Abstract
Chronic meningitis of unknown etiology is a vexing illness for patients and clinicians. Identification of the correct pathogen can be challenging and time consuming, leading to delays in appropriate treatment. Although Sporothrix schenckii is a recognized and treatable cause of chronic meningitis, neurologists and infectious diseases physicians may not regularly evaluate for Sporothrix infection. We describe an immunocompetent patient with chronic meningitis who partially responded to empiric fluconazole. Prompted by a recent culture-confirmed case of meningeal sporotrichosis, we tested for S schenckii antibodies from the cerebrospinal fluid, which were positive. His clinical and functional status improved, and the S schenckii antibody titer decreased with itraconazole therapy. Clinicians should consider S schenckii in the differential diagnosis for chronic meningitis, even in immunocompetent patients, particularly when the clinical picture does not respond to standard empiric therapy.
Keywords: Sporothrix schenckii, sporotrichosis, chronic meningitis, fungal, central nervous system infection
Case Report
A 34-year-old-man with no past medical history presented with 3 months of fever, headache, and confusion. The patient was originally from Mexico but had lived in Northern California for over a decade. He had not traveled out of state but had driven through central California on multiple occasions. He worked on construction jobs involving roofing, laying tile, and removing carpet. A family member was recently diagnosed with tuberculosis. He denied alcohol, tobacco, or other substance use.
He presented to a local emergency department where a head computed tomography (CT) scan was normal. Lumbar puncture (LP) revealed 500 white blood cells (WBC)/mm3 (86% lymphocytes), low glucose, and elevated protein (Table 1). Cerebrospinal fluid (CSF) bacterial culture and herpes simplex virus 1 and 2 polymerase chain reaction were negative. He was treated empirically with acyclovir, ampicillin, vancomycin, and ceftriaxone for 7 days.
Table 1.
Lumbar Puncture Results Over the Course of Illness.
| Time Since Initial Presentation | Interval Events | Cerebrospinal Fluid (CSF) Profile | ||||
|---|---|---|---|---|---|---|
| WBC Cells/mm3, (% Lymphocytes) | Glucose, mg/dL | Protein, mg/dL | Opening Pressure, cm H20 | Sporothrix Schenckii Antibody Titer | ||
| 0 month | 3 months after symptom onset on initial presentation to local hospital | 500 (86) | 19 | 170 | – | – |
| 2 months | Received 7 days of empiric antibiotic therapy 2 months prior | 330 | 18 | 168 | 30 | – |
| 3 months | On admission to our hospital | 263 (69) | 32 | 184 | – | – |
| 5 months | Started antituberculous therapy and fluconazole 2 months prior | 2 | 33 | 98 | – | – |
| 18 months | Discontinued fluconazole 1 month prior | 183 (54) | 23 | 241 | – | – |
| 22 months | After restarting on fluconazole | 128 (77) | 16 | 466 | – | 1:8 |
| 25 months | Initiated itraconazole 2 months prior | 16 (89) | 34 | 114 | – | 1:4 |
| 37 months | After 1 year of itraconazole treatment | 3 (91) | 48 | 50 | – | 1:2 |
Abbreviation: WBC, white blood cells.
His headache and fever fluctuated over the next 2 months. He returned to a local hospital where LP demonstrated an elevated opening pressure of 30 cm H2O with ongoing lymphocyte-predominant pleocytosis, low glucose, and elevated protein. He was treated with various antimicrobial agents including fluconazole before transfer to our hospital.
Upon arrival to our hospital, 3 months after initial presentation, he was confused and disoriented. No skin lesions were present. Lumbar puncture again demonstrated a lymphocyte-predominant pleocytosis with persistently low, although improved, glucose and elevated protein. An extensive evaluation of the CSF and serum for an infectious etiology was negative (Table 2). Magnetic resonance imaging (MRI) of the brain and spine showed meningeal enhancement and subacute infarcts in the basal ganglia (Figure 1). A chest CT scan demonstrated a 6-mm nodule in the left upper lobe. Based on epidemiologic risk factors and the CSF profile, meningitis due to Mycobacterium tuberculosis and Coccidioides species were high on the differential diagnosis, although evaluation for these diagnoses was negative (Table 2).
Table 2.
Testing Performed on Cerebrospinal Fluid (CSF) and Serum as Part of the Chronic Meningitis Evaluation (All Negative or Within Normal Limits Unless Otherwise Indicated).a
| Specimen | Test |
|---|---|
| CSF |
|
| Serum |
|
| Other |
|
Abbreviations: CSF, cerebrospinal fluid; Ab, antibody; Ag, antigen; PCR, polymerase chain reaction; AFB, acid fast bacilli; RPR, rapid plasma reagin; VDRL, Venereal Disease Research Laboratory; anti-Sm, anti-Smith; anti-RNP, anti-ribonucleoprotein; anti-SSA, anti-Sjogren's syndrome antigen A; anti-SSB, anti-Sjogren's syndrome antigen B.
a Bold-faced type indicates an abnormal result.
