Summary
Immunotherapy is now experiencing unprecedented successes in treating various cancers based on new understandings of cancer immunopathogenesis. Nonetheless, although ageing is the biggest risk factor for cancer, the majority of cancer immunotherapy preclinical studies are conducted in young hosts. This review will explore age‐related changes in immunity as they relate to cancer immune surveillance, immunopathogenesis and responses to immunotherapy. Although it is recognized that declining T cell function with age poses a great challenge to developing effective age‐related cancer immunotherapies, examples of successful approaches to overcome this hurdle have been developed. Further, it is now recognized that immune functions do not simply decline with age, but rather change in ways than can be detrimental. For example, with age, specific immune cell populations with detrimental functions can become predominant (such as cells producing proinflammatory cytokines), suppressive cells can become more numerous or more suppressive (such as myeloid‐derived suppressor cells), drugs can affect aged immune cells distinctly and the aged microenvironment is becoming recognized as a significant barrier to address. Key developments in these and other areas will be surveyed as they relate to cancer immunotherapy in aged hosts, and areas in need of more study will be assessed with some speculations for the future. We propose the term ‘age‐related immune dysfunction’ (ARID) as best representative of age‐associated changes in immunity.
Keywords: aging, cancer, immunity, immunotherapy
OTHER ARTICLES PUBLISHED IN THIS REVIEW SERIES
Immunosenescence: the importance of considering age in health and disease. Clinical and Experimental Immunology 2017, 187: 1–3.
The convergence of senescence and nutrient sensing during lymphocyte ageing. Clinical and Experimental Immunology 2017, 187: 4–5.
Immune senescence: significance of the stromal microenvironment. Clinical and Experimental Immunology 2017, 187: 6–15.
Innate immune responses in the ageing lung. Clinical and Experimental Immunology 2017, 187: 16–25.
Age‐related alterations in immune responses to West Nile virus infection. Clinical and Experimental Immunology 2017, 187: 26–34.
Intracellular signalling pathways: targets to reverse immunosenescence. Clinical and Experimental Immunology 2017, 187: 35–43.
Ageing and inflammation in patients with HIV infection. Clinical and Experimental Immunology 2017, 187: 44–52.
Ageing and obesity similarly impair antibody responses. Clinical and Experimental Immunology 2017, 187: 64–70.
The life cycle of a T cell after vaccination – where does immune ageing strike? Clinical and Experimental Immunology 2017, 187: 71–81.
Herpes zoster and the search for an effective vaccine. Clinical and Experimental Immunology 2017, 187: 82–92.
Adult vaccination against tetanus and diphtheria: the European perspective. Clinical and Experimental Immunology 2017, 187: 93–99.
Introduction
The immune system is exquisitely able to identify specific antigens and eliminate cells expressing them. Tumours are quintessentially antigenic tissues as the result of their many genetic mutations. This antigenicity (expression of antigens), however, does not usually translate into clinically meaningful immunogenicity (the ability of these antigens to elicit useful immunity), as spontaneous rejection of clinically apparent tumours occurs rarely. Tumour‐specific and tumour‐associated antigens were identified more than 60 years ago, prompting strategies to attempt to boost anti‐tumour immunity paralleling successful approaches to boost anti‐pathogen immunity. It is now clear that the inability of endogenous immunity to eradicate clinically evident cancers derives from various factors, including tumour‐driven immune dysfunction, co‐evolution of anti‐tumour immunity as tumours mutate to escape immune elimination (termed ‘immunoediting’, discussed below) and because anti‐tumour immunity is autoimmunity 1, 2. Recent discoveries have helped thinking advance to develop more effective anti‐cancer immunotherapies. With age, some of these impediments are compounded, and new barriers to successful cancer immunotherapy emerge 3, 4, 5, 6.
Tumour immune surveillance is part of a more comprehensive process termed ‘immunoediting’ 7, associated with the ‘three Es’ 8. The first E stands for elimination of nascent cancer cells. The second E is equilibrium, in which growth of malignant cells escaping immunity is balanced by immunity that induces immune elimination. This selective pressure results in antigenic evolution of tumours that leads ultimately to immune escape, the third E is where the tumour has evaded the immune defences and becomes clinically evident in mouse models 9, 10, 11 and humans 12, 13.
Characterization of cancer properties led to the original six fundamental cancer hallmarks, but these did not include immunity 14. The updated eight fundamental hallmarks include lack of immune rejection 15. Chronic generalized inflammation is another newly appreciated cancer hallmark 16, 17, suggested as another fundamental hallmark 18, along with genomic instability 19, abnormal vasculature 20 and stem cell features 21.
As we will discuss, these cancer hallmarks (especially immune rejection and chronic inflammation) bear directly upon the challenges in developing age‐specific immunotherapy. Age effects on immunity extend far beyond simple declines in functions or reductions in cell numbers. We propose the term ‘age‐related immune dysfunction’ (ARID) to encompass the full range of age‐related alterations in immunity with advancing age. The following sections address major topics relating to age effects on cancer immunotherapy.
Tumour‐specific T cells
With ageing, a general reduction in T cell immunity results from various processes affecting T cell numbers, diversity, phenotype and function 22. For example, phenotypically naive T cells (CD45RA+CD62L+CD27+CD28+) are produced throughout life in the thymus but thymic production wanes with age 23, 24, 25, reducing global T cell repertoire diversity even in healthy individuals, as overall peripheral T cell numbers remain relatively constant 26. Reduced haematopoietic stem cell production of T cell precursors 27 also contributes. Even as naive T cell numbers decrease, T cells with a memory phenotype, including cytokine‐producing cytotoxic CD8+ T cells, increase with age. Dysfunctional, terminally differentiated effector cells also increase, especially virus‐reactive cells, with highly reduced T cell receptor repertoire diversity and with limited proliferative ability 28. Thus, most T cells are memory/effector cells and low‐level chronic inflammation is characteristic of an aged immune system. In addition, T cell signalling declines with age 29.
Therapeutic strategies have been developed to lessen these age‐related defects and help to elicit effective T cell immunity. For example, CD4+ T cell functions decline with age, but function loss is mitigated by giving cytokines, including tumour necrosis factor (TNF)‐α or interleukin (IL)‐6 30. Defective age‐related T cell priming can be rescued using agonist αCD137 antibodies 31. Tumour‐specific immunity can also be enhanced. For example, OX40‐enhanced tumour rejection and effector T cell differentiation decreases with age 32, 33, but aged mice developed protective anti‐tumour immunity to a lymphoid tumour with an agonist αCD40 antibody 34. We showed that aged mice develop significant anti‐tumour immunity to aggressive and poorly immunogenic B16 melanoma, comparable to young mice, with similar clinical effect by simultaneous depletion of suppressive myeloid and T cells 5, discussed in detail below.
