Abstract
Motivation: With the rapid increase of infection resistance to antibiotics, it is urgent to find novel infection therapeutics. In recent years, antimicrobial peptides (AMPs) have been utilized as potential alternatives for infection therapeutics. AMPs are key components of the innate immune system and can protect the host from various pathogenic bacteria. Identifying AMPs and their functional types has led to many studies, and various predictors using machine learning have been developed. However, there is room for improvement; in particular, no predictor takes into account the lack of balance among different functional AMPs.
Results: In this paper, a new synthetic minority over-sampling technique on imbalanced and multi-label datasets, referred to as ML-SMOTE, was designed for processing and identifying AMPs’ functional families. A novel multi-label classifier, MLAMP, was also developed using ML-SMOTE and grey pseudo amino acid composition. The classifier obtained 0.4846 subset accuracy and 0.16 hamming loss.
Availability and Implementation: A user-friendly web-server for MLAMP was established at http://www.jci-bioinfo.cn/MLAMP.
Contacts: linweizhong@jci.edu.cn or xudong@missouri.edu
1 Introduction
With rapid increase in the infection resistance of antibiotics, it is urgent to find novel infection therapeutics. Over the past decade antimicrobial peptides (AMPs) have been utilized as potential alternatives for fighting infectious diseases. AMPs are key components of the innate immune system and can protect the host from various pathogenic bacteria. In invertebrates and vertebrates, AMPs have dual roles: rapid microbial killing and subsequent immune modulation (Wang, 2014). These effects result from AMP inducing multiple damages in bacteria by disrupting bacteria membranes (Malmsten, 2014), by inhibiting proteins, DNA and RNA synthesis, or by interacting with certain intracellular targets (Bahar and Ren, 2013). Therefore, AMPs were developed increasingly for new drugs. Some examples of using AMPs in therapeutics have been reported. Popovic et al. (2012) found that peptides with antimicrobial and anti-inflammatory activities had therapeutic potential for treatment of acne vulgaris. Yancheva et al. (2012) synthesized a novel didepsipeptide with antimicrobial activity against four of five tested bacterial strains of Escherichia coli. Conlon et al. demonstrated that peptides with antimicrobial activity from frog skin could stimulate insulin release, and hence had potential as an incretin-based therapy for Type 2 diabetes mellitus (Conlon et al., 2014). In addition, AMPs have been used as anticancer peptides in cancer therapy (Gaspar et al., 2013).
A surge in research on AMPs has promoted the development of various databases and prediction tools. APD2 (Wang et al., 2009) is a system dedicated to establishing a glossary, nomenclature, classification, information search, prediction, design, and statistics of AMPs. It gathered 2544 AMPs from the literature. CAMP (Thomas et al., 2010; Waghu et al., 2013) holds 6756 antimicrobial sequences and 682 3D structures of AMPs, together with prediction and sequence analysis tools. Niarchou et al. (2013) tested all subsequences ranging from 5 to 100 amino acids of the plant proteins in UniProKB/Swiss-prot and constructed an AMP database for plant species, named C-PAmP. Zhao et al. (2013) developed LAMP, a database used to aid the discovery and design of AMPs as new antimicrobial agents. The database contains 3904 natural AMPs and 1643 synthetic peptides. DBAASP was a manually curated database built by Gogoladze et al. (2014), and it collected those peptides for which antimicrobial activities against particular targets have been evaluated experimentally.
Generally, AMPs are short peptides with 10–50 amino acids (Malmsten, 2014) and have very low sequence homology to one another. So it is challenging to identify AMPs and its activities by automatic tools. Researchers made considerable efforts in this regard. In these studies, the support vector machine (SVM) was usually used as prediction engine (Joseph et al., 2012; Khosravian et al., 2013; Lata et al., 2010; Niarchou et al., 2013). Besides, nearest neighbor (Wang et al., 2011) or k-nearest neighbor algorithm (Xiao et al., 2013), random forests (RFs) (Joseph et al., 2012), decision tree model (Lira et al., 2013) and hidden Markov models (HMMs) (Fjell et al., 2007) were also applied as classifiers. Some predictors were only used to identify whether novel peptides are AMPs (Khosravian et al., 2013; Lata et al., 2007; Vishnepolsky and Pirtskhalava, 2014; Wang et al., 2011). In addition to these simple binary classifiers, there were some multi-class classifiers. Lira et al. (2013) created a decision tree model to classify the antimicrobial activities of synthetic peptides into four classes: none, low, medium and high. Joseph developed ClassAMP to predict the propensity of a peptide sequence to have antibacterial, antifungal, or antiviral activity (Joseph et al., 2012). Khamis et al. studied 14 AMP families and sub-families. They selected a specific description of AMP amino acid sequence, and identified compositional and physicochemical properties of amino acids to distinguish each AMP family (Khamis et al., 2015). Furthermore, Xiao et al. (2013) proposed a two-level multi-label classifier, iAMP-2L, which identifies not only whether a peptide is an AMP, but also its functional activities.
Although these methods have their own advantages and did play an important role in the research, they have following problems. First, most models only identified whether a new sequence is AMP, but not its type. Second, it is hard to search short peptides in the database because AMPs usually have only 5–50 amino acids. Methods based on Blast search and gene ontology (Lin et al., 2013) are often ineffective. Last but not least, classifying AMPs’ functions is a multi-label classification (MLC), especially when the number of AMPs with different activities does not distribute evenly. From APD2 (Wang et al., 2009), it is seen that antibacterial peptides occupy more than 90% of all AMPs, which is a highly unbalanced MLC. None of aforementioned automatic models considered the unbalanced amounts among various activities.
