Abstract
In the present study, the effect and mechanism of periostin on renal proliferation and extracellular matrix accumulation of lupus mice were investigated. MRL/lpr mice, known as lupus mice, were revealed to show enhanced periostin, proliferating cell nuclear antigen (PCNA), and extracellular matrix accumulation in the kidney accompanied by increased serum platelet-derived growth factor (PDGF). Again, cultured mouse mesangial cells (MMCs) were treated with PDGF, then periostin, and PCNA and secreted fibronectin were detected. The results showed that intracellular periostin and PCNA were respectively enhanced by 2.691 and 2.308 times in PDGF-treated MMC cells at 6 h after stimulation. In addition, secreted fibronectin was increased by 1.442 times. Next, the transfection of periostin shRNA vector in PDGF-stimulated MMC cells effectively suppressed periostin, PCNA and secreted fibronectin by 45.27%, 47.75%, and 39.95%, compared with PDGF-stimulated cells transfected with control vector. Furthermore, it was found that PDGF increased the expression of phospho-Akt (Ser 473) from 30 min to 6 h in MMCs. LY294002 effectively inhibited phospho-Akt (Ser 473) expression caused by PDGF stimulation. Then, periostin, PCNA, and fibronectin were respectively decreased by 69.61%, 46.00%, and 46.20%. In the end, phosphoinositide 3-kinase/protein kinase B/periostin was suggested to mediate PDGF-induced cell proliferation and extracellular matrix production in lupus nephritis.
Keywords: Periostin, platelet-derived growth factor, proliferating cell nuclear antigen, phosphoinositide 3-kinase/protein kinase B, extracellular matrix, lupus nephritis
Introduction
Systemic lupus erythematosus (SLE) is distinguished by the production of autoantibodies which drive immune-complex-related inflammation and consequent tissue damage by binding to autoantigens in various tissues and organs including the skin, joints, kidneys, and nervous system.1 Renal injury affects more than 60% of SLE patients and renal failure contributes mainly to morbidity and mortality. Currently, emerging evidence revealed that lupus nephritis was an important cause of chronic kidney disease (CKD).2 The pathological manifestation of lupus nephritis includes renal mesangial hypercellularity, mesangial matrix content accumulation, endocapillary proliferative lesions, thickening of the glomerular basement membrane, renal vein thrombosis, tubuloreticular inclusion, and renal sclerosis.3 Among these lesions, the most typical features are mesangial cell proliferation and cumulative extracellular matrix, accompanied with inflammatory cell infiltration. Renal mesangial cells are the most active intrinsic cells and can be stimulated by various cytokines, leading to the accumulation of an extracellular matrix, abnormal ingredients, and fibrosis.4
The extracellular matrix is a mesh that consists of glycosaminoglycans and fibrous proteins. In detail, the extracellular matrix components include hyaluronic acid, heparin sulfate, chondroitin sulfate, fibronectin, and collagen.5 At present, more and more studies focus on a kind of extracellular matrix-related molecule, matricellular protein that dynamically expressed non-structural protein and had a regulatory role.6 Matricellular proteins include thrombospondins (TSPs), fibulin, CCN family, and osteopontin.7 Among these, osteoblast-specific factor-2 (periostin), which is involved in tooth and bone remodeling, was named for expressing in the periosteum and periodontal ligament. Periostin was an osteoblast-specific factor involved in osteoblast recruitment and cell adhesion. The structure of periostin composed of an N-terminal emilin (EMI) domain, four tandem repetitive fasciclin-1 domain, and C-terminal heparin-binding site domain.8 Periostin distributes not only inside the cell, but also outside the cell. The different locations serve periostin as a multiple function protein. Periostin usually interacts with extracellular matrix molecules intracellularly,9 and promotes cell motility and migration extracellularly.10 In addition, it was reported that periostin regulates many cell physiological behavior such as cellular signal transduction, proliferation, differentiation,11 epithelial-mesenchymal transition (EMT), and renal interstitial fibrosis.12
Currently, Wantanasiri et al. analyzed periostin staining by an immunohistochemical method in the kidneys of lupus nephritis patients. A positive signal was found in the periglomerulus, the tubules, the sclerosed glomeruli, and interstitial fibrosis. Furthermore, they revealed that periostin was correlated with worsening renal function.13 However, the exact role and mechanism of periostin in lupus nephritis is still not clear. Therefore, lupus nephritis mice (MRL/lpr mice) and mouse mesangial cells (MMCs) were chosen to determine the relationship and mechanism between periostin and renal ECM accumulation, and cell hyperplasia.