Regulatory T cells (Tregs)
As Tregs are key mediators of tumour immune dysfunction, reducing Treg function or numbers is a rational cancer immunotherapy strategy 35, 36, 37, 38. Reports of Treg contributions to age‐related decline in immune responses are contradictory, with some studies showing increases in Treg prevalence and/or function with age in humans and mice 39, 40, 41, 42, whereas others show no changes or reduced Treg contributions 43, 44.
Increased Treg prevalence in lymphoid organs but not blood or thymus have been shown in aged mice 5, 45, 46. The effect of age on Treg functional properties is complex, depending on the experimental setting and the function assessed. Some functions appear to be reduced 47, such as suppression of delayed‐type hypersensitivity responses in vivo 41 or inhibition of T helper type 17 (Th17) function 45. In other studies, Tregs from aged mice appear to have similar or greater suppressive function versus young mice 5, 48. Few studies have assessed the changes in Treg prevalence in elderly humans 49, but they could increase in circulation with age 50. Tregs from young and elderly individuals similarly inhibited the proliferation of responder cells, whereas the production of the anti‐inflammatory cytokine IL‐10 was reduced in cells from aged subjects 51.
Although Treg depletion is an effective approach to improving anti‐tumour immunity and responses to immunotherapy 36, 38, conflicting studies report the effect of Treg depletion as cancer immunotherapy in aged hosts 52, 53. One early study correlated defective tumour clearance to increased Treg prevalence and used αCD25 to deplete Tregs and improve anti‐cancer immunity 53. We used denileukin diftitox to deplete Tregs in mice bearing B16 melanoma 5. While denileukin diftitox depleted Tregs similarly in young and aged hosts, slowed tumour growth and improved tumour‐specific immunity was observed only in young mice. Denileukin diftitox‐mediated Treg depletion affected interferon (IFN)‐γ‐ and IL‐17 producing T cells differentially in young versus aged mice. Tumour‐bearing aged mice had more CD11b+Gr‐1hi myeloid‐derived suppressor cells (MDSC) that were more suppressive. Treg depletion resulted in a further increase in MDSC numbers. When MDSC depletion using anti‐Gr‐1 antibody was added to Treg depletion, anti‐tumour immunity was restored in the aged mice resulting in slowed tumour growth similar to young hosts. This strategy did not improve treatment further in young mice as their MDSC did not increase with denileukin diftitox, representing the first cancer immunotherapy to work in aged but, to our knowledge, not young hosts 5. An illustration to represent events is shown in Fig. 1. Thus, mitigation of aged‐specific immune suppressive mechanisms allows successful cancer immunotherapy even in aged hosts.
Figure 1.
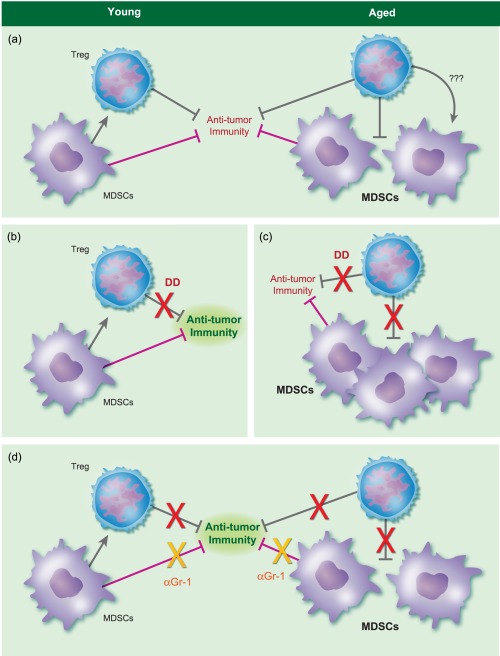
Example of an age‐specific immune effect that can be ameliorated to allow effective anti‐meanoma immunity. (a) Young mice challenged with B16 melanoma experience increased regulatory T cells (Tregs), but not myeloid derived suppressor cells (MDSC), whereas aged mice experience increased Tregs and MDSC. In the aged, the increased MDSC could be due to poor Treg control of them directly, or through indirect mechanisms (denoted by question marks). (b) In young mice, denileukin diftitox (DD) reduces Tregs with little MDSC effect, improving anti‐tumour immunity [increased interferon (IFN)‐γ+ T cells]. By contrast, in aged mice (c), DD‐mediated Treg reduction reduces Tregs but without significant increase in IFN‐γ+ T cells. Interleukin (IL)−17+, potentially detrimental T cells, increase, as do deleterious MDSC, thereby inhibiting beneficial anti‐tumour immunity. Green anti‐tumour immunity means good immunity, whereas red means less effective immunity. (d) By adding anti‐granulocyte‐differentiation antigen‐1 (αGr‐1) antibody to DD in aged mice, the MDSC increase is blunted and aged mice now mount anti‐tumour immunity comparable to young hosts receiving DD alone, with comparable clinical efficacy. Adding αGr‐1 to DD in young hosts does not improve immune or clinical effects further, as MDSC were not increased further by DD. The red ‘X’ denotes DD effects to reduce Treg inhibition.
Innate immunity
Declines in T cell function and chronic low‐level inflammation with age can promote expansion of suppressive myeloid cells. Thus, as anti‐tumour T cells may not be optimally functional in elderly cancer patients, targeting innate cells could represent a better strategy 54.
Dendritic cells (DCs)
DCs are antigen‐presenting cells that play a key role in mediating T cell immunity 55. The tumour environment promotes expansion of dysfunctional DC subsets that hamper effective anti‐tumour immune responses and immunotherapy 56, 57.
Circulating DC precursors and skin Langerhans cells decrease with age. Age effects on DC function depend on the function assessed and are reported variably as decreased or unchanged 58. As DC function declines, any related impaired T cell function or increased inflammation could contribute to age‐related cancer risk. Augmenting DC antigen‐presenting abilities, which is reported inconsistently to decline with age, induces strong tumour‐specific cytotoxic T cell immunity. For example, CD40L or agonist anti‐CD40 antibodies can boost DC activation in animal and human studies 59. In older cancer patients, a vaccine using CD40L linked to specific antigens has potential 60.
Macrophages
In contrast to reduced lymphopoiesis in age, myelopoeisis increases 61, increasing myeloid cell numbers. Macrophages are an important component of tumour stroma and tumour‐associated immune dysfunction 62. Tumour‐resident macrophages are characterized as proinflammatory M1 or anti‐inflammatory M2 macrophages and can switch phenotypes. M1 macrophages secrete proinflammatory cytokines, such as TNF‐α and IL‐12, that boost anti‐tumour immunity. M2 macrophages produce anti‐inflammatory cytokines (e.g. IL‐10, TGF‐β) that promote tumorigenesis 62.