In the past two decades, the topic of learning from multi-label datasets (MLDs) has drawn significant attention from researchers. Moreover, MLC methods are increasingly applied in various fields, such as semantic annotation of images (Zhang and Zhou, 2007), categorization (Liu and Chen, 2015) and bioinformatics (Chou, 2015; Chou and Shen, 2007, 2008; Chou et al., 2011, 2012, 2014; Sadasivam and Duraiswamy, 2015; Shen and Chou, 2007, 2009, 2010a,b; Wu et al., 2012; Yu et al., 2013). The existing MLC methods can be grouped into two categories: (i) problem transformation methods, which transform MLC either into one or more single-label classification or regression problem, and (ii) algorithm adaptation methods, which extend specific learning algorithms in order to handle MLDs directly (Tsoumakas et al., 2010). Numerous MLC algorithms were proposed, such as Adaboost.MH and Adaboost.MR (Schapire and Singer, 2000), ML-KNN (Zhang and Zhou, 2007), Classifier chains (Read et al., 2009, 2011) and Multi-label Naïve Bayes (Zhang et al., 2009).
MLC often has serious issues of unbalanced datasets, in which the numbers of samples from minority classes are substantially fewer than from majority classes. For example, in subcellular localization prediction (Lin et al., 2013), the number of the cytoplasm proteins is 44 times the number of the melanosome proteins. A similar situation occurs in many studies (Liu and Chen, 2015; Wan et al., 2012; Wu et al., 2011; Xiao et al., 2011). Standard machine learning algorithms often cannot achieve ideal performance when trained on unbalanced dataset. One approach to address this issue is to adapt existing classifier learning algorithms to strengthen learning with regard to the minority class (Xu et al., 2013). Another approach is to artificially sample the class distribution (Dong and Wang, 2011; Luengo et al., 2011; Tahir et al., 2012). Combining both approaches can also achieve strong classifiers (Zhang et al., 2012). Unquestionably, the sampling approach continues to be popular (Chawla, 2010). Various over- and under-sampling methods have been proposed (Bunkhumpornpat et al., 2009; Chawla, 2010; Chawla et al., 2002; Dong and Wang, 2011; Gao et al., 2011a,b; Luengo et al., 2011; Seiffert et al., 2008; Zhang et al., 2012). Among them, SMOTE (Synthetic Minority Oversampling TEchinque) (Chawla, 2010; Chawla et al., 2002) is a state-of-art over-sampling methods. Chawla (2010) argued that SMOTE creates effective regions for learning the minority class rather than being subsumed by the majority class samples around them. In bioinformatics, some studies have applied SMOTE to balance the skewing benchmark datasets (Jia et al., 2016a,b; Liu et al., 2015; Xiao et al., 2015). In addition, similar approaches have been recently introduced to handle the unbalanced datasets, such as Monte Carlo sampling (Jia et al., 2016c) and fusion ensemble approach (Liu et al., 2016a; Qiu et al., 2016).
Although the aforementioned methods have some success in addressing unbalanced datasets, they have not achieved a satisfactory result in processing multi-labeled and imbalanced datasets simultaneously. Few works address the imbalance problem in MLC. He et al. (2012) took into account the imbalance in predicting subcellular localization of human proteins. Charte et al. (2015) built an under-sampling and oversampling algorithm on MLDs. Those studies improved the multi-label classification performance; however, they have some drawbacks in how to address the multi-label character of the new synthetic instance. In this paper, we tackle the imbalanced problem by a novel oversampling model referred to as ML-SMOTE, which is a synthetic minority oversampling on MLDs. We developed a new tool as a two-level AMP predictor based on ML-SMOTE. For a peptides sequence, we first identify whether it is an AMP. If yes, we then predict what potential activities it has. The first-level is a binary predictor, and the second-level predictor is an unbalanced and multi-labeled multi-classes predictor. The result shows ML-SMOTE can adjust the label set distribution to improve the performance of the predictor.
2 Methods
2.1 Benchmark dataset
The benchmark dataset used in this study was taken from Xiao et al. (2013). The dataset can be formulated as
| (1) |
where contains 879 AMPs, and contains 2405 non-AMPs. The 879 AMPs are formulated as
| (2) |
where contains 770 antibacterial peptides, 140 anti-cancer/tumor peptides, 366 antifungal peptides, 84 anti-HIV peptides and 124 antiviral peptides.
2.2 Sequence encoding scheme
To develop a powerful method for classifying AMPs and their functional families according to the sequence information, one of the keys is to formulate the peptides with an effective mathematical expression that can truly reflect the intrinsic correlation with the target to be identified. However, when comparing with other protein functional predictions, the challenge is identifying how AMPs deal with shorter peptides. For a peptides sample P of L amino acids
| (3) |
where represents the ith residue, L is usually between 5 and 50.
In this study, we formulated an amino acids sequence by using Chou’s PseAAC(Chou, 2001, 2005) with the grey model (GM) (Deng, 1989). According to Chou’s general PseAAC formula (Chou, 2009, 2011), the peptides P in Eq. 3 can be represented as
| (4) |
where T is a transpose operator, while the subscript Ω is an integer and its value as well as the components p1, p2, … depend on how to extract the desired information from the amino acid sequence of P.