Materials and methods
Ethics statement
The Institutional Animal Care and Use Committee at Hebei Medical University approved the research procedures.
Reagents
Rabbit polyclonal anti-periostin antibody was purchased from Abcam Co. (Cambridge, MA). Rabbit polyclonal anti-fibronectin and anti-proliferating cell nuclear antigen (anti-PCNA) antibodies were bought from Protein Tech Group, Inc. (Chicago, IL). Rabbit monoclonal anti-Akt and anti-phospho-Akt (Ser 473) antibodies were bought from CST, Inc. (Boston, MA). Anti-β-actin antibody was obtained from Epitomics Co. (Burlingame, CA). Recombinant platelet-derived growth factor-BB (PDGF-BB) was bought from PeproTech, Inc. (Rocky Hill, NJ). Enzyme-linked immunosorbent assay (ELISA) kits for PDGF and fibronectin were acquired from Blue Gene Biotech Co. (Shanghai, China). Periostin shRNA vector (pYr-1.1-musPostn-sh), control vector (pYr-1.1-NC), periostin expression vector (pYr-ads-4-musPostn), and control vector (pYr-adshuttle-4) were constructed by Changsha Yingrun Biotechnology Co. Ltd (Changsha, Hunan, China). LY294002 was purchased from Sigma Chemical (St. Louis, MO). Lipofectamine 2000 was bought from Invitrogen (Carlsbad, CA). Periodic acid Schiff (PAS) staining kit was purchased from Baso Diagnostics Inc. (Zhuhai, Guangdong, China).
Mice
Female MRL/MpJ-FASlpr/J (MRL/lpr) mice known as lupus mice models were supplied by the Shanghai Experimental Animal Center (SLAC), Chinese Science Academy (Shanghai, China). Similar-aged female MRL/MpJ mice were chosen for control. All mice were housed according to the standard procedures, and were sacrificed at 24 weeks old before blood and urine were obtained for testing of BUN, Cr, PDGF, and urine protein. Then the kidneys were excised for protein extraction and section making.
Cell culture
MMCs stored in our lab were cultured in F12/DMEM medium containing 10% fetal bovine serum, 1% penicillin, and streptomycin. Cells were passaged at 70% confluency using trypsin digestion. For the periostin expression vector experiment, MMC cells were grouped as untransfection, blank vector, and periostin expression vector groups. Cell transfection was carried out using Lipofectamine 2000 (Invitrogen). In detail, 4 µg DNA and 10 µL lipofectamine 2000 were mixed and added into MMCs (90% confluence), before changing medium with complete medium at 6 h after transfection, followed by related analysis after 6 h. For the PDGF treatment (10 ng/mL) experiment, MMCs were grouped as 0 h, 2 h, 4 h, 6 h, and 12 h groups. For the periostin shRNA vector experiment, MMC cells were grouped as follows: blank control group, PDGF-treatment group, PDGF plus shRNA control vector group, and PDGF plus periostin shRNA vector group. Cells of the blank control group were incubated with complete medium without PDGF at 6 h after transfection. On the contrary, cells of the other three groups were incubated with the medium containing 10 ng/mL PDGF. Six hours later, periostin, PCNA, and fibronectin were detected. MMC cells were randomly grouped as follows: blank control group, PDGF treatment (10 ng/mL) group, PDGF (10 ng/mL) plus dimethyl sulfoxide (DMSO) group, and PDGF (10 ng/mL) plus LY294002 (10 µmol/L) treatment group in the LY294002 treatment experiment. At 6 h after treatment, the above tests were carried out.
Immunohistochemistry
Formalin-fixed, paraffin-embedded kidney slides were treated with a pressure cooker for antigen retrieval after they were deparaffinized and rehydrated. Goat serum was used to block non-specific binding sites, and then slides were respectively incubated with specific rabbit anti-PCNA antibody diluted at 1:200 overnight at 4℃, subsequently followed by a 30-min incubation with a biotin-labeled secondary antibody at 37℃. A phosphate-buffered saline (PBS) was applied to wash the slides three times before the incubation with horseradish peroxidase (HRP)-conjugated serum protein (SP) complex at 37℃ for 30 min. After being treated with DAB for 1 min, tissues were counterstained with hematoxylin for 3 min. A PBS was applied to replace the primary antibody as a negative control. Six images of each slide were captured with a microscope and quantitatively analyzed using Image Pro-Plus 5.0 software (Media Cybernetics, Silver Spring, MD), according to the integrated optical density (IOD) of the positive region.