Age effects on macrophage differentiation and function are complex. Macrophage prevalence in lymphoid organs increases in aged mice 63. M1 macrophage function could increase through production of age‐related reactive oxygen species (ROS), but other studies show reduced function with age in M1 macrophages 64, which could be from increased IL‐10‐producing M2 macrophages 62. Thus, macrophages from ageing hosts could promote tumour growth.
Tumour‐associated macrophages from elderly, but not young, mice produce high levels of immune‐suppressive TGF‐β, consistent with an M2‐type phenotype 63. Detrimental M2 macrophages can be converted to beneficial M1 using IL‐12 or poly‐(cysteine 5′ to guanine) (CpG) plus an αIL‐10 receptor 65. Thus, targeting tumour‐associated age‐related M2 macrophages could be useful to treat cancers in aged hosts.
MDSC
MDSC are immature, immunosuppressive, myeloid cells that increase in inflammatory diseases, particularly tumours 66, 67, 68, 69, and suppress anti‐tumour immunity 70. MDSCs produce inhibitory factors (e.g. IL‐10, arginase) that inhibit T cells and promote Tregs and detrimental M2 macrophages 71. MDSCs increase during ageing in human blood 72 and in lymphoid organs in mice 5, 63. MDSC contribute to immunopathology in aged hosts, including in cancer 73, 74. Treg depletion with denileukin diftitox increased MDSCs in aged mice, suggesting Treg control over MDSC 5, which could include indirect effects of Treg‐controlled cytokines that alter MDSC mobilization, proliferation or differentiation. In a CT26 colon cancer model, a Lentinula edodes mycelia extract reduced MDSC infiltration with a whole tumour cell vaccine in aged mice by suppressing the inflammatory MDSC‐promoting cytokines IL‐6 and TNF‐α 75. This extract also improved vaccine‐induced in‐vivo priming of tumour‐specific cytotoxic T cells. Targeting MDSC is another potentially effective approach to reverse cancer‐associated immune dysfunction in aged hosts.
Immune check‐point inhibitors
Immune check‐point receptor blockade has become one of the most successful immunotherapy strategies for various cancers 76, 77. None the less, the impact of those novel approaches in treating elderly patients has been little reported.
Immune check‐point molecules are those that control the degree of immune responses either positively (activating immunity) or negatively (dampening immunity), which is often the case in cancers. Immune check‐point inhibitor antibodies block these negative signals to improve anti‐cancer immunity 76, 77. The expression of regulatory immune check‐points on T cells increases with age in humans and mice 5, 78, 79, consistent with accumulation of hyporesponsive memory‐like T cells that express these molecules. The immune check‐point molecules identifying exhausted (poorly functional) T cells, such as programmed death 1 (PD‐1), lymphocyte activation gene 3 (Lag‐3) and T‐cell immunoglobulin and mucin‐domain containing 3 (Tim‐3) also increase with age. These and other related molecules on T cells are targets for immune check‐point blockade anti‐cancer immunotherapies. Other immune check‐point receptors, such as PD‐L1, are more prominent on myeloid cells or B cells in young hosts, but can be expressed at high levels on tumour cells and on CD8+ T cells in aged mice 80. Defining age‐specific strategies to reduce those inhibitory signals and reverse the hyporesponsiveness or exhaustion of aged T cells while reducing potential cytotoxic effects is important in developing these promising immunotherapies.
PD‐1
PD‐1 is immunopathogenic in cancers by impeding anti‐tumour PD‐1+ T cells. Monoclonal αPD‐1 antibodies (αPD‐1) have demonstrated remarkable clinical efficacy against a variety of cancers and two distinct αPD‐1 antibodies are US Food and Drug Administration (FDA)‐approved to treat melanoma, renal cell carcinoma, non‐small‐cell lung cancer, lymphoma and head and neck cancer.
PD‐1 is expressed preferentially on the surface of effector‐memory (CD44hiCD62Llo) T cells that increase with ageing. CD4+PD‐1+ T cells from old mice exhibit proliferative hyporesponsiveness, suggesting that the up‐regulation of surface‐expressed PD‐1 could contribute to the age‐dependent functional decline in effector‐memory T cells 81. PD‐1 increases on T cells with age 82. αPD‐1 improves T cell functions in aged mice 83, although this strategy has not been reported in aged hosts with cancer to our knowledge. We showed that rapamycin reduces age‐related T cell PD‐1 expression, and PD‐1+ cells in rapamycin‐treated mice were more functional versus PD‐1+ T cells in untreated, aged mice 82, suggesting that rapamycin or another mammalian target of rapamycin (mTOR) inhibitor could improve anti‐tumour immunity in aged hosts, as was shown for the ability of the related molecule, everolimus, to improve B cell immunity to influenza vaccine in aged humans 84.
PD‐ligand 1 (PD‐L1)
PD‐L1 is an immune co‐signalling molecule that signals through PD‐1. Like αPD‐1, αPD‐L1 is thought to work as cancer immunotherapy by protecting PD‐1+ anti‐tumour T cells from inhibition by tumour PD‐L1 expression 77, 85, 86, 87, 88.
Most old naive CD8+ T cells in mice are reportedly PD‐L1+ versus 25% in young mice. Aged CD8+ T cells showed reduced proliferation, but αPD‐L1 improved their proliferation to a level similar to young CD8+ T cells in vitro. αPD‐L1 improved anti‐tumour immunity in aged hosts to young host levels in a mouse lymphoma model 80.
We showed that αPD‐L1 treats B16 melanoma in young but not aged mice, but that treatment efficacy was partially restored by combining αPD‐L1 with anti‐cytotoxic T lymphocyte antigen 4 (αCTLA‐4) 89. The above study 80 tested αPD‐L1 in a lymphoma model in BALB/c mice, whereas we studied a carcinoma in BL6 mice. Thus, either lymphoma versus carcinoma or host genetic background, among other considerations, could help to explain different results with αPD‐L1.
CTLA‐4
The anti‐CTLA‐4 antibody ipilimumab was the first immune check‐point inhibitor agent that clearly benefited human cancer by improving overall and disease‐free survival in metastatic melanoma 90. There are no reports, to our knowledge, of published studies of age effects of anti‐CTLA‐4 in human cancer. We showed that αCTLA‐4 was the only single‐agent immunotherapy effective in aged mice in the B16 melanoma model 89.
Immune check‐point agonists
OX40 agonists and CD137 agonists are discussed above under T cells 31, 34.
