Abstract
The vascular supply to the human bladder is derived mainly from the superior and inferior vesical arteries, the latter being directly connected to the internal iliac artery. Aging is associated with an impairment of blood vessel function and changes may occur in the vasculature at the molecular, cellular and functional level. Pelvic arterial insufficiency may play an important role in the development of bladder dysfunctions such as detrusor overactivity (DO) and the overactive bladder syndrome. Chronic ischemia-related bladder dysfunction may progress to bladder underactivity and it would be desirable to treat not only lower urinary tract symptoms (LUTS) induced by chronic ischemia, but also the progression of the morphological bladder changes. Studies in experimental models in rabbits and rats have shown that pelvic arterial insufficiency may result in significant bladder ischemia with reduced bladder wall oxygen tension. In turn, this will lead to oxidative stress associated with upregulation of oxidative stress-sensitive genes, increased muscarinic receptor activity, ultrastructural damage, and neurodegeneration. The phosphodiesterase type 5 (PDE5) inhibitor tadalafil, the α1-adrenoceptor (AR) blocker silodosin, the β3-AR agonist mirabegron, and the free radical scavenger melatonin, exerted a protecting effect on urodynamic parameters, and on functional and morphological changes of the bladder demonstrable in vitro. Since the agents tested are used clinically for relieving LUTS, the results from the animal models seem to have translational value, and may be of relevance for designing clinical studies to demonstrate if the drugs may prevent progression of ischemia-related functional and morphological bladder changes.
Keywords: aging, bladder blood flow, bladder dysfunction, ischemia, LUTS, pharmacological treatment
Introduction
A high prevalence of aging-related bladder dysfunctions and associated lower urinary tract symptoms (LUTS) has been well documented in both male and female patients [Irwin et al. 2006]. A number of cardiovascular, metabolic, and endocrine factors can be associated with the development of LUTS [Andersson, 2003; Kaplan, 2006; Mariappan and Chong, 2006; Andersson, 2007; Rosen et al. 2009]. Even if LUTS may be multifactorial, evidence from epidemiologic, clinical, and basic research suggests that aging-associated changes in the pelvic vasculature may be an important contributing factor. Several investigators have concluded that vascular risk factors for atherosclerosis, eventually resulting in bladder ischemia, may play a role in the development of LUTS in both men and women [Ponholzer et al. 2006]. A close correlation between history of cardiovascular disease and incidence of lower urinary tract (LUT) dysfunction has been demonstrated [Diokno et al. 1986]. Smoking is a known vascular risk factor and has been associated with LUTS in men [Fultz and Herzog, 1996]. A close association between LUTS and erectile dysfunction (ED) has also been documented [Rosen et al. 2003]. Recent studies have more directly shown that many cases of LUTS in elderly men and women may be associated with bladder ischemia. Thus, transrectal color Doppler ultrasonography of elderly patients with LUTS showed a significant decrease in bladder blood flow in comparison with asymptomatic younger controls [Pinggera et al. 2008a, 2008b]. Improvement of LUTS with α1-adrenoceptor (AR) blocker treatment was associated with a significant increase in bladder blood flow [Pinggera et al. 2008a, 2008b].
Studies of experimental models have shown that pelvic arterial insufficiency and outlet obstruction may result in significant bladder ischemia. These ischemic conditions were shown to reduce the bladder wall oxygen tension, lead to oxidative stress and were associated with upregulation of oxidative stress-sensitive genes, muscarinic receptor over-reactivity, ultrastructural damage, and neurodegeneration [Azadzoi et al. 1999a, 1999b, 2008, 2010, Nomiya et al. 2012].
These observations suggest that bladder ischemia and subsequent hypoxia may be important factors contributing to LUTS, particularly in the elderly population. The mechanisms behind the changes in bladder function caused by chronic bladder ischemia, and the time course of the progression of these changes, are incompletely known. In this review, based on evidence from available literature, we discuss the blood flow to the bladder, both in normally functioning bladders and in bladder dysfunctions, the effects of aging on the vasculature, and how aging and chronic ischemia affects bladder function with particular emphasis on chronic ischemia associated with bladder outlet obstruction (BOO) and atherosclerosis. We also discuss possible therapeutic interventions, and suggest some directions for future research.
Bladder vasculature: adaptation to filling and voiding
The main arterial supply to the bladder comes from the internal iliac arteries and normally enters the bladder through between one and four superior vesical arteries, a single inferior vesical artery (of variable origin) and a vesiculo-deferential artery [Braithwaite, 1952; Shehata, 1976]. The bladder base and urethra receive a separate supply from branches of the inferior vesical arteries. The bladder is also variably supplied by small branches of several other arteries arising from the internal iliac arteries. Sarma studied the micro-anatomy of blood vessels in male (n = 12) and female (n = 8) bladders obtained from post mortem examinations and known to have been free from any bladder disease [Sarma, 1981]. He used radiographs of thin slices of the bladder wall after injection of the vessels with radiopaque contrast and described three plexuses of blood vessels that freely interconnected across the bladder wall, an intramural, an extramural and a submucosal (suburothelial) plexus. The vessels were seen to form an intricate plexus which extended from the extramural plane through the musculature to the lamina propria. There was considerable regional variation in the density of the plexus. Brading and colleagues used an antibody against von Willebrand factor to selectively stain the endothelial cells in blood vessel walls in order to study the micro-anatomy of the vessels in thick cryostat sections of normal pig and human bladders [Brading et al. 1999]. They found that the density of blood vessels appeared to be greater in the lamina propria than in the muscular wall, and that arteries and veins run predominantly in the connective tissue between the muscle bundles, with mainly capillaries penetrating between the smooth muscle fascicles. In thick sections the tortuous course of the blood vessels was clearly apparent. This was also conspicuous in the corrosion casting study on the human bladder wall performed by Miodoński and Litwin [Miodoński and Litwin, 1999]. They identified two major vascular plexuses (adventitial/serosal and mucosal), and distinguished two distinct capillary networks (muscular and subepithelial) in the successive layers of the wall. The striking feature of almost all bladder vessels, except the capillaries, was their tortuosity ranging from waviness to tight coiling. The mucosal plexus consisted of some capillaries, thin arteries (50–100 µm) and more numerous, thicker veins (80–250 µm), showing a tortuous appearance and frequent interlacements; it formed a distinct vascular layer parallel to the inner surface of the bladder and following the profiles of mucosal folds. The rich mucosal plexus followed the folds parallel to their surface and gave off short, straight, mostly perpendicular twigs communicating with the subepithelial capillary network. The subepithelial capillary network showed extreme density and uneven contours of the capillaries, and only in less folded areas of trigone and urethral orifice the network was looser and capillaries thinner. In contrast, the capillary system of the muscularis was poorly developed.
The subepithelial capillary network has been studied in detail by scanning electron microscopy of the rat [Inoue and Gabella, 1991] and human [Congiu et al. 2004] bladder. It was found in rat that the capillaries were located not just close to the urothelium, but within grooves of its basal surface so that some of them were surrounded by urothelial cells. It was suggested that there was a specific physiologic interaction between blood flow and urothelium, the significance of which was unclear [Inoue and Gabella, 1991]. Similar findings have been demonstrated in the bladders of rabbits [Hossler and Monson, 1995], dogs [Hossler and Kao, 2007], and mice [Hossler et al. 2013]. The morphological and functional properties of the suburothelial microvessels in the rat and mouse bladders have been extensively studied [Hashitani et al. 2011, 2012; Mitsui and Hashitani, 2013, Shimizu et al. 2014] (see below).
Since the barrier function of the urothelium and contractile functions of the detrusor depend on adequate supply of oxygen and nutrients from the blood, the blood vessels in the bladder wall must be capable of adaptation to the spatial changes resulting from the filling/voiding cycle without compromising the blood flow, and it is reasonable to assume that the arrangement of the bladder wall vessels should make it possible to maintain blood flow during normal filling.
However, blood flow in the bladder is not well understood. It is influenced by several mechanical factors: muscle contraction, compression of urine content, and stretch of tissue layers with bladder distension [Inoue and Gabella, 1991]. Measurements of bladder blood flow during filling, using different methodologies, have shown divergent results with both increased and decreased flow [Brading et al. 1999]. In human bladders without obstruction, there was a two-fold increase in bladder blood flow associated with filling, compared with the empty state, as measured with laser Doppler in the posterior wall [Kershen et al. 2002]. Blood flow did not start to decrease until 75% of maximum capacity was reached. Andersson and colleagues found in cats that filling of the bladder induced a sustained increase in vesical blood flow which was related to the bladder volume [Andersson et al. 1985]. Injection of increasing volume of saline decreased bladder vascular resistance. This vasodilation occurred despite a steady state increase in bladder pressure by 25–50 cm H2O. Blood flow decreased towards control values when the bladder was emptied. The response was unaffected by blockade of autonomic receptors, but was eliminated by papaverine, indicating that the response was not due to a rearrangement of the vessels at large bladder volumes. The authors speculated that local mechanisms, possibly involving release of prostaglandins, were involved in the vasodilation following bladder distension, thus ensuring good perfusion of the bladder during the vascular rearrangement necessary with an increased surface area.
