Abstract
Apolipoprotein E ɛ4 allele is a common susceptibility gene for late-onset Alzheimer's disease. Brain vascular and metabolic deficits can occur in cognitively normal apolipoprotein E ɛ4 carriers decades before the onset of Alzheimer's disease. The goal of this study was to determine whether early intervention using rapamycin could restore neurovascular and neurometabolic functions, and thus impede pathological progression of Alzheimer's disease-like symptoms in pre-symptomatic Apolipoprotein E ɛ4 transgenic mice. Using in vivo, multimodal neuroimaging, we found that apolipoprotein E ɛ4 mice treated with rapamycin had restored cerebral blood flow, blood–brain barrier integrity and glucose metabolism, compared to age- and gender-matched wild-type controls. The preserved vasculature and metabolism were associated with amelioration of incipient learning deficits. We also found that rapamycin restored the levels of the proinflammatory cyclophilin A in vasculature, which may contribute to the preservation of cerebrovascular function in the apolipoprotein E ɛ4 transgenics. Our results show that rapamycin improves functional outcomes in this mouse model and may have potential as an effective intervention to block progression of vascular, metabolic and early cognitive deficits in human Apolipoprotein E ɛ4 carriers. As rapamycin is FDA-approved and neuroimaging is readily used in humans, the results of the present study may provide the basis for future Alzheimer's disease intervention studies in human subjects.
Keywords: Brain imaging, rapamycin, APOE4, cerebral blood flow, cerebral glucose metabolism, cognition, blood–brain barrier, inflammation, Alzheimer’s disease
Introduction
The Apolipoprotein E ɛ4 allele (APOE4) is the major genetic risk factor for Alzheimer’s disease (AD).1 Individuals that possess one or two APOE4 alleles have a 4- to 8-fold increased risk of developing AD, with an age of onset of AD occurring 7–15 years earlier when compared to non-carriers.2 Cross-sectional studies in healthy young APOE4 carriers, who have intact memory and are free of amyloid beta (Aβ) or tau pathology, have reported reductions in cerebral metabolic rate of glucose (CMRGlc) in brain areas later vulnerable to AD decades before the possible onset of symptoms.3–7 Longitudinal studies further showed that regional cerebral blood flow (CBF) is reduced in an accelerated manner in cognitively healthy APOE4 carriers.3 Collectively, these findings indicate that brain physiology is altered in APOE4 carriers years before clinical markers such as Aβ, tau pathology and memory deficits appear.
Cerebrovascular impairments were proposed to be an initiating event that leads to neuronal activity alteration, proinflammatory cytokine production, Aβ/tau deposition, and memory loss.8–11 Therefore, preserving cerebrovascular functions early in life in APOE4 carriers may be critical for preserving metabolic and cognitive functions, slowing down AD progression, and even preventing the onset of AD. The therapeutic and preventive potential of preserving cerebrovascular function was highlighted by our recent studies. We showed that rapamycin, a drug that extends lifespan by delaying aging, restored cerebrovascular functions, including CBF and vascular density in mice modeling AD.12,13 We also showed that the vascular restoration was associated with reduced Aβ and improved spatial learning and memory in AD transgenic mice.12 These results suggested that rapamycin could be an effective treatment to restore cerebrovascular function and block or attenuate the progression of established AD-like deficits in mice modeling AD.
In this study, our goal was to determine whether rapamycin administered early in disease progression would restore vascular and metabolic functions in the young mice expressing human APOE4 genes. Particularly, we wanted to identify if we could rescue these functions in AD pre-symptomatic mice. We hypothesize that declines of CBF and CMRGlc, in addition to increased blood–brain barrier (BBB) leakage, will precede cognitive impairments, and rapamycin can rescue the vascular and metabolic deficits in the young APOE4 carriers.
Materials and methods
Animals
One-month-old female wild-type (WT, C57BL/6) and APOE4 transgenic mice were purchased from the Jackson Laboratory (Bar Harbor, Maine, USA). These mice express human APOE4 under the direction of the human glial fibrillary acidic protein (GFAP) promoter and do not express endogenous mouse APOE. We chose females because they have higher incidence for getting AD than males.14 WT mice were fed with control diet (WT-control), whereas APOE4 transgenic mice were fed with either control diet containing only microencapsulating materials or with diet supplemented with microencapsulated rapamycin at 14 mg per kg food, which is roughly equivalent to 2.24 mg/kg/mouse/day based on the assumption that an average mouse weights 30 g and consumes 5 g of food per day. Diet was given for six months. Body weight was measured once a week. Twenty-one mice per group were used in the study. Six mice per group were used for imaging; the other 15 were used for both behavioral and biochemical assays. All animal experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio, and in compliance with the ARRIVE guidelines.15
Study timeline
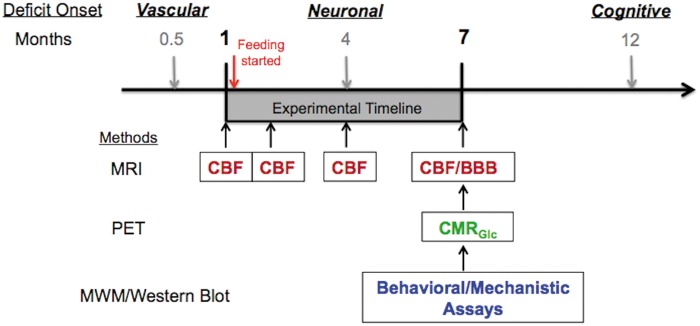
Figure 1 shows the study timeline. We designed the study based on the pathological developments reported in previous studies: vascular defects begin to show at two weeks of age, followed by neuronal dysfunction at four months of age,8,16 and memory decline at 12 months of age.17,18 Rapamycin was given to the APOE4 mice after baseline CBF was measured. Post-treatment CBF was measured longitudinally after 1, 3, and six months of feeding. BBB integrity was evaluated at the final time point (seven months of age). Neuronal function was assessed by measuring CMRGlc at seven months of age. Behavioral tests began at seven months of age and were completed by eight months. After completion of behavioral studies, mice were euthanized and their tissues were dissected for use in biochemical determinations.
