Abstract
Background:
Nodular sclerosis Hodgkin lymphoma (NS-HL) is the most common subtype of HL and usually has a good prognosis. A variant of NS, the syncytial variant (SV) has well-established histopathologic features but little is known about its clinical behavior. Small case series have suggested that SV patients present with advanced disease and have a comparatively aggressive course. The objective of this study was to determine the clinical characteristics and outcome of SV patients
Methods:
A total of 167 adult patients with NS-HL including 43 patients with SV and 124 patients with typical NS (t-NS) were included in our analysis following institutional review board (IRB) approval. The Kaplan–Meier method was used to calculate the progression-free survival (PFS) and overall survival (OS). Log-rank test was used to determine the differences in survival.
Results:
Of the 167 patients, 43 were confirmed as SV based on morphology and immunophenotype. Doxorubicin, bleomycin, vinblastine and dacarbazine (ABVD) was the most frequent induction regimen administered in 91% of all patients. The rate of complete response (CR) in the SV group was 74% versus 87% in the t-NS group (p = 0.05). At 49 months follow up, the PFS was 17 months in the SV group and not reached in the t-NS group [p < 0.0001; hazard ratio (HR) = 3.695; 95% confidence interval (CI) = 3.0, 11.07]. The median OS was not reached in both groups (p = 0.32).
Conclusions:
Our results show that SV histology represents a poor risk group with lower CR rate and shorter PFS and this should be considered in the risk stratification of classical HL patients.
Keywords: Hodgkin lymphoma, nodular sclerosis, outcome, syncytial variant
Introduction
Approximately 9000 cases of classical Hodgkin lymphoma (cHL) are diagnosed each year in the United States. CHL is a lymphoid malignancy characterized by the presence of Hodgkin Reed Sternberg (HRS) cells in a background of inflammatory infiltrate [Swerdlow, 2008]. Advancements in chemotherapy and radiation fields have improved the long-term survival of patients of cHL. Using the current standard risk stratification methods and treatment regimens, more than 85% of patients with early stage cHL will be cured of the disease [Kuruvilla et al. 2011; Ansell, 2014]. The 5-year overall survival (OS) is 95% for early stage and 82% for advanced disease but long-term toxicity remains a concern in survivors.
The identification of higher-risk subgroups can potentially guide alternative treatment strategies for these patients, including the incorporation of newer agents, with the goal to increase the cure rates and avoid long-term toxicities.
Overall, four subtypes of cHL have been described, largely based on the characteristics of the background inflammatory infiltrate and fibrosis. These include nodular sclerosis (NS), mixed cellularity, lymphocyte rich, and lymphocyte- depleted HL. The immunophenotype and the genetic features of these subtypes are identical but they differ in their clinical behavior and frequency of Epstein–Barr Virus (EBV) association [Weiss et al. 1987; Swerdlow, 2008]. Mixed cellularity and lymphocyte-depleted subtypes are more commonly associated with EBV.
NS-HL is the most common subtype of HL and accounts for about 70% of cHL cases [Pileri et al. 2002]. It is characterized by the presence of thick interlacing collagen bands that separate the cellular portion of the infiltrate into discrete nodules. A variant of NS known as the syncytial variant (SV) of NS-HL was first well described with immune confirmation in the 1980s [Strickler et al. 1986]. SV represents 5–16% of all cases of NS based on data from small studies [Strickler et al. 1986; Ben-Yehuda-Salz et al. 1990]. It is characterized by the presence of HRS cells in cohesive sheets and clusters. This variant may be mistaken for metastasis of carcinoma, melanoma or large cell lymphoma by morphology, and immunohistochemistry helps in the differential diagnosis.
