Abstract
Background and Purpose:
The ideal strategy to prevent infections in patients with external ventricular drains (EVDs) is unclear.
Methods:
We conducted a cross-sectional survey of members of the Neurocritical Care Society on infection prevention practices for patients with EVDs between April and July 2015.
Results:
The survey was completed by 52 individuals (5% response rate). Catheter selection, use of prolonged prophylactic systemic antibiotics (PPSAs), cerebrospinal fluid (CSF) collection policies, location of EVD placement, and performance of routine EVD exchanges varied. Antibiotic-impregnated catheters (AICs) and conventional catheters (CCs) were used with similar frequency, but no respondents reported routine use of silver-impregnated catheters (SICs). The majority of respondents were either neutral or disagreed with the need for PPSA with all catheter types (CC: 75%, AIC: 85%, and SIC: 87%). Despite this, 55% of the respondents reported PPSAs were routinely administered to patients with EVDs at their institutions. The majority (80%) of the respondents reported CSF collection only on an as-needed basis. The EVD placement was restricted to the operating room at 27% of the respondents’ institutions. Only 2 respondents (4%) reported that routine EVD exchanges were performed at their institution.
Conclusion:
Practice patterns demonstrate that institutions use varying strategies to prevent ventriculostomy-related infections. Identification and further study of optimum care for these patients are essential to decrease the risk of complications and to aid development of practice standards.
Keywords: cerebrospinal fluid, external ventricular drain, ventriculitis, ventriculostomy-related infection
Background
As the frequency of external ventricular drain (EVD) placement in neurosurgical patients has risen, practitioners have worked to minimize the risk of iatrogenic infections associated with these devices.1 Reported rates of ventriculostomy-related infections (VRIs) vary greatly (0%-45%). As with other health-care-associated infections, the target VRI rate is 0%2,3 because the incidence of VRI is a marker of the quality of care in an intensive care unit (ICU).4 There have been numerous publications addressing areas of improvement to prevent VRI, including catheter selection,5-11 use of prolonged prophylactic systemic antibiotics (PPSA) throughout the duration of EVD placement,7,9,10,12-16 frequency of cerebrospinal fluid (CSF) sampling,17-19 venue for EVD placement,17,20,21 and the benefit of prophylactic EVD exchange.5,22,23
We hypothesized that there is marked variation in EVD management strategies to prevent VRI. We sought to evaluate practice patterns in regard to these issues by surveying members of the Neurocritical Care Society about EVD management at their institutions.
Materials and Methods
An electronic survey on infection prevention for patients with EVDs was designed using Open RedCap (Research Electronic Data Capture, a secure, web-based application program hosted at NYU Langone Medical Center)24 and distributed to approximately 1000 members of the Neurocritical Care Society via advertisement on the Society’s website and in the Society’s electronic newsletters from April to July 2015. Reminders were sent on a monthly basis throughout the study period. Respondents were asked about demographic data, institutional EVD practices, and their personal beliefs on the need for PPSA with each catheter type (Appendix A, please note the survey also included questions about practices pertaining to other types of neurosurgical drains, but these results were reviewed separately).
Study data were analyzed using descriptive statistics and χ2 tests as appropriate. Intraclass correlation coefficient was calculated to assess the degree of variation in respondents’ beliefs about the need for PPSA with each catheter type. All statistical analyses were performed using standard software packages (SPSS 21; IBM, Armonk, New York). A P value of less than .05 was considered statistically significant. The institutional review board at NYU Langone Medical Center did not require submission of this study for approval.