Figure 1.
Experimental design and timeline. APOE4 transgenic mice can have vascular defects as early as two weeks (0.5 months of age);8 metabolic/synaptic dysfunctions at four months of age;8 memory decline at 12 months of age.17,18 We obtained female mice at one month of age to test the hypothesis that restoring vascular functions can further impede the decline of metabolic and cognitive functions. After baseline cerebral blood flow (CBF) was measured, rapamycin diet was continuously supplied for six months. CBF was measured longitudinally using MRI after 1, 3, and 6 months of feeding. At the end-point of the study (i.e. mice at 7 months of age), blood–brain barrier (BBB) integrity was evaluated using MRI and cerebral metabolic rate of glucose (CMRGlc) was measured by PET (N = 6). A separate group of mice (N = 15) underwent behavioral assessment with Morris water maze (MWM). After MWM, mice were sacrificed and brain tissues were used for mechanistic pathway analyses using Western blot.
Vascular and metabolic neuroimaging
Mice (N = 6 per group) were anesthetized with 4.0% isoflurane for induction and then maintained in a 1.2% isoflurane and air mixture using a facemask. Heart rate (90–130 bpm), respiration rate, and rectal temperature (37 ± 0.5℃) were continuously monitored. A water bath with circulating water at 45–50℃ was used to maintain the body temperature. Heart rate and blood oxygen saturation level were recorded using a MouseOx system (STARR Life Science, Oakmont, PA, USA) and maintained within normal physiological ranges.
Magnetic resonance imaging (MRI) experiments were performed on a 7T magnet (Bruker Biospec, Billerica, MA, USA). CBF was measured using the continuous arterial spin labeling techniques. Paired images were acquired with field of view (FOV) = 12.8 × 12.8 mm2, matrix = 64 × 64, slice thickness = 1 mm, 12 slices, labeling duration = 2100 ms, repetition time (TR) = 3000 ms per segment, and echo time (TE) = 15 ms.12 We used manganese (Mn)-enhanced MRI to determine BBB integrity as Mn does not penetrate BBB under normal conditions. Manganese (II) chloride tetrahydrate (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in saline and injected intraperitoneally in the volume of 0.25 ml and dosage of 70 mg/kg. The animals were then released back to their cage with free access to water and food. Imaging was acquired after 5–6 h of Mn injection using RARE: FOV = 12.8 × 12.8 mm2, matrix = 128 × 128, slice thickness = 1 mm; 20 slices, TR = 170 ms, and TE = 5 ms. BBB integrity was determined by comparing the impaired and normal area (ratio) of the region of interest.
We used 18FDG positron emission tomography (PET) (Focus 220 MicroPET, Siemens, Nashville, TN, USA) to determine CMRglc. 0.1 mCi of 18FDG dissolved in 1 ml of physiologic saline solution was injected through the tail vein. Forty minutes were allowed for 18FDG uptake before scanning. Emission data were acquired for 20 min in a three-dimensional (3D) list mode with intrinsic resolution of 1.5 mm. The 3D PET data were then rebinned into multiple frames of 1-s duration and reconstructed for each frame using a 2D filtered back projection algorithm. Decay and dead time corrections were applied to the reconstruction process. CMRGlc was determined using the mean standardized uptake value calculation.19
Behavioral testing
Spatial memory was assessed using the Morris water maze paradigm. Mice (N = 15 per group) were pre-screened for neurodevelopmental deficits and were admitted into the study only if they exhibited intact vision, swimming, and climbing abilities and had no other overt sensorimotor deficits as determined by a battery of neurobehavioral tasks performed before testing. Experimenters were blind with respect to genotype and treatment. Briefly, mice were subjected to a series of four trials in which they were released into a light-colored tank filled with opaque water whitened by the addition of non-toxic paint at 24.0 ± 1.0℃. Their task was to locate a 12 × 12 cm submerged platform (1 cm below the water surface) by utilizing visual cues. The water tank was surrounded by opaque dark panels with black-and-white geometric designs, as well as with different geometric designs placed at four locations at the inner edge of the pool 10 cm above the edge of the water to serve as internal cues. Animals were guided to the platform if they failed to locate it within 60 s, and they were required to remain on the platform for 15 to 20 s. Animals were then removed and placed in a dry cage under a warm heating lamp. Approximately 15 to 20 min later, each animal was subjected to a second trial using a different release position, and this was repeated for a total of four trials per day. This procedure was repeated for four days maintaining the position of the platform constant but changing the release points used each day. Twenty-four hours after completing training, a 45-s probe trial was administered in which the platform was removed from the pool. The number of times that each animal crossed the former location of the platform was determined as a measure of retention. During the course of testing, animals were monitored daily and their weights recorded weekly. Performance was recorded by a computer-based video tracking system (Water2020, HVS Image, Buckingham, UK). Data were analyzed offline by using HVS Image and processed with Microsoft Excel.