However, several studies have shown a decreased blood flow in both the mucosa and the detrusor muscle during filling. Nemeth and colleagues, using a microsphere technique in dogs, found a greater fall in the mucosal than in the muscularis blood flow [Nemeth et al. 1977]. This would mean that the mucosa, which has a metabolic rate three times higher than that of the detrusor [Hypolite et al. 1993], should be the most vulnerable part of the bladder wall. Since the urothelium and suburothelial vessels are located in the periphery of the bladder wall circulation, they may be more readily affected by ischemia than the outer detrusor muscle layers. Hashitani and colleagues therefore proposed that suburothelial microvessels may well have intrinsic properties that maintain blood flow to the urothelium/lamina propria [Hashitani et al. 2011]. A large part of afferent nerves is located to the lamina propria, and they may be activated to signal ischemic insult (e.g. outflow obstruction). Indeed, this seems to be the case. Azadzoi and colleagues showed that in the rabbit bladder, atherosclerosis-induced ischemia caused alterations of neurokinin (NK)2 receptor reactivity and gene expression, an increased number of tachykinin immunopositive nerves, and greater epithelial tachykinin immunoreactivity [Azadzoi et al. 2008].
The distribution of the blood vessels with a dense mucosal blood supply may play a role in ensuring that the deeper smooth muscles are protected from exposure to any ions or metabolites that may diffuse through the urothelium from the urine [Brading et al. 1999; Shimizu et al. 2014]. The ability of the blood vessels to supply a greatly increased surface area of bladder wall during filling clearly results from the coiled nature of the vessels in the empty bladder. It seems clear that perfusion can be maintained at a normal level as long as the intravesical pressure does not rise more than a few cm H2O as it fills. In addition, the bladder wall seems to possess well-developed mechanisms for producing local vasodilatation in response to reduced blood flow.
Aging-related vascular changes
Aging is associated with an impairment of blood vessel function and changes may occur in the vasculature on the molecular, cellular and functional level [Ungvari et al. 2010; Bachschmid et al. 2013; Oakley and Tharakan, 2014; Rubio-Ruiz et al. 2014]. Pathophysiologically, vascular aging is mainly characterized by endothelial dysfunction [el Assar et al. 2012, 2013; Bachschmid et al. 2013]. Vascular aging already starts in young adults by slow and progressive vascular remodeling [Kotsis et al. 2011; Nilsson et al. 2013], and early signs of declining endothelial function manifest before the fourth decade of life. The aging vasculature displays typical morphological and molecular alterations leading to increased vascular stiffness, reduced compliance, hyperpermeability, and the loss of vascular homeostasis [Kotsis et al. 2011; Nilsson et al. 2013; Bachschmid et al. 2013; Oakley and Tharakan, 2014]. Morphological changes involve intima–media thickening and vascular hypertrophy/hyperplasia accompanied by reorganization of the extracellular matrix [Bentzon et al. 2014].
Among the proposed mechanisms that contribute to age-dependent endothelial dysfunction are (a) reduction of nitric oxide (NO) bioavailability by diminished NO synthesis or augmented NO scavenging, (b) oxidative stress, leading to peroxynitrate formation, (c) imbalance in the production of vasoconstrictor/ vasodilator factors, (d) low-grade pro-inflammatory environment, (e) impaired angiogenesis, and (f) endothelial cell senescence [el Assar et al. 2012, 2013; Rubio-Ruiz et al. 2014]. In turn such changes will lead altered replicating potential, change in cellular phenotype, changes in responsiveness to contracting and relaxing mediators, and changes in intracellular signaling functions [Rubio-Ruiz et al. 2014].
Ischemia, induced by atherosclerosis, is a common cause of disorders in the elderly, including bladder dysfunction. Intimal thickening in atherosclerosis involves the invasion and de-differentiation of macrophages to lipid-laden foam cells as well as recruitment of vascular smooth muscle cells and neointima formation. This process causes severe luminal reduction, and unstable plaques may promote thrombus formation and arterial occlusive disease [Bentzon et al. 2014].
Aging-related changes in bladder function
Blood flow changes
Saito and colleagues compared blood flow to the bladder and detrusor function in vivo and in vitro in young (6-month-old) and aged (24-month-old) male Sprague-Dawley rats [Saito et al. 1999]. They found that in both young and old rats, blood flow to the bladder, as measured by a laser Doppler flowmeter, decreased as intravesical volume increased and was smaller in old rats than in young rats. Cystometrograms performed under anesthesia revealed that old rats had smaller voiding pressure and larger bladder capacity than young rats. In isolated bladders, the pressure increase in response to bethanechol and low-frequency field stimulation was impaired by aging. Volume-pressure studies showed that in isolated bladders of old rats, compliance was greater and peak response to field stimulation was observed at a larger capacity. These findings indicate that bladders of older rats have a larger capacity with good compliance, but less contractility. Aging changes correlated with a decrease in blood flow to the bladder.
Also in humans, bladder blood flow seems to be reduced with aging. Using transrectal color Doppler ultrasonography, Pinggera and colleagues showed that elderly patients with LUTS had a significant lower bladder blood flow compared with with asymptomatic younger controls [Pinggera et al. 2008a, 2008b].
Bladder receptor changes
In rodents, the muscarinic receptor function has been compared in vitro in bladder tissue from old versus young animals, and also in vivo, most often by cystometry. The results have been contradictory, which may partly be due to strain-specific differences [Andersson, 2011; Bjorling et al. 2015]. Whatever age-related changes in muscarinic receptor functions have been demonstrated in animal bladders, they do not seem to be predictive for what is occurring in humans. A study by Mansfield and colleagues using a radioligand binding assay, showed that the total number of muscarinic receptors in the human male detrusor decreased with age [Mansfield et al. 2005]. They also found a decrease in mRNA expression of M3 receptors with age in both male and female subjects, while M2 receptors expression did not change. Due to the lack of highly specific antibodies for the muscarinic receptor subtypes, it was not possible to determine if these changes in mRNA expression were accompanied by a change in protein expression. The functional consequences of the findings were not reported. In detrusor muscle from patients undergoing radical cystectomy, Wuest and colleagues found no age-related changes in muscarinic receptor expression, nor in contractility [Wuest et al. 2005]. Similarly, Fry and colleagues found no evidence for a decline of detrusor smooth muscle contractility or excitability as a function of age, nor any gender difference or presence of pathology [Fry et al. 2011]. If there are any consistent age-related changes in muscarinic receptor function in the human bladder, they do not seem to be associated with a change in the response to antimuscarinics: no evidence for reduced therapeutic benefits from such treatment in older patients has been found [Michel et al. 2002; Dubeau et al. 2014].
Yoshida and colleagues found a significant positive correlation between age and the purinergic component of human bladder preparation contraction, and a significant negative correlation between age and the cholinergic component of human bladder preparation contraction [Yoshida et al. 2001, 2004]. The authors studied the neurotransmitter release from the detrusor during electrical field stimulation (EFS), using high-performance liquid chromatography. They found that acetylcholine release and age were significantly negatively correlated, while ATP release and age were positively correlated. In support of this finding, Chua and colleagues found that there was an age-related decrease in P2X1 mRNA expression in bladders from males undergoing cystoscopy for annual bladder cancer review or for investigation of asymptomatic hematuria, and suggested that this downregulation might occur as a result of an increased component of neural ATP release in the aging bladder [Chua et al. 2007]. Interestingly, no downregulation was found in obstructed bladders.
Wuest and colleagues studied the putative age-dependence of concentration-response curves to the purinergic agonists ATP and α-β-methylene-ATP in human detrusor muscle strips [Wuest et al. 2005]. In accordance with the results of Yoshida and colleagues they found that the sensitivity to α-β-methylene-ATP increased with age [Yoshida et al. 2001].
Clinical symptoms and urodynamic changes
The aging process of both sexes can be associated with significant changes in bladder function and clinical symptomatology. Most LUTS have been suggested to be age-dependent, however, the pathophysiology underlying the various dysfunctions resulting in LUTS is sometimes difficult to establish, since it is often difficult to separate what can be attributed to ‘normal’ aging from what is caused by co-morbidities. Uroflow studies have demonstrated an age-dependent decrease in Qmax [Jørgensen et al. 1992, 1993], which was confirmed and shown to be similar in both sexes [Madersbacher et al. 1998; Pfisterer et al. 2006]. However, this was not demonstrable in symptomatic elderly men with nonobstructive voiding dysfunction [Ameda et al. 1999].
Detrusor underactivity [Miyazato et al. 2013; Drake et al. 2014; Osman et al. 2014; Osman and Chapple, 2014], leading to emptying difficulties and symptoms sometimes overlapping with those of DO, may have many underlying causes. Some of the most frequently discussed are impaired detrusor contractility and decreased sensation [Smith, 2010; Smith et al. 2015]. The related condition, ‘detrusor hyperactivity with impaired contractile function’ (DHIC) [Resnick and Yalla, 1987] can also present with advancing age.
Urodynamic assessment in older patients of both sexes without overt neurological disease have shown varying results. Malone-Lee and Wahedna showed higher residual volumes and lower detrusor shortening velocities, but no changes in isometric detrusor function [Malone-Lee and Wahedna, 1993]. In a series of patients, where the bladder capacity at first void was taken as measure of bladder sensation, this parameter showed a progressive increase with age suggesting an age-dependent decrease in bladder sensation [Collas and Malone-Lee, 1996]. This finding has been confirmed by several other investigators [Kenton et al. 2007; Pfisterer et al. 2006]. In a clinical study of patients referred for LUTS or UI, Madersbacher and colleagues found an increase in postvoid residual, along with a decrease of flow rates, voided volumes and bladder capacity associated with increasing age [Madersbacher et al. 1998]. These findings were similar in both sexes. Pfisterer and colleagues assessed a group of 85 female volunteers aged between 20–90 years with a bladder diary, uroflowmetry and detailed videourodynamics [Pfisterer et al. 2006]. Bladder capacity did not change with age, but was smaller in women with DO on urodynamics. Urine production and urine frequency did not differ significantly with age. Bladder sensation, detrusor contraction strength, maximal flow rate and maximum urethral closure pressure were all negatively associated with age. It was concluded that there is a normal functional decline seen with aging in otherwise asymptomatic women. This study thus suggested a progressive decrease in detrusor contraction strength, which was in line with the findings of van Mastrigt, who demonstrated a statistically significant age-related decrease of the detrusor contractility parameter, Wmax, in both sexes [van Mastrigt, 1992]. Other investigators were unable to show any correlations between bladder contractility and age in symptomatic elderly men with nonobstructive bladder dysfunction [Ameda et al. 1999], or between maximum detrusor pressure and detrusor pressure at peak flow rate and age in LUTS patients of both sexes [Madersbacher et al. 1998].