Results
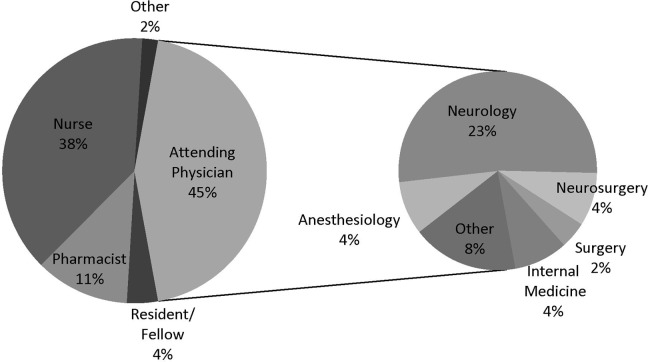
The survey was completed by 52 people (5% response rate). The majority of respondents was younger than 40 years (58%), female (52%), and worked at institutions in the United States (79%) with 750 or fewer beds (52%). The institutions represented were 67% academic, 21% community, 8% private, and 4% government, and 85% had a dedicated neurosciences ICU. Additional data on the respondents’ roles and fields are shown in Figure 1.
Figure 1.
The first pie graph illustrates the role of respondents by percentage. The second pie graph shows the specialties of the attending physicians.
Routine use of EVDs was reported by every respondent except 1. The manner in which the remaining 51 respondents’ institutions addressed VRI prevention is shown in Table 1. Antibiotic-impregnated catheter (AIC) and conventional catheter (CC) use were reported with similar frequency (53% vs 47%), but no respondents reported routine use of silver-impregnated catheters (SICs). Although most respondents (69%) reported routine use of antibiotics at the time of EVD placement, administration of PPSA varied, with nearly half of the respondents indicating PPSAs were not used with EVDs at their institution. Nearly all (80%) respondents reported CSF collection only on an as-needed basis. Only 2 respondents reported that routine EVD exchanges were performed at their institution. There was no significant relationship between institution type and catheter selection (P = .59), antibiotic usage (at the time of EVD placement [P = .99] or PPSA [P = .65]), CSF sampling (P = .89), or venue for EVD placement (P = .98). Institution size was also not significantly related to catheter selection (P = .77), antibiotic usage (at the time of EVD placement [P = .76] or PPSA [P = .71]), CSF sampling (P = .14), or venue for EVD placement (P = .32). The presence of a dedicated neurosciences ICU was not significantly related to catheter selection (P = .76), antibiotic usage (at the time of EVD placement [P = .84] or PPSA [P = .87]), CSF sampling (P = .83), or venue for EVD placement (P = .90).
Table 1.
External Ventricular Drain (EVD) Practice Patterns.
| Management Strategy | Number of Respondents, N = 51 (%) |
|---|---|
| Catheter selection | |
| Antibiotic-impregnated catheter | 27 (53%) |
| Conventional catheter | 24 (47%) |
| Silver-impregnated catheter | 0 (0%) |
| Antibiotic usage | |
| At the time of EVD placement | |
| Yes | 35 (69%) |
| No | 16 (31%) |
| Prolonged prophylactic systemic antibiotics | |
| Yes | 28 (55%) |
| No | 23 (45%) |
| Cerebrospinal fluid sampling | |
| Daily | 4 (8%) |
| Routinely (less frequently than daily) | 6 (12%) |
| As needed if there is concern for ventriculitis | 41 (80%) |
| Venue for EVD placement | |
| Operating room only | 14 (27%) |
| Routine placement permitted outside the operating room | 37 (73%) |
| Prophylactic EVD exchange | |
| Yes | 2 (4%) |
| No | 49 (96%) |
The majority of respondents were either neutral or disagreed with the need for PPSA with all catheter types (CC: 75%, AIC: 85%, and SIC: 87%), as shown in Figure 2. The intraclass correlation coefficient was 0.843, indicating that respondents generally felt similarly about the need for PPSA regardless of the catheter type. Although there was no significant relationship between respondent role and belief that PPSA was indicated for any catheter type (P = .16 for CC, P = .06 for AIC, and P = .41 for SIC), the 6 pharmacist respondents universally disagreed or disagreed strongly with the need for PPSA with AIC. There was no significant relationship between age and belief that PPSA was indicated for any catheter type (P = .93 for CC, P = .19 for AIC, and P = .99 for SIC).