In our study, we use the GM(1,1) model, which is an important and generally used model in GM. GM(1,1) firstly converts a series without any obvious regularity into a strict monotonic increasing series by using the accumulative generation operation (AGO). This process can reduce the randomness and enhance the smoothness of the series and minimize any interference from the random information. Let us assume that
| (5) |
is a non-negative original series of real numbers with an irregular distribution. Then
| (6) |
is viewed as the first-order accumulative generation operation (1-AGO) series for X(0), and the components in X(1) are given by
| (7) |
The GM(1,1) model can be expressed by the following grey differential equation with one variable:
| (8) |
where and b are elements of parameters vector , that is
| (9) |
In Eq. 8, is the developing coefficient and b the influence coefficient. They can be solved using a least square estimator.
| (10) |
where
| (11) |
| (12) |
The coefficients and b should carry some intrinsic information contained in the discrete data sequence X(0) sampled from the system investigated. In view of this, we incorporate these coefficients into the general form of PseAAC (Eq. 4) to reflect the correlation between the peptide sequence and prediction labels. In order to translate an amino acid sequence expressed with alphabets in Eq. 3 into a non-negative real series in Eq. 5, we need the amino acid numerical codes. In the same manner as that shown in (Xiao et al., 2013), we also use the numerical value of the following five physical-chemical properties for each of the 20 amino acids: (1) hydrophobicity; (2) pk1 (); (3) pk2 (NH3); (4) PI (25 °C); and (5) molecular weight. Finally, we used a 30-D features vector to represent a peptide; i.e. instead of Eq. 4, we now have
| (13) |
where are the frequencies of 20 amino acids; and p21 and p22 are the coefficients of Eq. 10 when amino acids are coded by hydrophobicity numerical values; p23 and p24 are the coefficients of Eq. 10 when amino acids are coded by pk1 numerical values, and so on.
2.3 ML-SMOTE algorithm
In Eq. 2, the AMP function family dataset is an unbalanced MLD, in which the antibacterial peptides have nine times the amount of the anti-HIV peptides. How to handle the MLC in unbalanced MLD is essential for improving prediction performance.
Let denote an m-dimensions real vector of instance and let
| (14) |
be a class label set. MLD can be represented as
| (15) |
We define the sample set with the j-th label as
| (16) |
If, the class is a majority class and the class is a minority class.
Different from SMOTE (Chawla et al., 2002) in a single label dataset, the new synthetic instance maybe have one or more labels. Hence, in (Charte et al., 2015), Charte et al. compared random undersampling (RUS) and random oversampling (ROS) based on Label Power-set (LP) and Multi-Label (ML), respectively. However, their LP-RUS and LP-ROS methods can only work well when the label density is low. Moreover, because their ML-ROS just clones the minority class samples, it is ineffective when these samples simultaneously have the majority class label, which happens often in MLD. In this study, we propose a novel oversampling model named ML-SMOTE. In the following algorithm description, we express a multi-label dataset (see Eq. 15) with N samples as
| (17) |
where
and the subset D(j) in which each sample is labeled lj class:
| (18) |
Algorithm ML-SMOTE algorithm’s pseudo-code
Inputs: Dataset: D with m features and q labels (see Eq. 17); k (the number of nearest neighbors)
Outputs: Preprocessed dataset S
(1) S = D
(2) MeanSize = (Ti is defined as Eq. 18)
(3) For j = 1→q
(4) If < meanSize
(5) For each sample in , do
(6) Find k-nearest neighbors set knn of sample in
(7) Randomly select a sample z from knn
(8) Get a random vector , where each element of r is a random number between 0 and 1.
(9) Calculate features of new sample:
Calculate labels of new sample:
where .* means array multiplying with element by element, and means round number.
(10) Add new sample (v, u) to S
(11) End for
(12) End if
(13) End for
For a new sample (v, u) synthesized from and its near neighbor z in,
| (19) |
3 Results
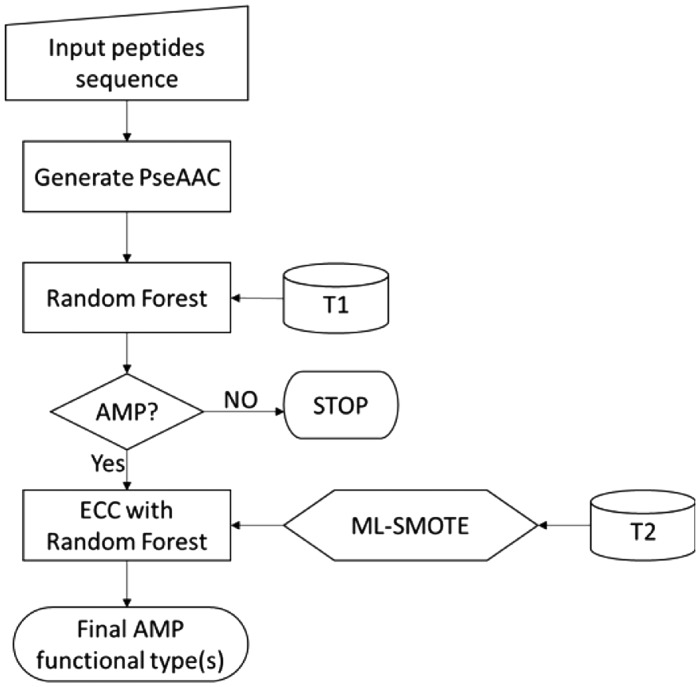
After the sequence feature retrieval and ML-SMOTE preprocessing as described above, a two-level AMP predictor named MLAMP was constructed, in which the Ensemble of Classifier Chains (ECC) algorithm (Waghu et al., 2014) was adopted as the prediction method (Fig. 1). We used the canonical implementation of ECC provided by the MULAN (Tsoumakas et al., 2010; Tsoumakas et al., 2011) multi-label learning in the Weka (Hall et al., 2009) library And for ECC, the binary and multi-class learners are implemented on the Weka platform using the Random Forest (RF) algorithm (Breiman, 2001).