Age-related changes in the LUT in patients with no signs or symptoms of LUT dysfunction, should be clearly differentiated from pathological alterations seen with conditions such as overactive bladder (OAB) or LUTS. The current available data from animal and human studies demonstrate that aging impacts the LUT function through ultrastructural and physiological alterations. The reported age-related changes in animals do not always correspond to what is found in humans, and should be interpreted with caution. Overall, in humans, bladder sensation and ability to empty the bladder seem to decrease with advancing age as a possible consequence of neuronal loss and remodeling of the bladder and urethra.
Consequences of ischemia/hypoxia
As mentioned previously, bladder ischemia and subsequent hypoxia may be important factors contributing to LUTS, particularly in the elderly population. Expression of hypoxia-inducible factors (HIFs) are considered good functional markers of hypoxia [Semenza, 2009, 2014]. HIFs are heterodimeric nuclear transcriptional factors consisting of two subunits that regulate transcription of genes mediating cellular homeostatic responses to altered oxygenation [Semenza, 2012]. HIF-1α is an oxygen-dependent transcriptional activator, and activates genes involved in glucose transport and metabolism, up-regulates a gene involved in cell survival and apoptosis, and interferes with extracellular matrix metabolism and epithelial homeostasis. HIF is a heterodimer composed of two subunits -1α and -1β. The HIF-α subunit (HIF-1α and HIF-2α) is oxygen-regulated, whereas HIF-β is expressed constitutively in the nucleus. HIF-1α is expressed in all nucleated cells, and HIF-2α expression is restricted to specific cell types, including vascular endothelial cells [Talks et al. 2000; Wiesener et al. 2003]. However, very few HIF-1α and HIF-2α proteins are detectable in normal tissues in normoxia [Talks et al. 2000]. The expression of HIF-α in many tissues increases exponentially as oxygen concentration decline [Semenza, 2009]. HIF-1α can also be stimulated by other cytokines in normoxic conditions, but it can also be activated in response to mechanical stress [Christiaansen et al. 2011].
Pathologic vascular wall remodeling of many common diseases of the blood vessels has been found to be associated with altered activity of the HIF pathway [Lim et al. 2013]. Galvin and colleagues demonstrated that isolated normal human detrusor cells responded to hypoxia by upregulation of HIF-1α [Galvin et al. 2004]. Hypoxia did not induce cell death, but significantly decreased the rate of proliferation. Koritsiadis and colleagues investigated the tissue distribution of ischemia in human detrusor in patients with BOO and nonobstructed controls, and correlated the results with clinical variables [Koritsiadis et al. 2008, 2010]. In the specimens from the control group only few rare cells showed weak, mainly cytoplasmatic, immunoreactivity to HIF-1α. HIF-1α immunoreactive cells were expressed mainly in the stroma, and the urothelium and detrusor muscle showed no positive staining. Bladder tissue from obstructed subjects showed high immunoreactivity to HIF-1α, and particularly in patients with urinary retention the number of immunoreactive cells was high. Interestingly, Koritsiadis and colleagues found that in patients treated with α-adrenceptor antagonists, the number of HIF-1α immunoreactive cells was the same as in controls, and concluded that medication did not influence tissue hypoxia in chronic bladder outflow obstruction [Koritsiadis et al. 2010]. Ekman and colleagues found that HIF activation in outlet obstruction involves mechanisms beyond the accumulation of HIF-1α protein and results in a switch of the energetic support of contraction to anaerobic glycolysis [Ekman et al. 2014]. In turn this metabolic adaptation encompasses increased expression of glucose transporters and glycolytic enzymes combined with mitochondrial remodeling, and together, these changes uphold contractility when mitochondrial respiration is limited.
Bladder ischemia
Studies in experimental models in rabbits and rats have shown that pelvic arterial insufficiency may result in significant bladder ischemia with reduced bladder wall oxygen tension [Tarcan et al. 1998; Azadzoi et al. 1999a, 1999b, 2008, 2010; Nomiya et al. 2012]. In turn, this will lead to oxidative stress associated with upregulation of oxidative stress-sensitive genes, increased muscarinic receptor activity, ultrastructural damage, and neurodegeneration [Azadzoi et al. 2010]. It has also been shown in rabbits that moderate ischemia causes bladder hyperactivity, whereas severe ischemia causes bladder underactivity [Azadzoi et al. 1999a, 1999b]. In an established rat model where chronic bladder ischemia was induced by iliac arterial injury + high cholesterol diet for 8 weeks, neointimal formation, luminal occlusion, and bladder ischemia could be demonstrated [Nomiya et al. 2012]. Urodynamically, micturition intervals were significantly decreased (Figure 1) and bladder capacity and voided volume significantly lower than in controls. In vitro, contractile responses of bladder strips to KCl, EFS and carbachol were significantly less than in controls. Bladders from the arterial injury animals also showed a significantly increased percentage of collagen deposition in the bladder wall.
Figure 1.
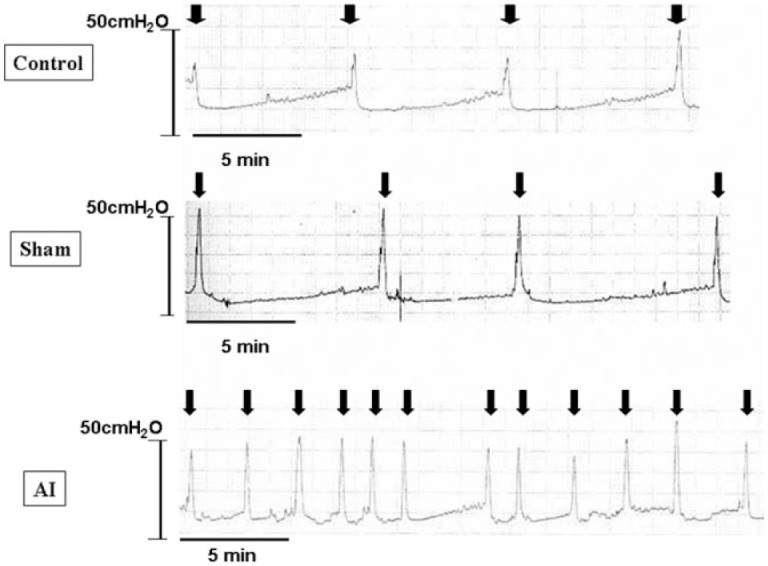
Cystogram obtained in conscious rats with chronic bladder ischemia induced by occlusion of the common internal iliac arteries (AI). Control: n = 8; Sham: n = 8; AI: n = 8.
It may be discussed whether the decreases in bladder blood flow during the normal micturition cycle demonstrated for example by Brading and colleagues could contribute to bladder injury in certain situations [Brading et al. 1999]. For example, in bladder outflow obstruction there may be repeated episodes of prolonged detrusor ischemia which may cause ischemia-reperfusion injury [Greenland and Brading, 2001; Greenland et al. 2000]. In an already ischemic bladder, it may be speculated that the decrease in bladder blood flow during a voiding cycle, particularly with high degrees of bladder filling, may create an ischemia-reperfusion episode. With time such repeated episodes could further damage the bladder.
OAB symptoms, with and without associated DO, are often associated with BOO and benign prostatic enlargement (BPE). In obstructed bladders, there is a reduction of blood flow due to the effect of raised intravesical pressure during voiding and/or the increased tissue pressure in the bladder wall during filling [Greenland et al. 2000; Greenland and Brading, 2001].
Hashitani and colleagues investigated the properties of suburothelial microvessels in the rat bladder by measuring changes in vessel diameter, membrane potential and intracellular Ca2+ dynamics [Hashitani et al. 2011]. They found that suburothelial venules showed spontaneous constrictions that primarily relied on periodic Ca2+ release from intracellular stores. Neighboring perivascular interstitial cells showed spontaneous Ca2+ transients that may well have a role in generating these spontaneous constrictions. In contrast, suburothelial arterioles were quiescent but could be constricted upon the stimulation of sympathetic nerves that released noradrenaline, which primarily acts on α1A-ARs. Further studies on the suburothelial vessels in the mouse supported these results [Hashitani et al. 2012; Mitsui and Hashitani, 2013]. It has been shown that normal bladders maintain suburothelial circulation during bladder filling, despite considerable stretch of the bladder wall, and only transient decreases in the blood supply are recorded during voiding [Greenland et al. 2000; Greenland and Brading, 2001]. However, during obstruction, there may be a prolonged decrease in blood flow during the filling phase, and since outflow obstruction is often associated with DO, there may be repeated episodes of ischemia and reperfusion. This may also be the case in aging and atherosclerosis. Since the urothelium and suburothelial vessels are located in the periphery of the bladder wall circulation, they may be more readily affected by ischemia than the outer detrusor muscle layers. Hashitani and colleagues therefore proposed that suburothelial microvessels may well have intrinsic properties that maintain their blood flow [Hashitani et al. 2011].