Figure 2.
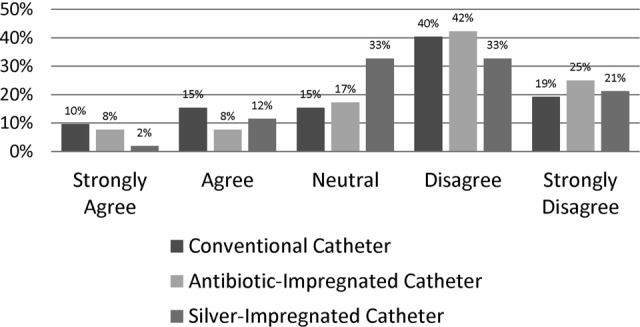
Graph showing respondents’ opinions on the statement: “Prolonged prophylactic systemic antibiotics should be used after external ventricular drain placement.”
Discussion
Catheter Selection
Use of CC and AIC was evenly divided among our respondents. A prospective randomized controlled trial (RCT) at 6 centers showed that bacterial growth from CSF cultures was 7 times less frequent in patients with AIC in comparison to those with standard catheters.8 Wang et al performed a systematic review and meta-analysis on the efficacy of AIC and looked at 4 RCTs and 4 nonrandomized prospective trials and found a significant reduction in CSF infections with the use of AIC.9 Another meta-analysis of 4 randomized and 10 observational studies noted a risk reduction of 62% (P < .00001) in patients with AIC in comparison to CC.25 A review of the literature from 1966 to 2011 revealed 11 in vivo and 4 in vitro studies and concluded that AICs decrease the incidence of CSF pathogen growth but noted that the relationship between the presence of positive bacterial cultures and the development of clinical ventriculitis is unclear.5 A cost analysis of 6 studies in the literature (2 RCTs and 4 prospective cohort trials) demonstrated that in comparison to CC, AIC may be associated with 2.7 fewer deaths, 82 fewer hospital days due to infection, and a savings of US$264 069 per 100 patients.26
Although none of the survey respondents reported the use of SIC, Silver Impregnated Line Versus EVD Randomized Trial, a double-blind prospective RCT showed that in comparison to CC, SICs were associated with a lower risk of infection (P = .04).6 A pooled analysis of 1 RCT and 1 nonrandomized prospective study also showed reduction in rate of infections with SIC, but this result was not significant (P = .18).9
A systematic review and meta-analysis looked at 36 studies (16 796 procedures) and concluded that both AIC and SIC were associated with reduction in CSF infections in comparison to CC. Two studies (an RCT and a prospective cohort study) compared SIC and AIC and found no difference in rate of infection in either study or in the pooled analysis. Non-CC use was associated with increased methicillin-resistant Staphylococcus aureus, nonstaphylococcal, and gram-negative bacterial infections,11 but a separate meta-analysis showed similar rates of resistant infections in patients with CC and AIC. Notably, the use of PPSA was inconsistent among studies reviewed.9
Despite these data, nearly half of our respondents reported routine use of CC. Given the multitude of studies that demonstrated decreased morbidity, mortality, and rate of VRI with AIC,9,25,26 we believe these catheters should be favored over CC. Further research is warranted to determine whether AICs are superior to SIC.