Fig.1.
This flowchart shows the training process of MLAMP. T1 represents the data taken from the dataset for training the 1st-level predictor; T2 represents those from the dataset for training the 2nd-level predictor
MLAMP is a two-level prediction engine (See Fig. 1). The first level of MLAMP predicts a query peptide as AMP or non-AMP by using the RF algorithm. It belongs to the case of single-label classification. The following four measures were used for examining the performance of a single-label predictor, they are: (i) overall accuracy or Acc; (ii) Mathew’s correlation coefficient or MCC; (iii) sensitivity or Sn; and (iv) specificity or Sp.
| (20) |
where TP represents the true positive; TN, the true negative; FP, the false positive; FN, the false negative.
Although Eq. 20 was often used in the literature to measure the prediction quality of a method, they often lack intuitiveness, especially to biologists, particularly the MCC. According to Chou’s formulation, these four measures can be expressed as (Chen et al., 2016; Lin et al., 2014)
| (21) |
where stands for the total number of AMP samples investigated, whereas for the number of AMP samples incorrectly predicted to be of non-AMP; for the total number of non-AMP samples investigated, whereas for the number of non-AMP samples incorrectly predicted to be of AMP. With such a formulation as given in Eq. 21, the meanings of sensitivity, specificity, overall accuracy and Mathew’s correlation coefficient and their scopes would become more intuitive and easier-to-understand, particularly for the Mathew’s correlation coefficient, as concurred by a series of studies published very recently (Jia et al., 2016 b,c,d; Lin et al., 2014 , Liu et al., 2016a,b,c; Qiu et al., 2016; Xiao et al., 2016)
If a query peptide is predicted as AMP, the second level of MLAMP will start to classify its functional families. This process belongs to the case of multi-label classification. Hamming loss, Subset Accuracy, Accuracy, Precision and Recall are the mostly used evaluation metrics for the performance of a multi-label classifier (Lin et al., 2013; Tsoumakas and Katakis, 2007; Tsoumakas et al., 2010; Xiao et al., 2013). Suppose is the subset that contains all the labels for the kth sample ; is the subset that contains all the predicted labels for the kth sample ; N is the total number of samples; and M is the total number of labels. In this study, N = 879 and M = 5. The five metrics have been clearly defined as follows (Chou, 2013):
| (22) |
where is the operator acting on the set therein to count the number of its elements, and
| (23) |
When assessing a predictor, the following three cross-validation methods are often used in the literature: independent dataset test, subsampling (K-fold cross-validation) test and jackknife test. However, as elaborated in (Chou and Zhang, 1995), among the three cross-validation methods, the jackknife test is deemed the least arbitrary and most objective because it can always yield a unique result for a given benchmark dataset. Hence, the jackknife test was adopted in this study to examine the anticipated success rates of the current predictor. The process of jackknife test can be explained as follows:
Input: multi-label dataset T = {Pi | 1≤i≤N}.
Output: predicted label set.
For i: 0→N
T is divided into testing dataset Ts = {Pi},
and training dataset Tr = T-Ts.
Generate new training dataset Tr’ by using ML-SOMTE on Tr.
Train model on Tr’ by using ECC algorithm.
Predict the label set of Pi by the model trained above.
End For
Table 1 compares the performance of MLAMP with an existing method iAMP-2L in the first-level result on the benchmark (Eq. 1), where overall accuracy Acc and MCC achieved by MLAMP are higher than those achieved by iAMP-2L.
Table 1.
Result obtained by MLAMP in identifying AMP in identifying AMP and non-AMP on benchmark
| Predictor | Sn | Sp | Acc | MCC |
|---|---|---|---|---|
| MLAMPa | 77.0% | 94.6% | 89.9% | 0.737 |
| iAMP-2L | 87.1% | 86.0% | 86.2% | 0.726 |
The two parameters, i.e. the number of trees and features used in Random forest were 500 and 6, respectively.
The bold font indicates the better performance in the category.
To further demonstrate the power of the MLAMP predictor, we compared it with other classical predictors on an independent dataset containing 920 AMPs and 920 non-AMPs. This comparison was used for independent testing in (Thomas et al., 2010; Xiao et al., 2013). The results listed in Table 2 were obtained by MLAMP, iAMP-2L (Xiao et al., 2013) and CAMP (Thomas et al., 2010) on . As shown in Table 2, the performances achieved by MLAMP is remarkably higher than the performances reported by iAMP-2L (Xiao et al., 2013) and CAMP (Thomas et al., 2010) in all metrics (Sn, Sp, Acc and MCC).
Table 2.