There is evidence from animal experiments of an age-related decrease of both motor and sensory innervation of the rat bladder [Warburton and Santer, 1994; Mohammed and Santer, 2001, 2002].
Nerves are highly sensitive to ischemia and hypoxia and chronic bladder ischemia secondary to BOO could lead to partial denervation. This was also demonstrated in the obstructed male human bladder, where a reduction of both motor and sensory innervation has been demonstrated [Chapple et al. 1991, 1992]. This might contribute to obstruction-induced male LUTS.
Possible therapeutic interventions
As mentioned previously, it is well established that the causes of LUTS, including the OAB syndrome, are multifactorial and involve many pathophysiologic mechanisms in both men and women [Roosen et al. 2009; Banakhar et al. 2012; Meng et al. 2012; Patra and Patra, 2015]. Particularly in the elderly, age-associated changes in pelvic vasculature, such as atherosclerosis, may be an important contributing factor in both sexes [Ponholzer et al. 2006; Cellek et al. 2014; Michel et al. 2015]. Intimal thickening in atherosclerosis involves the invasion and de-differentiation of macrophages to lipid-laden foam cells as well as recruitment of vascular smooth muscle cells and neointima formation. This process severely reduces vessel lumen diameter, and unstable plaques may promote thrombus formation and arterial occlusive disease.
Vascular endothelial dysfunction occurs with ageing and is an independent risk factor for the development of atherosclerosis and hypertension [Herrera et al. 2010]. Moreover, the abdominal aorta and its branches, especially the bifurcation of the iliac arteries, are particularly vulnerable to atherosclerotic lesions [Tarcan et al. 1998]. The vascular supply to the human genitourinary tract, including bladder, prostate, urethra and penis, is primarily derived from the iliac arteries, and atherosclerotic obstructive changes distal to the aortic bifurcation will have consequences for the distal vasculature and for LUT blood flow [Yamaguchi et al. 2014]. Pinggera and colleagues found that elderly patients with LUTS had a significant decrease in bladder blood flow in comparison with asymptomatic young individuals [Pinggera et al. 2008b]. These studies suggest that arterial occlusive disease and concomitant chronic bladder ischemia may produce bladder dysfunction, including DO. However, despite intensive study in various animal models, the mechanisms behind changes in bladder function caused by chronic ischemia are incompletely known, and there is no established treatment. It has been suggested by animal studies that chronic ischemia-related bladder dysfunction will progress to bladder underactivity [Sagawa et al. 2013; Nomiya et al. 2014], and there is experimental evidence that this may occur [Zhao et al. 2016]. As pointed out by el Assar and colleagues, vascular aging was previously considered an ‘immutable and inexorable risk factor’ [el Assar et al. 2012]. However, it is now viewed as a target process for intervention in order to achieve a healthier old age [Michel et al. 2015].
Treatment with α1-AR blockers and phosphodiesterase type 5 (PDE5) inhibitors, such as tadalafil, sildenafil and vardenafil, have been shown to be effective for treating LUTS associated with benign prostatic hyperplasia (BPH) [Soler et al. 2013] and also mirabegron, the β3-AR agonist, has proven to be an effective treatment of OAB [Andersson et al. 2013; Chapple et al. 2014]. Theoretically, free radical scavengers could also offer interesting treatment options for LUTS/OAB [Soler et al. 2013]. The different mechanisms of action of these drugs support a multifactorial pathogenesis of LUTS/OAB. It is interesting to note that the drugs principles shown to have efficacy in the treatment of LUTS, also shows efficacy in the model of chronic bladder ischemia in rats (Table 1).
Table 1.
Possible therapeutic interventions in pelvic ischemia.
| Drug/intervention | Mechanism of action | Clinical effect in OAB | Effect in animals |
|---|---|---|---|
| α1-AR blockers | Increase in blood flow | Documented (males) | Documented |
| PDE5 inhibitors | Unknown | Documented (males) | Documented |
| Free radical scavengers | Tissue protection? | ? | Documented |
| β3-AR agonists | Increase in blood flow? | Documented | Documented |
| Stem cells | Unknown | Unknown | Documented? |
AR, adrenoceptor; OAB, overactive bladder; PDE5, phosphodiesterase type 5.
α1-AR blockers
The LUT, including bladder, prostate, and urethra smooth muscle and the LUT vasculature, contains α-ARs that in various ways contribute its function [Michel and Vrydag, 2006; Andersson and Gratzke 2007; Yamada and Ito, 2011]. Generally, α1-ARs are predominating, and molecular clones have been isolated for three α1-subtypes (α1A, α1B, and α1D), and these subtypes have been functionally characterized. The α1A-AR mRNA is the most abundantly transcribed in the prostate, urethra and bladder neck of many species, including humans, but the expression pattern may change with pathologies (e.g. bladder outflow obstruction). Yono and colleagues compared 3 and 22-month-old male Fischer rats, male normotensive Wistar-Kyoto (WKY) rats and spontaneously-hypertensive rats (SHRs), and found that the 22-month-old rats and SHRs had significantly higher total α1-AR density in the internal iliac artery and lower blood flow to the bladder and penis than 3-month-old and WKY rats [Yono et al. 2011]. Analysis of mRNA by RT-PCR showed an age- and hypertension-related increase in the expression of α1B-AR mRNA in the internal iliac, vesical and internal pudendal arteries and a switch from α1A predominance in 3-month old and WKY rats to α1B > α1A in 22-month-old rats and SHRs. It was concluded that age and hypertension may cause alterations in vascular α1-AR subtype distribution and in blood flow to the rat bladder and penis. These findings suggest that pharmacological blockade particularly of the vascular α1B-AR could increase pelvic blood flow, which may contribute to improvement of bladder dysfunction associated with reduced blood flow.
By blocking the α1 ARs, selective α1-AR antagonists can increase blood flow to the LUT, relax ischemia-induced contraction of LUT smooth muscle, decrease afferent signaling and oxidative stress, and possibly indirectly affect the central nervous system [Michel and Vrydag, 2006; Andersson and Gratzke, 2007; Michel, 2010]. Antagonists for the α1-AR are currently the first-line therapy option for LUTS associated with BPH [Michel, 2010; Lepor et al. 2012], but have also been shown to improve LUTS without outflow obstruction [Lepor et al. 2012].
In 4 healthy men and in 19 patients with LUTS, treated with daily 0.4 mg tamsulosin for 5 weeks, urodynamic variables, vesical and prostatic blood flows were investigated before and after treatment using transrectal color Doppler ultrasound (TRCDUS) and measurement of the color pixel density (CPD) [Pinggera et al. 2008a]. It was found that in the healthy men, perfusion of the LUT increased considerably (157%) during filling of the bladder to a mean maximum cystometric capacity (Cmax). All the patients with LUTS had a reduced mean Cmax during filling. The mean CPD in the urinary bladder and the prostate were only increased by 58.4% during filling. After α1-AR-blocker therapy (tamsulosin) the mean Cmax during filling increased and perfusion of the LUT measured by CPD was significantly increased (132.8%). The authors concluded that LUTS are associated with chronic ischemia of the prostate and urinary bladder, and that α1-AR-blockers increase perfusion in the LUT and Cmax.
Goi and colleagues demonstrated in a rat model of chronic pelvic ischemia that α1-AR blockade with silodosin increased bladder blood flow, both in the empty bladder and during bladder filling [Goi et al. 2013]. Markers of oxidative stress were significantly decreased and the drug mitigated signs of bladder dysfunction in a metabolic cage study and on cystometry: micturition frequency was normalized, and the mean voided volume was increased, both in the absence of any change in urine production. These results suggest that restoration of bladder blood flow through α1-AR inhibition may improve bladder function caused by chronic bladder ischemia.
PDE5 inhibitors
In male LUTS, the therapeutic effects of PDE5 inhibitors such as tadalafil, sildenafil, and vardenafil have been well summarized in several reviews (e.g. [Lythgoe and McVary, 2013; Gacci et al. 2014]). Their mechanisms of action have not been established, but an increased pelvic organ blood flow/perfusion in the LUT has been suggested [Andersson et al. 2011; Giuliano et al. 2013; Gacci et al. 2016]. PDE5 inhibitors also appear to modulate afferent activity [Behr-Roussel et al. 2011; Minagawa et al. 2012] upregulate cyclic guanosine monophosphate (cGMP)/protein kinase G (PKG) activity [Lythgoe and McVary, 2013], downregulate Rho kinase activity [Vignozzi et al. 2014], and possibly reduce inflammation [Vignozzi et al. 2013].
In a randomized controlled trial (RCT), Pinggera and colleagues, using transrectal ultrasonography, compared the effects of tadalafil 5 mg/day and placebo given for 8 weeks to men with moderate to severe LUTS/BPH [Pinggera et al. 2014]. They found no differences between the treatments, but did not exclude that changes in blood flow may have occurred, which for several reasons could not be detected. Interestingly, Pinggera and colleagues, using TRCDUS , had previously and quite convincingly demonstrated that tamsulosin could increase perfusion to the LUT [Pinggera et al. 2008b].
In rats with chronic bladder ischemia, addition of tadalafil or mirodenafil protected bladder function and morphology, resulting in decreased bladder hyperactivity [Nomiya et al. 2013a; Choi et al. 2015]. Interestingly, Garcia-Barroso and colleaguesfound that in mice, tadalafil can cross the blood brain barrier and reverse cognitive function an Alzheimer’s disease [Garcia-Barroso et al. 2013]. If this is valid also for humans, tadalafil may be an interesting alternative for treatment of LUTS in the elderly.