Brain microvasculature isolation and Western blotting
After behavioral testing, animals were euthanized by isoflurane overdose followed by cervical dislocation. Microvasculature was isolated and prepared as described previously12 with the exception that penicillin was excluded from the MCDB131 media. Brain microvasculature lysates were used for Western blot analyses. Briefly, right hemibrains were removed and flash frozen on dry ice, then homogenized into powder in liquid nitrogen and finally solubilized via sonication in cell lysis buffer (Cell Signaling Technology, Danvers, WA, USA) containing protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN,USA). The homogenates were centrifuged at 12,000 r/min for 15 min. Protein concentrations of lysates were determined using the Bradford method (Bio-Rad Laboratories, Hercules, CA,USA). Lysates were resolved by SDS-PAGE (Invitrogen, Temecula, CA, USA) under reducing conditions and transferred to a nitrocellulose membrane, blocked with 5% bovine serum albumin in Tris-buffered saline containing 0.1% Tween 20 (TBS-T) at room temperature for 1 h, and subsequently incubated overnight at 4℃ in 5% BSA/TBS-T containing relevant primary antibodies as follows: Rabbit anti-NF-κB (Cell Signaling Technology, 8242, 1:1000), rabbit anti-GAPDH (Thermo Scientific, TAB1001, 1:2000), rabbit anti-cyclophilin A (CypA) (Abcam, ab42408, 1:1000). Blots were washed three times for 5 min in TBS-T and then incubated at room temperature with the appropriate horseradish peroxidase-conjugated secondary antibodies. Membranes were then washed four times for 5 min in TBS-T and incubated for 1 min with Amershaw ECL Western Blotting Detection Reagent (GE Healthcare, Waukesha, WI, USA) and exposed to film (Kodak, Rochester, NY, USA). Densitometry was performed using ImageQuant software (GE Healthcare, Waukesha, WI, USA).
Blood glucose measurement
Chronic rapamycin feeding has been reported to cause glucose intolerance in mice.20 To determine whether rapamycin might cause adverse effects on blood glucose, we collected blood samples when the mice were sacrificed. Blood sample was collected in 500 µl lithium heparin 12.5 IU Terumo Capiject Capillary blood collection tubes (Vacutainer K2 EDTA) to avoid blood coagulation. 1–2 µl of blood sample was used to measure blood glucose level using a blood glucose meter and a test strip (Clarity Plus, Boca Raton, FL).
Statistics
Statistical analyses were performed using GraphPad Prism (GraphPad, San Diego, CA, USA). Significance of differences among means was determined using one-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test. Significance of differences between means in pairwise comparisons between experimental groups was done using Student’s t-test. Values of P < 0.05 were considered significant.
Results
Rapamycin restored brain vascular functions of APOE4 mice
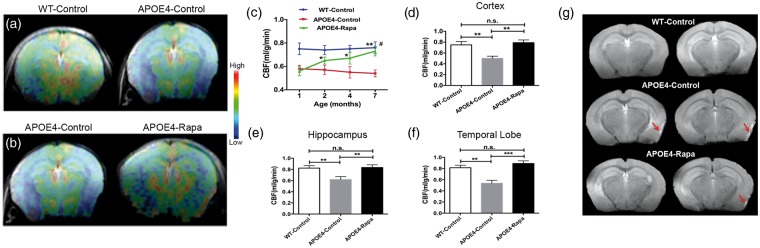
Figure 2(a) shows the CBF maps of the WT-control and pre-treated APOE4 mice at one month of age. Compared to the WT mice, the APOE4 mice had a 20% reduction in global CBF (P = 0.0049). After one month of rapamycin treatment, the APOE4-Rapa group showed significant increases in CBF (Figure 2(b)). The CBF increments were prominent over time – after six months of treatment, the APOE4-Rapa mice showed no significant difference in global CBF compared to the WT-control mice (Figure 2(c)). In contrast, CBF of APOE4-control mice remained low over time. We also did regional CBF analyses on cortex, hippocampus, and temporal lobe. Figure 2 (d) to (f) shows the results at the end-point. The APOE4-control group had dramatically decreased CBF in cortex (−33%, P = 0.0036), hippocampus (−25%, P = 0.0037), and temporal lobe (−38%, P = 0.0024), relative to the WT-control group. However, these regional CBF reductions were not found in the APOE4-Rapa mice.
Figure 2.
Rapamycin restoring brain vascular functions of APOE4 mice. (a) Representative baseline (pre-treated) CBF images of a WT-control and APOE4-control mice. The color code indicates the level of CBF in a linear scale; (b) comparison of CBF between pre- and one-month-post-treatment of a APOE4 mouse; (c) the time course of the global CBF changes among the three groups; (d) cortical CBF (in ml/g/min) of the mice at seven months of age; (e) hippocampal CBF (in ml/g/min) of the mice at seven months of age; (f) temporal lobe CBF (in ml/g/min) of the mice at seven months of age; (g) BBB leakage (indicated by the arrows) of the mice at seven months of age. Data are presented as mean ± standard error of the mean. *P < 0.05; **P < 0.01; ***P < 0.001;# no difference between WT-control and APOE4-Rapa.
n.s.: non-significant; APOE4: apolipoprotein E4.
