Abstract
Traumatic peripheral nerve damage is a major medical problem without effective treatment options. In repurposing studies on 4‐aminopyridine (4‐AP), a potassium channel blocker that provides symptomatic relief in some chronic neurological afflictions, we discovered this agent offers significant promise as a small molecule regenerative agent for acute traumatic nerve injury. We found, in a mouse model of sciatic crush injury, that sustained early 4‐AP administration increased the speed and extent of behavioral recovery too rapidly to be explained by axonal regeneration. Further studies demonstrated that 4‐AP also enhanced recovery of nerve conduction velocity, promoted remyelination, and increased axonal area post‐injury. We additionally found that 4‐AP treatment enables distinction between incomplete and complete lesions more rapidly than existing approaches, thereby potentially addressing the critical challenge of more effectively distinguishing injured individuals who may require mutually exclusive treatment approaches. Thus, 4‐AP singularly provides both a new potential therapy to promote durable recovery and remyelination in acute peripheral nerve injury and a means of identifying lesions in which this therapy would be most likely to be of value.
Keywords: 4‐aminopyridine, localized delivery, nerve conduction velocity, peripheral nerve injury, remyelination
Subject Categories: Neuroscience, Regenerative Medicine
Introduction
One of the most exciting opportunities for efficient development of new medical interventions is by repurposing existing therapeutic agents for novel applications. A particularly promising approach to such repurposing is to discover situations in which known properties of existing agents can be exploited to provide clinically relevant benefits in settings that are qualitatively different than their established use.
We have applied such a repurposing approach to the medical problem of enhancing functional recovery after traumatic peripheral nerve injuries. Such injuries occur in ~3% of all trauma patients (Taylor et al, 2008; Asplund et al, 2009; Fex Svennigsen & Dahlin, 2013; Sakuma et al, 2015) and can cause severe loss of both motor and sensory functions. A significant subset of injuries [representing, e.g. 45% of peripheral nerve injuries in British soldiers injured in Iraq or Afghanistan between 2005 and 2010 (Birch et al, 2012)] exhibit eventual spontaneous recovery, while others require surgical intervention if recovery is ever going to occur. Those injuries in which recovery can occur spontaneously, categorized as neurapraxic lesions, are thought to represent instances in which at least some axons retain continuity through the lesion site. In contrast, if all axons are transected, then recovery depends upon surgical interventions that require cutting of the nerve to present clean ends for rejoining as quickly as possible after injury (Campbell, 2008; Niver & Ilyas, 2014; Ljungquist et al, 2015). The decision whether to perform a surgical intervention or not poses a challenging situation for any surgeon. If the surgery is performed too early, any potential for spontaneous recovery is lost. On the other hand, categorization of an injury as neurapraxic is equally risky as the validity of such a diagnosis depends on a spontaneous recovery after weeks or months post‐injury (e.g. Shah & Jebson, 2008; Bishop & Ring, 2009; Birch et al, 2012). If no recovery occurs, the opportunity for prompt surgical intervention has been lost. Thus, the approaches of extended patient observation and surgical intervention present options that are effectively mutually exclusive.
There are two possible ways to address the above challenges: One is to enhance the speed of recovery in neurapraxic lesions, thus shortening the time required for spontaneous recovery to be observed, while a second is to provide prospective methods of identifying lesions in which axonal continuity exists. Enhancing the speed and/or extent of recovery in neurapraxic lesions is by itself an important medical goal that would improve the quality of life for injured individuals while decreasing costs of medical care and also potentially decreasing the secondary consequences that occur when nerve function is lost (e.g. lost wages, worker productivity). Moreover, the present inability to enhance recovery in neurapraxic lesions, or at least to identify such lesions more quickly, compromises the possible implementation of therapies to improve axonal regeneration in cut nerves by delaying the utilization of such therapies.
The importance of enhancing functional recovery after peripheral nerve injury has spurred multiple research efforts on this topic (e.g. Wan et al, 2010a,b; Makoukji et al, 2012; Fex Svennigsen & Dahlin, 2013; Stassart et al, 2013; McLean et al, 2014; Nishimoto et al, 2015; Tang et al, 2015) with one of the most extensively studied and promising approaches being transient electrical stimulation of the nerve. Electrical stimulation was first reported to increase the speed of functional recovery after experimental peripheral nerve injury over 30 years ago and can promote both axonal regeneration and repair of damage to myelin (e.g. Nix & Hopf, 1983; Pockett & Gavin, 1985; Al‐Majed et al, 2000; Brushart et al, 2002; Ahlborn et al, 2007; English et al, 2007; Geremia et al, 2007; Vivo et al, 2008; Haastert‐Talini et al, 2011; Huang et al, 2012; Singh et al, 2012). Despite the long‐standing interest in electrical stimulation as a potential therapy for peripheral nerve injury, however, implementing this approach has proven challenging (see e.g. Sakuma et al, 2015) and it remains relatively unused as a therapeutic strategy.
We hypothesized that if the benefits of electrical stimulation are due to promoting nerve conduction, then pharmacological promotion of impulse conduction should also prove beneficial. We investigated this hypothesis by repurposing of 4‐aminopyridine (4‐AP), a potassium channel blocker that has not previously been investigated in the context of acute application for traumatic nervous system injury, despite over three decades of study as a potential means of restoring neurological function in a variety of chronic afflictions (e.g. Lundh et al, 1977, 1979; Jones et al, 1983; Stefoski et al, 1987; Hayes et al, 1994; Polman et al, 1994; Segal & Brunnemann, 1998; Wolfe et al, 2001; DeForge et al, 2004; Grijalva et al, 2010; Claassen et al, 2013; Kremmyda et al, 2013; Jensen et al, 2014; Strupp et al, 2014).
In contrast to all previous published studies on 4‐AP, we now show that 4‐AP is a potent small molecule neuroregenerative agent that enhances both the speed and extent of functional recovery following acute peripheral nerve injury, promotes remyelination, and uniquely among experimental therapies, also enables rapid identification of lesions with axonal continuity. We thus provide a new approach to both treatment and diagnosis of peripheral nerve injuries, based on the discovery of unanticipated benefits of 4‐AP in such a setting. The fact that 4‐AP is already approved for clinical use for other purposes makes this a compelling candidate to consider for potential clinical studies.
Results
4‐AP rapidly enhances functional recovery in acute peripheral nerve injury
As restoration of motor function is the primary goal in treatment of peripheral nerve injury, we first examined the effects of 4‐AP administration on this outcome. We employed a standard compression model of sciatic nerve injury (e.g. Magill et al, 2007; Elfar et al, 2008; Savastano et al, 2014) so as not to prejudice experiments toward an absolute requirement for axonal regeneration. To test the clinical relevance of this approach, we initiated treatment 24 h post‐injury in order to provide a clinically relevant window of therapeutic opportunity. Mice were treated daily (in the afternoon) with a single injection of 10 μg 4‐AP (which approximates a relevant human dose calculated by body weight, but is only ~10% of the mouse body surface area equivalent (Reagan‐Shaw et al, 2008) of the dosage of 20 mg/day used in treating multiple sclerosis (e.g. Dunn & Blight, 2011; Krishnan & Kiernan, 2013). Motor function was assessed with standard sciatic function index (SFI) analysis (Inserra et al, 1998), using measurements of total footprint length, toe spread, and intermediate toe spread. To determine whether improvements were dependent on the presence of drug, all measurements were conducted 20–22 h post‐treatment, when the estimated biological half‐life of 4‐AP in rodents would have caused levels to decrease to < 0.01% of initial dosage (Capacio et al, 1996) and effects requiring the presence of 4‐AP are no longer observable.
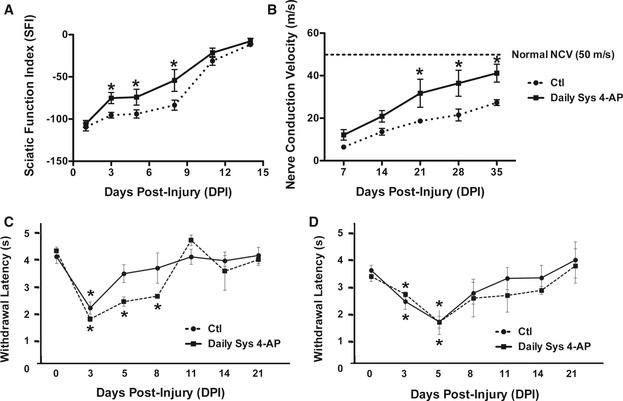
We found that once‐daily administration of 10 μg 4‐AP enhanced the speed of recovery from crush injury (Fig 1A). As early as 3 days post‐injury, mice treated daily (beginning 24 h post‐injury) already showed a significant > 25% improvement in gait function over vehicle‐treated animals. At 5 and 8 days post‐injury, 4‐AP‐treated mice showed statistically significant twofold greater levels of improvement than vehicle‐treated controls.