Figure 1.
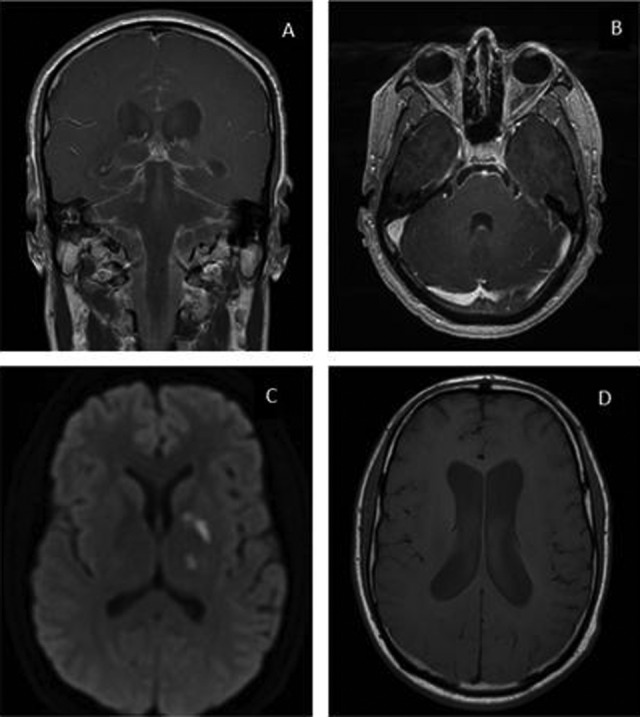
Magnetic resonance imaging (MRI) of the brain with contrast. Leptomeningeal enhancement was most pronounced in the basilar cisterns (A) and the bilateral fifth and sixth cranial nerves (B) as shown in these gadolinium-enhanced postcontrast T1 images. Foci of reduced diffusion were seen in the left caudothalamic groove, left globus pallidus, and left thalamus (C) as shown in the T2-weighted trace sequence. Moderate communicating hydrocephalus was also present (D) as shown in this T1 sequence.
He was empirically treated with standard 4-drug therapy (rifampin, isoniazid, pyrazinamide, and ethambutol) for tuberculous meningitis and fluconazole for coccidioidal meningitis with clinical improvement. Two months after initiation of therapy, a repeat LP demonstrated 2 WBC/mm3 low glucose and improved protein. The fluconazole was discontinued, which resulted in recurrent symptoms and worsened CSF profile. He was restarted on fluconazole but lost to follow-up. One year later, he was readmitted to our hospital with headaches after discontinuing fluconazole 1 month prior to admission. An LP 18 months after initial presentation again revealed a lymphocytic pleocytosis, low glucose, and elevated protein.
A few months prior, a 25-year-old-man with diabetes mellitus was admitted with headache and unsteady gait. He tended rose bushes and had 2 healthy cats at home. Brain MRI demonstrated marked ventricular dilatation with extensive meningeal enhancement. Lumbar puncture revealed 167 WBC/mm3 (70% lymphocytes), glucose 43 mg/dL, protein 388 mg/dL, and 4 oligoclonal bands unique to the CSF. The CSF acid-fast bacilli culture yielded growth identified by molecular testing as S schenckii. Sporothrix antibody titer by latex agglutination (LA) was 1:16 in the CSF and 1:8 in the serum (Mayo Medical Laboratories, Rochester, Minnesota). Influenced by the availability heuristic,1 this recent case of culture-positive Sporothrix meningitis prompted us to evaluate the CSF of our patient for antibodies to S schenckii. LA for antibodies to S schenckii in the CSF returned positive at a titer of 1:8 (Mayo Medical Laboratories).
The patient declined treatment with liposomal amphotericin B and was started on itraconazole. Two months later, repeat LP revealed improved CSF profile. Sporothrix antibody titer from the CSF was 1:4, and serum antibodies were negative. After 1 year of itraconazole therapy, LP demonstrated 3 WBC/mm3 (91% lymphocytes) with normal glucose and protein. Sporothrix antibodies from the CSF declined 4-fold from baseline to 1:2, and he has returned to work.