Provided that the chronic ischemia model mimics the situation in humans, available data support the use of PDE5 inhibitors in the treatment of bladder dysfunction induced by chronic ischemia. It also seems reasonable to suggest that PDE5 inhibition may also be useful in the prophylactic setting, to slow progression.
Free radical scavengers
The oxidative stress theory of aging states that accumulation of reactive oxygen species (ROS) occurs with age and leads to functional alterations and pathological conditions [Kregel & Zhang, 2007; Nocchi et al. 2014]. The antioxidant effects of melatonin, either administered at a low dose via drinking water (2.5 mg/kg/day) or at a high dose via gavage (20 mg/kg/day), have been tested on the ischemic rat bladder [Nomiya et al. 2013b]. While chronic treatment with melatonin did not prevent the development of arterial occlusive disease (although, in the high-dose group it was reduced), there were beneficial effects on bladder function and morphology. A prolonged micturition interval and an increase in bladder capacity were observed with melatonin treatment; while these benefits failed to reach statistical significance with low-dose melatonin, they were almost normalized at the high dose. High-dose melatonin also significantly improved markers of oxidative stress and NOS expression, and there were significant improvements in all the functional and morphological variables compared with the untreated iliac arterial ischemia group. It was suggested that chronic melatonin treatment helps to protect bladder function and morphology, probably through its antioxidative action. Whether these proof-of-concept data can be extrapolated to humans is unclear; the doses used are very high, and it is unlikely that high-dose melatonin will become a treatment for chronic bladder ischemia in humans.
β3-AR agonists
β3-AR agonists (e.g. mirabegron) stimulate adenylyl cyclase causing an increase in cAMP, which activates potassium channels causing hyperpolarization. Both these effects result in smooth muscle relaxation [Takasu et al. 2007; Hatanaka et al. 2013]. Thus, it can be assumed that these agents could prevent spontaneous bladder activity, thereby (1) increasing bladder compliance by decreasing bladder tone during filling; (2) increasing the threshold of the level of distension required to activate the micturition reflex, increasing bladder capacity; and (3) decreasing afferent activity, with a corresponding effect on symptoms. These agents appear to have little or no effect on voiding contraction, resulting in a reduced risk of urinary retention.
Sawada and colleagues showed that 8 weeks of treatment with mirabegron appears to protect bladder function and morphology, resulting in reduced bladder hyperactivity in the chronically ischemic rat bladder, with comparable effectiveness to that seen with PDE5 inhibitors [Sawada et al. 2013]. If these findings are valid for humans, they support β3-AR agonism as a potential treatment option for chronic ischemia-related bladder dysfunction.
Stem cell treatment
Based on the anti-inflammatory, anti-apoptotic, and antifibrotic actions of stem cells, Chen and colleagues injected a rat mesenchymal stem cell suspension into the common iliac artery of rats with chronic ischemia-induced bladder detrusor dysfunction, followed by intragastric administration of doxazosin mesylate [Chen et al. 2012]. They showed that transplanted stem cells regenerated the bladder tissue, increased the proportion of smooth muscle and nerve cells in the bladder wall, and improved the contractile function of the detrusor muscle. Interpretation of these results is, however, limited by the absence of a control group not treated with doxazosin. Whether these effects can be clinically applied to patients with LUTS/OAB is yet to be explored.
Future perspectives
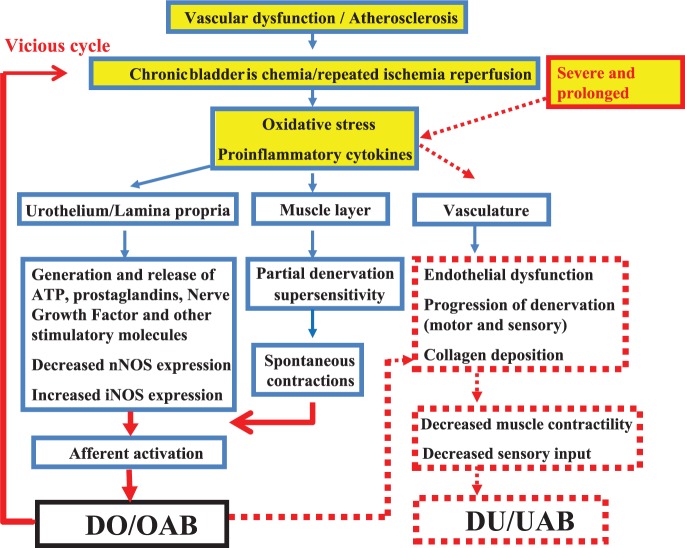
There is evidence that chronic bladder ischemia and oxidative stress may be important factors contributing to the development of LUTS (Figure 2), particularly in the elderly. Consequently, improvement of LUT perfusion and control of oxidative stress as therapeutic strategies may be expected to have beneficial effects on chronic ischemia-related bladder dysfunctions. It has been suggested by animal studies that chronic ischemia-related bladder dysfunction will progress from DO/OAB to Detrusor Underactivity (DU)/ Underactive Bladder (UAB), and that drugs with different mechanisms of action, such as α1-AR antagonists, PDE-5 inhibitors, free radical scavengers and β3-AR agonists may not only to protect against functional changes, but also to prevent the decrease in bladder muscle contractility and the detrimental structural changes. Future clinical studies are necessary to demonstrate the validity of these effects in humans.
Figure 2.
Possible mechanisms for LUTS induced by chronic bladder ischemia and oxidative stress. Severe and prolonged ischemia may induce progression from DO/OAB to DU/UAB.
DO, detrusor overactivity; DU, detrusor underactivity; iNOS, inducible nitric oxide; LUTS, lower urinary tract symptoms; nNOS, neuronal nitric oxide; OAB, overactive bladder; UAB, underactive bladder.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Karl-Erik Andersson, Institute of Clinical Medicine, Department of Obstetrics and Gynecology, Aarhus University Hospital, Palle Juul-Jensens Boulevard 99, DK 8200 Aarhus N, Denmark.
Donna B. Boedtkjer, Department of Biomedicine, Aarhus University, Denmark
Axel Forman, Department of Gynecology and Obstetrics, Aarhus University Hospital, Denmark.
References
- Ameda K., Sullivan M., Bae R., Yalla S. (1999) Urodynamic characterization of nonobstructive voiding dysfunction in symptomatic elderly men. J Urol 162: 142–146. [DOI] [PubMed] [Google Scholar]
- Andersson K. (2003) Storage and voiding symptoms: pathophysiologic aspects. Urology 62(Suppl. 2): 3–10. [DOI] [PubMed] [Google Scholar]
- Andersson K. (2007) LUTS treatment: future treatment options. Neurourol Urodyn 26(Suppl. 6): 934–947. [DOI] [PubMed] [Google Scholar]
- Andersson K. (2011) Muscarinic acetylcholine receptors in the urinary tract. Handb Exp Pharmacol 202: 319–314. [DOI] [PubMed] [Google Scholar]
- Andersson K., de Groat W., McVary K., Lue T., Maggi M., Roehrborn C., et al. (2011) Tadalafil for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia: pathophysiology and mechanism(s) of action. Neurourol Urodyn 30: 292–301. [DOI] [PubMed] [Google Scholar]
- Andersson K., Gratzke C. (2007) Pharmacology of alpha1-adrenoceptor antagonists in the lower urinary tract and central nervous system. Nat Clin Pract Urol 4: 368–378. [DOI] [PubMed] [Google Scholar]
- Andersson K., Martin N., Nitti V. (2013) Selective β3-adrenoceptor agonists for the treatment of overactive bladder. J Urol 190: 1173–1180. [DOI] [PubMed] [Google Scholar]
- Andersson P., Bloom S., Mattiasson A., Uvelius B. (1985) Changes in vascular resistance in the feline urinary bladder in response to bladder filling. J Urol 134: 1041–1046. [DOI] [PubMed] [Google Scholar]
- Azadzoi K., Tarcan T., Kozlowski R., Krane R., Siroky M. (1999a) Overactivity and structural changes in the chronically ischemic bladder. J Urol 162: 1768. [PubMed] [Google Scholar]
- Azadzoi K., Tarcan T., Siroky M., Krane R. (1999b) Atherosclerosis-induced chronic ischemia causes bladder fibrosis and non-compliance in the rabbit. J Urol 161: 1626. [PubMed] [Google Scholar]
- Azadzoi K., Radisavljevic Z., Siroky M. (2008) Effects of ischemia on tachykinin-containing nerves and neurokinin receptors in the rabbit bladder. Urology 71: 979. [DOI] [PubMed] [Google Scholar]
- Azadzoi K., Radisavljevic Z., Golabek T., Yalla S., Siroky M. (2010) Oxidative modification of mitochondrial integrity and nerve fiber density in the ischemic overactive bladder. J Urol 183: 362. [DOI] [PubMed] [Google Scholar]
- Bachschmid M., Schildknecht S., Matsui R., Zee R., Haeussler D., Cohen R., et al. (2013) Vascular aging: chronic oxidative stress and impairment of redox signaling-consequences for vascular homeostasis and disease. Ann Med 45: 17–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banakhar M., Al-Shaiji T., Hassouna M. (2012) Pathophysiology of overactive bladder. Int Urogynecol J 23: 975–982. [DOI] [PubMed] [Google Scholar]