Maintenance Antibiotics
In 1999, a survey of 36 university neurosurgical programs revealed that 72% of institutions queried started PPSA in patients with EVDs.14 Results from single-center studies showed mixed results, though, with some demonstrating a significant decrease in risk of infections with PPSA15,16 and some showing no significant difference in rate of VRI with or without PPSA.12,13,20,27 A systematic review of 3 RCTs and 7 observational studies showed a protective effect of PPSA but noted that there was no sufficient data to compare the benefits of PPSA with those of AIC or to definitively determine whether both therapies should be used in conjunction.7 In a prospective performance analysis, Murphy et al looked at 410 patients with AIC on PPSA and compared them to 135 patients with AIC and no systemic antibiotics, and they found no statistically significant difference in rates of VRI between the 2 groups.3 In a prospective RCT, Wong et al compared the use of PPSA with CC to the use of AIC without PPSA and found no significant difference in rate of CSF infection, ICU length of stay, or functional outcome.10 Discontinuation of PPSA resulted in a significant reduction in rates of nosocomial infections.3,27 Additionally, PPSAs have been associated with the growth of resistant or opportunistic pathogens.12,16,21 Cessation of PPSA use was estimated to lead to US$80 000 of annual savings in direct drug costs at 1 institution12 and US$162 516 of annual savings in both drug costs and costs of treatment for nosocomial infections at another.3
Although the data suggest that discontinuation of PPSA prevents complications and decreases cost of hospitalization without incurring increased risk of infection,3,10,12,13,20,27 55% of the respondents indicated their institutions still use PPSA. Notably, despite the fact that this issue remains controversial, the trend seems to be toward less frequent use of PPSA as compared to the 72% of respondents who reported the use of PPSA in the study of Prabhu et al in 1999.14 Respondents generally felt similarly about the need for PPSA regardless of the catheter type, but we found that pharmacists had the highest predilection for discontinuing PPSA in the setting of AIC. This indicates that pharmacists are up-to-date on this topic and demonstrate their potential to educate other health professionals on relevant data to influence practice.28
Cerebrospinal Fluid Sampling
Frequency of CSF sampling varied among respondents, but 80% indicated it was only performed on an as-needed basis and 20% reported it was performed either daily or routinely. Although Arabi et al demonstrated that the frequency of CSF sampling had no effect on rate of VRI,17 other studies have noted reduction in the rate of VRI with lower sample frequency.18,19 Frequent CSF sampling may increase the likelihood of introduction of bacteria into the sterile system.18 Furthermore, because CSF can be expected to be abnormal in patients with underlying diseases that require ventricular drainage, the value of routine CSF analysis for prediction of VRI is limited. Schade et al found that CSF glucose, protein, and leukocyte count were comparable in patients with infection and those without infection and noted that no CSF parameter had both sensitivity and specificity greater than 60% for the diagnosis of VRI.29
Venue for EVD Placement
Catheter placement outside the operating suite was shown to have a trend toward increased rate of VRI (P = .06) in a single-center prospective study.17 In a prospective multicenter Italian study, Citerio et al found a statistically significant (odds ratio: 4.01, P = .05) difference in the rate of VRI based on venue of EVD placement.30 However, Lozier et al noted in a review that in 5 studies on the relationship between venue of EVD placement and risk of VRI, there was no significant difference in infection rate regardless of whether the drain was placed in the ICU, operating room, or emergency department.21 With a strict infection prevention protocol, Murphy et al reported a very low incidence (0.92%) of VRI, despite the fact that catheters were predominantly placed in the ICU.3
The majority of survey respondents (73%) noted that EVDs were routinely placed outside the operating room at their institutions. This allows for the rapid ability to monitor intracranial pressure and treat hydrocephalus.31 Risk of VRI can be minimized under these circumstances via a multidisciplinary effort to create and adhere to a meticulous standardized protocol for EVD insertion, maintenance, and management.32-35
Prophylactic EVD Exchange
In 1984, Mayhall et al found a significantly higher risk of infection in patients who had EVDs present for greater than 5 days (P = .017) and concluded that if monitoring is required for more than 5 days, the catheter should be removed and reinserted at a different site.22 Holloway et al subsequently conducted a retrospective analysis at Medical College of Virginia and found that prophylactic exchange at 5 days did not reduce the rate of infection in comparison to exchange after 5 days.23 A review of the literature showed that of 17 studies evaluating the effect of duration of catheterization on risk for VRI, 10 reported longer duration was associated with increased risk of infection and 7 reported no association between duration of catheterization and risk of infection. Although the effect of EVD duration on risk of infection varied, the effect of prophylactic catheter exchange on the development of subsequent infections was unknown,21 so Wong et al conducted a prospective RCT involving planned catheter exchange after 5 days. The RCT showed a higher rate of VRI in patients with planned exchanges (7.8%) in comparison to those whose catheters were left in place as long as clinically indicated (3.8%), but this difference was not significant (P = .5).36 In line with these findings, 96% of respondents to our survey noted that EVDs were not routinely exchanged at their institutions.