Discussion
Sporotrichosis is caused by thermally dimorphic fungi belonging to the genus Sporothrix, which are found in soil and plant material throughout the world. Molecular methods have reclassified S schenckii into several different pathogenic species comprising the S schenckii complex.2 While the incidence of sporotrichosis may be on the rise in certain tropical and subtropical regions where the infection is endemic,3 data are limited regarding its incidence in the United States and other countries in which Sporothrix infection is less common and not a reportable disease.4
Infections usually result from traumatic inoculation of the skin with soil or vegetation contaminated by the fungus. Zoonotic transmission of Sporothrix through animal scratches or bites is well described,5 and veterinarians are a known risk group for zoonotic transmission from infected animals, especially cats.6
Most Sporothrix infections in immunocompetent individuals are limited to cutaneous and subcutaneous manifestations. Erythematous papulonodular lesions arise from sites of skin trauma and spread proximally along lymphatic vessels. Extracutaneous involvement occurs via hematogenous or contiguous spread of local disease. Musculoskeletal disease, including osteomyelitis and septic arthritis, is the most frequent extracutaneous site of infection.7 Pulmonary involvement arises after direct inhalation of the fungus or hematogenous spread from another source and can present with cavitary or noncavitary lesions.8
Meningeal sporotrichosis is rare and can present with associated complications of meningitis, such as infarcts and hydrocephalus.9 The CSF profile typically demonstrates a moderate lymphocyte-predominant pleocytosis with low glucose, elevated protein, immunoglobulin G index, and oligoclonal bands.9,10
Extracutaneous and disseminated sporotrichosis, including meningeal disease, are more common in individuals with immune compromise due to AIDS, alcohol use, cirrhosis, solid organ transplantation, use of immunosuppressive medications, or malignancy.4 Our patient had a negative HIV test, no history of heavy alcohol use, and no known immunosuppression. In 1 study of 1697 patients with sporotrichosis, Galhardo et al found that 2 of the 17 HIV-infected individuals in the cohort developed meningeal disease compared to none of the HIV-uninfected individuals.9 When they reviewed 21 cases of meningeal sporotrichosis in the literature, underlying immunosuppression was present in 15 patients. In only half of these cases (and in no individuals who were immunocompetent) was skin or joint involvement observed prior to or in conjunction with meningeal disease, suggesting that isolated meningitis without preceding skin involvement, as was the case with our patient, may not be an atypical presentation.
In addition to lack of awareness of S schenckii as a cause of chronic meningitis, diagnosis may be delayed in cases of isolated central nervous system (CNS) disease due to difficulty recovering the organism from the CSF. Culture is considered the gold standard for diagnosis; however, growth can take up to 1 month, and the fungus is challenging to isolate if fungal burden is low.3,4 In 1 study of 7 cases of meningeal sporotrichosis, the interval from symptom onset to positive culture ranged from 3 to 11 months.10
Antibody assays have not been validated for use in standard clinical practice but may assist in establishing a diagnosis. Antibody detection by enzyme immunoassay (EIA) and LA have varying degrees of utility. In 1 study, detection of serum antibodies to Sporothrix antigen by EIA was 100% sensitive in culture-proven cutaneous sporotrichosis; however, low cross-reactivity occurred with other infections.11 In another study of culture and mycological examination-confirmed Sporothrix cases compared with various controls, serum EIA was 89% sensitive and 82% specific overall and 100% sensitive for extracutaneous disease.12 Sensitivity and specificity of LA testing have been demonstrated to be as high as 94% and 100%, respectively, when compared to culture-confirmed cases of sporotrichosis and other infections.13
Systematic evaluation of serological methods to detect antibodies in specimens other than serum from patients with sporotrichosis has not been performed. In a case series of culture-confirmed meningeal sporotrichosis by Scott et al, antibodies were detected in the CSF and serum from all 7 cases using LA and EIA.10 No cross-reactivity occurred in individuals with meningeal infections due to other pathogens. In another study, Sporothrix antibodies were detected by EIA in the serum of 26 patients with extracutaneous disease including 4 with CNS involvement.14 Mayo Medical Laboratories uses an LA assay similar to that developed by Scott et al; for this assay, titers ≥1:8 in the serum and any detectable antibody in the CSF is considered positive.
Itraconazole is the treatment of choice for many forms of sporotrichosis and can be used relatively safely for long courses. A lipid formulation of amphotericin B is first line for the initial treatment of meningeal sporotrichosis followed by oral itraconazole for at least 12 months, although our patient has been successfully treated with oral azole therapy alone. Our patient had a partial response to empiric fluconazole but ultimately stopped responding. Fluconazole is only moderately active against S schenckii and should not be used as first-line therapy.15
Conclusion
A positive serological test does not constitute a definitive diagnosis of sporotrichosis, but the clinical syndrome and response to itraconazole therapy with subsequent decline in Sporothrix antibodies observed in our patient were highly suggestive of meningeal sporotrichosis. We may have avoided the 2-year delay in diagnosis for our patient if CSF and serum from patients with chronic meningitis were systematically tested for Sporothrix antibodies. Clinicians should consider serological testing for Sporothrix antibodies from the CSF and serum in patients with chronic meningitis, especially if routine studies have not determined the diagnosis or response to empiric therapy is incomplete.