While SV is a well-characterized pathologic entity, the data for the clinical behavior of this subset of cHL are limited. Earlier studies that examined the prognostic significance of grading of NS-HL based on the British National Lymphoma Investigation (BNLI) criteria [Bennett et al. 1981; MacLennan et al. 1989] showed grade II to be associated with a poorer prognosis [MacLennan et al. 1989; Wijlhuizen et al. 1989; Ferry et al. 1993]. Others discussed the significance to be less in the era of combination chemotherapy [van Spronsen et al. 1997]. The BNLI criteria for grading of NS-HL are based on three patterns: (1) degree of cellularity of the nodules; (2) the amount of sclerosis; and (3) the number and atypical neoplastic cells. The SV usually belongs to grade II due to the number of atypical cells. However, most grade II cases are not SV, even those based on increased HRS cells, because grade II requires a number of tumor cells but not necessarily cohesive and confluent distribution (Figure 1). There is a suggestion that along with its unique morphology, SV has distinct clinical manifestations often presenting with bulky mediastinal mass, B symptoms and advanced stage at diagnosis [Ben-Yehuda-Salz et al. 1990]. Based on previous small case series, SV may represent an adverse histopathological prognostic factor portending a more aggressive course [Darabi et al. 2015]. In the present study, we evaluated the clinical features, response to treatment and outcome of patients with SV and further compared them with typical NS (t-NS). To our knowledge, this is the largest study describing the disease characteristics and outcome in SV patients published in English literature.
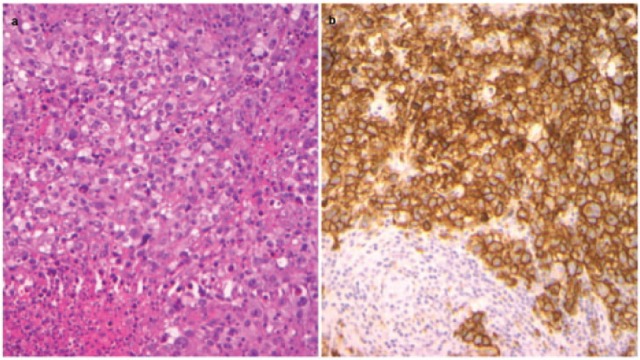
Figure 1.
Histopathologic distinction of SV and NS-HL (a) High power (40×) hematoxylin and eosin stain in SV HL, (b) High power (40×) CD30 immunostain outlining the HRS cells in sheets in SV HL.
HL, Hodgkin lymphoma; HRS, Hodgkin Reed Sternberg; NS-HL, nodular sclerosis Hodgkin lymphoma; SV, syncytial variant.
Materials and methods
Patient selection
After obtaining institutional review board (IRB) approval, we identified 167 patients with NS-HL evaluated at Vanderbilt University Medical Center, TN, USA between 1995 and 2014. Patients were at least 18 years of age with pathology confirmation of NS-HL reviewed by a pathologist at our institution. These patients were followed from the time of diagnosis to the date of last follow up or death. Clinical, laboratory and outcome data including relapse and death were extracted from the electronic medical record. Within the study cohort, 43 patients with SV were compared with 124 patients with t-NS with regards to patient characteristics, disease-related variables, treatment administered, as well as outcome.
Pathologic review
All initial histopathology data were collected retrospectively from pathology reports of specimens reviewed at our institution. The histopathology was independently reviewed by a second hematopathologist for our research cohort. The diagnosis in each of these cases was based on morphology and immunophenotypic characteristics as described. The diagnosis of NS-HL was based on the 2008 World Health Organization (WHO) classification criteria [Swerdlow, 2008]. NS-HL is characterized by collagen bands that surround at least one cellular nodule containing HRS cells. The diagnosis of SV was based on presence of sheets of cohesive HRS cells (syncytial growth pattern) in a majority of the nodules in contrast with scattered HRS cells in t-NS following the 2008 WHO criteria and previous literatures [Strickler et al. 1986; Ben-Yehuda-Salz et al. 1990; Swerdlow, 2008] The diagnosis of all cases was further confirmed by the combined results of a panel of immunohistochemical markers including CD3, CD20, PAX5, CD15, CD30, CD45 and EBER. The following diagnosis had been excluded based on extensive immunohistochemical workup: (1) anaplastic large cell lymphoma (ALCL), especially ALK1 negative type (cHL: T-marker negative, PAX5+, CD15+, CD45−; while ALCL was the opposite); (2) primary mediastinal large B-cell lymphoma (PMBCL) (CD20 diffuse+, CD45+, CD15−; while in cHL was the opposite). In addition, epithelial membrane antigen (EMA), cytokeratin and S-100 were also used to differentiate between metastatic carcinoma or melanoma.