Immunofluorescence
Immunofluorescence was performed for the detection of periostin and PCNA. After the cells were fixed and permeabilized, goat serum was used to block unspecific binding at 37℃. Subsequently, cells were incubated overnight with the first antibody (periostin 1:200, PCNA 1:100) at 4℃, and the first antibody was visualized with a DyLight 549 or 488 labeled secondary antibody (KPL Co., Gaithersburg, MD) at 37℃ for 2 h followed by the staining of cell nuclei with 4',6-diamidino-2-phenylindole (DAPI). Images were captured with a fluorescence optical microscope. Negative control was done with PBS replacing a primary antibody. Image Pro-Plus 5.0 software was used to statistically analyze the IOD of the positive signal.
PAS staining
Slides were deparaffinized, rehydrated, and placed in 0.5% periodic acid for 5 min. Schiff reagent was used to stain slides for 15 min after slides were washed with distilled water. Subsequently, counterstaining was performed in hematoxylin for 1 min, and then slides were dehydrated and mounted.
Enzyme-linked immunosorbent assay
A serum sample was collected from mice and supernatant was collected from in vitro cultured cells. The level of soluble fibronectin and serum PDGF were measured by ELISA kits in accordance with the manufacturer instructions. Absorbance was measured with a spectrophotometer at 450 nm. In order to reduce inter-assay and intra-assay variations, each experiment was repeated three times with each sample in triplicate.
Western blot
Tissue and cell protein were extracted using radioimmunoprecipitation assay (RIPA) buffer according to the standard protocol. Equal protein (30 µg) was loaded and electrophoresed followed by transferring to the polyvinylidene difluoride (PVDF) membrane. Blots were incubated with primary antibody (periostin 1:1000, PCNA 1:1000, phosphor-Akt Ser473 1:1000, Akt 1:1000, fibronectin 1:500, β-actin 1:1000) and then secondary antibody (1:5000 dilution in Tris-buffered saline). Proteins were revealed using an enhanced chemiluminescence detection kit following the manufacturer instructions. The internal control was β-actin to normalize the protein content. Each experiment was repeated three times. Bands were quantitatively evaluated with Gel-pro analyzer (Media Cybernetics, Silver Spring, MD) normalized for β-actin.
Statistical analysis
All values were presented as means ± SD, and differences were compared by t-test or one-way analysis of variance (ANOVA) followed by the Student–Newman–Keuls test. P < 0.05 was considered significant.
Results
Renal periostin, glomerular cell proliferation, and extracellular matrix production were increased in MRL/lpr mice
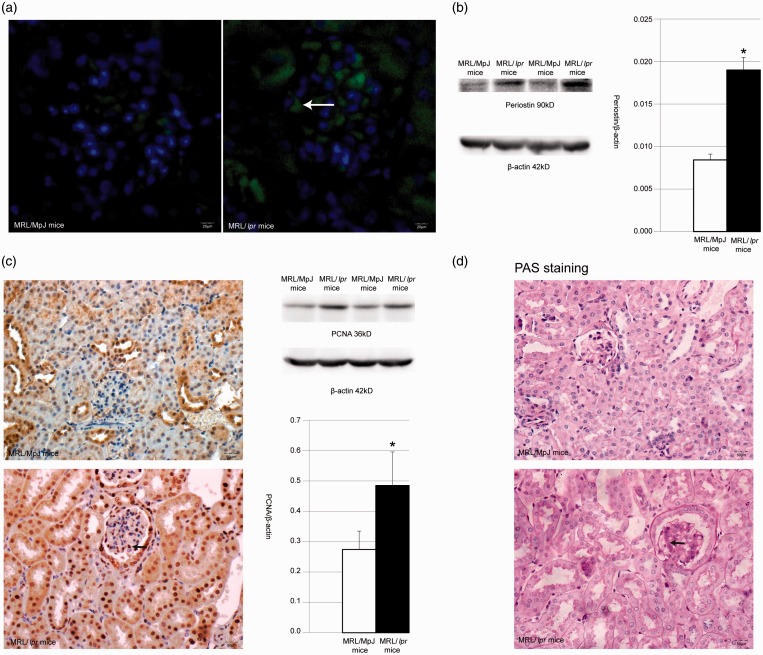
As seen in Table 1, MRL/lpr mice showed higher serum blood urea nitrogen (BUN) and urine protein compared with normal control mice (MRL/MpJ mice). Again, no significant difference in serum BUN between MRL/lpr mice and MRL/MpJ mice was found. Contrarily, the difference in urine protein between MRL/lpr and MRL/MpJ mice was significant (P < 0.05). The PDGF level of the serum was detected with an ELISA kit and the result revealed that PDGF was increased by 1.220 times in MRL/lpr mice versus controls (P < 0.05). Immunofluorescence and Western blot were used to determine the expression of periostin in the kidneys. The results of the immunofluorescence revealed an enhanced expression (indicated in green) of periostin in renal tubular and glomerular cells of MRL/lpr mice (Figure 1(a)). Furthermore, Western blot confirmed that periostin protein was increased by 2.254 times in MRL/lpr mice versus MRL/MpJ mice (Figure 1(b)).