In addition to CBF deficits, the APOE4-control mice also exhibited loss of BBB integrity at seven months of age, The BBB leakage, shown by manganese contrast, was found in the temporal lobe (Figure 2(g)). The mice showed an impaired/normal area ratio of 0.31 ± 0.04. Rapamycin significantly improved the BBB integrity – APOE4-Rapa mice reduced the ratio to 0.09 ± 0.01 (P = 0.0056).
Rapamycin restored brain metabolic functions of APOE4 mice
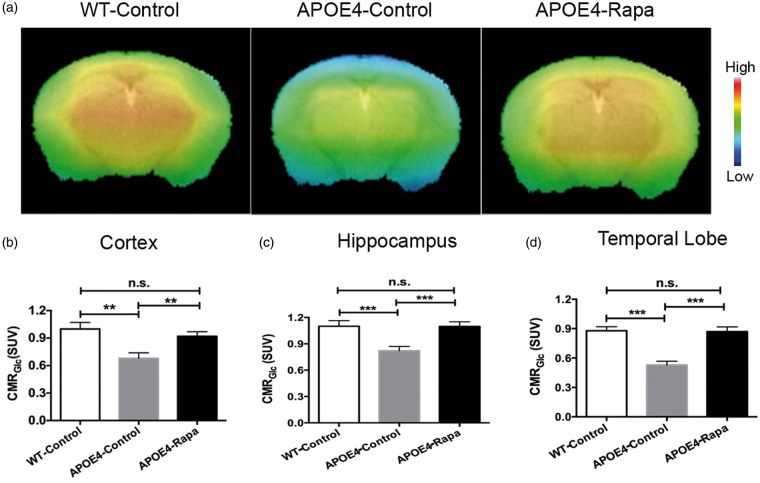
Figure 3(a) shows CMRGlc maps of the three groups obtained at seven months of age. Similar to the patterns found in CBF, APOE4-control mice had dramatic reductions of CMRGlc in the cortex (Figure 3(b)), hippocampus (Figure 3(c)), and temporal lobe (Figure 3(d)). Rapamycin was able to rescue the metabolic deficits. CMRGlc of the APOE4-Rapa mice had no significant differences in the three measured areas when compared to the WT-control mice.
Figure 3.
Rapamycin restoring brain metabolic functions of APOE4 mice. (a) CMRGlc maps of mice at seven months of age; the color code indicates the level of CMRGlc in a linear scale. Quantitative CMRGlc in the (b) cortex; (c) hippocampus; and, (d) temporal lobe of the three groups of mice. Data are presented as mean ± standard error of the mean. **P < 0.01; ***P < 0.001;
n.s.: non-significant; APOE4: apolipoprotein E4.
Rapamycin attenuated incipient spatial learning deficits of APOE4 mice
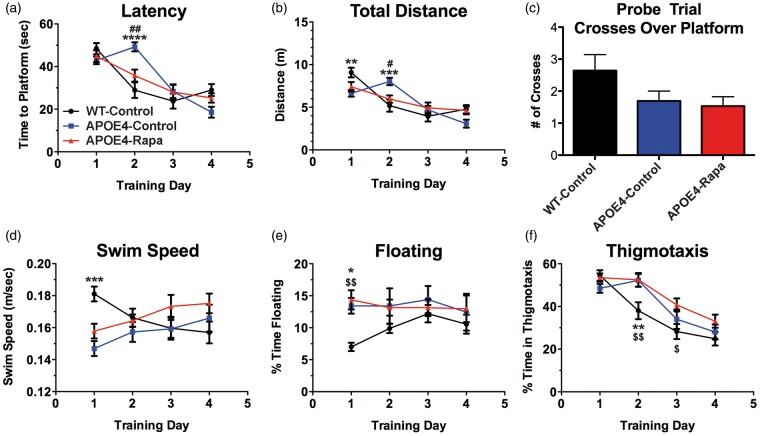
WT-control and APOE4-Rapa groups progressed indistinguishably with regard to a progressive decrease in latency to reach the platform, a measure of spatial learning, during the training phase of the Morris water maze task. APOE4-control animals, however, showed significantly increased latencies on day 2 compared to all other experimental groups (Figure 4(a)), a behavioral pattern common to other mouse models of AD12,13,21 and suggestive of delayed learning. All groups reached criterion (Figure 4 (a) and (b)) at day 3 and did not display significant deficits in their ability to retain the spatial information acquired (Figure 4(c)). Our data suggest that APOE4 transgenic mice started to show incipient learning deficits at seven months of age, and treatment with rapamycin early in the progression of AD-like disease may abrogate subtle signs of impaired spatial learning.
Figure 4.