Disease variables and treatment
Performance status was assessed using Eastern Cooperative Oncology Group (ECOG) scale. B symptoms were defined as fever, night sweats or weight loss >10% in a 6-month period. Advanced stage was defined as stage III/IV disease according to Cotswold’s modification of the Ann Arbor staging system. Bulky disease was defined as mass >10 cm or a mediastinal mass >1/3 of thoracic diameter at T5–T6. The treatment regimens were doxorubicin, bleomycin, vinblastine and dacarbazine (ABVD); doxorubicin, vinblastine and dacarbazine (AVD); doxorubicin, vinblastine, mechlorethamine, vincristine, bleomycin, etoposide, prednisone with or without radiation (Stanford V); cyclophosphamide, vincristine, procarbazine and prednisone (COPP); mechlorethamine, vincristine, procarbazine and prednisone (MOPP); doxorubicin, bleomycin and vinblastine (ABV). Response was assessed based on standard criteria [Cheson et al. 2014]. Response assessment was based on positron emission tomography/computerized tomography (CT) in 146 patients and CT with contrast in the remaining 21 patients.
Statistical analysis
Baseline patient characteristics and disease-related variables were described using median and range for continuous variables and percent of total for categorical variables. Progression-free survival (PFS) was defined as the time between date of diagnosis to date of progression (relapse) or last known clinical contact. OS was defined as the time between the date of diagnosis to death or last known clinical contact. PFS and OS (months) were estimated by the Kaplan–Meier method and compared using a log-rank test, Univariate analysis was performed with disease-related variables to see the effect on long-term outcome for patients. Chi-square test was used to determine the relationship between all categorical variables. Mann–Whitney U rank sum tests were used for association between continuous variables and categories. Multivariate analyses were performed using logistic regression or Cox proportional hazard regression models. The proportional hazards assumptions for all the variables were examined by testing time-dependent covariates. Statistical analysis was performed using SPSS version 22 software (IBM-SPSS, Chicago, IL, USA). Statistical significance was set at p < 0.05.
Results
Patient characteristics
The patient and disease characteristics are summarized in Table 1.
Table 1.
Patient characteristics.
| All patients (n = 167) |
Typical NS (n = 124) |
SV (n = 43) |
|
|---|---|---|---|
| Age, years | 31 | 30 | 31 |
| Median, range | 17–75 | 17–75 | 20–53 |
| Male | 85 (50%) | 60 (58%) | 25 (48%) |
| ECOG 0–1 | 161 (96%) | 119 (97%) | 42 (96%) |
| Stage (III/IV) | 65 (39%) | 48 (38%) | 17 (41%) |
| >3 node site | 44 (26%) | 34 (27%) | 10 (23%) |
| ⩽3 | 78 (47%) | 59 (48%) | 19 (44%) |
| N/A | 45 (27%) | 31 (25%) | 14 (33%) |
| B symptoms | 82 (49%) | 57 (46%) | 26 (60%)† |
| Bulky | 41 (25%) | 31 (25%) | 10 (23%) |
| Nonbulky | 91 (55%) | 70 (56%) | 21 (49%) |
| N/A | 35 (20%) | 23 (19%) | 12 (28%) |
| Treatment | |||
| ABVD | 152 (91%) | 113 (91%) | 39 (91%) |
| Stanford V | 4 (2.5%) | 3 (2.5%) | 1 (2%) |
| COPP/ABV | 6 (3.5%) | 3 (2.5%) | 3 (7%) |
| Other* | 5 (3%) | 5 (4%) | — |
| XRT | 62 (38%) | 45 (36.8%) | 17 (43%) |
| HDT/ASCT | 44 (27%) | 28 (23%) | 16 (37%)‡ |
| No ASCT | 114 (68%) | 93 (75%) | 21 (49%) |
| N/A | 9 (5%) | 3 (2%) | 6 (14%) |
Other, ABV, AVD, COPP and MOPP/ABV.