Table 1.
Serum BUN, Cr, PDGF, and urine protein in MRL/MpJ and MRL/lpr mice
| MRL/MpJ mice | MRL/lpr mice | |
|---|---|---|
| Serum BUN (mmol/L) | 11.330 ± 1.804 | 16.517 ± 2.982 |
| Serum Cr (U/mL) | 53.167 ± 5.913 | 56.667 ± 6.377 |
| Serum PDGF (pg/mL) | 1923.727 ± 152.962 | 2347.867 ± 111.649* |
| Urine protein (mg/24 h) | 0.510 ± 0.071 | 3.542 ± 0.542* |
Means P < 0.05 vs. MRL/MpJ mice.
Figure 1.
(a) The expression of periostin in kidney of MRL/MpJ mice and MRL/lpr mice by immunofluorescence. White arrow shows positive signal. (b) Western blot detection and statistical analysis for periostin in kidney of MRL/MpJ mice and MRL/lpr mice. *Means P < 0.05 vs. MRL/MpJ mice. (c) PCNA expression in kidney of MRL/MpJ mice and MRL/lpr mice by immunohistochemistry and Western blot. Black arrow shows positive signal in immunohistochemistry picture. *means P < 0.05 vs. MRL/MpJ mice. (d) PAS staining in kidney of MRL/MpJ mice and MRL/lpr mice. Black arrow shows PAS-positive extracellular matrix accumulation. (A color version of this figure is available in the online journal.)
PCNA was chosen to indicate the proliferation of renal cells using the method of immunohistochemistry. PCNA expression is mainly located in the nucleus of a variety of cells including renal mesangial cells, renal tubular cells, and renal capsule’s epithelial cells. The result of Western blot revealed a 1.769-time increase of PCNA in MRL/lpr mice versus control mice (Figure 1(c)). Again, PAS staining was used to explore the extracellular matrix accumulation, and it can be seen in Figure 1(d) that no obvious extracellular matrix deposit was found in the kidneys of control mice. Contrarily, renal glomeruli showed evident extracellular matrix accumulation in MRL/lpr mice.
Periostin expression vector caused directly enhanced PCNA and secreted fibronectin in MMCs
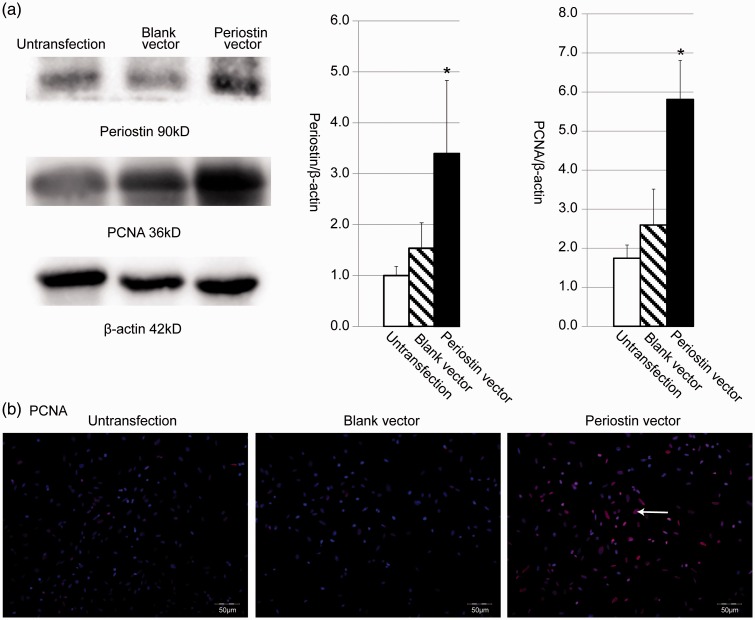
Periostin expression vector was transfected into MMCs and G418 was used to identify the cell clone that stably expressed periostin. Western blot data in Figure 2(a) show that periostin vector-transfected cells presented higher periostin and PCNA expression than untransfected cells and blank vector-transfected cells. In detail, periostin and PCNA proteins were respectively increased by 2.211 times and 2.239 times in periostin vector-transfected cells versus blank vector control cells. We further used an immunofluorescence technique to confirm PCNA expression, and the data are illustrated in Figure 2(b) that PCNA expression was higher in periostin vector-transfected MMC cells (shown in more pink fluorescence) than untransfected MMC cells and blank vector-transfected MMC cells.