Rapamycin ameliorates incipient learning phenotypes of APOE4 mice. (a) Time in seconds to reach a hidden platform. Time F (3, 117) = 37.51, P < 0.0001; Treatment F (2, 39) = 0.496, P = 0.613; Interaction F (6, 117) = 5.919, P < 0.0001; (b) total distance swam during trial. Distance F (3, 117) = 30.06, P < 0.0001; treatment F (2, 39) = 0.079, P = 0.024; interaction F (6, 117) = 5.704, P < 0.0001; (c) number of times mice crossed over the platform location in the probe trial. n.s., P = 0.091; (d) average swim speeds during training. Speed F (3, 117) = 0.416, P = 0.742; treatment F (2, 39) = 1.59, P = 0.217; interaction F (6, 117) = 4.986, P < 0.0001; (e) percent of trial spent floating. Time floating F (3, 117) = 0.821, P = 0.485; treatment F (2, 39) = 2.601, P = 0.087; interaction F (6, 117) = 1.19, P = 0.316; (f) percent of trial spent in thigmotaxis. Time in thigmotaxis F (3, 117) = 39.25, P < 0.0001; treatment F (2, 39) = 5.92, P = 0.006; interaction F (6, 117) = 2.212, P = 0.047. Data are presented mean ± standard error of the mean of 4 trials/animal/day. All asterisks (*) indicate a significant difference between WT-control vs. APOE4-control, all pound signs (#) indicate a significant difference between APOE4-control vs. APOE4-Rapa, and all money signs ($) indicate a significant difference between WT-control vs. APOE4-Rapa. Behavioral data were analyzed by two-way ANOVA followed by Tukey’s multiple comparisons test.
APOE4: apolipoprotein E4.
Rapamycin did not change anxiety level of APOE4 mice
Both groups of APOE4 transgenic animals exhibited a slower mean swim speed compared to WT-controls on day 1 (Figure 4(d)). This could be explained by increased floating, a measure of depressive-like behavior, or lack of motivation (Figure 4(e)). We also observed that APOE4 mice, regardless of treatment, spent a higher percentage of trial time engaged in thigmotactic swim on day 2, suggesting a genotype effect resulting in increased anxiety (Figure 4(f)).
Rapamycin did not affect body weight and blood glucose level of APOE4 mice
Table 1 shows the end-point body weight data (mice at 7–8 months of age) and the blood glucose level of the mice. Neither of the measures showed statistical differences among the three groups (P > 0.5).
Table 1.
Body weight and blood glucose level of the mice.
| Mice | Body weight (g) | Blood glucose (mg/dL) |
|---|---|---|
| WT-control | 27.3 ± 0.4 | 122.4 ± 11.3 |
| APOE4-control | 26.9 ± 0.6 | 109.9 ± 14.7 |
| APOE4-Rapa | 27.8 ± 0.2 | 117.7 ± 16.2 |
Note: Data are presented as Mean ± SEM.
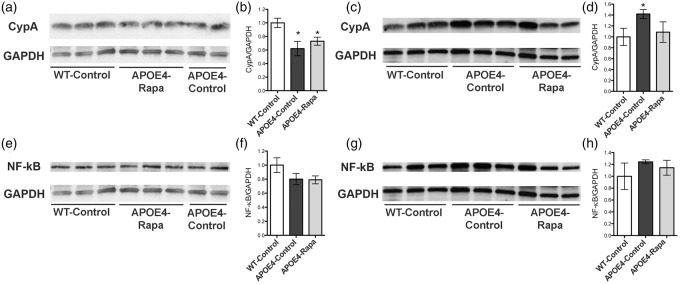
Rapamycin restored CypA levels in cerebrovasculature of APOE4 mice
Previous studies have shown that compared to other allele variants, APOE4 carriers exhibit weaker APOE binding to low-density lipoprotein 1 in brain, resulting in increased levels of CypA and nuclear factor-κB (NF-κB) and subsequent BBB breakdown in APOE4 transgenic mice.8 To determine whether rapamycin may restore BBB integrity by blocking this pathway, we measured CypA and NF-κB levels in cortical and in cerebrovascular compartments of WT as well as control- and rapamycin-treated APOE4 mice. We found that the total CypA levels (in tissue and vasculature) among the three groups were similar. However, the APOE4-control group had significantly lower CypA levels in brain tissue (Figure 5 (a) and (b)), but dramatically higher CypA levels in vasculature compared to the WT-control group (Figure 5(c) and (d)), suggesting a re-distribution of CypA, and an increased inflammation in the vasculature of mice expressing the APOE4 gene. Notably, cerebrovascular CypA levels, however, were restored to WT levels in APOE4-Rapa animals. A similar trend was also observed with NF-κB levels but did not reach statistical significance (Figure 5 (e) to (h)).
Figure 5.
Rapamycin restoring CypA levels in brain vasculature of APOE4 mice. (a,b) Immunoblots of cortical CypA lysates and the corresponding quantitative analyses; (c,d) immunoblots of microvascular CypA lysates and the corresponding quantitative analyses; (e,f) immunoblots of cortical NF-κB lysates and the corresponding quantitative analyses; (g,h) immunoblots of microvascular NF-κB lysates and the corresponding quantitative analyses. Data are presented as mean ± standard error of the mean. *P < 0.05.
CypA: cyclophilin A; NF-κB: nuclear factor-κb.