Comparison of MLAMP with iAMP-2L and CAMP on the independent dataset
| Predictor | Algorithm | Sn | Sp | Acc | MCC |
|---|---|---|---|---|---|
| MLAMP | Random forest | 97.3% | 92.1% | 94.7% | 0.895 |
| iAMP-2L | Fuzzy k-nearest neighbor | 97.2% | 86.3% | 92.2% | 0.845 |
| CAMP | Support vector machine | 88.4% | 66.6% | 77.5% | 0.55 |
| Random forest | 89.7% | 26.0% | 57.8% | 0.157 | |
| Discriminant analysis | 86.6% | 64.1% | 75.4% | 0.508 |
Furthermore, in the second level prediction, MLAMP also obtained better performance than iAMP-2L. Some different metrics were used from single-label classification, in particular-Hamming loss, Accuracy, Precision, Recall and Subset Accuracy (Tsoumakas et al., 2010) were commonly applied in MLC. Table 3 gives the detailed jackknife test results on the AMP dataset (Eq. 2). Especially MLAMP gained a 0.4846 success rate in the strict assessment of subset accuracy and this performance was 5% higher than that by iAMP-2L.
Table 3.
Performance metrics achieved at the 2nd-level by MLAMP on the AMP dataset .
| Predictor | Hamming loss | Accuracy | Precision | Recall | Subset Accuracy |
|---|---|---|---|---|---|
| MLAMP | 0.1595 | 0.6864 | 0.8338 | 0.7631 | 0.4846 |
| iAMP-2L | 0.1640 | 0.6687 | 0.8331 | 0.7570 | 0.4305 |
Why can these metrics be improved so remarkably by using MLAMP? There are two key reasons. The first reason is probably the new peptide feature coding model (see Eq. 13). Table 4 sorts the 30 features in decreasing order after analyzing the benchmark dataset by the feature selection tool minimal-redundancy-maximal-relevance (mRMR) (Kolde et al., 2016). As shown in Table 4, those features generated by the grey model include more information than amino acids frequency, especially their biochemical properties. And one can draw a conclusion that some physicochemical properties of amino acids may play an important role in AMP, such as molecular weight, PI and Pk2. The second reason points to the new ML-SMOTE model. The AMP dataset is an imbalance MLD, and previous studies did not take it into account. After processing the training dataset by the ML-SMOTE model, the balance property of the new synthetic training dataset was improved, which can help the machine learning obtained a better performance.
Table 4.
Features in the descending order of importance
| Features in Eq. 13 | Description of features |
|---|---|
| P11 | Frequency of Methionine |
| P29 | Molecular weight ① |
| P30 | Molecular weight ① |
| P27 | PI ② |
| P28 | PI ② |
| P26 | pk2 ③ |
| P25 | pk2 ③ |
| P23 | pk1 ④ |
| P21 | Hydrophobicity ⑤ |
| P17 | Frequency of Threonine |
| P20 | Frequency of Tyrosine |
| P15 | Frequency of Arginine |
| P18 | Frequency of Valine |
| P19 | Frequency of Tryptophan |
| P14 | Frequency of Glutamine |
| P12 | Frequency of Asparagine |
| P16 | Frequency of Serine |
| P13 | Frequency of Proline |
| P4 | Frequency of Glutamic Acid |
| P24 | pk1 ④ |
| P7 | Frequency of Histidine |
| P9 | Frequency of Lysine |
| P8 | Frequency of Isoleucine |
| P6 | Frequency of Glycine |
| P5 | Frequency of Phenylalanine |
| P3 | Frequency of Aspartic Acid |
| P22 | Hydrophobicity ⑤ |
| P10 | Frequency of Leucine |
| P2 | Frequency of Cysteine |
| P1 | Frequency of Alanine |
①: parameter of grey model built by molecular weight; ②: parameter of grey model built by PI; ③: parameter of grey model built by pk2 code; ④: parameter of grey model built by pk1 code; ⑤: parameter of grey model built by hydrophobicity code.
4 Conclusion
Due to increasing antibiotic resistance, AMPs, which are key components of innate immune system, are becoming more and more important in drug development. Efficiently and effectively identifying AMPs and their functional types has become an urgent research topic. The results reported in this study indicate that the novel predictor, MLAMP, provides an accurate and useful tool for researchers to find new infection therapeutics.
MLAMP obtained a better prediction performance than that of a previous method. The primary reason for our good performance is our formulation model’s peptide extraction features. Since AMPs usually have 5–50 amino acids, our model (Eq.13) is good for formulating short peptides. It includes the internal relationship of amino acids sequence in various physical-chemical properties. The second reason is the ML-SMOTE model, which does a good job of handling the lack of balance problem in multi-label datasets. Compared with other methods, the sample synthetized by using ML-SMOTE retains the multi label distributions. It not only accumulates minority samples but also keeps the label density of MLD. In the future, the MLSMOTE model can be extended to assist with imbalance and multi-label datasets for other problems.
For practical applications, a user-friendly web-server for MLAMP has been established at http://www.jci-bioinfo.cn/MLAMP, which allows users to easily obtain their desired results without the need to follow the complicated mathematical equations involved in developing the predictor. Users can submit a peptide sequence to the webserver and subsequently the webserver will return the predicted result in real time. Alternatively, users can choose the batch prediction by entering their e-mail address and their batch input file of many peptide sequences. They will quickly receive an email showing the predicted results from seconds to hours depending on the number of sequences.
Funding
This work has been supported by the National Natural Science Foundation of China (No. 61462047, 31560316) and by the US National Institutes of Health (GM100701)
Conflict of Interest: none declared.