Figure 1. Daily systemic 4‐AP administration (10 μg/day, i.p.) improves functional recovery of crushed sciatic nerve.
-
ADaily 10 μg 4‐AP enhanced recovery of sciatic nerve motor function as compared with treatment with vehicle at 3 days post‐injury (dpi) through 8 dpi. *: day 3 (D3), P = 0.0131; D5, P = 0.0475; D8, P = 0.0472; ANOVA with post hoc comparisons using two‐tailed unpaired t‐test. n = 6.
-
BDaily 10 μg 4‐AP administration also enhanced recovery of nerve conduction velocity (NCV) as observed beginning at 21 days post‐injury, eventually restoring NCV to near‐normal values while NCV in vehicle‐treated mice remained less than half that of uninjured animals. *: D21, P = 0.0454; D28, P = 0.0487; D35, P = 0.0475; ANOVA with post hoc comparisons using two‐tailed unpaired t‐test. n = 5.
-
C, DAnalysis of withdrawal latency in response to thermal (C) or mechanical (D) stimuli revealed that 4‐AP treatment did not worsen these diagnostics of neuropathic pain syndromes. In the case of response to thermal hyperalgesia, 4‐AP‐treated mice showed a significantly more rapid return to baseline levels. *: (C) D3, D5, D8: P < 0.001 saline versus baseline; D3, P = 0.006 4‐AP versus baseline; (D) D3, P = 0.014; D5, P = 0.008; saline versus baseline; D3, P = 0.006; D5, P = 0.0472; 4‐AP versus baseline; ANOVA with post hoc comparisons using two‐tailed unpaired t‐test. n = 10.
Daily 4‐AP administration enhances recovery of nerve conduction velocity and does not enhance neuropathic pain responses
As recovery of motor function was too rapid to be explained by axonal regeneration (which occurs at a speed of about 1 mm/day), we considered the possibility that recovery was due to restoration of nerve conduction by other means. To examine this possibility, we first investigated the effects of daily 4‐AP administration on restoration of nerve conduction velocity (NCV) after sciatic nerve crush. NCV decreases as a consequence of injury, and restoring the speed of impulse conduction to normal levels is a desired outcome of a regenerative therapy.
Daily 4‐AP treatment increased both the speed of improvement in NCV after sciatic nerve crush and the total amount of recovery seen over 35 days (Fig 1B). Nerve crush caused a > 80% decrease in NCV at 7 days post‐injury, with only partial recovery over 35 days to 55% of pre‐injury NCV. Small, but not yet significant, increases in NCV were seen as early as 7 days after initiation of 4‐AP treatment. By 21 days post‐injury, 4‐AP‐treated mice recovered 64% of the normal velocity of conduction followed by 73 and 82% of normal NCV at 28 and 35 days post‐injury, respectively (all of which improvements were statistically significant as compared with vehicle‐treated mice at all time points).
As peripheral nerve damage often causes neuropathic pain syndromes, and the injury model used in our studies is a standard model for causing such syndromes in the laboratory (e.g. Barriere et al, 2009), we also examined the effects of 4‐AP treatment on pain responses, with a primary concern of determining whether treatment worsened such responses. In these experiments, injured mice treated with 4‐AP were examined for thermal hyperalgesia (Hargreaves assay) or mechanical allodynia (von Frey assay) at 3, 5, 8, 11, 14, and 21 days post‐injury.
4‐AP treatment did not worsen symptoms of neuropathic pain and instead appeared to improve symptoms of thermal hyperalgesia (Fig 1C). Saline‐treated injured mice showed significantly increased thermal sensitivity at days 3, 5, and 8 post‐injury and returned to baseline sensitivity by day 11, while mice treated with 4‐AP only showed increased sensitivity on day 3. Both 4‐AP and saline‐treated mice showed significantly increased sensitivity to mechanical stimuli at days 3 and 5 post‐injury before returning to baseline values, with no differences between the two experimental groups (Fig 1D).
Moreover, there were no changes in response to either type of stimulus in the uninjured limb.
Localized slow‐release administration of 4‐AP promotes enhanced recovery
As one of the common features in studies on electrical stimulation is that each treatment period is limited in length (usually for 30 min, even when administered on multiple days (e.g. Wan et al, 2010a,b; McLean et al, 2014), it is possible that the pulsatile effects of transient stimulation are required to obtain benefit. As the short half‐life of 4‐AP would effectively constitute a pulsatile stimulation, we next utilized localized slow‐release formulations of 4‐AP to provide continuous administration.
To examine effects of sustained administration of 4‐AP, we developed localized slow‐release approaches to 4‐AP delivery based on encapsulation of 4‐AP in poly(lactic‐co‐glycolic acid) (PLGA, 50:50) microparticles and films, which exhibit different loading capacities and release rates. Loading capacities were 0.8–1.0 μg 4‐AP/mg PLGA for particle carriers and 60 μg 4‐AP/mg PLGA for films. The releasing profile of (4‐AP)‐PLGA microparticles in vitro was 25.8 μg/mg/day with continued release over ~30 days (Appendix Fig S1). This dosage, released over the course of 24 h, is < 1% of the already low daily dosages delivered by intraperitoneal injections in our first experiments. (4‐AP)‐PLGA films demonstrated a higher rate of release, with 70% of the 4‐AP (i.e. 210 μg) released over the first few days followed by a release rate of ~143 μg/mg/day. We also confirmed that (4‐AP)‐PLGA carriers labeled with rhodamine and implanted directly onto the crushed nerves (using PEG hydrogel to hold them in place) remained in place for at least 2 weeks as detected by noninvasive imaging (Appendix Fig S2). Recovery surgery at 3 weeks post‐implantation confirmed the continued presence of the PLGA carriers despite the natural motion and inflammatory response to crush injury over the treatment period (Appendix Fig S2). Finally, the bioactivity of 4‐AP released from the PLGA delivery systems was confirmed by in vitro collection of 4‐AP released from PLGA formulations, followed by administration in vivo. The 4‐AP released in vitro after encapsulation appeared to be identical in efficacy to 4‐AP that was freshly made up before administration (Appendix Fig S3). Further details on carrier fabrication, loading and analysis are provided in the Appendix.
Sustained release administration of 4‐AP was also effective in restoring function after crush injury to the sciatic nerve (Figs 2A and EV1A), even when the total amount of 4‐AP delivered was < 0.1% of the total daily dosage applied in our first experiments. In these experiments, either of the two PLGA formulations of 4‐AP or identical PLGA formulations not containing 4‐AP were placed on the lesion site at the time of injury (so as not to subject mice to a second surgery). As for i.p. administration, we examined motor recovery and NCV recovery.
Figure 2. Local administration of 4‐AP in PLGA films enhances functional and electrophysiological recovery after sciatic nerve crush.
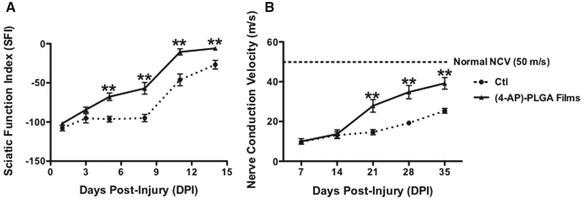
- Local 4‐AP‐treated crushed sciatic nerve (dashed line, vehicle PLGA films; solid line, (4‐AP)‐PLGA films) regained partial walking ability as early as 3 days post‐injury compared to vehicle‐treated group. **: D5, P = 0.0004; D8, P = 0.0012; D11, P = 0.003; D14, P = 0.0089; ANOVA with post hoc comparisons using two‐tailed unpaired t‐test. n = 6.
- Local 4‐AP‐treated crushed sciatic nerve (dashed line: vehicle PLGA films; solid line: (4‐AP)‐PLGA films) showed faster improvement in NCV restoration compared with vehicle‐treated mice, beginning at 21 days post‐injury. **: D21, P = 0.0002; D28, P = 0.00007; D35, P = 0.00019; ANOVA with post hoc comparisons using two‐tailed unpaired t‐test. n = 5. The figure shows representative outcomes from one of three replicated experiments.
Figure EV1. Local administration of 4‐AP in PLGA particles or films enhances functional and electrophysiological recovery after sciatic nerve crush.
