DOI] [PubMed] [Google Scholar]
- 125. Gray WL. Simian varicella virus: molecular virology. Curr Top Microbiol Immunol 2010; 342:291–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Felsenfeld AD, Schmidt NJ. Varicella‐zoster virus immunizes patas monkeys against simian varicella‐like disease. J Gen Virol 1979; 42:171–8. [DOI] [PubMed] [Google Scholar]
- 127. Mahalingam R, Smith D, Wellish M et al Simian varicella virus DNA in dorsal root ganglia. Proc Natl Acad Sci USA 1991; 88:2750–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Mahalingam R, Clarke P, Wellish M et al Prevalence and distribution of latent simian varicella virus DNA in monkey ganglia. Virology 1992; 188:193–7. [DOI] [PubMed] [Google Scholar]
- 129. Gray WL, Gusick NJ, Fletcher TM, Soike KF. Simian varicella virus antibody response in experimental infection of African green monkeys. J Med Primatol 1995; 24:246–51. [DOI] [PubMed] [Google Scholar]
- 130. Mahalingam R, Wellish M, Soike K, White T, Kleinschmidt‐DeMasters BK, Gilden DH. Simian varicella virus infects ganglia before rash in experimentally infected monkeys. Virology 2001; 279:339–42. [DOI] [PubMed] [Google Scholar]
- 131. Kolappaswamy K, Mahalingam R, Traina‐Dorge V et al Disseminated simian varicella virus infection in an irradiated rhesus macaque (Macaca mulatta). J Virol 2007; 81:411–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Mahalingam R, Traina‐Dorge V, Wellish M et al Simian varicella virus reactivation in cynomolgus monkeys. Virology 2007; 368:50–9. [DOI] [PubMed] [Google Scholar]
- 133. Messaoudi I, Barron A, Wellish M et al Simian varicella virus infection of rhesus macaques recapitulates essential features of varicella zoster virus infection in humans. PLOS Pathog 2009; 5:e1000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Mahalingam R, Traina‐Dorge V, Wellish M et al Latent simian varicella virus reactivates in monkeys treated with tacrolimus with or without exposure to irradiation. J Neurovirol 2010; 16:342–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Haberthur K, Engelmann F, Park B et al CD4 T cell immunity is critical for the control of simian varicella virus infection in a nonhuman primate model of VZV infection. PLOS Pathog 2011; 7:e1002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Meyer C, Kerns A, Barron A, Kreklywich C, Streblow DN, Messaoudi I. Simian varicella virus gene expression during acute and latent infection of rhesus macaques. J Neurovirol 2011; 17:600–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Haberthur K, Kraft A, Arnold N et al Genome‐wide analysis of T cell responses during acute and latent simian varicella virus infections in rhesus macaques. J Virol 2013; 87:11751–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Ouwendijk WJ, Mahalingam R, de Swart RL et al T‐Cell tropism of simian varicella virus during primary infection. PLOS Pathog 2013; 9:e1003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Ouwendijk WJ, Getu S, Mahalingam R, Gilden D, Osterhaus AD, Verjans GM. Characterization of the immune response in ganglia after primary simian varicella virus infection. J Neurovirol, in press 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Ouwendijk WJ, Abendroth A, Traina‐Dorge V et al T‐cell infiltration correlates with CXCL10 expression in ganglia of cynomolgus macaques with reactivated simian varicella virus. J Virol 2013; 87:2979–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Corey L, Langenberg AG, Ashley R et al Recombinant glycoprotein vaccine for the prevention of genital HSV‐2 infection: two randomized controlled trials. JAMA 1999; 282:331–40. [DOI] [PubMed] [Google Scholar]
- 142. Stanberry LR, Spruance SL, Cunningham AL et al Glycoprotein‐D‐adjuvant vaccine to prevent genital herpes. N Engl J Med 2002; 347:1652–61. [DOI] [PubMed] [Google Scholar]
- 143. Lu PJ, Euler GL, Jumaan AO, Harpaz R. Herpes zoster vaccination among adults aged 60 years or older in the United States, 2007: uptake of the first new vaccine to target seniors. Vaccine 2009; 27:882–7. [DOI] [PubMed] [Google Scholar]