References
- Bahar A.A., Ren D. (2013) Antimicrobial peptides. Pharmaceuticals, 6, 1543–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiman L. (2001) Random forests. Mach. Learn., 45, 5–32. [Google Scholar]
- Bunkhumpornpat C. et al. (2009) Safe-Level-SMOTE: safe-level-synthetic minority over-sampling TEchnique for handling the class imbalanced problem. Adv. Knowl. Discov. Data Min. Proc., 5476, 475–482. [Google Scholar]
- Charte F. et al. (2015) Addressing imbalance in multilabel classification: measures and random resampling algorithms. Neurocomputing, 163, 3–16. [Google Scholar]
- Chawla N. (2010) Data mining for imbalanced datasets: an overview In Maimon O., Rokach L. (eds) Data Mining and Knowledge Discovery Handbook. Springer, US, pp. 875–886. [Google Scholar]
- Chawla N.V. et al. (2002) SMOTE: synthetic minority over-sampling technique. J. Artif. Intell. Res., 16, 321–357. [Google Scholar]
- Chawla N.V. et al. (2003) SMOTEBoost: Improving prediction of the minority class in boosting. In Lavrac,N., et al (eds), Knowledge discovery in databases: PKDD 2003: 7th European Conference on Principles and Practice of Knowledge discovery in databases, Cavtat– Dubrovnik, Croatia, September 22-26, 2003. Proceedings. Springer Berlin Heidelberg, Berlin, Heidelberg, 107–119. [Google Scholar]
- Chen W. et al. (2016) iACP: a sequence-based tool for identifying anticancer peptides. Oncotarget, 7, 16895–16909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L. et al. (2014) Gene function prediction based on the gene ontology hierarchical structure. PLoS One, 9, e107187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou K.C. (2001) Prediction of protein cellular attributes using pseudo amino acid composition. Proteins Struct. Funct. Bioinf., 246–255. [DOI] [PubMed] [Google Scholar]
- Chou K.C. (2005) Using amphiphilic pseudo amino acid composition to predict enzyme subfamily classes. Bioinformatics, 21, 10–19. [DOI] [PubMed] [Google Scholar]
- Chou K.C. (2009) Pseudo amino acid composition and its applications in bioinformatics, proteomics and system biology. Curr. Proteomics, 262–274. [Google Scholar]
- Chou K.C. (2011) Some remarks on protein attribute prediction and pseudo amino acid composition. J. Theor. Biol., 273, 236–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou K.C. (2013) Some remarks on predicting multi-lable attributes in molecular biosystems. Mol. Biosyst., 1092–1100. [DOI] [PubMed] [Google Scholar]
- Chou K.C. (2015) Impacts of bioinformatics to medicinal chemistry. Med. Chem., 11, 218–234. [DOI] [PubMed] [Google Scholar]
- Chou K.C., Shen H.B. (2007) Euk-mPLoc: a fusion classifier for large-scale eukaryotic protein subcellular location prediction by incorporating multiple sites. J. Proteome Res., 6, 1728–1734. [DOI] [PubMed] [Google Scholar]
- Chou K.C., Shen H.B. (2008) Cell-PLoc: a package of Web servers for predicting subcellular localization of proteins in various organisms. Nat. Protoc., 3, 153–162. [DOI] [PubMed] [Google Scholar]
- Chou K.C., Zhang C.T. (1995) Prediction of protein structural classes. Crit. Rev. Biochem. Mol. Biol., 30, 275–349. [DOI] [PubMed] [Google Scholar]
- Chou K.C. et al. (2011) iLoc-Euk: a multi-label classifier for predicting the subcellular localization of singleplex and multiplex eukaryotic proteins. PLoS ONE, 6, e18258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou K.C. et al. (2012) iLoc-Hum: using the accumulation-label scale to predict subcellular locations of human proteins with both single and multiple sites. Mol. Biosyst., 8, 629–641. [DOI] [PubMed] [Google Scholar]
- Conlon J.M. et al. (2014) Potential therapeutic applications of multifunctional host-defense peptides from frog skin as anti-cancer, anti-viral, immunomodulatory, and anti-diabetic agents. Peptides, 57, 67–77. [DOI] [PubMed] [Google Scholar]
- Deng J.L. (1989) Introduction to grey system theory. J. Grey Syst., 1–24. [Google Scholar]
- Dong Y.J., Wang X.H. (2011) A new over-sampling approach: random-SMOTE for learning from imbalanced data sets. Knowl. Sci. Eng. Manag., 7091, 343–352. [Google Scholar]
- Fjell C.D. et al. (2007) AMPer: a database and an automated discovery tool for antimicrobial peptides. Bioinformatics, 23, 1148–1155. [DOI] [PubMed] [Google Scholar]
- Gao M. et al. (2011a) A combined SMOTE and PSO based RBF classifier for two-class imbalanced problems. Neurocomputing, 74, 3456–3466. [Google Scholar]
- Gao M. et al. (2011b) On combination of SMOTE and particle swarm optimization based radial basis function classifier for imbalanced problems. 2011 Int. Jt. Conf. Neural Netw. (IJCNN), 1146–1153. [Google Scholar]
- Gaspar D. et al. (2013) From antimicrobial to anticancer peptides. A review. Front. Microbiol., 4, 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogoladze G. et al. (2014) DBAASP: database of antimicrobial activity and structure of peptides. FEMS Microbiol. Lett., 357, 63–68. [DOI] [PubMed] [Google Scholar]
- Hall M. et al. (2009) The WEKA data mining software: An updata. SIGKDD Explor. Newsl., 11, 10–18. [Google Scholar]