Adoptive cell transfers
Older adoptive cell strategies (passive vaccination) using DC or tumour‐infiltrating lymphocytes to treat cancer were generally poorly effective. Chimeric antigen receptor (CAR) cell transfers have shown great promise more recently in haematological malignancies. To make a CAR‐expressing cell, an artificial antigen recognition receptor (the CAR) is engineered into a lymphocyte that is then transfused into the patient 91. To our knowledge, there are no published reports on the effects of aged T cells on generating CAR T cells, aside from the recognition that in‐vitro culture of T cells from older patients generally yields fewer CAR T cells versus younger patients. To the extent that aged T cells have reduced cytolytic or homing potential, they could generate less effective CAR T cells for cancer immunotherapy. Means to boost aged T cell functions discussed above could potentially be used to increase efficacy of CAR T cells made from aged cells. As natural killer (NK) cells and γδ T cells do not require major histocompatibility matching to kill their targets, CAR engineered into such cells could potentially be useful to treat aged cancer patients with CAR NK or CAR γδ T cells, as these strategies are under investigation in younger hosts. Similarly, to the extent that aged DC have reduced T cell‐activating or antigen‐presenting functions, their utility in DC adoptive cell transfers could be limited, although means to improve aged DC functions discussed above could be tested in this regard.
mTOR inhibitors
mTOR inhibitors at doses lower than used usually for immunosuppression can improve antigen‐specific immunity, including against cancers 92. We showed that rapamycin can reduce T cell PD‐1 expression and improves PD‐1+ T cell function in aged mice 47 and improves γδ T cell‐mediated anti‐cancer immunity 93. Rapamycin prolongs life in mice even when given late in life 94. Thus, mTOR inhibitors could be used as adjuncts to various cancer immunotherapy agents to improve aged immune function, as we showed is possible 82, with potentially additional life or health extension effects.
mTOR signals are critical to T cell differentiation, and it is well known that mTOR suppression can promote Treg cell function differentiation 82, 95. Thus, there is some concern that small molecule inhibitors could reduce anti‐tumour immunity by promoting Tregs, as these can be detrimental to anti‐tumour immunity 36. However, in appropriate settings and at sufficiently low doses, small molecule mTOR inhibitors produce net benefits to anti‐tumour immunity 47, 82, 92, 93 and do not appear to promote increased Treg numbers or function 82. We showed recently that low‐dose rapamycin could be beneficial to, whereas typical therapeutic rapamycin doses could impair, anti‐cancer immunity (Yang et al., in revision). More work on this important issue is needed.
Caloric restriction
Caloric restriction suppresses mTOR similar to rapamycin. When used during 6–8 months, caloric restriction improved αCD40 treatment responses in models of sarcoma and breast cancer when mice were aged 12 months. Tumour antigen‐specific CD4+ but not CD8+ T cell priming was similar to young controls 96. Certain types of caloric restriction are tolerable in aged patients, and in young subjects can improve anti‐tumour immunity 97. However, mTOR suppression can reduce immunity in the aged 24 and caloric restriction at too late an age or at too great a caloric reduction can harm aged hosts. This interesting intervention merits additional studies in aged hosts to mitigate cancer treatment symptoms, or prevent or treat cancer.
Tumour microenvironment
The tumour microenvironment consists of tumour, immune and stromal cells. Until recently, most studies of ageing and cancer have focused upon the tumour and the immune system, but tumour microenvironmental/stromal effects are now also recognized as important drug discovery areas. The aged tumour microenvironment could be more immunosuppressive than in young hosts. Factors include increased M2 macrophages and MDSC that result from chronic, low‐grade age‐related inflammation. This aged, protumorigenic tissue microenvironment could be compromised further by tumour cells that can attract detrimental MDSC, Tregs or neutrophils that not only inhibit anti‐tumour immunity but also produce protumorigenic factors 63, 98. Primary prostate stromal fibroblasts from older versus younger patients produce more proinflammatory factors 99.
A transgenic murine model of prostate cancer and benign prostate hyperplasia found an age‐related increase in TGF‐β1 that resulted in altered tissue architecture consistent with local inflammation 100, a result corroborated in prostates of aged normal mice 101. Aged primary fibroblasts attract CD4+ T cells that promote prostate epithelial cell proliferation. Macrophages, neutrophils and CD8+ T cells promote prostate cancer cell proliferation 90.
Fibroblasts in the aged melanoma microenvironment can contribute to melanoma growth and progression. Aged fibroblasts undergo senescence and can drive increased tumour metastases and angiogenesis through production of secreted frizzled‐related protein 2. Older fibroblasts also produced fewer ROS scavengers that can promote DNA damage 6. Finally, in studies of an agonist αCD40 antibody, aged mice failed to respond to cancer treatment from defective CD40 signalling in tumour microenvironmental CD40 signals, not to T cell‐intrinsic effects 33. Thus, therapeutically modifying stromal elements, in particular myeloid cells or their products, could be relevant for elderly cancer patients.
Toll‐like receptor (TLR) agonists
Bacille Calmette–Guérin (BCG) is an attenuated Mycobacterium bovis that is FDA‐approved to treat non‐muscle invasive bladder cancer. It is a strong TLR agonist. Although its mechanism of anti‐cancer action is incompletely understood, it is thought to promote anti‐cancer immunity. Observational data suggest that age could influence response to BCG therapy in bladder cancer. In a trial of BCG plus IFN‐α immunotherapy in bladder cancer, aged patients had increased relapse rates following BCG compared to younger patients 102. Of the patients who were aged 61–70 years versus patients older than 80 years, cancer‐free survival was 39 versus 61%, respectively, at a median follow‐up of 24 months (P = 0·002). In analyses of aged patients receiving BCG for non‐muscle invasive bladder cancer, age was an independent risk for progression and the 2‐year progression‐free survival was 87% among patients aged less than 75 years compared to 65% among patients aged greater than 75 years 103. In a large (n = 805) cohort of patients with bladder cancer age was found to have a small but measurable association with response to BCG therapy 104. Nevertheless, the influence of competing morbidities and selective surgical management of elderly patients versus immune effects is not clear from these studies. Thus, although BCG is the sole agent to our knowledge for which age‐specific immunotherapy effects in humans has been reported extensively, a clear conclusion on age‐related immune effects cannot be made from these data. As BCG is standard‐of‐care immunotherapy and bladder cancer patients tend to be older than for many other cancer types, these data merit follow‐up. There are no reports to our knowledge of age effects on efficacy of other approved or investigational TLR agonists for cancer treatment.