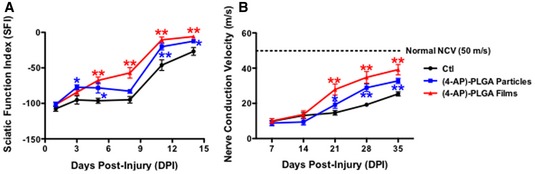
- Local 4‐AP‐treated crushed sciatic nerve (black, vehicle PLGA films; red, (4‐AP)‐PLGA films; blue, (4‐AP)‐PLGA particles) regained partial walking ability as early as 3 days post‐injury compared to vehicle‐treated group (*P < 0.05; **P < 0.01; ANOVA with post hoc comparisons using two‐tailed unpaired t‐test; N = 6 for each group).
- Local 4‐AP‐treated crushed sciatic nerve (black, vehicle PLGA films; red, (4‐AP)‐PLGA films; blue, (4‐AP)‐PLGA particles) showed faster improvement in nerve conduction velocity restoration compared with vehicle‐treated mice, beginning at 21 days post‐injury. In addition, mice treated with the higher dosage 4‐AP‐containing films showed greater improvement than mice treated with the lower dosage of local 4‐AP‐containing particles (*P < 0.05; **P < 0.01; ANOVA with post hoc comparison using two‐tailed unpaired t‐test: n = 6 for each group).
At 5 days post‐injury, mice treated with (4‐AP)‐PLGA carriers demonstrated significantly improved gait function compared to vehicle‐treated controls. Animals implanted with (4‐AP)‐PLGA films (containing 300 μg 4‐AP in 5 mg of PLGA film) had a 40% improvement compared to vehicle‐implanted control mice at 3 days post‐injury (not statistically different at this time point), while mice implanted with 4‐AP‐containing particles showed a statistically significant 45% improvement at this time point. Mice implanted with 4‐AP‐containing films showed a steady improvement in motor function over 14 days, while those implanted with particles showed a brief plateau before an improved recovery. Mice implanted with 4‐AP‐containing films showed a threefold improvement by day 5 and a 3.5‐fold improvement by day 8 post‐injury (which was statistically significant for both time points; Fig 2A). Administration of 4‐AP in PLGA films showed a trend toward being more effective than delivery in PLGA particles (Fig EV1A).
NCV in mice treated with local 4‐AP administration also improved more rapidly than in mice treated with vehicle alone. As with i.p. delivery of 4‐AP, the total amount of improvement in NCV in 4‐AP‐treated mice was greater than that seen in vehicle‐treated mice. Significantly greater improvement in NCV was apparent in mice treated with (4‐AP)‐PLGA particles by the third week post‐treatment, indicating that benefit was provided by local activity of 4‐AP (as the amount of drug released was so low that systemic effects would not have occurred). Larger improvements were seen in mice treated with (4‐AP)‐PLGA films (Figs 2B and EV1B; 14.6 ± 1.3 m/s versus 19.2 ± 2.1 m/s versus 27.9 ± 3.2 m/s, respectively, with differences being statistically significant for both Ctl versus particles and Ctl versus films). By 5 weeks post‐injury, mice treated with 4‐AP‐containing films had a 65% faster NCV than vehicle‐treated mice (39.2 ± 2.9 m/s versus 25.4 ± 3.6 m/s, a significant difference). As for systemic 4‐AP administration, and as expected from patterns of recovery from nerve injury seen clinically, the restoration of near‐normal NCV took longer to achieve than restoration of normal SFI.
Sustained 4‐AP treatment enhances neuronal area and myelination and number of myelinated axons
Changes in NCV require changes in axonal structural properties, and the two axonal properties known to contribute to the speed of impulse conduction are cross‐sectional area and myelination (e.g. Sanders & Whitteridge, 1946; Waxman, 1980; Ikeda & Oka, 2012 and references therein). We therefore next examined the effects of 4‐AP treatment on these parameters.
Injured mice treated with 4‐AP films (the most effective of our treatments) showed small but significant increases in average axonal area in nerves examined 21 days after injury and film application (as detected by ultrastructural analysis; Fig 3). This time point was examined because this was the earliest time point at which we observed significant NCV changes (Fig 2B). We measured axonal area rather than diameter due to the changes in cross‐sectional circularity associated with injury, and we focused attention on myelinated axons because of the role of these fibers in motor function. In uninjured axons, the average area of a myelinated axon (excluding the myelin) was 11.2 ± 1.3 μm2. In injured nerves treated with PLGA film only, the average area of myelinated axons decreased to 5.7 ± 0.2 μm2 (Fig 3B). In contrast, in nerves treated with 4‐AP‐containing films, the average area of myelinated axons was 6.7 ± 0.4 μm2, a significant improvement versus mice treated only with film. In addition, the proportion of axons with areas above the average value for uninjured nerves was 5 ± 3% in injured nerves treated with vehicle alone but showed a statistically significant threefold increase to 15 ± 2% in nerves treated with 4‐AP‐containing films.
Figure 3. Sustained local administration of 4‐AP increased axonal area following sciatic nerve crush.
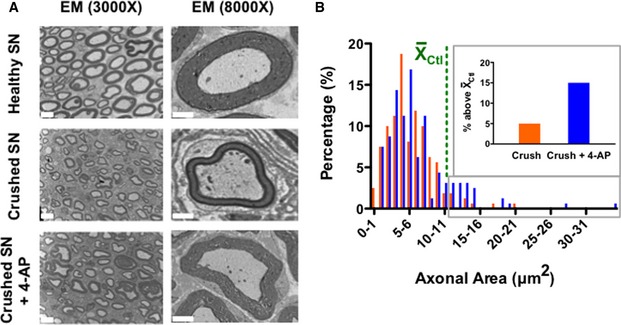
- Electron microscopy images of healthy, crushed, and 4‐AP‐treated crushed sciatic nerve at 21 days post‐injury. Scale bar at 3,000× = 5 μm; scale bar at 8,000× = 2 μm.
- Comparison of the axonal area of randomly chosen individual axons of vehicle‐treated and 4‐AP‐treated mice (n = 4 for each experimental group; 40 axons analyzed per mouse). 4‐AP‐treated sciatic nerve showed statistically greater axonal area compared to the vehicle‐treated group (P < 0.05; ANOVA; restricted‐maximum‐likelihood). Moreover, 4‐AP‐treated mice had a greater proportion of axons with areas greater than the mean value for uninjured mice (shown in the green line), with inset figure displaying all axons with values above this mean.
Ultrastructural analysis also revealed that localized 4‐AP treatment caused significant changes in myelination post‐injury. Mice treated with 4‐AP‐containing films exhibited an increased myelin thickness and area compared with mice treated with PLGA alone. On day 21 after injury, the average thickness of the myelin within the injured area was 0.53 ± 0.03 μm but showed a statistically significant increase to 0.95 ± 0.14 μm with localized 4‐AP administration, as compared with an average myelin thickness in uninjured nerves of 1.26 ± 0.08 μm. In 4‐AP‐treated mice, 21.4 ± 10.8% of axons had a myelin thickness above the average for uninjured nerve, while vehicle‐treated mice had no axons with a myelin thickness above the average. We also measured myelin area (Fig 4A) and found the average cross‐sectional area of myelin per myelinated large axon in uninjured nerve was 14.8 ± 1.7 μm2, was decreased to 4.9 ± 0.3 μm2 in injured nerve, and was significantly increased to 10.1 ± 0.7 μm2 with localized 4‐AP administration. In 4‐AP‐treated mice, 21.2 ± 9.3% of axons had myelin areas that were above the average for uninjured nerve, while this value in vehicle‐treated mice was only 1.9 ± 1.4%.
Figure 4. Long‐term/local 4‐AP treatment promoted remyelination and increased the number of myelinated axons.