- Behr-Roussel D., Oger S., Caisey S., Sandner P., Bernabé J., Alexandre L., et al. (2011) Vardenafil decreases bladder afferent nerve activity in unanesthetized, decerebrate, spinal cord-injured rats. Eur Urol 59: 272–279. [DOI] [PubMed] [Google Scholar]
- Bentzon J., Otsuka F., Virmani R., Falk E. (2014) Mechanisms of plaque formation and rupture. Circ Res 114: 1852–1866. [DOI] [PubMed] [Google Scholar]
- Bjorling D., Wang Z., Vezina C., Ricke W., Keil K., Yu W., et al. (2015) Evaluation of voiding assays in mice: impact of genetic strains and sex. Am J Physiol Renal Physiol 308: F1369–F1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A., Greenland J., Mills I., McMurray G., Symes S. (1999) Blood supply to the bladder during filling. Scand J Urol Nephrol 201(Suppl.): 25–31. [PubMed] [Google Scholar]
- Braithwaite J. (1952) The arterial supply of the male urinary bladder. Br J Urol 24: 64–71. [PubMed] [Google Scholar]
- Chapple C., Cardozo L., Nitti V., Siddiqui E., Michel M. (2014) Mirabegron in overactive bladder: a review of efficacy, safety, and tolerability. Neurourol Urodyn 33: 17–30. [DOI] [PubMed] [Google Scholar]
- Chapple C., Crowe R., Gilpin S., Gosling J., Burnstock G. (1991) The innervation of the human prostate gland—the changes associated with benign enlargement. J Urol 146: 1637–1644. [DOI] [PubMed] [Google Scholar]
- Chapple C., Milner P., Moss H., Burnstock G. (1992) Loss of sensory neuropeptides in the obstructed human bladder. Br J Urol 70: 373–381. [DOI] [PubMed] [Google Scholar]
- Christiaansen C., Sun Y., Hsu Y., Chai T. (2011) Alterations in expression of HIF-1α, HIF-2α, and VEGF by idiopathic overactive bladder urothelial cells during stretch suggest role for hypoxia. Urology 77: 1266.e7–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Zhang H., Zhang N., Li W., Shan H., Liu K., et al. (2012) Treatment for chronic ischaemia-induced bladder detrusor dysfunction using bone marrow mesenchymal stem cells: an experimental study. Int J Mol Med 29: 416–422. [DOI] [PubMed] [Google Scholar]
- Choi H., Bae J., Shim J., Park J., Moon du G., Lee J. (2015) Mirodenafil prevents bladder dysfunction induced by chronic bladder ischemia in rats. Int Neurourol J 19: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua W., Liu L., Mansfield K., Vaux K., Moore K., Millard R., et al. (2007) Age-related changes of P2X(1) receptor mRNA in the bladder detrusor from men with and without bladder outlet obstruction. Exp Gerontol 42: 686–692. [DOI] [PubMed] [Google Scholar]
- Cellek S., Cameron N., Cotter M., Fry C., Ilo D. (2014) Microvascular dysfunction and efficacy of PDE5 inhibitors in BPH-LUTS. Nat Rev Urol 11: 231–241. [DOI] [PubMed] [Google Scholar]
- Collas D., Malone-Lee J. (1996) Age-associated changes in detrusor sensory function in women with lower urinary tract symptoms. Int Urogynecol J Pelvic Floor Dysfunct 7: 24–29. [DOI] [PubMed] [Google Scholar]
- Congiu T., Radice R., Raspanti M., Reguzzoni M. (2004) The 3D structure of the human urinary bladder mucosa: a scanning electron microscopy study. J Submicrosc Cytol Pathol 36: 45–53. [PubMed] [Google Scholar]
- Diokno A., Brock B., Brown M., Herzog A. (1986) Prevalence of urinary incontinence and other urological symptoms in the noninstitutionalized elderly. J Urol 136: 1022. [PubMed] [Google Scholar]
- Drake M., Williams J., Bijos D. (2014) Voiding dysfunction due to detrusor underactivity: an overview. Nat Rev Urol 11: 454–464. [DOI] [PubMed] [Google Scholar]
- Dubeau C., Kraus S., Griebling T., Newman D., Wyman J., Johnson T., et al. (2014) Effect of fesoterodine in vulnerable elderly subjects with urgency incontinence: a double-blind, placebo controlled trial. J Urol 191: 395–404. [DOI] [PubMed] [Google Scholar]
- Ekman M., Uvelius B., Albinsson S., Swärd K. (2014) HIF-mediated metabolic switching in bladder outlet obstruction mitigates the relaxing effect of mitochondrial inhibition. Lab Invest 94: 557–568. [DOI] [PubMed] [Google Scholar]
- El Assar M., Angulo J., Vallejo S., Peiró C., Sánchez-Ferrer C., Rodríguez-Mañas L. (2012) Mechanisms involved in the aging-induced vascular dysfunction. Front Physiol 3: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Assar M., Angulo J., Rodríguez-Mañas L. (2013) Oxidative stress and vascular inflammation in aging. Free Radic Biol Med 65: 380–401. [DOI] [PubMed] [Google Scholar]
- Fry C., Bayliss M., Young J., Hussain M. (2011) Influence of age and bladder dysfunction on the contractile properties of isolated human detrusor smooth muscle. BJU Int 108(2 Pt 2): E91–E96. [DOI] [PubMed] [Google Scholar]
- Fultz N., Herzog A. (1996) Epidemiology of urinary symptoms in the geriatric population. Urol Clin North Am 23: 1. [DOI] [PubMed] [Google Scholar]
- Gacci M., Andersson K., Chapple C., Maggi M., Mirone V., Oelke M., et al. (2016) Latest evidences on the use of phosphodieasterase type 5 inhibitors for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. EurUrol 70: 124–133. [DOI] [PubMed] [Google Scholar]
- Gacci M., Carini M., Salvi M., Sebastianelli A., Vignozzi L., Corona G., et al. (2014) Management of benign prostatic hyperplasia: role of phosphodiesterase-5 inhibitors. Drugs Aging 31: 425–439. [DOI] [PubMed] [Google Scholar]
- García-Barroso C., Ricobaraza A., Pascual-Lucas M., Unceta N., Rico AJ., Goicolea MA., et al. (2013) Tadalafil crosses the blood-brain barrier and reverses cognitive dysfunction in a mouse model of AD. Neuropharmacology 64: 114–123. [DOI] [PubMed] [Google Scholar]
- Galvin D.J., Watson R.W., O’Neill A., Coffey R.N., Taylor C., Gillespie J.I. (2004) Hypoxia inhibits human bladder smooth muscle cell proliferation: a potential mechanism of bladder dysfunction. Neurourol Urodyn 23: 342–348. [DOI] [PubMed] [Google Scholar]
- Giuliano F., Ückert S., Maggi M., Birder L., Kissel J., Viktrup L. (2013) The mechanism of action of phosphodiesterase type 5 inhibitors in the treatment of lower urinary tract symptoms related to benign prostatic hyperplasia. Eur Urol 63: 506–516. [DOI] [PubMed] [Google Scholar]
- Goi Y., Tomiyama Y., Nomiya M., Sagawa K., Aikawa K., Yamaguchi O. (2013) Effects of silodosin, a selective α1A-adrenoceptor antagonist, on bladder blood flow and bladder function in a rat model of atherosclerosis induced chronic bladder ischemia without bladder outlet obstruction. J Urol 190: 1116–1122. [DOI] [PubMed] [Google Scholar]
- Greenland J., Brading A. (2001) The effect of bladder outflow obstruction on detrusor blood flow changes during the voiding cycle in conscious pigs. J Urol 165: 245–248. [DOI] [PubMed] [Google Scholar]
- Greenland J., Hvistendahl J., Andersen H., Jörgensen T., McMurray G., Cortina-Borja M., et al. (2000) The effect of bladder outlet obstruction on tissue oxygen tension and blood flow in the pig bladder. BJU Int 85: 1109–1114. [DOI] [PubMed] [Google Scholar]
- Hashitani H., Mitsui R., Shimizu Y., Higashi R., Nakamura K. (2012) Functional and morphological properties of pericytes in suburothelial venules of the mouse bladder. Br J Pharmacol 167: 1723–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H., Takano H., Fujita K., Mitsui R., Suzuki H. (2011) Functional properties of suburothelial microvessels in the rat bladder. J Urol 185: 2382–2391. [DOI] [PubMed] [Google Scholar]
- Hatanaka T., Ukai M., Watanabe M., Someya A., Ohtake A., Suzuki M., et al. (2013) In vitro and in vivo pharmacological profile of the selective β3-adrenoceptor agonist mirabegron in rats. Naunyn Schmiedebergs Arch Pharmacol 386: 247–253. [DOI] [PubMed] [Google Scholar]
- Herrera M., Mingorance C., Rodríguez-Rodríguez R., Alvarez de Sotomayor M. (2010) Endothelial dysfunction and aging: an update. Ageing Res Rev 9: 142–152. [DOI] [PubMed] [Google Scholar]
- Hossler F., Kao R. (2007) Microvasculature of the urinary bladder of the dog: a study using vascular corrosion casting. Microsc Microanal 13: 220–227. [DOI] [PubMed] [Google Scholar]
- Hossler F., Monson F. (1995) Microvasculature of the rabbit urinary bladder. Anat Rec 243: 438–448. [DOI] [PubMed] [Google Scholar]
- Hossler F., Lametschwandtner A., Kao R., Finsterbusch F. (2013) Microvascular architecture of mouse urinary bladder described with vascular corrosion casting, light microscopy, SEM, and TEM. Microsc Microanal 19: 1428–1435. [DOI] [PubMed] [Google Scholar]
- Hypolite J., Longhurst P., Gong C., Briscoe J., Wein A., Levin R. (1993) Metabolic studies on rabbit bladder smooth muscle and mucosa. Mol Cell Biochem 125: 35–42. [DOI] [PubMed] [Google Scholar]