Immunotherapy toxicity mitigation
Cancer immunotherapy in aged mice can generate rapid and/or lethal toxicities in distinct organs due to proinflammatory cytokine production, both systemically and locally in affected tissues. Proinflammatory effects could be driven by macrophages rather than T or NK cells. Myeloid cell depletion during therapy mitigates these toxicities without significantly compromising treatment efficacy. Aged macrophages from mice and normal humans produce higher proinflammatory cytokines, including TNF‐α and IL‐6 versus young macrophages. Blocking TNF‐α in aged mice also improves survival and reduces toxicities without loss of anti‐tumour effects 3, 4. As αTNF‐α, αIL‐6 and αIL‐6R antibodies are FDA‐approved, this concept merits further investigation. However, as other studies have shown that IL‐6 and TNF‐α administration can improve aged T cell function 30, much additional work is required to understand how such approaches could be incorporated safely and effectively into cancer immunotherapy strategies.
Microbiome effects
The gut microbiome affects systemic immunity and is affected by age 105, 106, 107. Two recent reports showed that gut microbes affected anti‐cancer immunotherapy in human subjects receiving αPD‐L1 108 or αCTLA‐4 109. There is currently much justified interest in understanding microbial effects on anti‐cancer immunity although, to our knowledge, age effects are not yet reported in cancer immunotherapy. As the gut microbiome can affect anti‐cancer treatments through immune effects 110, including modulation of tumour microenvironmental myeloid cells 111 which themselves are also affected by age 82, it is reasonable for there to be age‐associated microbial effects on anti‐cancer immunotherapy, an area deserving of additional studies.
Conclusions
Immune therapy for cancer, including in elderly people, has a strong scientific rationale. Recent breakthroughs in understanding the basis for cancer‐driven immune dysfunction have led to the development of highly successful anti‐cancer immunotherapies. Nonetheless, much of our understanding of tumour immunity derives from studies of younger hosts. There is now a growing, but still relatively small, body of literature demonstrating age effects on cancer immunity and responses to immunotherapies, which have led in some instances to demonstrations of novel approaches to improving anti‐cancer immunotherapy in aged hosts most at risk for cancer.
‘Immune decline’ is an inaccurate term, and ‘immunosenescence’ and ‘inflammageing’ describe specific attributes of an aged immune system. We propose the term ‘age related immune dysfunction’ (ARID) to describe the totality of age‐related immune changes to include increased or decreased numbers of certain immune cells, appearance of novel immune cell populations, increased proinflammatory or immune suppressive functions, restricted T cell receptor (TCR) repertoire and the myriad other changes that age brings to immunity. To the extent that underlying ARID can be reversed in aged cancer patients, clinically relevant anti‐tumour immunity could potentially be achieved, as has now been demonstrated clearly.
Challenges in developing effective age‐appropriate cancer immunotherapy
Improved understanding of tumour‐specific immunopathology and related age‐specific effects will help to guide more effective treatment approaches. Individual immunotherapy agents are unlikely to treat most cancers effectively, and thus optimal means to combine agents and approaches need to be defined, including specific agents, doses and scheduling. We need to identify biomarkers that differentiate aged patients with sufficiently responsive tumour‐specific immunity from those in whom additional adjuncts will be needed. Adjuncts for those patients with impaired immunity could include adoptive transfer of rejuvenated or young CAR‐expressing cells, novel antigen‐presenting cells, thymic transplants, gene therapy [including with caspase 9/clustered regularly interspaced short palindromic repeats (CRISPR) approaches] or other approaches.
Toxicity mitigation strategies that do not compromise clinical efficacy are also needed, as is identification of rational combination therapies to reduce dosage of potentially harmful individual agents. Studies of the effect of age on the human microbiome are in their infancy, and could help understanding of the heterogeneity of responses to immunotherapy observed both in humans and in preclinical models. Tumour mutational burden clearly affects the efficacy of many types of cancer immunotherapies. Mutational burden will be dictated, among other considerations, by immune editing. We thus need a clearer understanding of age effects on immune editing and tumour mutational burden, which could produce related or individual contributions to cancer immunotherapy efficacy. Finally, we need to deepen understanding of age effects on tumour stroma, including vasculature effects that can alter immune cell trafficking and drug delivery. An illustration of some challenges is shown in Fig. 2.
Figure 2.
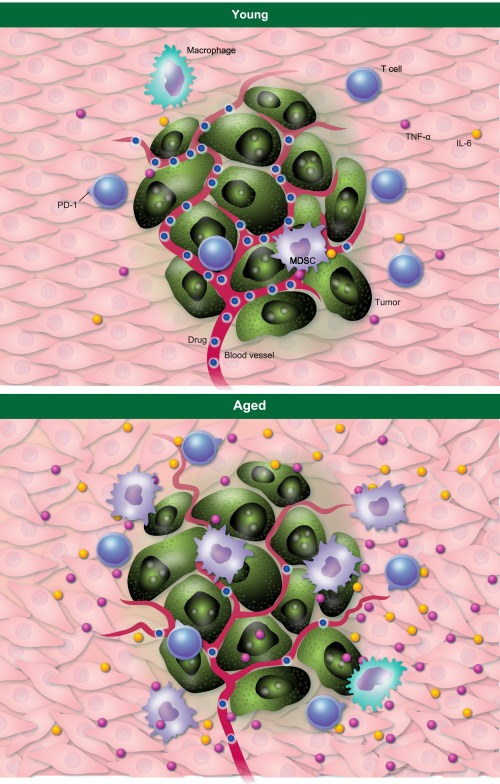
Examples of age effects in the tumour microenvironment. The aged tumour microenvironment can have elevated levels of proinflammatory molecules that reduce anti‐tumour immunity such as interleukin (IL)‐6 (yellow dots) and tumour necrosis factor (TNF)‐α (purple dots). Green cells = tumour. Myeloid cells, including myeloid‐derived suppressor cells (MDSC) (light purple) and macrophages (light green) that inhibit anti‐tumour immunity can also be increased in the aged tumour microenvironment. These cells can be a source of detrimental molecules. Local IL‐6 and other factors can increase MDSC. T cells programmed death 1 (PD)‐1 expression can be increased, suggesting reduced T cell functions. As the Wnt antagonist secreted frizzled‐related protein 2 (sFRP2) can promote angiogenesis, we have taken the liberty of showing bigger and more numerous blood vessels in the aged microenvironment, although more work is needed in this area. Drug delivery could be reduced from abnormal vasculature.
As we use ever more powerful but costly and potentially harmful approaches in ever‐older cancer patients, economic, feasibility and ethical issues will need to be addressed.
Disclosure
None to declare.
Author contributions
V. H., R. S. S. and T. J. C. wrote the manuscript. V. H. and A. P. performed some experiments described.
Acknowledgements
Financial support is acknowledged for T. C. (CA170491, CA54174, CDMRP, The Holly Beach Public Library, The Owens Foundation, The Barker Foundation and the Skinner endowment).