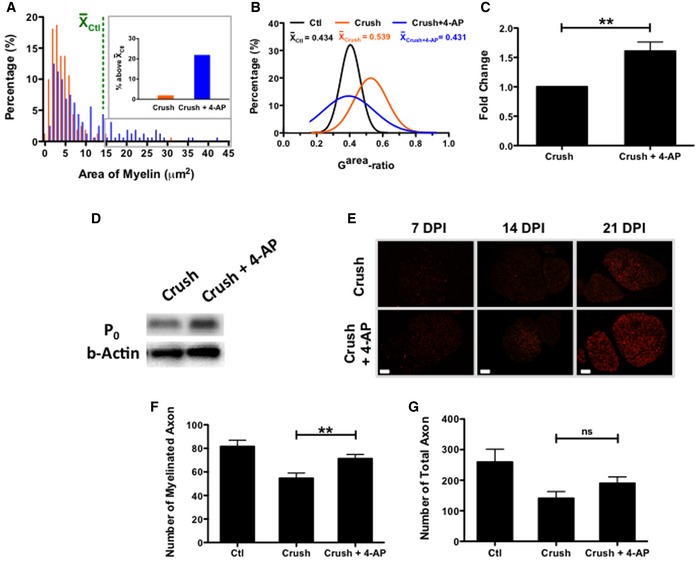
-
A, BSustained local 4‐AP administration was associated with increased myelin area and close‐to‐normal garea‐ratio compared to untreated group, as determined by analysis of 40 randomly chosen myelinated axons from sections of 4 nerves for each group (P < 0.0001 for saline‐treated vs. 4‐AP treated nerves; ANOVA with post hoc comparisons using two‐tailed unpaired t‐test). 4‐AP‐treated mice had a greater proportion of axons for which the associated myelin area was greater than the mean value for uninjured mice (shown in the green line), with inset figure displaying all axons with values above this mean.
-
C, D4‐AP‐treated nerves also showed increases in the levels of P0 protein as detected by Western blot analysis (**P < 0.01; ANOVA with post hoc comparisons using two‐tailed unpaired t‐test).
-
EIncreases in P0 protein over time also were observed by immunofluorescence analysis at different time points, with P0 protein expression increasing to a greater extent in nerves of mice treated with 4‐AP. Scale bars = 200 μm.
-
F, G4‐AP treatment increased the number of myelinated axons (**P = 0.002; ANOVA with post hoc comparisons using two‐tailed unpaired t‐test; n = 4). Even though the 4‐AP‐treated group also exhibited a greater number of total axons, this difference was not statistically significant. All myelinated and total axons were counted in five randomly chosen grids from each of 4 nerves for each group. This experiment represents a single group of mice of one of three replicates on NCV recovery.
Benefits of treatment were also observed by analysis of the ratio of the area of the axon to the area of the axon plus associated myelin. This garea‐ratio, a variant of the usually employed g‐ratio, was calculated due to the decreased circularity in injured nerves. We found that, at 21 days after injury, the average garea‐ratio in crushed nerves increased significantly from 0.43 ± 0.01 (healthy sciatic nerve) to 0.54 ± 0.06 (crushed and vehicle‐treated sciatic nerve). However, with local 4‐AP treatment, the garea‐ratio improved significantly and was in the normal range of 0.43 ± 0.09 (Fig 4B). In addition, in 4‐AP‐treated mice, 54.4 ± 18.5% of myelinated axons showed a garea‐ratio less than the average for uninjured mice, while this value in vehicle‐treated mice was 14.4 ± 1.4%.
Increases in myelin were also observed by immunofluorescence and Western blot analysis for the myelin‐specific P0 protein. Analysis of tissue lysates at 21 days post‐injury/treatment (Fig 4C and D) showed that 4‐AP‐treated nerves contained 61 ± 15% more P0 protein in the lesion area than seen in the nerves in vehicle‐treated animals, a significant increase. Levels of P0 protein increased over time as determined by immunofluorescence analysis in sections of crushed nerves treated with vehicle alone or with 4‐AP (Fig 4E). The number of myelinated axons was also significantly greater in 4‐AP‐treated mice examined at 21 days post‐injury, as determined by ultrastructural analysis (Fig 4F). The number of total myelinated axons per TEM grid examined (2,310 μm2) was 82 ± 5 axons in undamaged nerve, as compared with 55 ± 4 axons in injured vehicle‐treated mice and 71 ± 4 axons in 4‐AP‐treated mice, a significant increase over vehicle‐treated mice) Similar trends were seen for total axons (Fig 4F; 259 ± 42 versus 140 ± 22 versus 190 ± 21, respectively) and for unmyelinated axons (177 ± 44 versus 86 ± 18 versus 118 ± 19, respectively; not shown), but these differences did not reach statistical significance.
4‐AP administration in acute injuries enables rapid identification of lesions with axonal continuity
We also examined the possibility that activities of 4‐AP could be harnessed to provide a novel solution to the problem of prospectively identifying individuals with axonal continuity. Specifically, in traumatic injuries, it would be valuable to discriminate quickly between patients in which some axonal continuity exists and those for whom complete axonal transection has occurred because these two groups represent distinct clinical populations that require mutually exclusive interventions. In the former case, preservation and/or enhancement of function of existing axonal connections (e.g. by promoting remyelination) may be able to bring significant benefits. In contrast, in the latter case, benefit can only be achieved if some form of axonal regeneration occurs. Current electro‐diagnostic approaches to categorizing nerve injury typically distinguish neurapraxia retrospectively. In the best circumstances, diagnosis can be made 1–6 weeks post‐injury, but often requires still longer times (e.g. Robinson, 2000; Lee et al, 2004; Perry, 2005; Campbell, 2008; Shah & Jebson, 2008; Bishop & Ring, 2009; Birch, 2010; Birch et al, 2012; Kimura, 2013; Ljungquist et al, 2015). This delay in diagnosis eliminates opportunities for prompt surgical intervention when such treatment is required.
In comparison with the long periods needed to analyze axonal continuity by electrophysiological approaches, when we examined mice within 1 h of a single injection of 10 μg 4‐AP (i.p. 24 h post‐injury), we observed significantly improved SFI function in mice with crush injuries, but not in mice with transected nerves (Fig 5). All effects were transient (< 4 h), as predicted from known clearance rates for 4‐AP (Uges et al, 1982). To examine whether these short‐term effects might be the result of 4‐AP's ability to enhance synaptic efficacy (e.g. Vizi et al, 1977; Lundh, 1978), we also treated mice with neostigmine, a cholinesterase inhibitor that causes increased concentrations of acetylcholine at the neuromuscular junction (e.g. Treffers et al, 1988). Neostigmine treatment, however, did not cause any changes in SFI (Fig 5C).
Figure 5. Systemic 4‐AP administration transiently enhances sciatic nerve motor function after crush injury.
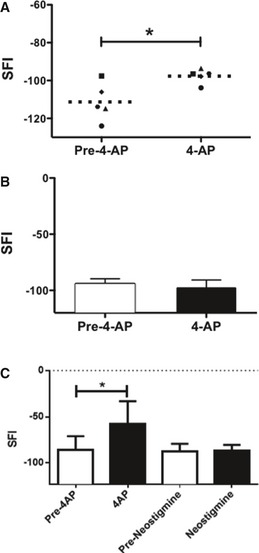
- At 1 day post‐injury, a single dose of 10 μg 4‐AP significantly improved walking function as determined by SFI analysis. Horizontal dashed lines represent the mean of each experiment. Each symbol represents a different mouse (n = 5; *P = 0.001; ANOVA with post hoc comparisons using two‐tailed unpaired t‐test).
- In contrast, even higher doses of 4‐AP administration (50 μg, i.p.) had no effect on SFI in mice with transected nerves (n = 10).
- In contrast to effects of 4‐AP, treatment with neostigmine did not cause improvements in SFI (*P = 0.0024 for 4‐AP versus saline; 1‐way ANOVA with Tukey's multiple comparison test; n = 8).
Thus, acute administration of a single treatment with 4‐AP enables a rapid distinction to be made between injuries in which the damaged nerve contained axons that traversed the lesion site and those in which all axons were transected.
Discussion
Sustained administration of 4‐AP after acute traumatic peripheral nerve injury offers a novel approach to addressing currently unmet medical needs and provides new uses of this therapeutic agent in settings qualitatively different from its established utility. Despite the lack of prior indications for utility of 4‐AP in acute injuries or for enhancing tissue repair, we found that acute initiation of sustained 4‐AP treatment enhanced both the speed and extent of recovery of normal motor behavior after crush injury to the sciatic nerve, as analyzed by SFI. Moreover, sustained 4‐AP treatment enhanced both the speed and extent of restoration of normal NCV and caused regenerative increases in axonal area, myelin thickness, and levels of the myelin‐specific P0 protein. These benefits were not associated with increases in neuropathic pain, as determined by analysis of response to thermal and mechanical stimuli. Indeed, 4‐AP treatment appeared to enhance recovery of a normal response to thermal stimuli. Acute transient administration of 4‐AP additionally provides a new approach to the identification of nerve injuries in which at least some axons still traverse the lesion site, and thus has additional potential utility as a new approach to diagnosis of peripheral nerve injuries in a manner relevant to initiation of appropriate therapeutic approaches.