Figure 2.
Expression of periostin and PCNA were determined by Western blot and immunofluorescence in MMC cells transfected with periostin expression vector. (a) Western blot analysis quantified by densitometry for periostin and PCNA in untransfected cells, blank vector-transfected cells, and periostin vector-transfected cells. *Means P < 0.05 vs. blank vector-transfected cells. (b) PCNA protein was detected by immunofluorescence with counterstain by DAPI in MMC cells. White arrow shows PCNA positive expression. (A color version of this figure is available in the online journal.)
Moreover, it was proven by ELISA that secreted fibronectin was increased by about 9.493 times in MMC cells that stably expressed periostin protein, compared with cells that were transfected with blank control vector (P < 0.05). Again, there was no significant difference in secreted fibronectin between untransfected MMC cells and blank vector-transfected MMC cells found (Table 2).
Table 2.
The effect of periostin expression vector on secreted fibronectin in mouse mesangial cells by ELISA
| Groups | Secreted fibronectin (ng/mL) |
|---|---|
| Untransfection | 0.591 ± 0.127 |
| Blank vector | 0.524 ± 0.145 |
| Periostin expression vector | 4.971 ± 0.232* |
Means P < 0.05 vs. blank vector group.
In vitro cultured MMCs stimulated by PDGF showed increased periostin, PCNA, and secreted fibronectin
Western blot revealed that PDGF increased periostin expression in a time-dependent manner. At 2 h after the addition of PDGF, periostin began to increase and peaked at 6 h. Then periostin began to decrease from 6 h after stimulation and showed similar expression at 12 h as 0 h (Figure 3(a)). Immunofluorescence also confirmed that PDGF could promote periostin expression (indicated in red) in renal mesangial cells (Figure 3(b)). Subsequently, PCNA expression was detected by Western blot and immunofluorescence. We can see in Figure 3(c) that PCNA (detected in pink) was increased strongly in the nucleus of renal mesangial cells stimulated by PDGF, while control group cells showed a weak expression of PCNA. Western blot revealed that PDGF increased the PCNA protein level by 2.308 times at 6 h after stimulation (P < 0.05) (Figure 3(d)).
Figure 3.
The expression of periostin and PCNA by Western blot and immunofluorescence in MMC cells stimulated by PDGF with different time points. (a) Western blot detection and statistical analysis for periostin, *Means P < 0.05 vs. cells of 0 h group. (b) Immunofluorescence of periostin in cells treated by PDGF (red fluorescence). White arrow shows positive signal. (c) Immunofluorescence of PCNA in PDGF-treated cells of 0 h group and 6 h group. White arrow shows positive signal. (d) PCNA expression by Western blot in MMC cells stimulated by PDGF from 0 to 12 h. *means P < 0.05 vs. cells of 0 h group. (A color version of this figure is available in the online journal.)
Table 3 shows the result of ELISA that PDGF promoted the production of more secreted fibronectin in supernatant of cultured MMC cells. Statistical analysis revealed that compared with normal control cells fibronectin was increased by 1.442 times in PDGF-treated cells at 6 h after treatment.
Table 3.
ELISA of secreted fibronectin in mouse mesangial cells stimulated with PDGF
| Time point | Secreted fibronectin (ng/mL) |
|---|---|
| 0 h | 0.969 ± 0.160 |
| 2 h | 1.297 ± 0.070* |
| 4 h | 1.605 ± 0.240* |
| 6 h | 1.398 ± 0.123* |
| 12 h | 1.257 ± 0.058* |
Means P < 0.05 vs. 0 h group.