p = 0.07; ‡p = 0.016.
ABV, doxorubicin, bleomycin and vinblastine; ABVD, adriamycin, bleomycin, vinblastine, dacarbazine; ASCT, autologous stem cell transplant; AVD, doxorubicin, vinblastine and dacarbazine; COPP, cyclophosphamide, vincristine, procarbazine and prednisone; HDT, high dose therapy; MOPP, mechlorethamine, vincristine, procarbazine and prednisone; N/A, not available; NS, nodular sclerosis; Stanford V, doxorubicin, vinblastine, mechlorethamine, vincristine, bleomycin, etoposide, and prednisone; SV, syncytial variant; XRT, radiation.
A total of 167 patients were included in our final analysis. EBER results were available for 11/43 SV patients and all were negative. The median age at diagnosis for all patients was 31 years (17–75 years.). A total of 85 patients were male (51%). Overall, 96% patients had an ECOG performance scale of 0–1. A total of 49% had B symptoms and 39% had advanced stage disease. There were 25% of patients noted to have bulky disease at presentation and 26% had more than three involved nodal sites. ABVD was the most frequent induction regimen administered in 91% of all patients, followed by COPP/ABV (3.5%), Stanford V (2.5%) and other regimens (3%). The other regimens included ABV, AVD, COPP and MOPP/ABV. A total of 38% of patients received radiation (XRT) to the disease site. Of the patients who needed salvage treatment, 8% had primary refractory disease and 92% were relapsed after an initial response. There were 27% of all patients in the study cohort that received high-dose therapy followed by autologous stem cell transplant (ASCT) after relapse. Overall, 3/4 of the patients who had relapsed or primary refractory disease underwent ASCT.
There were no significant differences noted in age, sex, performance status, stage, bulky disease, number of nodal sites and chemotherapy regimens between the two groups (t-NS and SV, p > 0.05). In the SV group, a higher proportion of patients presented with B symptoms (Table 1).
Treatment response, PFS and OS
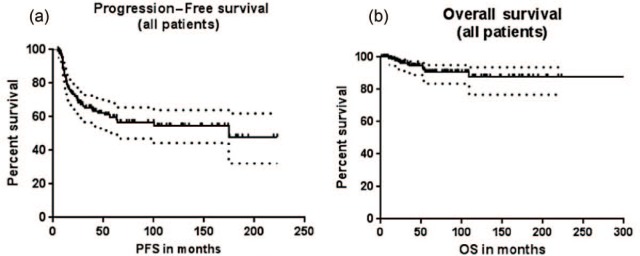
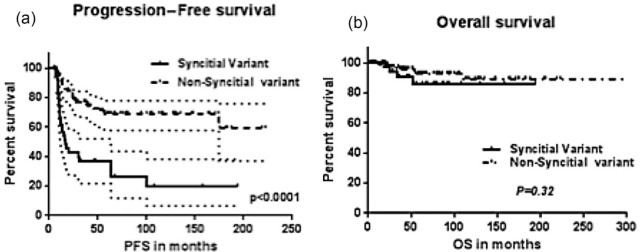
The rate of complete response (CR) in the SV group was 74% versus 87% in the t-NS group with the difference approaching significance (p = 0.05). The median PFS for the entire cohort was 174.7 months (Figure 2a). At a median follow up of 49 months, OS was not reached (Figure 2b). The median PFS (Figure 3a) in the SV group was 17.02 months which was significantly shorter compared with that of the t-NS group (not reached) [p < 0.0001; hazard ratio (HR) = 3.695; 95% confidence interval (CI) = 3.0, 11.07]. The median OS (Figure 3b) was not reached in both groups and was not statistically different (p = 0.32).