Despite being studied for over 30 years in settings of chronic neurological illness, there are no prior indications that 4‐AP would provide durable improvements that are essential for regenerative applications (e.g. Lundh et al, 1977, 1979; Jones et al, 1983; Stefoski et al, 1987; Hayes et al, 1994; Polman et al, 1994; Segal & Brunnemann, 1998; Wolfe et al, 2001; DeForge et al, 2004; Grijalva et al, 2010; Claassen et al, 2013; Kremmyda et al, 2013; Jensen et al, 2014; Strupp et al, 2014). Moreover, the only two published examinations of 4‐AP in acute settings provide no evidence for utility in experimental SCI (Haghighi et al, 1995) or in acute vestibular failure (Beck et al, 2014).
We hypothesized that if the widely observed benefits of electrical stimulation in models of peripheral nerve injury are due to simulating nerve conduction, then pharmacologically enabling impulse conduction should also prove beneficial. The only pharmacological agent that is clinically approved for other purposes and that might be useful to achieve this outcome is 4‐AP, which enables impulse conduction in demyelinated axons, theoretically by blocking K+ channels that allow leakage of K+ from these axons and thereby enabling axons to restore the level of depolarization required for propagation of action potentials (e.g. Sherratt et al, 1980; Bostock et al, 1981; Targ & Kocsis, 1985; Blight, 1989; Davis et al, 1995; Hayes, 2004).
Several of our findings suggest that 4‐AP treatment is an attractive candidate for clinical consideration as a treatment for acute peripheral nerve injury. First, durable and significant improvements in motor function occurred very rapidly (within 3 days post‐injury and after just 2 days of treatment), with a speed much greater than could be explained by axonal regeneration. These improvements are unlikely to be due to the presence of residual 4‐AP as a single dose of this agent only caused a transient functional improvement, after which SFI returned to pretreatment values. Improvements in NCV also occurred more quickly and were greater than seen in vehicle‐treated mice. Although improvements in NCV occurred more slowly than behavioral improvements, such an outcome is consistent with clinical observations. Electro‐diagnostic improvement may depend on a greater proportion of neurons acting in a particular way than is required to observe changes in motor function. If 4‐AP improved motor function by enabling activity of a relatively small number of demyelinated neurons, this may not be observable by electrophysiological analysis.
The observations that the time course of recovery was too rapid to be due to axonal regeneration led to the unexpected discovery that sustained 4‐AP treatment promoted remyelination after crush injury. Remyelination was observed by TEM and by analysis of P0 expression, and improvements in NCV were also consistent with remyelination. Improvements in NCV might also be due, at least in part, to increases in axonal area. As axonal area is increased during myelination (Starr et al, 1996), however, it might be that this change in area is primarily due to repair of myelin damage.
Still a further clinically relevant benefit provided by 4‐AP was the ability to cause improved motor function even after a single dose of this agent, delivered 24 h after injury, an observation that could have important consequences for classification and treatment of injuries. The ability of 4‐AP to so rapidly improve motor function requires the presence of axonal continuity through the lesion site and appears more likely to be due to enabling axonal conduction than increasing synaptic efficacy, as treatment with the cholinesterase inhibitor neostigmine had no effect on motor behavior. Use of 4‐AP to identify lesions with such properties offers the possibility of complementing current approaches to lesion diagnosis, which are dependent on retrospective analysis of recovery, with a means of prospectively identifying lesions in which at least some axonal continuity exists. This would have the dual benefits of being able to assign appropriate patients for treatment with remyelination therapies and also could enable more rapid identification of patients for whom recovery is dependent on surgical intervention. Even if a single 4‐AP treatment did not provide these outcomes in clinical settings, shortening the time required to observe recovery would also improve the ability to identify lesions that warrant surgical intervention. Such improvements would be of great importance, as current approaches to diagnosis that rely on a combination of electrodiagnosis and observation of lesions over an extensive period can delay clinical management decisions for as long as 4–6 months, with electrodiagnosis at 7 weeks considered to be an early time point for use of electrodiagnosis in radial nerve injury caused by bone fracture, for example (Shah & Jebson, 2008; Bishop & Ring, 2009). As more rapid repair yields superior outcomes over delayed repair (Campbell, 2008; Niver & Ilyas, 2014; Ljungquist et al, 2015), this also would be a useful application of 4‐AP in acute injury. The use of 4‐AP to identify lesions with axonal continuity more quickly than is currently possible may also offer a valuable complementary diagnostic classification system to the Seddon and Sutherland classifications originally proposed in 1943 and 1978, respectively (e.g. Campbell, 2008).
Thus, 4‐AP administration appears to provide a solution to two of the biggest challenges in treating peripheral nerve injury, by enhancing the speed of recovery in neurapraxic lesions and providing a potential means of even more rapidly identifying lesions in which axonal continuity exists. 4‐AP is unique in providing an ability to achieve both of these goals with a single agent and thus differs from other attempts to promote remyelination (e.g. Makoukji et al, 2012; Stassart et al, 2013). Moreover, even if it is correct that 4‐AP promotes remyelination by pharmacologically causing similar effects as electrical stimulation (Wan et al, 2010a,b; McLean et al, 2014), there are several reasons why 4‐AP may offer a more attractive candidate for clinical studies. First, implantation of sustained release formulations of 4‐AP provides clear benefit, while repeated application of electrical stimulation may cause adverse outcomes (Gigo‐Benato et al, 2010). 4‐AP also is already approved for clinical use, and the studies leading to this approval provide extensive information relevant to potential use of 4‐AP in new settings. 4‐AP also has the advantage of distributing effectively throughout the body and thus could be used to treat injuries in multiple locations with a single intervention, while use of electrical stimulation has to be applied to defined lesion sites. Even when the location of a lesion is established, our discovery that localized slow‐release administration of 4‐AP is also therapeutically effective may provide advantages over electrical stimulation by virtue of being applicable as a one‐time treatment without requiring multiple clinical visits. We do not yet know if 4‐AP can, like electrical stimulation, promote axonal regeneration (a topic of future studies), but even if it does not provide this benefit, its use still would promote remyelination and would enable more rapid identification of lesions in which the surgical interventions required to enable axonal regeneration to occur can be conducted.
Finally, it is important to consider that 4‐AP provides a potent example of the greater importance of the activity of an agent, as contrasted with the exact mechanism of action, in developing novel therapeutic interventions. Despite over 30 years of clinical and research studies, it is still unclear whether the benefits provided to individuals with multiple sclerosis are due to enabling conduction in demyelinated axons, enhancing synaptic efficacy, or both. Indeed, it is not yet clear if the concentrations of 4‐AP achieved in vivo are high enough to bind to the K+ channels thought to be 4‐AP's targets (Dunn & Blight, 2011). Yet, 4‐AP has proven to be a safe and effective therapy for many individuals with multiple sclerosis (e.g. Dunn & Blight, 2011; Blight et al, 2014; Jensen et al, 2014). If its use were held back until such mechanism‐related questions were satisfactorily solved, patients would be denied such benefits. We suggest the same considerations are likely to apply to the potential use of 4‐AP in treating and diagnosing acute traumatic injury to peripheral nerves.
Materials and Methods
Further details are provided in the Appendix.
Reagents and antibodies
4‐Aminopyridine, rhodamine‐B, poly(D,L‐lactide‐co‐glycolide) (50:50, acid terminated, average MW 38,000–54,000) were from Sigma‐Aldrich, anti‐P0 monoclonal antibodies from Aves Labs Inc.; anti‐β‐actin antibodies from Santa Cruz Biotechnology; Hydrogel PEGDM was kindly provided by Danielle S.W. Benoit (University of Rochester).
Mouse model of peripheral nerve injury
All animal experiments described were approved by the University Committee on Animal Resources (IACUC) at the University of Rochester Medical Center. Anesthetized 10‐week‐old female C57BL6 mice had the sciatic nerve bluntly exposed directly posterior to the femur. Mice randomly then underwent wound closure without manipulation of the nerve (sham‐surgery group), or crush injury of 30‐s duration (crush‐injury group), as described in, for example (Magill et al, 2007; Elfar et al, 2008). All experiments were repeated at least three times with a minimum of 5 mice (and generally 8 mice) per experimental group.
For systemic application of 4‐AP, drug or saline was injected (10 μg once per day, i.p.) for the duration of the experiment. For localized application of 4‐AP, 5 mg of (4‐AP)‐PLGA particles (containing ~10 μg 4‐AP) were suspended in 20 μl PEG hydrogel then photopolymerized in a plastic tube mold (0.02 inches in diameter) to form a (4‐AP)‐PLGA particles/PEG hydrogel ribbon. This ribbon was placed at the crushed site of the injured nerve immediately after the surgery. For (4‐AP)‐PLGA films, 5 mg of film (containing 300 μg 4‐AP) was shredded to yield fragments of ~1 mm × 3 mm size, which were placed at the crushed site of the sciatic nerve in 20 μl PEG hydrogel, followed by wound closure procedure. Fabrication and release kinetics of (4‐AP)‐PLGA carriers are discussed in the Appendix.