Gene silencing aimed at periostin-reversed, PDGF-caused increased PCNA and secreted fibronectin in MMCs
It can be seen in Figure 4(a) that PDGF elevated periostin protein expression in cultured MMC cells compared with the blank control group. Moreover, the transfection of periostin shRNA vector in PDGF-stimulated MMC cells caused about a 60.38% decrease of periostin protein expression compared with PDGF-stimulated untransfected cells (P < 0.05). Again, there was no evident difference in periostin expression between PDGF-stimulated untransfection MMC cells and PDGF-stimulated shRNA control vector MMC cells found. Furthermore, immunofluorescence revealed similar data to Western blot that periostin was mainly located in the MMC cells’ cytoplasm, indicated in red, and was increased by the addition of PDGF, which was reversed by the transfection with periostin shRNA vector (Figure 4(b)). Similarly, the results of Figure 4(c) demonstrate that PDGF-caused increased PCNA in MMC cells compared with non-PDGF-stimulated blank control cells. Then, it was prevented by the transfection of periostin shRNA vector without any change in PDGF-stimulated control vector-transfected MMC cells.
Figure 4.
The expression of periostin and PCNA in PDGF-treated MMC cells transfected with periostin shRNA vector by Western blot and immunofluorescence. (a) Western blot detection and statistical analysis for periostin, *means P < 0.05 vs. cells of blank control group, #means P < 0.05 vs. cells of PDGF plus control vector group. (b) Immunofluorescence of periostin in MMC cells, white arrows show periostin positive expression (red fluorescence). (c) Immunofluorescence of PCNA counterstained by DAPI in MMC cells transfected with periostin shRNA vector. White arrows show positive signal. (A color version of this figure is available in the online journal.)
The data of Table 4 show that compared with blank control MMC cells, secreted fibronectin was enhanced in MMC cells of the PDGF group and the PDGF plus control vector group (P < 0.05). Moreover, secreted fibronectin in the PDGF plus control vector MMC cells was decreased by 39.95% in MMC cells of the PDGF plus periostin shRNA vector transfection group.
Table 4.
The effect of periostin shRNA vector on secreted fibronectin in PDGF-stimulated mouse mesangial cells
| Groups | Secreted fibronectin (ng/mL) |
|---|---|
| Blank control | 0.969 ± 0.199 |
| PDGF | 2.323 ± 0.155* |
| PDGF plus control vector | 2.195 ± 0.625* |
| PDGF plus periostin shRNA vector | 1.318 ± 0.077# |
Means P < 0.05 vs. blank control group.
Means P < 0.05 vs. PDGF plus control vector group.
PDGF led to enhanced periostin, PCNA, and fibronectin in MMCs via the PI3K/Akt pathway
First, we detected the effect of PDGF on the phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) pathway in MMC cells. It can be seen in Figure 5(a) that PDGF increased the expression of phospho-Akt (Ser 473) from 30 min to 6 h. Then phospho-Akt (Ser 473) expression began to decrease from 6 h and reached equal levels at 0 h and at 12 h. However, no difference in Akt was seen among six groups.
Figure 5.
Inhibition of the PI3K/Akt pathway decreased PDGF-induced increased periostin, PCNA and fibronectin in MMC cells. (a) Western blot and statistical analysis for the effect of PDGF on phospho-Akt (Ser 473) and Akt in time-dependent manner. *means P < 0.05 vs. cells of 0 min group. (b) Western blot and statistical analysis for the effect of LY294002 (10 µmol/L) on phospho-Akt (Ser 473), Akt, periostin, PCNA, and fibronectin in MMC cells. *Means P < 0.05 vs. blank control group, #means P < 0.05 vs. PDGF plus DMSO group
Second, we determined the influence of the PI3K/Akt pathway inhibition on PDGF-induced increased phospho-Akt (Ser 473) periostin, PCNA, and fibronectin in MMC cells. The results showed that LY294002 effectively inhibited phospho-Akt (Ser 473) expression caused by PDGF stimulation. Similarly, periostin, PCNA, and fibronectin expression were respectively decreased by 69.61%, 46.00%, and 46.20% in the PDGF plus LY294002 group, compared with the PDGF plus DMSO group (P < 0.05) (Figure 5(b)).
Discussion
As we know, there are two different localizations of periostin: intracellular localization and extracellular localization. Our study revealed that periostin mainly expressed in the cytoplasm of renal mesangial cells and tubular epithelial cells. Similarly, cytoplasmic periostin has been reported in various cells such as M3T3-E1 osteoblast-like cells,14 human corneal fibroblast cell lines,15 human breast cancer cells,16 renal tubular cells of 5/6 nephrectomy, and in unilateral ureteral obstruction.12 Some researchers speculated that the increased cytoplasmic periostin indicated damage of the cell secretory system.12 Jackson-Boeters et al. found that skin pathological remodeling caused the switch of periostin from cells to the ECM.17 Therefore, we speculated that no significant extracellular periostin in this present study might be due to a mild interstitial lesion that was not enough to trigger the switch. However, these hypotheses required confirmation by further experimentation.