Figure 2.
PFS (a) and OS (b) of all patients with NS-HL.
OS, overall survival; PFS, progression-free survival.
Figure 3.
PFS (a) and OS (b) of SV compared with typical NS-HL (non SV).
NS-HL, nodular sclerosis Hodgkin lymphoma; OS, overall survival; PFS, progression-free survival; SV, syncytial variant.
Univariate and multivariate analyses
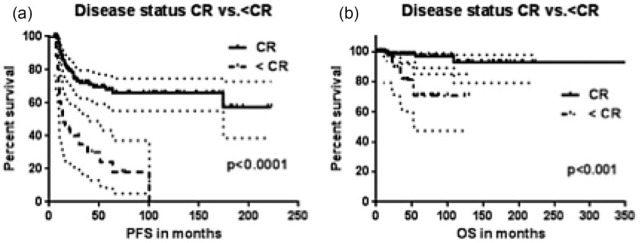
In the univariate analysis, stage of disease and achievement of CR were predictive of PFS (Stage, p = 0.002; CR, p = 0.003) and OS (Stage, p = 0.007; CR, p = 0.009) in the two groups (Table 2). In the corresponding multivariate analysis (Table 3) the achievement of CR following completion of treatment (Figure 4) predicted a difference in PFS (p < 0.0001; HR, 4.22; CI, 4.6, 23.8) and was the only independent predictor of OS (p < 0.01; HR 7.61; CI 4.35, 90). SV was an independent predictor of PFS (p < 0.01; HR, 3.69; CI, 3.0, 11.07) irrespective of stage.
Table 2.
Univariate analysis.
| Variable | n (%) | OS | PFS |
|---|---|---|---|
| Age | |||
| <median | 85 (51) | p = 0.92 | p = 0.68 |
| >median | 82 (49) | ||
| Sex | |||
| Male | 85 (50) | p = 0.23 | p = 0.97 |
| Syncytial | 43 (26) | p = 0.01 | p = 0.016 |
| Nonsyncytial | 124 (74) | ||
| Stage | |||
| I/II | 99 (59) | p = 0.007 | p = 0.002 |
| III/IV | 65 (39) | ||
| Bulky, ⩾10 cm | |||
| Yes | 41 (25) | p = 0.64 | p = 0.99 |
| No | 91 (55) | ||
| Disease status | |||
| CR | 127 (76) | p = 0.009 | p = 0.003 |
| <CR | 15 (9) | ||
CR, complete response; OS, overall survival; PFS, progression-free survival.
Table 3.
Multivariate analysis.
| Variable | OS |
PFS |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| SV | 0.54 | 0.12–1.98 | 0.34 | 3.69 | 3.0–11.07 | <0.0001 |
| Disease status (CR versus <CR) |
7.61 | 4.35–90.0 | 0.001 | 4.22 | 4.6–23.8 | <0.0001 |
CI, confidence interval; CR, complete response; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; SV, syncytial variant.
Figure 4.
PFS (a) and OS (b) of patients who achieved a CR after first line treatment versus patients who did not achieve a CR (<CR).
CR, complete response; OS, overall survival; PFS, progression-free survival.
Discussion
SV was originally described in 1980 in a small case series that associated this variant with an aggressive clinical behavior presenting with B symptoms and advanced disease [Strickler et al. 1986]. While there have been some indications that SV may represent a poor prognostic subtype of NS-HL [Darabi et al. 2015], to our knowledge there have been no large studies comparing the presentation and outcome of SV and t-NS. In this study, we present our observations on the clinical course of adult patients with the SV and describe the results of long-term follow up of patients with NS-HL. We observed that the rate of CR was lower in the SV variant as compared with t-NS with standard induction therapy. Patients with SV also had a shorter PFS and experienced disease relapse. This, however, did not compromise the OS as most of these patients were successfully salvaged with salvage chemotherapy ± ASCT.