References
- 1. Pardoll D. T cells and tumours. Nature 2001; 411:1010–2. [DOI] [PubMed] [Google Scholar]
- 2. Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol 2003; 21:807–39. [DOI] [PubMed] [Google Scholar]
- 3. Bouchlaka MN, Murphy WJ. Impact of aging in cancer immunotherapy: the importance of using accurate preclinical models. Oncoimmunology 2013; 2:e27186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bouchlaka MN, Sckisel GD, Chen M et al Aging predisposes to acute inflammatory induced pathology after tumor immunotherapy. J Exp Med 2013; 210:2223–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hurez V, Daniel BJ, Sun L et al Mitigating age‐related immune dysfunction heightens the efficacy of tumor immunotherapy in aged mice. Cancer Res 2012; 72:2089–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaur A, Webster MR, Marchbank K et al sFRP2 in the aged microenvironment drives melanoma metastasis and therapy resistance. Nature 2016; 532:250–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011; 331:1565–70. [DOI] [PubMed] [Google Scholar]
- 8. Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol 2004; 22:329–60. [DOI] [PubMed] [Google Scholar]
- 9. Koebel CM, Vermi W, Swann JB et al Adaptive immunity maintains occult cancer in an equilibrium state. Nature 2007; 450:903–7. [DOI] [PubMed] [Google Scholar]
- 10. Matsushita H, Vesely MD, Koboldt DC et al Cancer exome analysis reveals a T‐cell‐dependent mechanism of cancer immunoediting. Nature 2012; 482:400–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DuPage M, Mazumdar C, Schmidt LM, Cheung AF, Jacks T. Expression of tumour‐specific antigens underlies cancer immunoediting. Nature 2012; 482:405–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Strauss DC, Thomas JM. Transmission of donor melanoma by organ transplantation. Lancet Oncol 2010; 11:790–6. [DOI] [PubMed] [Google Scholar]
- 13. Stephens JK, Everson GT, Elliott CL et al Fatal transfer of malignant melanoma from multiorgan donor to four allograft recipients. Transplantation 2000; 70:232–6. [PubMed] [Google Scholar]
- 14. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100:57–70. [DOI] [PubMed] [Google Scholar]
- 15. Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell 2012; 21:297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Demaria S, Pikarsky E, Karin M et al Cancer and inflammation: promise for biologic therapy. J Immunother 2010; 33:335–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140:883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer‐related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 2009; 30:1073–81. [DOI] [PubMed] [Google Scholar]
- 19. Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability – an evolving hallmark of cancer. Nat Rev Mol Cell Biol 2010; 11:220–8. [DOI] [PubMed] [Google Scholar]
- 20. De Bock K, Cauwenberghs S, Carmeliet P. Vessel abnormalization: another hallmark of cancer? Molecular mechanisms and therapeutic implications. Curr Opin Genet Dev 2010; 21:73–9. [DOI] [PubMed] [Google Scholar]
- 21. Teschendorff AE, Menon U, Gentry‐Maharaj A et al Age‐dependent DNA methylation of genes that are suppressed in stem cells is a hallmark of cancer. Genome Res 2010; 20:440–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hakim FT, Flomerfelt FA, Boyiadzis M, Gress RE. Aging, immunity and cancer. Curr Opin Immunol 2004; 16:151–6. [DOI] [PubMed] [Google Scholar]
- 23. Haynes BF, Sempowski GD, Wells AF, Hale LP. The human thymus during aging. Immunol Res 2000; 22:253–61. [DOI] [PubMed] [Google Scholar]
- 24. Goldberg EL, Romero‐Aleshire MJ, Renkema KR et al Lifespan‐extending caloric restriction or mTOR inhibition impair adaptive immunity of old mice by distinct mechanisms. Aging Cell 2015; 14:130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang H, Youm YH, Vandanmagsar B et al Obesity accelerates thymic aging. Blood 2009; 114:3803–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunol Rev 2006; 211:154–63. [DOI] [PubMed] [Google Scholar]
- 27. Anderlini P, Przepiorka D, Seong C et al Factors affecting mobilization of CD34+ cells in normal donors treated with filgrastim. Transfusion 1997; 37:507–12. [DOI] [PubMed] [Google Scholar]
- 28. Fulop T, Larbi A, Pawelec G. Human T cell aging and the impact of persistent viral infections. Front Immunol 2013; 4:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fulop T, Witkowski JM, Le Page A, Fortin C, Pawelec G, Larbi A. Intracellular signalling pathways: targets to reverse immunosenescence. Clin Exp Immunol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haynes L, Eaton SM, Burns EM, Rincon M, Swain SL. Inflammatory cytokines overcome age‐related defects in CD4 T cell responses in vivo. J Immunol 2004; 172:5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bansal‐Pakala P, Croft M. Defective T cell priming associated with aging can be rescued by signaling through 4‐1BB (CD137). J Immunol 2002; 169:5005. [DOI] [PubMed] [Google Scholar]
- 32. Ruby CE, Weinberg AD. OX40‐enhanced tumor rejection and effector T cell differentiation decreases with age. J Immunol 2009; 182:1481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ruby CE, Weinberg AD. The effect of aging on OX40 agonist‐mediated cancer immunotherapy. Cancer Immunol Immunother 2009; 58:1941–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lustgarten J, Dominguez AL, Thoman M. Aged mice develop protective antitumor immune responses with appropriate costimulation. J Immunol 2004; 173:4510–5. [DOI] [PubMed] [Google Scholar]
- 35. Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest 2007; 117:1167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Curiel TJ. Regulatory T cells and treatment of cancer. Curr Opin Immunol 2008; 20:241–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Curiel TJ, Coukos G, Zou L et al Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004; 10:942. [DOI] [PubMed] [Google Scholar]
- 38. Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol 2006; 6:295–307. [DOI] [PubMed] [Google Scholar]
- 39. Zhang H, Podojil JR, Luo X, Miller SD. Intrinsic and induced regulation of the age‐associated onset of spontaneous experimental autoimmune encephalomyelitis. J Immunol 2008; 181:4638–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rosenkranz D, Weyer S, Tolosa E et al Higher frequency of regulatory T cells in the elderly and increased suppressive activity in neurodegeneration. J Neuroimmunol 2007; 188:117–27. [DOI] [PubMed] [Google Scholar]
- 41. Zhao L, Sun L, Wang H, Ma H, Liu G, Zhao Y. Changes of CD4+CD25+Foxp3+ regulatory T cells in aged Balb/c mice. J Leukoc Biol 2007; 81:1386–94. [DOI] [PubMed] [Google Scholar]