Other Methods to Prevent VRI
It is important to note that in addition to the aforementioned aspects of care that can impact rates of VRI, variation in other practices including aseptic technique, hair removal, skin preparation, and dressings also affect the frequency of VRI. It is ideal to have a standardized institutional protocol for EVD insertion, maintenance, and management. It is also necessary to ensure that EVDs are monitored to ensure compliance with the protocol and that education about VRI prevention occurs regularly.37
Limitations
There are inherent limitations to the results of practice surveys, and our results represent current practice but not necessarily best practice or even desired practice. We only asked respondents their opinions on PPSA usage, but it is feasible that a respondent disagreed with institutional practice (for example, a respondent who wanted to use AIC but did not have them available at their institution). This desire would not have been captured by our survey questions.
Our cohort includes only a small sample of the membership of the Neurocritical Care Society, but we feel that the variety of responses we received effectively demonstrates that there is a diverse range of VRI prevention strategies. Although the group was very heterogeneous and only 49% of respondents were physicians (and of the physicians, only a few were neurosurgeons), we believe that as members of the Neurocritical Care Society, all respondents were in positions that would allow them to be well versed in their institutional EVD-related policies and practices. The survey was voluntary, so we believe that respondents would only complete it if they were familiar with EVD management at their institutions, but this could introduce a voluntary response bias to our findings. Because every survey question was mandatory and we did not include an “I don’t know” option for any questions, if a respondent did not know the answer to a question, they may have chosen to arbitrarily select a response, resulting in a response bias. This could compromise the quality, credibility, and actuality of our findings. The majority of the respondents worked at academic institutions in the United States, which may limit generalizability.
We did not collect data on respondents’ institutions, so there was no mechanism to control for multiple responses from a single institution, which could introduce sample bias. In addition, we did not ask respondents whether their institutions had local protocols on EVD management and if they were personally involved in directing these protocols.
Conclusion
Practice patterns demonstrate that institutions use varying strategies to prevent VRIs. We found that there was the greatest disparity in catheter selection and use of PPSA. Venue of EVD placement and frequency of CSF collection provoked less varied responses. Routine catheter exchange was nearly universally not performed at respondents’ institutions. Optimizing care for these patients through creation of practice standards is essential to decrease the risk of complications.
Acknowledgments
The authors would like to thank the Neurocritical Care Society, specifically Dr DaiWai Olson, for their assistance in facilitating and distributing this survey.
Appendix A
Infection Prevention in Neurocritical Care
The following is a brief survey about infection prophylaxis practices in neurocritical care patients. All responses are anonymous. The goal of this survey is to identify trends in practice for infection prevention in neurocritical care. The results of the survey will be used to produce a manuscript for presentation/publication. The survey should take less than 10 minutes to complete.