Sciatic function index (SFI) determined by walking track analysis
Assessment of motor function recovery was performed by calculating the sciatic function index (SFI) (de Medinaceli et al, 1982), as described previously (Elfar et al, 2008). Briefly, individual footprints were obtained by painting each foot prior to mice walking a 50 cm path down a narrow corridor lined with paper. Gait was measured from the metrics of resulting footprints: (i) toe spread (TS) (first through fifth toes), (ii) total print length (PL), and (iii) intermediate toe spread (ITS) (second, third, and fourth toes) of both limbs. If motor dysfunction was so severe as to cause overlap of toe prints, footprints were magnified to enable analysis. All three measurements from three clearly inked randomly chosen footprints per trial were taken from the normal (N) and experimental (E) sides, and the SFI was calculated using the following formula: SFI = −38.3((EPL − NPL)/NPL) + 109.5((ETS − NTS)/NTS) + 13.3((EIT − NIT)/NIT) – 8.8, where E is the injured limb and N is the control limb as in previous studies (Gladman et al, 2012).
Nerve conduction velocity and response to noxious stimuli
Nerve conduction studies were performed by electrical stimulation of a nerve and recording the compound muscle action potential (CMAP) from needle electrodes overlying a muscle supplied by that nerve, as in, for example (Gupta & Steward, 2003; Osuchowski et al, 2009). The EMG method was performed with subdermal stainless steel needle electrode placed into the hindlimbs (6 V, 0.1 ms, 1 Hz, 5–15 mA). The stimulating electrode was placed in resting muscle on gluteal fold to obtain the first CMAP. Then, the stimulating electrode was moved to popliteal fossa with a 10 mm fixed distance from gluteal fold to get the second record of CMAP. Nerve conduction velocity (NCV) was determined from the latencies of the potentials and the distance between two stimulating positions (10 mm). Mechanical allodynia and thermal hyperalgesia were determined in injured and uninjured limbs by Von Frey and Hargreaves analyses, as described in the Appendix. Uninjured limbs showed no effect of 4‐AP treatment.
Transmission electron microscopy
Sciatic nerves were immersion fixed and processed by standard procedures (see Appendix). Grids were examined using a Hitachi 7650 TEM and photographed using an attached Gatan Erlangshen 11 megapixel digital camera system.
Immunofluorescence analyses
Experimental and contralateral (uninjured) sciatic nerves from each test group were harvested at specific time points during healing and recovery and fixed in 4% paraformaldehyde (3 h) and embedded in paraffin. Slides were pretreated with 0.01 M citrate buffer (pH 6.0) for antigen retrieval. Nonspecific blocking was performed with 1:20 diluted serum for 30 min. Sequentially sectioned slides were incubated with primary antibody overnight, followed by incubation with a fluorochrome‐labeled secondary antibody for 1 h.
Immunoblotting analyses
The crushed site of the sciatic nerve at 21 days post‐injury was collected and lysed in cell extraction buffer (Invitrogen). Samples were resolved on SDS–PAGE gels and transferred to PVDF membranes (PerkinElmer Life Science, Wellesley, MA, USA). After blocking in 5% bovine serum albumin in PBS containing 0.1% Tween‐20, membranes were incubated with a primary antibody, followed by incubation with HRP‐conjugated secondary antibody (Santa Cruz Biotechnology).
Image analysis
Images of cross‐sectioned sciatic nerve taken by TEM were processed by ImageJ (US National Institutes of Health, Bethesda, Maryland, USA) to determine myelin area, myelin thickness, and garea‐ratio on myelinated axonal fibers. Axonal area, axonal circularity, and the number of myelinated and total axons were also counted. For analyzing myelin thickness, 15 randomly chosen myelinated axons were analyzed for each mouse, and 6 thicknesses of myelin sheath (at equal degrees of separation around a central point) were measured on each axon. For determining the axonal area, myelin area and garea‐ratio: 40 randomly chosen axons were analyzed in each mouse. For analyzing number of myelinated and unmyelinated axons, all axons were counted in each TEM image. Immunofluorescence images of P0 expression in cross‐sectioned sciatic nerve were analyzed by ImageJ to determine the average fluorescence intensity of axons‐associated P0 labeling.
Author contributions
K‐CT, HL, AC, and LS conducted experiments; K‐CT, AC, and LS conducted primary data analysis; K‐CT, MN, and JE conducted further data analysis; and K‐CT, MZ, MN, and JE wrote the paper.
Conflict of interest
The authors declare that they have no conflict of interest.
The paper explained.
Problem
Traumatic peripheral nerve damage is a major medical problem without effective treatment options and in which diagnostic approaches have been static for decades. Even in lesions with the potential for spontaneous recovery, functional restoration occurs slowly, thus impacting quality of life for these individuals and increasing the time to identification of individuals in whom prompt surgical intervention is needed if recovery will ever occur.
Results
Results provided in this manuscript indicate that 4‐aminopyridine (4‐AP), a potassium channel blocker long studied in the context of chronic neurological afflictions, offers significant promise as a small molecule regenerative agent following acute traumatic nerve injury. In crush injuries of the mouse sciatic nerve, 4‐AP treatment accelerated behavioral and electrophysiological recovery and enhanced remyelination post‐injury. In addition, 4‐AP treatment enabled distinction between incomplete and complete lesions more rapidly than existing approaches, thus offering the possibility of more effectively distinguishing between injuries that may require distinct therapeutic approaches.
Impact
The ability of 4‐AP to promote durable recovery and remyelination following acute traumatic nerve injury offers a potentially valuable new use of this agent as a small molecule regenerative agent able to enhance endogenous repair. As constant daily 4‐AP administration is already approved to improve chronic walking disability in multiple sclerosis, transient use for regenerative purposes offers a compelling opportunity for future clinical studies. The additional ability of 4‐AP to enable rapid distinction between incomplete and complete nerve injuries means this one drug can potentially be used to identify lesions in which short‐term treatment with 4‐AP to promote durable recovery would be most likely to be beneficial and also to more rapidly identify individuals for whom timely surgical intervention is required to enhance the likelihood of recovery.
Supporting information
Appendix
Expanded View Figures PDF
Review Process File
Acknowledgements
The authors thank Margot Mayer‐Proschel, Chris Proschel and Hartmut Land for comments, and Karen Bentley for her assistance in the Transmission Electron Microscopy Core. The research was supported, in part, by a grant from the National Institutes of Health to JE (NIH K08 AR060164), by the Friends of Nancy Lieberman Fund (MN) and by the New York State Department of Health Spinal Cord Injury Research Program (C030178).
EMBO Mol Med (2016) 8: 1409–1420
Contributor Information
Mark Noble, Email: mark_noble@urmc.rochester.edu.
John Elfar, Email: openelfar@gmail.com.