- He J. et al. (2012) Imbalanced multi-modal multi-label learning for subcellular localization prediction of human proteins with both single and multiple sites. PLoS ONE, 7, e37155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J. et al. (2016a) iPPBS-Opt: a sequence-based ensemble classifier for identifying protein-protein binding sites by optimizing imbalanced training datasets. Molecules, 21, E95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J. et al. (2016b) iSuc-PseOpt: identifying lysine succinylation sites in proteins by incorporating sequence-coupling effects into pseudo components and optimizing imbalanced training dataset. Anal. Biochem., 497, 48–56. [DOI] [PubMed] [Google Scholar]
- Jia J. et al. (2016c) iCar-PseCp: identify carbonylation sites in proteins by Monto Carlo sampling and incorporating sequence coupled effects into general PseAAC. Oncotarget, 7, 34558–34570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J. et al. (2016d) pSuc-Lys: predict lysine succinylation sites in proteins with PseAAC and ensemble random forest approach. J. Theor. Biol., 394, 223–230. [DOI] [PubMed] [Google Scholar]
- Joseph S. et al. (2012) ClassAMP: a prediction tool for classification of antimicrobial peptides. IEEE/ACM Trans. Comput. Biol. Bioinform., 9, 1535–1538. [DOI] [PubMed] [Google Scholar]
- Khamis A.M. et al. (2015) Distinct profiling of antimicrobial peptide families. Bioinformatics, 31, 849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravian M. et al. (2013) Predicting antibacterial peptides by the concept of Chou's pseudo-amino acid composition and machine learning methods. Protein Pept. Lett., 20, 180–186. [DOI] [PubMed] [Google Scholar]
- Kolde R. et al. (2016) seqlm: an MDL based method for identifying differentially methylated regions in high density methylation array data. Bioinformatics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lata S. et al. (2007) Analysis and prediction of antibacterial peptides. BMC Bioinformatics, 8, 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lata S. et al. (2010) AntiBP2: improved version of antibacterial peptide prediction. BMC Bioinformatics, 11, S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W.Z. et al. (2013) iLoc-Animal: a multi-label learning classifier for predicting subcellular localization of animal proteins. Mol. Biosyst., 9, 634–644. [DOI] [PubMed] [Google Scholar]
- Lin H. et al. (2014) iPro54-PseKNC: a sequence-based predictor for identifying sigma-54 promoters in prokaryote with pseudo k-tuple nucleotide composition. Nucleic Acids Res., 42, 12961–12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lira F. et al. (2013) Prediction of antimicrobial activity of synthetic peptides by a decision tree model. Appl. Environ. Microbiol., 79, 3156–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.M., Chen J.H. (2015) A multi-label classification based approach for sentiment classification. Expert. Syst. Appl., 42, 1083–1093. [Google Scholar]
- Liu Z. et al. (2015) iDNA-Methyl: identifying DNA methylation sites via pseudo trinucleotide composition. Anal. Biochem., 474, 69–77. [DOI] [PubMed] [Google Scholar]
- Liu B. et al. (2016a) iDHS-EL: identifying DNase I hypersensitive sites by fusing three different modes of pseudo nucleotide composition into an ensemble learning framework. Bioinformatics, 32, 2411–2418. [DOI] [PubMed] [Google Scholar]
- Liu B. et al. (2016b) iEnhancer-2L: a two-layer predictor for identifying enhancers and their strength by pseudo k-tuple nucleotide composition. Bioinformatics, 32, 362–369. [DOI] [PubMed] [Google Scholar]
- Liu Z. et al. (2016c) pRNAm-PC: predicting N(6)-methyladenosine sites in RNA sequences via physical-chemical properties. Anal. Biochem., 497, 60–67. [DOI] [PubMed] [Google Scholar]
- Luengo J. et al. (2011) Addressing data complexity for imbalanced data sets: analysis of SMOTE-based oversampling and evolutionary undersampling. Soft Comput., 15, 1909–1936. [Google Scholar]
- Malmsten M. (2014) Antimicrobial peptides. Upsala J. Med. Sci., 119, 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niarchou A. et al. (2013) C-PAmP: large scale analysis and database construction containing high scoring computationally predicted antimicrobial peptides for all the available plant species. PLoS One, 8, e79728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic S. et al. (2012) Peptides with antimicrobial and anti-inflammatory activities that have therapeutic potential for treatment of acne vulgaris. Peptides, 34, 275–282. [DOI] [PubMed] [Google Scholar]
- Qiu W.R. et al. (2016) iPhos-PseEvo: identifying human phosphorylated proteins by incorporating evolutionary information into general PseAAC via Grey System Theory. Mol Inform, in press. [DOI] [PubMed] [Google Scholar]
- Read J. et al. (2009) Classifier chains for multi-label classification. Lect. Notes Comput. Sci., 5782, 254–269. [Google Scholar]
- Read J. et al. (2011) Classifier chains for multi-label classification. Mach. Learn., 85, 333–359. [Google Scholar]
- Sadasivam R., Duraiswamy K. (2015) MLDSS: an algorithm to mine multi-label disease spreading sequence using spatio-time interval database. J. Med. Imag. Health, 5, 17–26. [Google Scholar]
- Schapire R.E., Singer Y. (2000) BoosTexter: a boosting-based system for text categorization. Mach. Learn., 39, 135–168. [Google Scholar]
- Seiffert C. et al. (2008) RUSBoost: improving classification performance when training data is skewed. In: Int C Patt Recog, pp. 3650–3653. [Google Scholar]