Discussion
We demonstrated that cerebrovascular deficits occur early in mice with the APOE4 genotype. At one month of age, the APOE4 mice already show significant CBF reductions in the whole brain and in regions involved in higher order of cognitive functions, including hippocampus and temporal lobe. BBB impairment was also found in the temporal lobe. This is consistent with human studies, which suggest that AD pathology evolves early in temporal lobe.22,23 We also found significantly decreased glucose metabolism in the APOE4 mice. This is in good agreement with an ample body of literature demonstrating that APOE4 carriers exhibit mitochondrial dysfunction and an overall reduction of glucose metabolism in the brain.4,5,7 As glucose metabolism is highly associated with neuronal activity,24 our results indicate that the APOE4 mice displayed both neuronal and synaptic dysfunctions, consistent with previous reports.8 Neuronal integrity is critical for cognitive functions, including memory and learning ability.25 In line with this, we observed a transient but significant delay in learning difficulty in the MWM in the APOE4-control mice, though it was overcome with repeated training. Collectively, consistent with human studies, we found that vascular and metabolic defects occur early in life and long before irreversible memory loss in APOE4 mice.3–7
We further demonstrated that rapamycin was sufficient to restore brain vascular functions in young APOE4 mice. The APOE4-Rapa group had restored CBF and BBB integrity after six months of treatment. These vascular restorations were associated with the reduced CypA levels in the vasculature. CypA is a proinflammatory cytokine and that has previously been shown to have deleterious effects on the vascular system in mice lacking murine APOE with aortic aneurysms and atherosclerosis.8 CypA causes BBB breakdown by activating the NF-κB-matrix-metalloproteinase-9 (MMP9) pathway.8,11,26 We also observed a trend toward increased vascular NF-κB in the APOE4-control group, though it did not reach statistical significance when compared to that of the WT-control and APOE4-Rapa groups. This could be because the mice were still young and the pathology was not yet fully developed. Our results suggest that down-regulating CypA activity in cerebrovasculature alone has significant protective effects on vascular integrity and that these changes may be sufficient to improve cognitive outcomes.
Our findings indicate that rapamycin was able to inhibit endothelial CypA and restore its distribution between brain tissue and the vasculature. This is consistent with previous findings that rapamycin binds to and inhibits the rotamase activity of FKBP-12 of cyclophilin.27 Using cyclosporin A, an immunosuppressant similar to rapamycin, Bell et al.8 also observed CypA inhibition and subsequent restoration of cerebrovascular functions in mice with the APOE4 genotype.
In addition to endothelial CypA inhibition, we previously showed that rapamycin restores vascular functions via mammalian target of rapamycin (mTOR) pathway.12 In symptomatic hAPP (J20) mice, a mouse model of AD, mTOR inhibition with rapamycin increased the activity of endothelial nitric oxide synthase (eNOS) and led to the release of NO, a vasodilator. The subsequent increase in CBF observed in the hAPP (J20) mice was dependent on NOS activity. In that study, we also found that restoration of CBF and cerebrovascular density were associated with improved cognitive function and lower brain Aβ levels.12 The findings of the present study are a nice compliment to our previous findings as here we demonstrate that attenuation of mTOR restores vascular function12,24 in APOE4 transgenic mice. That APOE is intricately linked to Aβ clearance only furthers our suggestion that mTOR-mediated regulation of cerebral vasculature may be a crucial target in mitigating AD-like cognitive deficits.
Cerebrovascular integrity is highly coupled with brain glucose metabolism.28–30 In line with this, we observed restored CMRGlc in the APOE4-Rapa mice. Glucose utilization plays a critical role in sustaining neurotransmission31 and memory formation.32 As such, rapamycin-mediated restoration of glucose metabolism may play a role in the restoration of the observed incipient learning and memory deficits in APOE4 mice.
Notably, rapamycin did not reduce anxiety level of APOE4 mice, however. Regardless of treatment, APOE4 mice displayed increased floating in the MWM on day 1 and spent a longer amount of time in thigmotactic swim on day 2. Interestingly, despite increased anxiety compared to the WT-controls, the APOE4-Rapa mice did not express the learning deficits exhibited by the APOE4-control mice. We suggest that these differences are due to the rapamycin-induced vascular and metabolic restorations. Previous studies showed that cognition is highly associated with CBF and CMRGlc levels,33–36 and preserving brain hemodynamics and metabolism are critical for optimizing brain health.37,38 Consistent with the literature, we found that preserved cognition was reflected by the preservation of both CBF and CMRGlc in the APOE4-Rapa mice.
Taken together, our results suggest that rapamycin may delay or even block early events in the progression of physiological and cognitive deficits in APOE4 mice. In the future, it will be important to investigate whether rapamycin could also restore memory in old APOE4 mice after the onset of significant memory loss. One limitation of the study was that the APOE4 transgenic mice do not generate Aβ, a hallmark of AD. Thus, the pathology observed in the mouse model does not fully reflect that of human AD. In the future, transgenic mouse models with APOE4-specific changes in Aβ accumulation would be helpful to further identify rapamycin effects on clearing Aβ in APOE4 carriers.39
This study may have tremendous translational potential since rapamycin has been approved by the FDA and vascular/metabolic neuroimaging is readily used in humans.40–42 Moreover, rapamycin has been applied for various uses in humans since 1999 and as such the toxicity profiles are well characterized.43,44 Similar findings were also recently reported in humans. Mannick et al.45 reported that low doses of rapamycin significantly improved immune functions in the elderly with minimal side effects.45 This information, combined with the fact that rapamycin and rapalogues have been used for cancer treatment,46,47 make rapamycin therapy an attractive candidate for future applications from normal aging to age-related neurodegenerative disorders.48
In summary, our findings indicate that abnormal vascular and metabolic functions precede irreversible cognitive decline in APOE4 carriers. Thus, identifying the timeframe of brain physiology changes and intervening early are critical to impede the development of AD pathology and thus prevent the onset of AD. The studies herein suggest rapamycin may be effective at mitigating the early vascular deficits associated with AD. Using neuroimaging, we were further able to non-invasively detect the vascular and metabolic changes that precede cognitive dysfunction in young APOE4 mice and were also able to restore these physiological and cognitive functions through mTOR inhibition. As rapamycin is FDA-approved and neuroimaging is readily used in humans, this study may have tremendous translational potential regarding future prevention trials.