References
- Ahlborn P, Schachner M, Irintchev A (2007) One hour electrical stimulation accelerates functional recovery after femoral nerve repair. Exp Neurol 208: 137–144 [DOI] [PubMed] [Google Scholar]
- Al‐Majed AA, Neumann CM, Brushart TM, Gordon T (2000) Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci 20: 2602–2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asplund M, Nilsson M, Jacobsson A, von Holst H (2009) Incidence of traumatic peripheral nerve injuries and amputations in Sweden between 1998 and 2006. Neuroepidemiology 32: 217–228 [DOI] [PubMed] [Google Scholar]
- Barriere H, Bagdany M, Bossard F, Okiyoneda T, Wojewodka G, Gruenert D, Radzioch D, Lukacs GL (2009) Revisiting the role of cystic fibrosis transmembrane conductance regulator and counterion permeability in the pH regulation of endocytic organelles. Mol Biol Cell 20: 3125–3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck R, Gunther L, Xiong G, Potschka H, Boning G, Bartenstein P, Brandt T, Jahn K, Dieterich M, Strupp M et al (2014) The mixed blessing of treating symptoms in acute vestibular failure–evidence from a 4‐aminopyridine experiment. Exp Neurol 261: 638–645 [DOI] [PubMed] [Google Scholar]
- Birch R (2010) Surgical disorders of the peripheral nerve, 2nd edn London, UK: Springer; [Google Scholar]
- Birch R, Misra P, Stewart MP, Eardley WG, Ramasamy A, Brown K, Shenoy R, Anand P, Clasper J, Dunn R et al (2012) Nerve injuries sustained during warfare: part I‐Epidemiology. J Bone Joint Surg 94: 523–528 [DOI] [PubMed] [Google Scholar]
- Bishop J, Ring D (2009) Management of radial nerve palsy associated with humeral shaft fracture: a decision analysis model. J Hand Surgery 34: 991–996.e1 [DOI] [PubMed] [Google Scholar]
- Blight AR (1989) Effect of 4‐aminopyridine on axonal conduction‐block in chronic spinal cord injury. Brain Res Bull 22: 47–52 [DOI] [PubMed] [Google Scholar]
- Blight AR, Henney HR III, Cohen R (2014) Development of dalfampridine, a novel pharmacologic approach for treating walking impairment in multiple sclerosis. Ann N Y Acad Sci 1329: 33–44 [DOI] [PubMed] [Google Scholar]
- Bostock H, Sears TA, Sherratt RM (1981) The effects of 4‐aminopyridine and tetraethylammonium ions on normal and demyelinated mammalian nerve fibres. J Physiol 313: 301–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM, Hoffman PN, Royall RM, Murinson BB, Witzel C, Gordon T (2002) Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron. J Neurosci 22: 6631–6638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WW (2008) Evaluation and management of peripheral nerve injury. Clin Neurophysiol 119: 1951–1965 [DOI] [PubMed] [Google Scholar]
- Capacio BR, Byers CE, Matthews RL, Chang FC (1996) A method for determining 4‐aminopyridine in plasma: pharmacokinetics in anaesthetized guinea pigs after intravenous administration. Biomed Chromatogr 10: 111–116 [DOI] [PubMed] [Google Scholar]
- Claassen J, Spiegel R, Kalla R, Faldon M, Kennard C, Danchaivijitr C, Bardins S, Rettinger N, Schneider E, Brandt T et al (2013) A randomised double‐blind, cross‐over trial of 4‐aminopyridine for downbeat nystagmus–effects on slowphase eye velocity, postural stability, locomotion and symptoms. J Neurol Neurosurg Psychiatry 84: 1392–1399 [DOI] [PubMed] [Google Scholar]
- Davis FA, Stefoski D, Quandt FN (1995) Mechanism of action of 4‐aminopyridine in the symptomatic treatment of multiple sclerosis. Ann Neurol 37: 684 [DOI] [PubMed] [Google Scholar]
- DeForge D, Nymark J, Lemaire E, Gardner S, Hunt M, Martel L, Curran D, Barbeau H (2004) Effect of 4‐aminopyridine on gait in ambulatory spinal cord injuries: a double‐blind, placebo‐controlled, crossover trial. Spinal Cord 42: 674–685 [DOI] [PubMed] [Google Scholar]
- Dunn J, Blight A (2011) Dalfampridine: a brief review of its mechanism of action and efficacy as a treatment to improve walking in patients with multiple sclerosis. Curr Med Res Opin 27: 1415–1423 [DOI] [PubMed] [Google Scholar]
- Elfar JC, Jacobson JA, Puzas JE, Rosier RN, Zuscik MJ (2008) Erythropoietin accelerates functional recovery after peripheral nerve injury. J Bone Joint Surg Am 90: 1644–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AW, Schwartz G, Meador W, Sabatier MJ, Mulligan A (2007) Electrical stimulation promotes peripheral axon regeneration by enhanced neuronal neurotrophin signaling. Dev Neurobiol 67: 158–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fex Svennigsen A, Dahlin LB (2013) Repair of the peripheral nerve‐Remyelination that works. Brain Sci 3: 1182–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geremia NM, Gordon T, Brushart TM, Al‐Majed AA, Verge VM (2007) Electrical stimulation promotes sensory neuron regeneration and growth‐associated gene expression. Exp Neurol 205: 347–359 [DOI] [PubMed] [Google Scholar]
- Gigo‐Benato D, Russo TL, Geuna S, Domingues NR, Salvini TF, Parizotto NA (2010) Electrical stimulation impairs early functional recovery and accentuates skeletal muscle atrophy after sciatic nerve crush injury in rats. Muscle Nerve 41: 685–693 [DOI] [PubMed] [Google Scholar]
- Gladman SJ, Huang W, Lim SN, Dyall SC, Boddy S, Kang JX, Knight MM, Priestley JV, Michael‐Titus AT (2012) Improved outcome after peripheral nerve injury in mice with increased levels of endogenous omega‐3 polyunsaturated fatty acids. J Neurosci 32: 563–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grijalva I, Garcia‐Perez A, Diaz J, Aguilar S, Mino D, Santiago‐Rodriguez E, Guizar‐Sahagun G, Castaneda‐Hernandez G, Maldonado‐Julian H, Madrazo I (2010) High doses of 4‐aminopyridine improve functionality in chronic complete spinal cord injury patients with MRI evidence of cord continuity. Arch Med Res 41: 567–575 [DOI] [PubMed] [Google Scholar]
- Gupta R, Steward O (2003) Chronic nerve compression induces concurrent apoptosis and proliferation of Schwann cells. J Comp Neurol 461: 174–186 [DOI] [PubMed] [Google Scholar]
- Haastert‐Talini K, Schmitte R, Korte N, Klode D, Ratzka A, Grothe C (2011) Electrical stimulation accelerates axonal and functional peripheral nerve regeneration across long gaps. J Neurotrauma 28: 661–674 [DOI] [PubMed] [Google Scholar]
- Haghighi SS, Pugh SL, Perez‐Espejo MA, Oro JJ (1995) Effect of 4‐aminopyridine in acute spinal cord injury. Surg Neurol 43: 443–447 [DOI] [PubMed] [Google Scholar]
- Hayes KC, Potter PJ, Wolfe DL, Hsieh JT, Delaney GA, Blight AR (1994) 4‐Aminopyridine‐sensitive neurologic deficits in patients with spinal cord injury. J Neurotrauma 11: 433–446 [DOI] [PubMed] [Google Scholar]
- Hayes KC (2004) The use of 4‐aminopyridine (fampridine) in demyelinating disorders. CNS Drug Rev 10: 295–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Lu L, Zhang J, Hu X, Zhang Y, Liang W, Wu S, Luo Z (2012) Electrical stimulation to conductive scaffold promotes axonal regeneration and remyelination in a rat model of large nerve defect. PLoS ONE 7: e39526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Oka Y (2012) The relationship between nerve conduction velocity and fiber morphology during peripheral nerve regeneration. Brain Behav 2: 382–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inserra MM, Bloch DA, Terris DJ (1998) Functional indices for sciatic, peroneal, and posterior tibial nerve lesions in the mouse. Microsurgery 18: 119–124 [DOI] [PubMed] [Google Scholar]
- Jensen HB, Ravnborg M, Dalgas U, Stenager E (2014) 4‐Aminopyridine for symptomatic treatment of multiple sclerosis: a systematic review. Ther Adv Neurol Disord 7: 97–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RE, Heron JR, Foster DH, Snelgar RS, Mason RJ (1983) Effects of 4‐aminopyridine in patients with multiple sclerosis. J Neurol Sci 60: 353–362 [DOI] [PubMed] [Google Scholar]
- Kimura J (2013) Electrodiagnosis in diseases of nerve and muscle: principles and practice, 4th edn Oxford, UK: Oxford University Press; [Google Scholar]
- Kremmyda O, Zwergal A, la Fougere C, Brandt T, Jahn K, Strupp M (2013) 4‐Aminopyridine suppresses positional nystagmus caused by cerebellar vermis lesion. J Neurol 260: 321–323 [DOI] [PubMed] [Google Scholar]