While a majority of these patients can be successfully salvaged with high-dose therapy and ASCT, studies with upfront use of novel agents such as conjugated antibodies or immunotherapeutic agents should be considered in these patients to improve initial CR rates thereby avoiding the need for more toxic salvage therapies.
Earlier studies report a 5–16% incidence of SV in all cases of NS. In our study, a quarter of the patients with NS had the SV. We cannot rule out the possibility of a referral bias to our institution for relapsed/ refractory disease. Darabi and colleagues in their study involving 10 SV and 53 t-NS patients also reported an inferior PFS for SV [Darabi et al. 2015]. This study also reported a lower OS in this group. In reference to other past studies, the BNLI group introduced histological grading criteria of NS-HL in the early 1980s which subclassified these patients into grade I and II NS-HL based on the cellularity of the nodules, necrosis, sclerosis and number and atypia of neoplastic cells [Bennett et al. 1981,1983]. The clinical relevance of NS-HL grading has remained controversial [Wijlhuizen et al. 1989; Ferry et al. 1993; Hess et al. 1994].
A few studies grouped SV patients with NS grade II. These studies described that NS grade II patients presented with higher stage disease and a more aggressive course including a shorter survival [MacLennan et al. 1989; Ferry et al. 1993]. The largest published clinicopathologic study that exclusively evaluated SV patients included eight patients treated between 1982 and 1988 and reported a possible trend towards a more advanced disease at diagnosis and huge mediastinal masses. The study however, was too small to determine any statistically significant differences [Ben-Yehuda-Salz et al. 1990]. In our study cohort, we did not find a statistically significant difference at presentation for the patients with SV versus t-NS with regards to the traditional adverse factors including stage of diagnosis, B symptoms as well as bulky disease. This may imply that the relatively adverse outcome for these may be driven by distinct disease biology from the rest of the NS-HL patients rather than due to more advanced disease.
There are possible explanations for the distinct disease biology of SV including differences in activated genetic pathways, constitution of the tumor microenvironment or the abundance of Reed-Sternberg (RS) cells in SV compared with t-NS.
In conclusion, our study demonstrates that patients with SV experience a lower than expected rate of CR with a shorter progression-free interval as compared with those with t-NS with standard first-line therapy. This highlights the need for recognition of SV as a high-risk subgroup amongst the traditionally well-behaved NS subtype of cHL. While further prospective validation will help establish the prognostic significance of SV, we suggest that this should be included as a histopathologic correlation in future trials involving novel agents to assess response in these patients.
Acknowledgments
We would like to thank Jonathan Douds, MD, Department of Hematopathology, Vanderbilt University Medical Center, TN, USA for providing histopathology pictures (Figure 1).
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: TS, VN, SL, DM and JG have nothing to declare NR declares an interest in Celgene (research funding, advisory board); Seattle Genetics, Pharmacyclics, Gilead, AbbVie (advisory board). There are no direct financial or personal interests to disclose pertinent to the article.
Contributor Information
Tarsheen Sethi, Vanderbilt University Medical Center, Nashville, TN, USA.
Van Nguyen, Vanderbilt University Medical Center, Nashville, TN, USA.
Shaoying Li, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
David Morgan, Vanderbilt University Medical Center, Nashville, TN, USA.
John Greer, Vanderbilt University Medical Center, Nashville, TN, USA.
Nishitha Reddy, Vanderbilt University, 3927 The Vanderbilt Clinic, 1301 Medical Center Drive, Nashville, TN 37232, USA.