Acknowledgements
We thank Mrs. Paula Thomason and Rachel Armstrong of the Sanders-Brown Center on Aging of the University of Kentucky for editing the article.
Funding
The work was supported by funding from the National Institute on Aging (NIA) of NIH (K01AG040164 to A-LL), NIH CTSA at the University of Kentucky (UL1TR0000117; pilot grant to A-LL), 5I01BX002211-01A2 Veterans Administration Research and Development Merit Award, the Robert L. Bailey and Daughter Lisa K. Bailey Alzheimer’s Fund, the William & Ella Owens Medical Research Foundation and the JMR Barker Foundation to VG. JBJ was supported by NIA Training Grant 2T32AG021890-11.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
A-LL and AR designed research; A-LL, JBJ, WZ, ND and PR performed research; A-LL, VG, JBJ, WZ, ND and PR analyzed data; A-LL, JBJ, VG and AR wrote the paper.
References
- 1.Liu CC, Kanekiyo T, Xu H, et al. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nature Rev Neurol 2013; 9: 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 1993; 261: 921–923. [DOI] [PubMed] [Google Scholar]
- 3.Thambisetty M, Beason-Held L, An Y, et al. APOE epsilon4 genotype and longitudinal changes in cerebral blood flow in normal aging. Arch Neurol 2010; 67: 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reiman EM, Chen K, Alexander GE, et al. Correlations between apolipoprotein E epsilon4 gene dose and brain-imaging measurements of regional hypometabolism. Proc Natl Acad Sci USA 2005; 102: 8299–8302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reiman EM, Caselli RJ, Chen K, et al. Declining brain activity in cognitively normal apolipoprotein E epsilon 4 heterozygotes: a foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer's disease. Proc Natl Acad Sci USA 2001; 98: 3334–3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleisher AS, Chen K, Liu X, et al. Apolipoprotein E epsilon4 and age effects on florbetapir positron emission tomography in healthy aging and Alzheimer disease. Neurobiol Aging 2013; 34: 1–12. [DOI] [PubMed] [Google Scholar]
- 7.Reiman EM, Chen K, Alexander GE, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proc Natl Acad Sci USA 2004; 101: 284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bell RD, Winkler EA, Singh I, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 2012; 485: 512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Austin BP, Nair VA, Meier TB, et al. Effects of hypoperfusion in Alzheimer's disease. J Alzheimer's Dis 2011; 26(Suppl 3): 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nature Rev Neurosci 2011; 12: 723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alata W, Ye Y, St-Amour I, et al. Human apolipoprotein E varepsilon4 expression impairs cerebral vascularization and blood-brain barrier function in mice. J Cereb Blood Flow Metab 2015; 35: 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin AL, Zheng W, Halloran JJ, et al. Chronic rapamycin restores brain vascular integrity and function through NO synthase activation and improves memory in symptomatic mice modeling Alzheimer's disease. J Cereb Blood Flow Metab 2013; 33: 1412–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spilman P, Podlutskaya N, Hart MJ, et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer's disease. PloS One 2010; 5: e9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer's disease: assessing sex and gender differences. Clin Epidemiol 2014; 6: 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilkenny C, Browne W, Cuthill IC, et al. Animal research: reporting in vivo experiments – the ARRIVE guidelines. J Cereb Blood Flow Metab 2011; 31: 991–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zlokovic BV. Cerebrovascular effects of apolipoprotein E: implications for Alzheimer disease. JAMA Neurol 2013; 70: 440–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salomon-Zimri S, Boehm-Cagan A, Liraz O, et al. Hippocampus-related cognitive impairments in Young apoE4 targeted replacement mice. Neurodegener Dis 2014; 13: 86–92. [DOI] [PubMed] [Google Scholar]
- 18.Yin JX, Turner GH, Lin HJ, et al. Deficits in spatial learning and memory is associated with hippocampal volume loss in aged apolipoprotein E4 mice. J Alzheimer's Dis 2011; 27: 89–98. [DOI] [PubMed] [Google Scholar]
- 19.Pulliam DA, Deepa SS, Liu Y, et al. Complex IV-deficient Surf1(-/-) mice initiate mitochondrial stress responses. Biochem J 2014; 462: 359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Diaz V, Fernandez E, et al. Rapamycin-induced metabolic defects are reversible in both lean and obese mice. Aging 2014; 6: 742–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galvan V, Gorostiza OF, Banwait S, et al. Reversal of Alzheimer's-like pathology and behavior in human APP transgenic mice by mutation of Asp664. Proc Natl Acad Sci USA 2006; 103: 7130–7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brommelhoff JA, Sultzer DL. Brain structure and function related to depression in Alzheimer's disease: contributions from neuroimaging research. J Alzheimer's Dis 2015; 45: 689–703. [DOI] [PubMed] [Google Scholar]