- 42. Kryczek I, Liu R, Wang G et al FOXP3 defines regulatory T cells in human tumor and autoimmune disease. Cancer Res 2009; 69:3995–4000. [DOI] [PubMed] [Google Scholar]
- 43. Kozlowska E, Biernacka M, Ciechomska M, Drela N. Age‐related changes in the occurrence and characteristics of thymic CD4(+) CD25(+) T cells in mice. Immunology 2007; 122:445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thomas DC, Mellanby RJ, Phillips JM, Cooke A. An early age‐related increase in the frequency of CD4+ Foxp3+ cells in BDC2.5NOD mice. Immunology 2007; 121:565–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sun L, Hurez VJ, Thibodeaux SR et al Aged regulatory T cells protect from autoimmune inflammation despite reduced STAT3 activation and decreased constraint of IL‐17 producing T cells. Aging Cell 2012; 11:509–19. [DOI] [PubMed] [Google Scholar]
- 46. Lages CS, Suffia I, Velilla PA et al Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. J Immunol 2008; 181:1835–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tsaknaridis L, Spencer L, Culbertson N et al Functional assay for human CD4+CD25+ Treg cells reveals an age‐dependent loss of suppressive activity. J Neurosci Res 2003; 74:296–308. [DOI] [PubMed] [Google Scholar]
- 48. Garg SK, Delaney C, Toubai T et al Aging is associated with increased regulatory T‐cell function. Ageing Cell 2014; 13:441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fessler J, Ficjan A, Duftner C, Dejaco C. The impact of aging on regulatory T‐cells. Front Immunol 2013; 4:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gregg R, Smith CM, Clark FJ et al The number of human peripheral blood CD4+ CD25high regulatory T cells increases with age. Clin Exp Immunol 2005; 140:540–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hwang KA, Kim HR, Kang I. Aging and human CD4(+) regulatory T cells. Mech Ageing Dev 2009; 130:509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dominguez AL, Lustgarten J. Implications of aging and self‐tolerance on the generation of immune and antitumor immune responses. Cancer Res 2008; 68:5423–31. [DOI] [PubMed] [Google Scholar]
- 53. Sharma S, Dominguez AL, Lustgarten J. High accumulation of T regulatory cells prevents the activation of immune responses in aged animals. J Immunol 2006; 177:8348–55. [DOI] [PubMed] [Google Scholar]
- 54. Jackaman C, Dye DE, Nelson DJ. IL‐2/CD40‐activated macrophages rescue age and tumor‐induced T cell dysfunction in elderly mice. Age (Dordr) 2014; 36:9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer 2012; 12:265–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zou W, Machelon V, Coulomb‐L'Hermin A et al Stromal‐derived factor‐1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med 2001; 7:1339–46. [DOI] [PubMed] [Google Scholar]
- 57. Curiel TJ, Wei S, Dong H et al Blockade of B7‐H1 improves myeloid dendritic cell‐mediated antitumor immunity. Nat Med 2003; 9:562–7. [DOI] [PubMed] [Google Scholar]
- 58. Agrawal A, Agrawal S, Tay J, Gupta S. Biology of dendritic cells in aging. J Clin Immunol 2008; 28:14–20. [DOI] [PubMed] [Google Scholar]
- 59. Khong A, Nelson DJ, Nowak AK, Lake RA, Robinson BW. The use of agonistic anti‐CD40 therapy in treatments for cancer. Int Rev Immunol 2012; 31:246–66. [DOI] [PubMed] [Google Scholar]
- 60. Tang YC, Thoman M, Linton PJ, Deisseroth A. Use of CD40L immunoconjugates to overcome the defective immune response to vaccines for infections and cancer in the aged. Cancer Immunol Immunother 2009; 58:1949–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Geiger H, Rudolph KL. Aging in the lympho‐hematopoietic stem cell compartment. Trends Immunol 2009; 30:360–5. [DOI] [PubMed] [Google Scholar]
- 62. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer‐related inflammation. Nature 2008; 454:436–44. [DOI] [PubMed] [Google Scholar]
- 63. Jackaman C, Radley‐Crabb HG, Soffe Z, Shavlakadze T, Grounds MD, Nelson DJ. Targeting macrophages rescues age‐related immune deficiencies in C57BL/6J geriatric mice. Aging Cell 2013; 12:345–57. [DOI] [PubMed] [Google Scholar]
- 64. Mahbub S, Deburghgraeve CR, Kovacs EJ. Advanced age impairs macrophage polarization. J Interferon Cytokine Res 2012; 32:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Watkins SK, Egilmez NK, Suttles J, Stout RD. IL‐12 rapidly alters the functional profile of tumor‐associated and tumor‐infiltrating macrophages in vitro and in vivo . J Immunol 2007; 178:1357–62. [DOI] [PubMed] [Google Scholar]
- 66. Ostrand‐Rosenberg S, Sinha P. Myeloid‐derived suppressor cells: linking inflammation and cancer. J Immunol 2009; 182:4499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer‐expanded myeloid‐derived suppressor cells induce energy of NK cells through membrane‐bound TGF‐beta 1. J Immunol 2009; 182:240–9. [DOI] [PubMed] [Google Scholar]
- 68. Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid‐derived suppressor cells in tumour‐bearing mice. J Immunol 2008; 181:5791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor‐induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev 2008; 222:162–79. [DOI] [PubMed] [Google Scholar]
- 70. Huang B, Pan PY, Li Q et al Gr‐1+CD115+ immature myeloid suppressor cells mediate the development of tumor‐induced T regulatory cells and T‐cell energy in tumor‐bearing host. Cancer Res 2006; 66:1123–31. [DOI] [PubMed] [Google Scholar]
- 71. Marvel D, Gabrilovich DI. Myeloid‐derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest 2015; 125:3356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Verschoor CP, Johnstone J, Millar J et al Blood CD33(+)HLA‐DR(–) myeloid‐derived suppressor cells are increased with age and a history of cancer. J Leukoc Biol 2013; 93:633–7.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Grizzle WE, Xu X, Zhang S et al Age‐related increase of tumor susceptibility is associated with myeloid‐derived suppressor cell mediated suppression of T cell cytotoxicity in recombinant inbred BXD12 mice. Mech Ageing Dev 2007; 128:672–80. [DOI] [PubMed] [Google Scholar]
- 74. Enioutina EY, Bareyan D, Daynes RA. A role for immature myeloid cells in immune senescence. J Immunol 2011; 186:697–707. [DOI] [PubMed] [Google Scholar]
- 75. Ishikawa S, Matsui Y, Wachi S, Yamaguchi H, Harashima N, Harada M. Age‐associated impairment of antitumor immunity in carcinoma‐bearing mice and restoration by oral administration of Lentinula edodes mycelia extract. Cancer Immunol Immunother 2016; 65:961–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pardoll D, Drake C. Immunotherapy earns its spot in the ranks of cancer therapy. J Exp Med 2012; 209:201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12:252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Canaday DH, Parker KE, Aung H, Chen HE, Nunez‐Medina D, Burant CJ. Age‐dependent changes in the expression of regulatory cell surface ligands in activated human T‐cells. BMC Immunol 2013; 14:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Channappanavar R, Twardy BS, Krishna P, Suvas S. Advancing age leads to predominance of inhibitory receptor expressing CD4 T cells. Mech Ageing Dev 2009; 130:709–12. [DOI] [PubMed] [Google Scholar]