References
- Ansell S. (2014) Hodgkin lymphoma: 2014 update on diagnosis, risk stratification, and management. Am J Hematol 89: 771–779. [DOI] [PubMed] [Google Scholar]
- Bennett M., Maclennan K., Easterling M., Vaughan Hudson B., Jelliffe A., Vaughan Hudson G. (1983) The prognostic significance of cellular subtypes in nodular sclerosing Hodgkin’s disease: an analysis of 271 non-laparotomised cases (BNLI report no. 22). Clin Radiol 34: 497–501. [DOI] [PubMed] [Google Scholar]
- Bennett M., Tu A., Hudson G. (1981) Analysis of grade 1 Hodgkin’s disease (Report no 6). Clin Radiol 32: 491–498. [DOI] [PubMed] [Google Scholar]
- Ben-Yehuda-Salz D., Ben-Yehuda A., Polliack A., Ron N., Okon E. (1990) Syncytial variant of nodular sclerosing Hodgkin’s disease. A new clinicopathologic entity. Cancer 65: 1167–1172. [DOI] [PubMed] [Google Scholar]
- Cheson B., Fisher R., Barrington S., Cavalli F., Schwartz L., Zucca E., et al. (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the lugano classification. J Clin Oncol 32: 3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darabi K., Tester W., Daskal I., Cohn J. (2015) Syncytial variant of nodular sclerosing Hodgkin’s disease. Blood 104: 4533–4533. [Google Scholar]
- Ferry J., Linggood R., Convery K., Efird J., Eliseo R., Harris N. (1993) Hodgkin disease, nodular sclerosis type. Implications of histologic subclassification. Cancer 71: 457–463. [DOI] [PubMed] [Google Scholar]
- Hess J., Bodis S., Pinkus G., Silver B., Mauch P. (1994) Histopathologic grading of nodular sclerosis Hodgkin’s disease. Lack of prognostic significance in 254 surgically staged patients. Cancer 74: 708–714. [DOI] [PubMed] [Google Scholar]
- Kuruvilla J., Keating A., Crump M. (2011) How I treat relapsed and refractory Hodgkin lymphoma. Blood 117: 4208–4217. [DOI] [PubMed] [Google Scholar]
- Maclennan K., Bennett M., Tu A., Hudson B., Easterling M., Hudson G., et al. (1989) Relationship of histopathologic features to survival and relapse in nodular sclerosing Hodgkin’s disease. A study of 1659 patients. Cancer 64: 1686–1693. [DOI] [PubMed] [Google Scholar]
- Pileri S., Ascani S., Leoncini L., Sabattini E., Zinzani P., Piccaluga P., et al. (2002) Hodgkin’s lymphoma: the pathologist’s viewpoint. J Clin Pathol 55: 162–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickler J., Michie S., Warnke R., Dorfman R. (1986) The “syncytial variant” of nodular sclerosing Hodgkin’s disease. Am J Surg Pathol 10: 470–477. [DOI] [PubMed] [Google Scholar]
- Swerdlow S., Campo E., Harris N., Jaffe E., Pileri S., Stein H., et al. (2008) Who Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press. [Google Scholar]
- Van Spronsen D., Vrints L., Hofstra G., Crommelin M., Coebergh J., Breed W. (1997) Disappearance of prognostic significance of histopathological grading of nodular sclerosing Hodgkin’s disease for unselected patients, 1972–92. Br J Haematol 96: 322–327. [DOI] [PubMed] [Google Scholar]
- Weiss L., Strickler J., Warnke R., Purtilo D., Sklar J. (1987) Epstein-Barr viral DNA in tissues of Hodgkin’s disease. Am J Pathol 129: 86–91. [PMC free article] [PubMed] [Google Scholar]
- Wijlhuizen T., Vrints L., Jairam R., Breed W., Wijnen J., Bosch L., et al. (1989) Grades of nodular sclerosis (NSI-NSII) in Hodgkin’s disease. Are they of independent prognostic value? Cancer 63: 1150–1153. [DOI] [PubMed] [Google Scholar]