Footnotes
Authors’ Note: Informed consent was obtained from the patient.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work has been supported by the National Center for Advancing Translational Sciences of the NIH under Award Number KL2TR000143.
References
- 1. Vickrey BG, Samuels MA, Ropper AH. How neurologists think: A cognitive psychology perspective on missed diagnoses. Ann Neurol. 2010;67(4):425–433. doi:10.1002/ana.21907. [DOI] [PubMed] [Google Scholar]
- 2. Marimon R, Cano J, Gene J, Sutton DA, Kawasaki M, Guarro J. Sporothrix brasiliensis, S.globosa, and S. mexicana, three new Sporothrix species of clinical interest. J Clin Microbiol. 2007;45(10):3198–3206. doi:10.1128/JCM.00808-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pappas PG, Tellez I, Deep AE, Nolasco D, Holgado W, Bustamante B. Sporotrichosis in Peru: description of an area of hyperendemicity. Clin Infect Dis. 2000;30(1):65–70. doi:10.1086/313607. [DOI] [PubMed] [Google Scholar]
- 4. de Lima Barros MB, de Almeida Paes R, Schubach AO. Sporothrix schenckii and Sporotrichosis. Clin Microbiol Rev. 2011;24(4):633–654. doi:10.1128/CMR.00007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alves SH, Boettcher CS, Oliveira DC, et al. Sporothrix schenckii associated with armadillo hunting in Southern Brazil: epidemiological and antifungal susceptibility profiles. Rev Soc Bras Med Trop. 2010;43(5):523–525. doi:10.1590/S0037-86822010000500010. [DOI] [PubMed] [Google Scholar]
- 6. Reed KD, Moore FM, Geiger GE, Stemper ME. Zoonotic transmission of sporotrichosis: case report and review. Clin Infect Dis. 1993;16(3):384–387. [DOI] [PubMed] [Google Scholar]
- 7. Lesperance M, Baumgartner D, Kauffman CA. Polyarticular arthritis due to Sporothrix schenckii. Mycoses. 1988;31(12):599–603. [PubMed] [Google Scholar]
- 8. Aung AK, Teh BM, McGrath C, Thompson PJ. Pulmonary sporotrichosis: case series and systematic analysis of literature on clinico-radiological patterns and management outcomes. Med Mycol. 2013;51(5):534–544. doi:10.3109/13693786.2012.751643. [DOI] [PubMed] [Google Scholar]
- 9. Galhardo MC, Silva MT, Lima MA, et al. Sporothrix schenckii meningitis in AIDS during immune reconstitution syndrome. J Neurol Neurosurg Psychiatr. 2010;81(6):696–699. doi:10.1136/jnnp.2009.173187. [DOI] [PubMed] [Google Scholar]
- 10. Scott EN, Kaufman L, Brown AC, Muchmore HG. Serologic studies in the diagnosis and management of meningitis due to Sporothrix schenckii. N Engl J Med. 1987;317(15):935–940. doi:10.1056/NEJM198710083171505. [DOI] [PubMed] [Google Scholar]
- 11. Penha CV, Bezerra LM. Concanavalin A-binding cell wall antigens of Sporothrix schenckii: a serological study. Med Mycol. 2000;38(1):1–7. [PubMed] [Google Scholar]
- 12. Bernardes-Engemann AR, de Lima Barros M, Zeitune T, Russi DC, Orofino-Costa R, Lopes-Bezerra LM. Validation of a serodiagnostic test for sporotrichosis: a follow-up study of patients related to the Rio de Janeiro zoonotic outbreak. Med Mycol. 2015;53(1):28–33. doi:10.1093/mmy/myu058. [DOI] [PubMed] [Google Scholar]
- 13. Blumer SO, Kaufman L, Kaplan W, McLaughlin DW, Kraft DE. Comparative evaluation of five serological methods for the diagnosis of sporotrichosis. Appl Microbiol. 1973;26(1):4–8. doi:10.1093/mmy/myu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Scott EN, Muchmore HG. Immunoblot analysis of antibody responses to Sporothrix schenckii. J Clin Microbiol. 1989;27(2):300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kauffman CA, Bustamante B, Chapman SW, Pappas PG. Clinical Practice Guidelines for the Management of Sporotrichosis: 2007 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45(10):1255–1265. doi:10.1086/522765. [DOI] [PubMed] [Google Scholar]