- 23.Kehoe EG, McNulty JP, Mullins PG, et al. Advances in MRI biomarkers for the diagnosis of Alzheimer's disease. Biomarker Med 2014; 8: 1151–1169. [DOI] [PubMed] [Google Scholar]
- 24.Lin AL, Zhang W, Gao X, et al. Caloric restriction increases ketone bodies metabolism and preserves blood flow in aging brain. Neurobiol Aging 2015; 36: 2296–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron 2004; 44: 109–120. [DOI] [PubMed] [Google Scholar]
- 26.Halliday MR, Rege SV, Ma Q, et al. Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer's disease. J Cereb Blood Flow Metab Epub ahead of print 11 March 2015. DOI: 10.1038/jcbfm.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steiner JP, Connolly MA, Valentine HL, et al. Neurotrophic actions of nonimmunosuppressive analogues of immunosuppressive drugs FK506, rapamycin and cyclosporin A. Nat Med 1997; 3: 421–428. [DOI] [PubMed] [Google Scholar]
- 28.Fox PT, Raichle ME, Mintun MA, et al. Nonoxidative glucose consumption during focal physiologic neural activity. Science 1988; 241: 462–464. [DOI] [PubMed] [Google Scholar]
- 29.Lin AL, Gao JH, Duong TQ, et al. Functional neuroimaging: a physiological perspective. Front Neuroenergetics 2010; 2: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin AL, Fox PT, Hardies J, et al. Nonlinear coupling between cerebral blood flow, oxygen consumption, and ATP production in human visual cortex. Proc Natl Acad Sci USA 2010; 107: 8446–8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin AL, Coman D, Jiang L, et al. Caloric restriction impedes age-related decline of mitochondrial function and neuronal activity. J Cereb Blood Flow Metab 2014; 34: 1440–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dash PK, Orsi SA, Moore AN. Spatial memory formation and memory-enhancing effect of glucose involves activation of the tuberous sclerosis complex-Mammalian target of rapamycin pathway. J Neurosci 2006; 26: 8048–8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poels MM, Ikram MA, Vernooij MW, et al. Total cerebral blood flow in relation to cognitive function: the Rotterdam Scan Study. J Cereb Blood Flow Metab 2008; 28: 1652–1655. [DOI] [PubMed] [Google Scholar]
- 34.Cunnane S, Nugent S, Roy M, et al. Brain fuel metabolism, aging, and Alzheimer's disease. Nutrition 2011; 27: 3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagata K, Buchan RJ, Yokoyama E, et al. Misery perfusion with preserved vascular reactivity in Alzheimer's disease. Ann N Y Acad Sci 1997; 826: 272–281. [DOI] [PubMed] [Google Scholar]
- 36.Nagata K, Kondoh Y, Atchison R, et al. Vascular and metabolic reserve in Alzheimer's disease. Neurobiol Aging 2000; 21: 301–307. [DOI] [PubMed] [Google Scholar]
- 37.Stranahan AM, Mattson MP. Metabolic reserve as a determinant of cognitive aging. J Alzheimer's Dis 2012; 30(Suppl 2): S5–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin AL, Pulliam DA, Deepa SS, et al. Decreased in vitro mitochondrial function is associated with enhanced brain metabolism, blood flow, and memory in Surf1-deficient mice. J Cereb Blood Flow Metab 2013; 33: 1605–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Youmans KL, Tai LM, Nwabuisi-Heath E, et al. APOE4-specific changes in Abeta accumulation in a new transgenic mouse model of Alzheimer disease. J Biol Chem 2012; 287: 41774–41786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin AL, Laird AR, Fox PT, et al. Multimodal MRI neuroimaging biomarkers for cognitive normal adults, amnestic mild cognitive impairment, and Alzheimer's disease. Neurol Res Int 2012; 2012: 907409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin AL, Rothman DL. What have novel imaging techniques revealed about metabolism in the aging brain?. Future Neurol 2014; 9: 341–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uh J, Lin AL, Lee K, et al. Validation of VASO cerebral blood volume measurement with positron emission tomography. Magn Reson Med 2011; 65: 744–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soefje SA, Karnad A, Brenner AJ. Common toxicities of mammalian target of rapamycin inhibitors. Target Oncol 2011; 6: 125–129. [DOI] [PubMed] [Google Scholar]
- 44.Hurez V, Dao V, Liu A, et al. Chronic mTOR inhibition in mice with rapamycin alters T, B, myeloid, and innate lymphoid cells and gut flora and prolongs life of immune-deficient mice. Aging Cell Epub ahead of print 28 August 2015. DOI: 10.1111/acel.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mannick JB, Del Giudice G, Lattanzi M, Valiante NM, Praestgaard J, Huang B, et al. mTOR inhibition improves immune function in the elderly. Science translational medicine 2014; 6(268): 268ra179. [DOI] [PubMed] [Google Scholar]
- 46.Alvarado Y, Mita MM, Vemulapalli S, et al. Clinical activity of mammalian target of rapamycin inhibitors in solid tumors. Target Oncol 2011; 6: 69–94. [DOI] [PubMed] [Google Scholar]
- 47.Mita MM, Mita A, Rowinsky EK. The molecular target of rapamycin (mTOR) as a therapeutic target against cancer. Cancer Biol Therapy 2003; 2(4 Suppl 1): S169–S177. [PubMed] [Google Scholar]
- 48.Richardson A, Galvan V, Lin AL, et al. How longevity research can lead to therapies for Alzheimer's disease: the rapamycin story. Exp Gerontol 2014; 68: 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]