In this study, we also revealed that up-regulation of periostin in MMC cells caused cell proliferation and enhanced secreted fibronectin. Similarly, Mael-Ainin et al. reported that mice with wild-type unilateral ureteral obstruction showed a progressive increase in periostin and renal lesions in the obstructed kidney. In contrast, less renal interstitial fibrosis and inflammation were revealed in mice lacking the periostin gene. In vitro, overexpression of collagen I was found in renal epithelial cells treated with periostin.18 Wallace et al.’s study on autosomal-dominant polycystic kidney disease (ADPKD) presented that periostin was increased in cyst-lining epithelial cells and bound to alphaVbeta3 and alphaVbeta5 integrins, leading to integrin-linked kinase activation and cell proliferation compared with normal tubule cells.19 Besides, other researchers also demonstrated the influence of periostin on cell proliferation in neural stem cells following hypoxia–ischemia,20 keloid fibroblast,21 and atrioventricular valve cushions cells.22 The above data suggested that periostin was a multiple functional regulator on extracellular matrix metabolism and cell proliferation.
PDGF was one of growth factors that regulated cell proliferation23 and was proven to be responsible for mesangial proliferation, crescent formation, and progressive glomerulosclerosis.24 In the present study, we also proved that PDGF was an activator to increase periostin expression, cell proliferation, and extracellular matrix metabolism in kidneys with lupus nephritis. Therefore, inhibition of serum PDGF may be an effective method to delay the progress of lupus nephritis. Furthermore, we investigated the mechanism involved in PDGF-induced increased periostin in MMC cells. PI3Ks are a family of enzymes that regulate intracellular signal transduction and are involved in diverse cellular functions by activating protein kinase B including cell proliferation, intracellular trafficking, differentiation, and motility.25 Our research revealed that the blockade of the PI3K/Akt pathway prevented PDGF-induced increased periostin, PCNA, and fibronectin in MMC cells. In the end, our study suggested that periostin, known as regulatory matricellular protein, plays a key role in regulating cell proliferation and extracellular matrix metabolism in lupus nephritis. Again, the PI3K/Akt pathway mediated PDGF-induced periostin expression, cell proliferation, and extracellular matrix production in lupus nephritis.
Acknowledgments
This study was supported by research grants from National Natural Science Foundation of China (No. 81370780).
Author contributions
HD designed the experiments. XZ, FL performed animal experiments. XZ, JH performed cell culture and biological detections. XZ, ZR performed immunohistochemical, immunofluorescence tests, and histopathology preparations. HD, JH wrote the manuscript. The final manuscript was read and approved by authors listed.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Mak A, Kow NY. The pathology of T cells in systemic lupus erythematosus. J Immunol Res 2014; 2014: 419029–419029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng L, Sinniah R, Hsu SI. Pathogenic role of NF-kappaB activation in tubulointerstitial inflammatory lesions in human lupus nephritis. J Histochem Cytochem 2008; 56: 517–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz MM. The pathology of lupus nephritis. Semin Nephrol 2007; 27: 22–34. [DOI] [PubMed] [Google Scholar]
- 4.Seret G, Le Meur Y, Renaudineau Y, Youinou P. Mesangial cell-specific antibodies are central to the pathogenesis of lupus nephritis. Clin Dev Immunol 2012; 2012: 579670–579670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abedin M, King N. Diverse evolutionary paths to cell adhesion. Trends Cell Biol 2010; 20: 734–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sen K, Lindenmeyer MT, Gaspert A, Eichinger F, Neusser MA, Kretzler M, Segerer S, Cohen CD. Periostin is induced in glomerular injury and expressed de novo in interstitial renal fibrosis. Am J Pathol 2011; 179: 1756–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Acharya C, Yik JH, Kishore A, Van Dinh V, Di Cesare PE, Haudenschild DR. Cartilage oligomeric matrix protein and its binding partners in the cartilage extracellular matrix: interaction, regulation and role in chondrogenesis. Matrix Biol 2014; 37: 102–11. [DOI] [PubMed] [Google Scholar]