| What country do you practice in? | |
| Which of the following describes you? |
|
| What field did you train in? |
|
| How many years have you been out of training? |
|
| What sex are you? |
|
| How old are you? |
|
| What type of institution do you practice in? |
|
| How many beds are in your institution? |
|
| Does your institution have a dedicated neurointensivecare unit? |
|
| Are external ventricular drains (EVDs) routinely used at your institution? |
|
| Except under emergent circumstances, are EVDs ever placed outside of the operating room at your institution? |
|
| What type of external ventricular drain catheter does your institution use on a routine basis? |
|
| Are intravenous antibiotics routinely given at the time of external ventricular drain placement at your institution? |
|
| Are intravenous antibiotics routinely used as prophylaxis throughout the time that the external ventricular drain is in place? |
|
| How often do you send cerebrospinal fluid samples from external ventricular drains? |
|
| Are external ventricular drain catheters routinely exchanged at your institution for the goal of trying to decrease risk of infection? |
|
| In which of the following scenarios, are prophylactic intravenous MAINTENANCE antibiotics routinely administered after device placement at your drain institution? (Check all that are applicable) |
|
| Do you BELIEVE prophylactic maintenance antibiotics should be used after STANDARD external ventricular drain placement? |
|
| Do you BELIEVE prophylactic maintenance antibiotics should be used after SILVER external ventricular drain placement? |
|
| Do you BELIEVE prophylactic maintenance antibiotics should be used after ANTIBIOTIC IMPREGNATED external ventricular drain placement? |
|
| Do you BELIEVE prophylactic maintenance antibiotics should be used after lumbar drain placement? |
|
| Do you BELIEVE prophylactic maintenance antibiotics should be used after intraparenchymal monitor placement? |
|
| Do you BELIEVE prophylactic maintenance antibiotics |
|
| Do you BELIEVE prophylactic maintenance antibiotics should be used after subgaleal drain placement? |
|
| Do you BELIEVE prophylactic maintenance antibiotics should be used after spinal drain placement in cases WITH instrumentation? |
|
| Do you BELIEVE prophylactic maintenance antibiotics WITHOUT instrumentation? |
|
| Do you BELIEVE prophylactic maintenance antibiotics should be used after nasal packing placement? |
|
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Aaron Lord receives support from the NYU-HHC Clinical and Translational Science Institute via grant UL1 TR000038 from the National Center for Advancing Translational Sciences of the National Institutes of Health.
References
- 1. Srinivasan VM, O’Neill BR, Jho D, Whiting DM, Oh MY. The history of external ventricular drainage. J Neurosurg. 2014;120(1):228–236. [DOI] [PubMed] [Google Scholar]
- 2. Bader MK, Littlejohns L, Palmer S. Ventriculostomy and intracranial pressure monitoring: in search of a 0% infection rate. Heart Lung. 1995;24(2):166–172. [DOI] [PubMed] [Google Scholar]
- 3. Murphy RKJ, Liu B, Srinath A, et al. No additional protection against ventriculitis with prolonged systemic antibiotic prophylaxis for patients treated with antibiotic-coated external ventricular drains. J Neurosurg. 2015;122(5):1120–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Le Roux P, Menon DK, Citerio G, et al. Consensus summary statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Neurocrit Care. 2014;21(suppl 2):S1–S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Babu MA, Patel R, Marsh WR, Wijdicks EFM. Strategies to decrease the risk of ventricular catheter infections: a review of the evidence. Neurocrit Care. 2012;16(1):194–202. [DOI] [PubMed] [Google Scholar]