- Inoue T., Gabella G. A. (1991) Vascular network closely linked to the epithelium of the urinary bladder of the rat. Cell Tissue Res 263: 137–143. [DOI] [PubMed] [Google Scholar]
- Irwin D., Milsom I., Hunskaar S., Reilly K., Kopp Z., Herschorn S., et al. (2006) Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol 50: 1306. [DOI] [PubMed] [Google Scholar]
- Jørgensen J., Jensen K., Mogensen P. (1992) Age-related variation in urinary flow variables and flow curve patterns in elderly males. Br J Urol 69: 265–271. [DOI] [PubMed] [Google Scholar]
- Jørgensen J., Jensen K., Mogensen P. (1993) Longitudinal observations on normal and abnormal voiding in men over the age of 50 years. Br J Urol 72: 413–420. [DOI] [PubMed] [Google Scholar]
- Kaplan S. (2006) Male pelvic health: a urological call to arms. J Urol 176: 2351. [DOI] [PubMed] [Google Scholar]
- Kenton K., Lowenstein L., Simmons J., Brubaker L. (2007) Aging and overactive bladder may be associated with loss of urethral sensation in women. Neurourol Urodyn 26: 981–984. [DOI] [PubMed] [Google Scholar]
- Kershen R., Azadzoi K., Siroky M. (2002) Blood flow, pressure and compliance in the male human bladder. J Urol 168: 121–125. [PubMed] [Google Scholar]
- Koritsiadis G., Stravodimos K., Koutalellis G., Agrogiannis G., Koritsiadis S., Lazaris A., et al. (2008) Immunohistochemical estimation of hypoxia in human obstructed bladder and correlation with clinical variables. BJU Int 102: 328–332. [DOI] [PubMed] [Google Scholar]
- Koritsiadis G., Tyritzis S., Koutalellis G., Lazaris A., Stravodimos K. (2010) The effect of alpha-blocker treatment on bladder hypoxia inducible factor-1 alpha regulation during lower urinary tract obstruction. Int Braz J Urol 36: 86–94. [DOI] [PubMed] [Google Scholar]
- Kotsis V., Stabouli S., Karafillis I., Nilsson P. (2011) Early vascular aging and the role of central blood pressure. J Hypertens 29: 1847–1853. [DOI] [PubMed] [Google Scholar]
- Kregel K., Zhang H. (2007) An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol 292: R18–R36. [DOI] [PubMed] [Google Scholar]
- Lepor H., Kazzazi A., Djavan B. (2012) α-Blockers for benign prostatic hyperplasia: the new era. Curr Opin Urol 22: 7–15. [DOI] [PubMed] [Google Scholar]
- Lim C., Kiriakidis S., Sandison A., Paleolog E., Davies A. (2013) Hypoxia-inducible factor pathway and diseases of the vascular wall. J Vasc Surg 58: 219–230. [DOI] [PubMed] [Google Scholar]
- Lythgoe C., McVary K. (2013) The use of PDE-5 inhibitors in the treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Curr Urol Rep 14: 585–594. [DOI] [PubMed] [Google Scholar]
- Madersbacher S., Pycha A., Schatzl G., Mian C., Klingler C., Marberger M. (1998) The aging lower urinary tract: a comparative urodynamic study of men and women. Urology 51: 206–212. [DOI] [PubMed] [Google Scholar]
- Malone-Lee J., Wahedna I. (1993) Characterisation of detrusor contractile function in relation to old age. Br J Urol 72: 873–880. [DOI] [PubMed] [Google Scholar]
- Mansfield K.J., Liu L., Mitchelson F.J., Moore K.H., Millard R.J., Burcher E. (2005) Muscarinic receptor subtypes in human bladder detrusor and mucosa, studied by radioligand binding and quantitative competitive RT-PCR: changes in ageing. Br J Pharmacol 144(8): 1089–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariappan P., Chong W. (2006) Prevalence and correlations of lower urinary tract symptoms, erectile dysfunction and incontinence in men from a multiethnic Asian population: Results of a regional population-based survey and comparison with industrialized nations. BJU Int 98: 1264. [DOI] [PubMed] [Google Scholar]
- Michel M. (2010) The forefront for novel therapeutic agents based on the pathophysiology of lower urinary tract dysfunction: alpha-blockers in the treatment of male voiding dysfunction - how do they work and why do they differ in tolerability? J Pharmacol Sci 112: 151–157. [DOI] [PubMed] [Google Scholar]
- Michel M., Chess-Williams R., Hegde S. (2015) Are blood vessels a target to treat lower urinary tract dysfunction? Naunyn Schmiedebergs Arch Pharmacol 388: 687–694. [DOI] [PubMed] [Google Scholar]
- Michel M., Schneider T., Krege S., Goepel M. (2002) Does gender or age affect the efficacy and safety of tolterodine? J Urol 168:1027–1031. [DOI] [PubMed] [Google Scholar]
- Michel M., Vrydag W. (2006) Alpha1-, alpha2- and beta-adrenoceptors in the urinary bladder, urethra and prostate. Br J Pharmacol 147(Suppl. 2): S88–S119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa T., Aizawa N., Igawa Y., Wyndaele J. (2012) Inhibitory effects of phosphodiesterase 5 inhibitor, tadalafil, on mechanosensitive bladder afferent nerve activities of the rat, and on acrolein-induced hyperactivity of these nerves. BJU Int 110: e259–e266. [DOI] [PubMed] [Google Scholar]
- Miodoński A., Litwin J. (1999) Microvascular architecture of the human urinary bladder wall: a corrosion casting study. Anat Rec 254: 375–381. [DOI] [PubMed] [Google Scholar]
- Mitsui R., Hashitani H. (2013) Immunohistochemical characteristics of suburothelial microvasculature in the mouse bladder. Histochem Cell Biol 140: 189–200. [DOI] [PubMed] [Google Scholar]
- Miyazato M., Yoshimura N., Chancellor M.B. (2013) The other bladder syndrome: underactive bladder. Rev Urol 15: 11–22. [PMC free article] [PubMed] [Google Scholar]
- Meng E., Lin W., Lee W., Chuang Y. (2012) Pathophysiology of overactive bladder. LUTS 4: 38–55. [DOI] [PubMed] [Google Scholar]
- Mohammed H., Santer R. (2001) Distribution and changes with age of nitric oxide synthase-immunoreactive nerves of the rat urinary bladder, ureter and in lumbosacral sensory neurons. Eur J Morphol 39: 137–144. [DOI] [PubMed] [Google Scholar]
- Mohammed H., Santer R. (2002) Distribution and changes with age of calcitonin gene-related peptide- and substance P-immunoreactive nerves of the rat urinary bladder and lumbosacral sensory neurons. Eur J Morphol 40: 293–301. [DOI] [PubMed] [Google Scholar]
- Nemeth C., Khan R., Kirchner P., Adams R. (1977) Changes in canine bladder perfusion with distension. Invest Urol 15: 149–150. [PubMed] [Google Scholar]
- Nilsson P., Boutouyrie P., Cunha P., Kotsis V., Narkiewicz K., Parati G., et al. (2013) Early vascular ageing in translation: from laboratory investigations to clinical applications in cardiovascular prevention. J Hypertens 31: 1517–1526. [DOI] [PubMed] [Google Scholar]
- Nocchi L., Daly D., Chapple C., Grundy D. (2014) Induction of oxidative stress causes functional alterations in mouse urothelium via a TRPM8-mediated mechanism: implications for aging. Aging Cell 13: 540–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomiya M., Burmeister D., Sawada N., Campeau L., Zarifpour M., Keys T., et al. (2013a) Prophylactic effect of tadalafil on bladder function in a rat model of chronic bladder ischemia. J Urol 189: 754–761. [DOI] [PubMed] [Google Scholar]
- Nomiya M., Burmeister D., Sawada N., Campeau L., Zarifpour M., Yamaguchi O., et al. (2013b) Effect of melatonin on chronic bladder-ischaemia-associated changes in rat bladder function. BJU Int 112: E221–E230. [DOI] [PubMed] [Google Scholar]
- Nomiya M., Yamaguchi O., Andersson K., Sagawa K., Aikawa K., Shishido K., et al. (2012) The effect of atherosclerosis-induced chronic bladder ischemia on bladder function in the rat. Neurourol Urodyn 31: 195–200. [DOI] [PubMed] [Google Scholar]
- Nomiya M., Yamaguchi O., Akaihata H., Hata J., Sawada N., Kojima Y., et al. (2014) Progressive vascular damage may lead to bladder underactivity in rats. J Urol 191(5): 1462–1469. [DOI] [PubMed] [Google Scholar]
- Oakley R., Tharakan B. (2014) Vascular hyperpermeability and aging. Aging Dis 5: 114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman N., Chapple C., Abrams P., Dmochowski R., Haab F., Nitti V., et al. (2014) Detrusor underactivity and the underactive bladder: a new clinical entity? A review of current terminology, definitions, epidemiology, aetiology, and diagnosis. Eur Urol 65: 389–398. [DOI] [PubMed] [Google Scholar]
- Osman N., Chapple C. (2014) Contemporary concepts in the aetiopathogenesis of detrusor underactivity. Nat Rev Urol 11: 639–648. [DOI] [PubMed] [Google Scholar]
- Patra P., Patra S. (2015) Research findings on overactive bladder. Curr Urol 8: 1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfisterer M., Griffiths D., Schaefer W., Resnick N. (2006) The effect of age on lower urinary tract function: a study in women. J Am Geriatr Soc 54: 405–412. [DOI] [PubMed] [Google Scholar]