- 8.Kudo A. Periostin in fibrillogenesis for tissue regeneration: periostin actions inside and outside the cell. Cell Mol Life Sci 2011; 68: 3201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kii I, Nishiyama T, Li M, Matsumoto K, Saito M, Amizuka N, Kudo A. Incorporation of tenascin-C into the extracellular matrix by periostin underlies an extracellular meshwork architecture. J Biol Chem 2010; 285: 2028–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillan L, Matei D, Fishman DA, Gerbin CS, Karlan BY, Chang DD. Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5) integrins and promotes cell motility. Cancer Res 2002; 62: 5358–64. [PubMed] [Google Scholar]
- 11.Liu AY, Zheng H, Ouyang G. Periostin, a multifunctional matricellular protein in inflammatory and tumor microenvironments. Matrix Biol 2014; 37: 150–6. [DOI] [PubMed] [Google Scholar]
- 12.Satirapoj B, Wang Y, Chamberlin MP, Dai T, LaPage J, Phillips L, Nast CC, Adler SG. Periostin: novel tissue and urinary biomarker of progressive renal injury induces a coordinated mesenchymal phenotype in tubular cells. Nephrol Dial Transplant 2012; 27: 2702–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wantanasiri P, Satirapoj B, Charoenpitakchai M, Aramwit P. Periostin: a novel tissue biomarker correlates with chronicity index and renal function in lupus nephritis patients. Lupus 2015; 24: 835–45. [DOI] [PubMed] [Google Scholar]
- 14.Litvin J, Selim AH, Montgomery MO, Lehmann K, Rico MC, Devlin H, Bednarik DP, Safadi FF. Expression and function of periostin-isoforms in bone. J Cell Biochem 2004; 92: 1044–61. [DOI] [PubMed] [Google Scholar]
- 15.Kim BY, Olzmann JA, Choi SI, Ahn SY, Kim TI, Cho HS, Suh H, Kim EK. Corneal dystrophy-associated R124H mutation disrupts TGFBI interaction with periostin and causes mislocalization to the lysosome. J Biol Chem 2009; 284: 19580–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puglisi F, Puppin C, Pegolo E, Andreetta C, Pascoletti G, D'Aurizio F, Pandolfi M, Fasola G, Piga A, Damante G, Di Loreto C. Expression of periostin in human breast cancer. J Clin Pathol 2008; 61: 494–8. [DOI] [PubMed] [Google Scholar]
- 17.Jackson-Boeters L, Wen W, Hamilton DW. Periostin localizes to cells in normal skin, but is associated with the extracellular matrix during wound repair. J Cell Commun Signal 2009; 3: 125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mael-Ainin M, Abed A, Conway SJ, Dussaule JC, Chatziantoniou C. Inhibition of periostin expression protects against the development of renal inflammation and fibrosis. J Am Soc Nephrol 2014; 25: 1724–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallace DP, White C, Savinkova L, Nivens E, Reif GA, Pinto CS, Raman A, Parnell SC, Conway SJ, Fields TA. Periostin promotes renal cyst growth and interstitial fibrosis in polycystic kidney disease. Kidney Int 2014; 85: 845–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma SM, Chen LX, Lin YF, Yan H, Lv JW, Xiong M, Li J, Cheng GQ, Yang Y, Qiu ZL, Zhou WH. Periostin promotes neural stem cell proliferation and differentiation following hypoxic-ischemic injury. PloS One 2015; 10: e0123585–e0123585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Z, Nie F, Kang C, Chen B, Qin Z, Ma J, Ma Y, Zhao X. Increased periostin expression affects the proliferation, collagen synthesis, migration and invasion of keloid fibroblasts under hypoxic conditions. Int J Mol Med 2014; 34: 253–61. [DOI] [PubMed] [Google Scholar]
- 22.Ghatak S, Misra S, Norris RA, Moreno-Rodriguez RA, Hoffman S, Levine RA, Hascall VC, Markwald RR. Periostin induces intracellular cross-talk between kinases and hyaluronan in atrioventricular valvulogenesis. J Biol Chem 2014; 289: 8545–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin F, Zhu J, Tonnesen MG, Taira BR, McClain SA, Singer AJ, Clark RA. Fibronectin peptides that bind PDGF-BB enhance survival of cells and tissue under stress. J Invest Dermatol 2014; 134: 1119–27.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Zubiria Salgado A, Herrera-Diaz C. Lupus nephritis: an overview of recent findings. Autoimmune Dis 2012; 2012: 849684–849684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niswender KD, Gallis B, Blevins JE, Corson MA, Schwartz MW, Baskin DG. Immunocytochemical detection of phosphatidylinositol 3-kinase activation by insulin and leptin. J Histochem Cytochem 2003; 51: 275–83. [DOI] [PubMed] [Google Scholar]