- 6. Keong NCH, Bulters DO, Richards HK, et al. The SILVER (Silver Impregnated Line Versus EVD Randomized Trial): a double-blind, prospective, randomized, controlled trial of an intervention to reduce the rate of external ventricular drain infection. Neurosurgery. 2012;71(2):394–403. [DOI] [PubMed] [Google Scholar]
- 7. Sonabend AM, Korenfeld Y, Crisman C, et al. Prevention of ventriculostomy-related infections with prophylactic antibiotics and antibiotic-coated external ventricular drains: a systematic review. Neurosurgery. 2011;68(4):996–1005. [DOI] [PubMed] [Google Scholar]
- 8. Zabramski JM, Whiting D, Darouiche RO, et al. Efficacy of antimicrobial-impregnated external ventricular drain catheters: a prospective, randomized, controlled trial. J Neurosurg. 2003;98(4):725–730. [DOI] [PubMed] [Google Scholar]
- 9. Wang X, Dong Y, Qi X, et al. Clinical review: efficacy of antimicrobial-impregnated catheters in external ventricular drainage—a systematic review and meta-analysis. Crit Care. 2013;17(4):234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wong GKC, Ip M, Poon WS, Mak CWK, Ng RYT. Antibiotics-impregnated ventricular catheter versus systemic antibiotics for prevention of nosocomial CSF and non-CSF infections: a prospective randomised clinical trial. J Neurol Neurosurg Psychiatry. 2010;81(10):1064–1067. [DOI] [PubMed] [Google Scholar]
- 11. Konstantelias AA, Vardakas KZ, Polyzos KA, Tansarli GS, Falagas ME. Antimicrobial-impregnated and -coated shunt catheters for prevention of infections in patients with hydrocephalus: a systematic review and meta-analysis. J Neurosurg. 2015;122(5):1096–1112. [DOI] [PubMed] [Google Scholar]
- 12. Alleyne CH, Hassan M, Zabramski JM. The efficacy and cost of prophylactic and periprocedural antibiotics in patients with external ventricular drains. Neurosurgery. 2000;47(5):1124–1127. [DOI] [PubMed] [Google Scholar]
- 13. Blomstedt GC. Results of trimethoprim-sulfamethoxazole prophylaxis in ventriculostomy and shunting procedures. A double-blind randomized trial. J Neurosurg. 1985;62(5):694–697. [DOI] [PubMed] [Google Scholar]
- 14. Prabhu VC, Kaufman HH, Voelker JL, et al. Prophylactic antibiotics with intracranial pressure monitors and external ventricular drains: a review of the evidence. Surg Neurol. 1999;52(3):226–237. [DOI] [PubMed] [Google Scholar]
- 15. Wyler AR, Kelly WA. Use of antibiotics with external ventriculostomies. J Neurosurg. 1972;37(2):185–187. [DOI] [PubMed] [Google Scholar]
- 16. Poon WS, Ng S, Wai S. CSF antibiotic prophylaxis for neurosurgical patients with ventriculostomy: a randomised study. Acta Neurochir Suppl. 1998;71:146–148. [DOI] [PubMed] [Google Scholar]
- 17. Arabi Y, Memish ZA, Balkhy HH, et al. Ventriculostomy-associated infections: incidence and risk factors. Am J Infect Control. 2005;33(3):137–143. [DOI] [PubMed] [Google Scholar]
- 18. Williamson RA, Phillips-Bute BG, McDonagh DL, et al. Predictors of extraventricular drain-associated bacterial ventriculitis. J Crit Care. 2014;29(1):77–82. [DOI] [PubMed] [Google Scholar]
- 19. Williams T, Leslie GD, Dobb GJ, Roberts B, van Heerden PV. Decrease in proven ventriculitis by reducing the frequency of cerebrospinal fluid sampling from extraventricular drains. J Neurosurg. 2011;115(5):1040–1046. [DOI] [PubMed] [Google Scholar]
- 20. Clark CW, Muhlbauer MS, Lowrey R, Hartman M, Ray MW, Watridge CB. Complications of intracranial pressure monitoring in trauma patients. Neurosurgery. 1989;25(1):20–24. [DOI] [PubMed] [Google Scholar]
- 21. Lozier AP, Sciacca RR, Romagnoli MF, Connolly ES. Ventriculostomy-related infections: a critical review of the literature. Neurosurgery. 2002;51(1):170–181. [DOI] [PubMed] [Google Scholar]