- Pinggera G., Frauscher F., Paduch D., Bolyakov A., Efros M., Kaminetsky J., et al. (2014) Effect of tadalafil once daily on prostate blood flow and perfusion in men with lower urinary tract symptoms secondary to benign prostatic hyperplasia: a randomized, double-blind, multicenter, placebo-controlled trial. Urology 84: 412–419. [DOI] [PubMed] [Google Scholar]
- Pinggera G., Mitterberger M., Pallwein L., Schuster A., Herwig R., Frauscher F., et al. (2008a) alpha-Blockers improve chronic ischaemia of the lower urinary tract in patients with lower urinary tract symptoms. BJU Int 101: 319–324. [DOI] [PubMed] [Google Scholar]
- Pinggera G., Mitterberger M., Steiner E., Pallwein L., Frauscher F., Aigner F., et al. (2008b) Association of lower urinary tract symptoms and chronic ischaemia of the lower urinary tract in elderly women and men: assessment using colour doppler ultrasonography. BJU Int 102: 470–474. [DOI] [PubMed] [Google Scholar]
- Ponholzer A., Temml C., Wehrberger C., et al. (2006) The association between vascular risk factors and lower urinary tract symptoms in both sexes. Eur Urol 50: 581. [DOI] [PubMed] [Google Scholar]
- Resnick N., Yalla S. (1987) Detrusor hyperactivity with impaired contractile function. An unrecognized but common cause of incontinence in elderly patients. J Am Med Assoc 257: 3076–3081. [DOI] [PubMed] [Google Scholar]
- Rosen R., Altwein J., Boyle P., Kirby R., Lukacs B., Meuleman E., et al. (2003) Lower urinary tract symptoms and male sexual dysfunction: the multinational survey of the aging male (MSAM-7). Eur Urol 44: 637. [DOI] [PubMed] [Google Scholar]
- Rosen R.C. Link C.L. O’Leary M.P. Giuliano F. Aiyer L.P. Mollon P. (2009) Lower urinary tract symptoms and sexual health: the role of gender, lifestyle and medical comorbidities. BJU Int 103(Suppl. 3): 42–47. [DOI] [PubMed] [Google Scholar]
- Roosen A., Chapple C., Dmochowski R., Fowler C., Gratzke C., Roehrborn C., et al. (2009) A refocus on the bladder as the originator of storage lower urinary tract symptoms: a systematic review of the latest literature. Eur Urol 56: 810–819. [DOI] [PubMed] [Google Scholar]
- Rubio-Ruiz M., Pérez-Torres I., Soto M., Pastelín G., Lans V. (2014) Aging in Blood Vessels. medicinal agents FOR systemic Arterial Hypertension in the elderly. Ageing Res Rev 18: 132–147. [DOI] [PubMed] [Google Scholar]
- Saito M., Ohmura M., Kondo A. (1999) Effect of ageing on blood flow to the bladder and bladder function. Urol Int 62: 93–98. [DOI] [PubMed] [Google Scholar]
- Sagawa K., Aikawa K., Nomiya M., Ogawa S., Akaihata H., Takahashi N., et al. (2013) Impaired detrusor contractility in a rat model of chronic bladder ischemia. Urology 81: 1379, e9–e14. [DOI] [PubMed] [Google Scholar]
- Sawada N., Nomiya M., Hood B., Koslov D., Zarifpour M., Andersson K. (2013) Protective effect of a β3-adrenoceptor agonist on bladder function in a rat model of chronic bladder ischemia. Eur Urol 64: 664–671. [DOI] [PubMed] [Google Scholar]
- Semenza G. (2009) Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 24: 97–106. [DOI] [PubMed] [Google Scholar]
- Semenza G. (2014) Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol 9: 47–71. [DOI] [PubMed] [Google Scholar]
- Semenza G. (2012) Hypoxia-inducible factors in physiology and medicine. Cell 148: 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehata R. (1976) The arterial supply of the urinary bladder. Acta Anat (Basel) 96: 128–134. [DOI] [PubMed] [Google Scholar]
- Sarma K. (1981) Microangiography of the bladder in health. Br J Urol 53: 237–240. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Mochizuki S., Mitsui R., Hashitani H. (2014) Neurohumoral regulation of spontaneous constrictions in suburothelial venules of the rat urinary bladder. Vascul Pharmacol 60: 84–94. [DOI] [PubMed] [Google Scholar]
- Smith P. (2010) Aging and the underactive detrusor: a failure of activity or activation? Neurourol Urodyn 29: 408–412. [DOI] [PubMed] [Google Scholar]
- Smith P.P., Chalmers D.J., Feinn R.S. (2015) Does defective volume sensation contribute to detrusor underactivity? Neurourol Urodyn November; 34(8):752–6. [DOI] [PubMed] [Google Scholar]
- Smith P.P., Chalmers D.J., Feinn R.S. (2015) Does defective volume sensation contribute to detrusor underactivity? Neurourol Urodyn 34(8): 752–756. [DOI] [PubMed] [Google Scholar]
- Smith P, Chalmers D., Feinn R. (2015) Does defective volume sensation contribute to detrusor underactivity? Neurourol Urodyn 34: 752–756. [DOI] [PubMed] [Google Scholar]
- Soler R., Andersson K., Chancellor M., Chapple C., de Groat W., Drake M., et al. (2013) Future direction in pharmacotherapy for non-neurogenic male lower urinary tract symptoms. Eur Urol 64: 610–621. [DOI] [PubMed] [Google Scholar]
- Takasu T., Ukai M., Sato S., Matsui T., Nagase I., Maruyama T., et al. (2007) Effect of (R)-2-(2-aminothiazol-4-yl)-4’-{2-[(2-hydroxy-2-phenylethyl)amino]ethyl} acetanilide (YM178), a novel selective beta3-adrenoceptor agonist, on bladder function. J Pharmacol Exp Ther 321: 642–647. [DOI] [PubMed] [Google Scholar]
- Tarcan T., Azadzoi K., Siroky M., Goldstein I., Krane R. (1998) Age-related erectile and voiding dysfunction: the role of arterial insufficiency. Br J Urol 82(Suppl. 1): 26–33. [DOI] [PubMed] [Google Scholar]
- Talks K., Turley H., Gatter K., Maxwell P., Pugh C., Ratcliffe P., et al. (2000) The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol 157: 411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z., Kaley G., de Cabo R., Sonntag W., Csiszar A. (2010) Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci 65: 1028–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Mastrigt R. (1992) Age dependency of urinary bladder contractility. Neurourol Urodyn 11: 315–317. [Google Scholar]
- Vignozzi L., Gacci M., Cellai I., Morelli A., Maneschi E., Comeglio P., et al. (2013) PDE5 inhibitors blunt inflammation in human BPH: a potential mechanism of action for PDE5 inhibitors in LUTS. Prostate 73: 1391–1402. [DOI] [PubMed] [Google Scholar]
- Vignozzi L., Filippi S., Comeglio P., Cellai I., Morelli A., Maneschi E., et al. (2014) Tadalafil effect on metabolic syndrome-associated bladder alterations: an experimental study in a rabbit model. J Sex Med 11: 1159–1172 [DOI] [PubMed] [Google Scholar]
- Warburton A., Santer R. (1994) Sympathetic and sensory innervation of the urinary tract in young adult and aged rats: a semi-quantitative histochemical and immunohistochemical study. Histochem J 26: 127–133. [DOI] [PubMed] [Google Scholar]
- Wiesener M., Jurgensen J., Rosenberger C., Scholze C., Horstrup J., Warnecke C., et al. (2003) Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. FASEB J 17: 271–273. [DOI] [PubMed] [Google Scholar]
- Wuest M., Morgenstern K., Graf E., Braeter M., Hakenberg O., Wirth M., et al. (2005) Cholinergic and purinergic responses in isolated human detrusor in relation to age. J Urol 173: 2182–2189. [DOI] [PubMed] [Google Scholar]
- Yamada S., Ito Y. (2011) α(1)-Adrenoceptors in the urinary tract. Handb Exp Pharmacol 202: 283–306. [DOI] [PubMed] [Google Scholar]
- Yamaguchi O., Nomiya M., Andersson K. (2014) Functional consequences of chronic bladder ischemia. Neurourol Urodyn 33: 54–58. [DOI] [PubMed] [Google Scholar]
- Yono M., Tanaka T., Tsuji S., Irie S., Sakata Y., Otani M., et al. (2011) Effects of age and hypertension on α1-adrenoceptors in the major source arteries of the rat bladder and penis. Eur J Pharmacol 670: 260–265. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Homma Y., Inadome A., Yono M., Seshita H., Miyamoto Y., et al. (2001) Age-related changes in cholinergic and purinergic neurotransmission in human isolated bladder smooth muscles. Exp Gerontol 36: 99–109. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Miyamae K., Iwashita H., Otani M., Inadome A. (2004) Management of detrusor dysfunction in the elderly: changes in acetylcholine and adenosine triphosphate release during aging. Urology 63(Suppl. 1): 17–23. [DOI] [PubMed] [Google Scholar]
- Zhao Z., Azad R., Yang J., Siroky M., Azadzoi K. (2016) Progressive changes in detrusor function and micturition patterns with chronic bladder ischemia. Investig Clin Urol 57: 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]