- 80. Mirza N, Duque MA, Dominguez AL, Schrum AG, Dong H, Lustgarten J. B7‐H1 expression on old CD8+ T cells negatively regulates the activation of immune responses in aged animals. J Immunol 2010; 184:5466–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Shimada Y, Hayashi M, Nagasaka Y, Ohno‐Iwashita Y, Inomata M. Age‐associated up‐regulation of a negative co‐stimulatory receptor PD‐1 in mouse CD4+ T cells. Exp Gerontol 2009; 44:517–22. [DOI] [PubMed] [Google Scholar]
- 82. Hurez V, Dao V, Liu A et al Chronic mTOR inhibition in mice with rapamycin alters T, B, myeloid, and innate lymphoid cells and gut flora and prolongs life of immune‐deficient mice. Aging Cell 2015; 14:945–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lages CS, Lewkowich I, Sproles A, Wills‐Karp M, Chougnet C. Partial restoration of T cell function in aged mice by in vitro blockade of the PD‐1/PD‐L1 pathway. Aging Cell 2010; 9:785–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mannick JB, Del Giudice G, Lattanzi M et al mTOR inhibition improves immune function in the elderly. Sci Transl Med 2014; 6:268ra179. [DOI] [PubMed] [Google Scholar]
- 85. Topalian SL, Hodi FS, Brahmer JR et al Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N Engl J Med 2012; 366:2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Dong H, Strome SE, Salomao DR et al Tumor‐associated B7‐H1 promotes T‐cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8:793–800. [DOI] [PubMed] [Google Scholar]
- 87. Brahmer JR, Tykodi SS, Chow LQ et al Safety and activity of anti‐PD‐L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366:2455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Taube JM, Anders RA, Young GD et al Colocalization of inflammatory response with B7‐h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012; 4:127ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Figueiredo ASP, Hurez V, Liu A. TJCJI. Age and sex affect αCTLA‐4 efficacy alone and combined with αB7‐H1 or regulatory T cell depletion in a melanoma model. J Immunol 2016; 196:213.4. [Google Scholar]
- 90. Hodi FS, O'Day SJ, McDermott DF et al Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. June CH, Riddell SR, Schumacher TN. Adoptive cellular therapy: a race to the finish line. Sci Transl Med 2015; 7:280ps7. [DOI] [PubMed] [Google Scholar]
- 92. Pedicord VA, Cross JR, Montalvo‐Ortiz W, Miller ML, Allison JP. Friends not foes: CTLA‐4 blockade and mTOR inhibition cooperate during CD8+ T cell priming to promote memory formation and metabolic readiness. J Immunol 2015; 194:2089–98. [DOI] [PubMed] [Google Scholar]
- 93. Dao V, Liu Y, Pandeswara S et al Immune stimulatory effects of rapamycin are mediated by stimulation of antitumor γδ T cells. Cancer Res, 2016; 76:5970–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hasty P, Livi CB, Dodds SG et al eRapa restores a normal life span in a FAP mouse model. Cancer Prev Res (Phila) 2014; 7:169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Pollizzi KN, Patel CH, Sun IH et al mTORC1 and mTORC2 selectively regulate CD8+ T cell differentiation. J Clin Invest 2015; 125:2090–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Farazi M, Nguyen J, Goldufsky J et al Caloric restriction maintains OX40 agonist‐mediated tumor immunity and CD4 T cell priming during aging. Cancer Immunol Immunother 2014; 63:615–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Di Biase S, Lee C, Brandhorst S et al Fasting‐mimicking diet reduces HO‐1 to promote T cell‐mediated tumor cytotoxicity. Cancer Cell 2016; 30:136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Jackaman C, Nelson DJ. Are macrophages, myeloid derived suppressor cells and neutrophils mediators of local suppression in healthy and cancerous tissues in aging hosts? Exp Gerontol 2014; 54:53–7. [DOI] [PubMed] [Google Scholar]
- 99. Begley LA, Kasina S, MacDonald J, Macoska JA. The inflammatory microenvironment of the aging prostate facilitates cellular proliferation and hypertrophy. Cytokine 2008; 43:194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Barron DA, Strand DW, Ressler SJ et al TGF‐beta1 induces an age‐dependent inflammation of nerve ganglia and fibroplasia in the prostate gland stroma of a novel transgenic mouse. PLOS ONE 2010; 5:e13751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bianchi‐Frias D, Vakar‐Lopez F, Coleman IM, Plymate SR, Reed MJ, Nelson PS. The effects of aging on the molecular and cellular composition of the prostate microenvironment. PLOS ONE 2010; 5:e12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Joudi FN, Smith BJ, O'Donnell MA, Konety BR. The impact of age on the response of patients with superficial bladder cancer to intravesical immunotherapy. J Urol 2006; 175:1634–9. discussion 9‐40. [DOI] [PubMed] [Google Scholar]
- 103. Margel D, Alkhateeb SS, Finelli A, Fleshner N. Diminished efficacy of bacille Calmette–Guerin among elderly patients with nonmuscle invasive bladder cancer. Urology 2011; 78:848–54. [DOI] [PubMed] [Google Scholar]
- 104. Herr HW. Age and outcome of superficial bladder cancer treated with bacille Calmette–Guerin therapy. Urology 2007; 70:65–8. [DOI] [PubMed] [Google Scholar]
- 105. Yatsunenko T, Rey FE, Manary MJ et al Human gut microbiome viewed across age and geography. Nature 2012; 486:222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Pitt JM, Vetizou M, Waldschmitt N et al Fine‐tuning cancer immunotherapy: optimizing the gut microbiome. Cancer Res 2016; 76:4602–7. [DOI] [PubMed] [Google Scholar]
- 107. Zitvogel L, Ayyoub M, Routy B, Kroemer G. Microbiome and anticancer immunosurveillance. Cell 2016; 165:276–87. [DOI] [PubMed] [Google Scholar]
- 108. Sivan A, Corrales L, Hubert N et al Commensal Bifidobacterium promotes antitumor immunity and facilitates anti‐PD‐L1 efficacy. Science 2015; 350:1084–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Vetizou M, Pitt JM, Daillere R et al Anticancer immunotherapy by CTLA‐4 blockade relies on the gut microbiota. Science 2015; 350:1079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Viaud S, Saccheri F, Mignot G et al The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013; 342:971–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Iida N, Dzutsev A, Stewart CA et al Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013; 342:967–70. [DOI] [PMC free article] [PubMed] [Google Scholar]