- 22. Mayhall CG, Archer NH, Lamb VA, et al. Ventriculostomy-related infections. A prospective epidemiologic study. N Engl J Med. 1984;310(9):553–559. [DOI] [PubMed] [Google Scholar]
- 23. Holloway KL, Barnes T, Choi S, et al. Ventriculostomy infections: the effect of monitoring duration and catheter exchange in 584 patients. J Neurosurg. 1996;85(3):419–424. [DOI] [PubMed] [Google Scholar]
- 24. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cui Z, Wang B, Zhong Z, et al. Impact of antibiotic- and silver-impregnated external ventricular drains on the risk of infections: a systematic review and meta-analysis. Am J Infect Control. 2015;43(7):e23–e32. [DOI] [PubMed] [Google Scholar]
- 26. Edwards NC, Engelhart L, Casamento EMH, McGirt MJ. Cost-consequence analysis of antibiotic-impregnated shunts and external ventricular drains in hydrocephalus. J Neurosurg 2015;122(1):139–147. [DOI] [PubMed] [Google Scholar]
- 27. Dellit TH, Chan JD, Fulton C, et al. Reduction in Clostridium difficile infections among neurosurgical patients associated with discontinuation of antimicrobial prophylaxis for the duration of external ventricular drain placement. Infect Control Hosp Epidemiol. 2014;35(5):589–590. [DOI] [PubMed] [Google Scholar]
- 28. Waterfield J. Is pharmacy a knowledge-based profession? Am J Pharm Educ. 2010;74(3):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schade RP, Schinkel J, Roelandse FWC, et al. Lack of value of routine analysis of cerebrospinal fluid for prediction and diagnosis of external drainage-related bacterial meningitis. J Neurosurg. 2006;104(1):101–108. [DOI] [PubMed] [Google Scholar]
- 30. Citerio G, Signorini L, Bronco A, Vargiolu A, Rota M, Latronico N; Infezioni LIquorali Catetere Correlate Study Investigators. External ventricular and lumbar drain device infections in ICU patients: a prospective multicenter Italian study. Crit Care Med. 2015;43(8):1630–1637. [DOI] [PubMed] [Google Scholar]
- 31. Foreman PM, Hendrix P, Griessenauer CJ, Schmalz PGR, Harrigan MR. External ventricular drain placement in the intensive care unit versus operating room: evaluation of complications and accuracy. Clin Neurol Neurosurg. 2015;128:94–100. [DOI] [PubMed] [Google Scholar]
- 32. Dasic D, Hanna SJ, Bojanic S, Kerr RS. External ventricular drain infection: the effect of a strict protocol on infection rates and a review of the literature. Br J Neurosurg. 2006;20(5):296–300. [DOI] [PubMed] [Google Scholar]
- 33. Flint AC, Rao V, Renda NC, Faigeles BS, Lasman TE, Sheridan W. A simple protocol to prevent external ventricular drain infections. Neurosurgery. 2013;72(6):993–999. [DOI] [PubMed] [Google Scholar]
- 34. Chatzi M, Karvouniaris M, Makris D, et al. Bundle of measures for external cerebral ventricular drainage-associated ventriculitis. Crit Care Med. 2014;42(1):66–73. [DOI] [PubMed] [Google Scholar]
- 35. Leverstein-van Hall M, Hopmans TEM, van der Sprenkel JW, et al. A bundle approach to reduce the incidence of external ventricular and lumbar drain-related infections. J Neurosurg. 2010;112(2):345–353. [DOI] [PubMed] [Google Scholar]
- 36. Wong GK, Poon WS, Wai S, Yu LM, Lyon D, Lam JM. Failure of regular external ventricular drain exchange to reduce cerebrospinal fluid infection: result of a randomised controlled trial. J Neurol Neurosurg Psychiatry. 2002;73(6):759–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hepburn-Smith M, Dynkevich I, Spektor M, Lord A, Czeisler B, Lewis A. Establishment of an external ventricular drain best practice guideline: the quest for a comprehensive, universal standard for external ventricular drain care. J Neurosci Nurs. 2016;48(1):54–65. [DOI] [PubMed] [Google Scholar]