- Shen H.B., Chou K.C. (2007) Hum-mPLoc: an ensemble classifier for large-scale human protein subcellular location prediction by incorporating samples with multiple sites. Biochem. Biophys. Res. Commun., 355, 1006–1011. [DOI] [PubMed] [Google Scholar]
- Shen H.B., Chou K.C. (2009) Gpos-mPLoc: a top-down approach to improve the quality of predicting subcellular localization of Gram-positive bacterial proteins. Protein Pept. Lett., 16, 1478–1484. [DOI] [PubMed] [Google Scholar]
- Shen H.B., Chou K.C. (2010a) Gneg-mPLoc: a top-down strategy to enhance the quality of predicting subcellular localization of Gram-negative bacterial proteins. J. Theor. Biol., 264, 326–333. [DOI] [PubMed] [Google Scholar]
- Shen H.B., Chou K.C. (2010b) Virus-mPLoc: a fusion classifier for viral protein subcellular location prediction by incorporating multiple sites. J. Biomol. Struct. Dyn., 28, 175–186. [DOI] [PubMed] [Google Scholar]
- Tahir M.A. et al. (2012) Inverse random under sampling for class imbalance problem and its application to multi-label classification. Pattern Recogn., 45, 3738–3750. [Google Scholar]
- Thomas S. et al. (2010) CAMP: a useful resource for research on antimicrobial peptides. Nucleic Acids Res., 38, D774–D780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoumakas G., Katakis l. (2007) Multi-label classification: an overview. Int. J. Data Warehousing Min., 3, 13. [Google Scholar]
- Tsoumakas G. et al. (2010) Mining multi-label data In Maimon O., Rokach L. (eds) Data Mining and Knowledge Discovery Handbook. Springer, US, pp. 667–685. [Google Scholar]
- Tsoumakas G. et al. (2011) MULAN: A java library for multi-label learning. J. Mach. Learn. Res., 12, 2411–2414. [Google Scholar]
- Vishnepolsky B., Pirtskhalava M. (2014) Prediction of linear cationic antimicrobial peptides based on characteristics responsible for their interaction with the membranes. J. Chem. Inf. Model., 54, 1512–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waghu F.H. et al. (2013) CAMP: Collection of sequences and structures of antimicrobial peptides. Nucleic Acids Res., 42, D1154–D1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S. et al. (2012) mGOASVM: multi-label protein subcellular localization based on gene ontology and support vector machines. BMC Bioinformatics, 13, 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. (2014) Human antimicrobial peptides and proteins. Pharmaceuticals, 7, 545–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. et al. (2009) APD2: the updated antimicrobial peptide database and its application in peptide design. Nucleic Acids Res., 37, D933–D937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P. et al. (2011) Prediction of antimicrobial peptides based on sequence alignment and feature selection methods. Plos One, 6, e18476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z.C. et al. (2011) iLoc-Plant: a multi-label classifier for predicting the subcellular localization of plant proteins with both single and multiple sites. Mol. Biosyst., 7, 3287–3297. [DOI] [PubMed] [Google Scholar]
- Wu Z.C. et al. (2012) iLoc-Gpos: a multi-layer classifier for predicting the subcellular localization of singleplex and multiplex Gram-positive bacterial proteins. Protein Pept. Lett., 19, 4–14. [DOI] [PubMed] [Google Scholar]
- Xiao X. et al. (2011) iLoc-Virus: a multi-label learning classifier for identifying the subcellular localization of virus proteins with both single and multiple sites. J. Theor. Biol., 284, 42–51. [DOI] [PubMed] [Google Scholar]
- Xiao X. et al. (2013) iAMP-2L: a two-level multi-label classifier for identifying antimicrobial peptides and their functional types. Anal. Biochem., 168–177. [DOI] [PubMed] [Google Scholar]
- Xiao X. et al. (2015) iDrug-Target: predicting the interactions between drug compounds and target proteins in cellular networking via benchmark dataset optimization approach. J. Biomol. Struct. Dyn., 33, 2221–2233. [DOI] [PubMed] [Google Scholar]
- Xiao X. et al. (2016) iROS-gPseKNC: Predicting replication origin sites in DNA by incorporating dinucleotide position-specific propensity into general pseudo nucleotide composition. Oncotarget, 7, 34180–34189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z.Y. et al. (2013) Optimization support vector machine. Front. Artif. Intel. Ap., 255, 371–379. [Google Scholar]
- Yancheva D. et al. (2012) Synthesis, structure and antimicrobial activity of 6-(propan-2-yl)-3-methyl-morpholine-2,5-dione. J. Mol. Struct., 1016, 147–154. [Google Scholar]
- Yu G. et al. (2013) Protein function prediction using multi-label ensemble classification. IEEE/ACM Trans. Comput. Biol. Bioinf., 10, 1–1. [DOI] [PubMed] [Google Scholar]
- Zhang M.L., Zhou Z.H. (2007) ML-KNN: A lazy learning approach to multi-label learning. Pattern Recogn., 40, 2038–2048. [Google Scholar]
- Zhang M.L. et al. (2009) Feature selection for multi-label naive Bayes classification. Inf. Sci., 179, 3218–3229. [Google Scholar]
- Zhang Y. et al. (2012) Using ensemble methods to deal with imbalanced data in predicting protein–protein interactions. Comput. Biol. Chem., 36, 36–41. [DOI] [PubMed] [Google Scholar]
- Zhao X. et al. (2013) LAMP: a database linking antimicrobial peptides. PLoS One, 8, e66557. [DOI] [PMC free article] [PubMed] [Google Scholar]