- Krishnan AV, Kiernan MC (2013) Sustained‐release fampridine and the role of ion channel dysfunction in multiple sclerosis. Mult Scler 19: 385–391 [DOI] [PubMed] [Google Scholar]
- Lee DH, Claussen GC, Oh S (2004) Clinical nerve conduction and needle electromyography studies. J Am Acad Orthop Surg 12: 276–287 [DOI] [PubMed] [Google Scholar]
- Ljungquist KL, Martineau P, Allan C (2015) Radial nerve injuries. J Hand Surg 40: 166–172 [DOI] [PubMed] [Google Scholar]
- Lundh H, Nilsson O, Rosen I (1977) 4‐aminopyridine–a new drug tested in the treatment of Eaton‐Lambert syndrome. J Neurol Neurosurg Psychiat 40: 1109–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundh H (1978) Effects of 4‐aminopyridine on neuromuscular transmission. Brain Res 153: 307–318 [DOI] [PubMed] [Google Scholar]
- Lundh H, Nilsson O, Rosen I (1979) Effects of 4‐aminopyridine in myasthenia gravis. J Neurol Neurosurg Psychiat 42: 171–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magill CK, Tong A, Kawamura D, Hayashi A, Hunter DA, Parsadanian A, Mackinnon SE, Myckatyn TM (2007) Reinnervation of the tibialis anterior following sciatic nerve crush injury: a confocal microscopic study in transgenic mice. Exp Neurol 207: 64–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makoukji J, Belle M, Meffre D, Stassart R, Grenier J, Shackleford G, Fledrich R, Fonte C, Branchu J, Goulard M et al (2012) Lithium enhances remyelination of peripheral nerves. Proc Natl Acad Sci USA 109: 3973–3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean NA, Popescu BF, Gordon T, Zochodne DW, Verge VM (2014) Delayed nerve stimulation promotes axon‐protective neurofilament phosphorylation, accelerates immune cell clearance and enhances remyelination in vivo in focally demyelinated nerves. PLoS ONE 9: e110174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Medinaceli L, Freed WJ, Wyatt RJ (1982) An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp Neurol 77: 634–643 [DOI] [PubMed] [Google Scholar]
- Nishimoto S, Tanaka H, Okamoto M, Okada K, Murase T, Yoshikawa H (2015) Methylcobalamin promotes the differentiation of Schwann cells and remyelination in lysophosphatidylcholine‐induced demyelination of the rat sciatic nerve. Front Cell Neurosci 9: 298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niver GE, Ilyas AM (2014) Management of radial nerve palsy following fractures of the humerus. Orthop Clin North Am 44: 419–424 [DOI] [PubMed] [Google Scholar]
- Nix WA, Hopf HC (1983) Electrical stimulation of regenerating nerve and its effect on motor recovery. Brain Res 272: 21–25 [DOI] [PubMed] [Google Scholar]
- Osuchowski MF, Teener J, Remick D (2009) Noninvasive model of sciatic nerve conduction in healthy and septic mice: reliability and normative data. Muscle Nerve 40: 610–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JD (2005) Electrodiagnosis in musculo‐skeletal disease. Best Pract Res Clin Rheumatol 19: 453–466 [DOI] [PubMed] [Google Scholar]
- Pockett S, Gavin RM (1985) Acceleration of peripheral nerve regeneration after crush injury in rat. Neurosci Lett 59: 221–224 [DOI] [PubMed] [Google Scholar]
- Polman CH, Bertelsmann FW, van Loenen AC, Koetsier JC (1994) 4‐aminopyridine in the treatment of patients with multiple sclerosis. Long‐term efficacy and safety. Arch Neurol 51: 292–296 [DOI] [PubMed] [Google Scholar]
- Reagan‐Shaw S, Nihal M, Ahmad N (2008) Dose translation from animal to human studies revisited. FASEB J 22: 659–661 [DOI] [PubMed] [Google Scholar]
- Robinson LR (2000) Role of neurophysiologic evaluation in diagnosis. J Am Acad Orthop Surg 8: 190–199 [DOI] [PubMed] [Google Scholar]
- Sakuma M, Minev IR, Gribi S, Singh B, Woolf CJ, Lacour SP (2015) Chronic electrical nerve stimulation as a therapeutic intervention for peripheral nerve repair. Bioelectron Med 2: 43–48 [Google Scholar]
- Sanders FK, Whitteridge D (1946) Conduction velocity and myelin thickness in regenerating nerve fibres. J Physiol 105: 152–174 [PubMed] [Google Scholar]
- Savastano LE, Laurito SR, Fitt MR, Rasmussen JA, Gonzalez Polo V, Patterson SI (2014) Sciatic nerve injury: a simple and subtle model for investigating many aspects of nervous system damage and recovery. J Neurosci Methods 227: 166–180 [DOI] [PubMed] [Google Scholar]
- Segal JL, Brunnemann SR (1998) 4‐Aminopyridine alters gait characteristics and enhances locomotion in spinal cord injured humans. J Spinal Cord Med 21: 200–204 [DOI] [PubMed] [Google Scholar]
- Shah A, Jebson PJ (2008) Current treatment of radial nerve palsy following fracture of the humeral shaft. J Hand Surg 33: 1433–1434 [DOI] [PubMed] [Google Scholar]
- Sherratt RM, Bostock H, Sears TA (1980) Effects of 4‐aminopyridine on normal and demyelinated mammalian nerve fibres. Nature 283: 570–572 [DOI] [PubMed] [Google Scholar]
- Singh B, Xu QG, Franz CK, Zhang R, Dalton C, Gordon T, Verge VM, Midha R, Zochodne DW (2012) Accelerated axon outgrowth, guidance, and target reinnervation across nerve transection gaps following a brief electrical stimulation paradigm. J Neurosurg 116: 498–512 [DOI] [PubMed] [Google Scholar]
- Starr R, Attema B, DeVries GH, Monteiro MJ (1996) Neurofilament phosphorylation is modulated by myelination. J Neurosci Res 44: 328–337 [DOI] [PubMed] [Google Scholar]
- Stassart RM, Fledrich R, Velanac V, Brinkmann BG, Schwab MH, Meijer D, Sereda MW, Nave KA (2013) A role for Schwann cell‐derived neuregulin‐1 in remyelination. Nat Neurosci 16: 48–54 [DOI] [PubMed] [Google Scholar]
- Stefoski D, Davis FA, Faut M, Schauf CL (1987) 4‐Aminopyridine improves clinical signs in multiple sclerosis. Ann Neurol 21: 71–77 [DOI] [PubMed] [Google Scholar]
- Strupp M, Feil K, Bardins S, Waidelich R (2014) 4‐aminopyridine improves lower urinary tract symptoms in a patient with benign prostatic hyperplasia and downbeat nystagmus syndrome. Int Neurol J 18: 221–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YD, Zheng XS, Ying TT, Yuan Y, Li ST (2015) Nimodipine‐mediated re‐myelination after facial nerve crush injury in rats. J Clin Neurosci 22: 1661–1668 [DOI] [PubMed] [Google Scholar]
- Targ EF, Kocsis JD (1985) 4‐Aminopyridine leads to restoration of conduction in demyelinated rat sciatic nerve. Brain Res 328: 358–361 [DOI] [PubMed] [Google Scholar]
- Taylor CA, Braza D, Rice JB, Dillingham T (2008) The incidence of peripheral nerve injury in extremity trauma. Am J Phys Med Rehabil 87: 381–385 [DOI] [PubMed] [Google Scholar]
- Treffers R, Frankhuyzen AL, Booij LH (1988) Effects of neostigmine, edrophonium, 4‐aminopyridine and their combinations. Acta Anaesthesiol Belg 39: 55–58 [PubMed] [Google Scholar]
- Uges DR, Sohn YJ, Greijdanus B, Scaf AH, Agoston S (1982) 4‐Aminopyridine kinetics. Clin Pharmacol Ther 31: 587–593 [DOI] [PubMed] [Google Scholar]
- Vivo M, Puigdemasa A, Casals L, Asensio E, Udina E, Navarro X (2008) Immediate electrical stimulation enhances regeneration and reinnervation and modulates spinal plastic changes after sciatic nerve injury and repair. Exp Neurol 211: 180–193 [DOI] [PubMed] [Google Scholar]
- Vizi ES, van Dijk J, Foldes FF (1977) The effect of 4‐aminopyridine on acetylcholine release. J Neural Transmission 41: 265–274 [DOI] [PubMed] [Google Scholar]
- Wan L, Xia R, Ding W (2010a) Short‐term low‐frequency electrical stimulation enhanced remyelination of injured peripheral nerves by inducing the promyelination effect of brain‐derived neurotrophic factor on Schwann cell polarization. J Neurosci Res 88: 2578–2587 [DOI] [PubMed] [Google Scholar]
- Wan LD, Xia R, Ding WL (2010b) Electrical stimulation enhanced remyelination of injured sciatic nerves by increasing neurotrophins. Neuroscience 169: 1029–1038 [DOI] [PubMed] [Google Scholar]
- Waxman SG (1980) Determinants of conduction velocity in myelinated nerve fibers. Muscle Nerve 3: 141–150 [DOI] [PubMed] [Google Scholar]
- Wolfe DL, Hayes KC, Hsieh JT, Potter PJ (2001) Effects of 4‐aminopyridine on motor evoked potentials in patients with spinal cord injury: a double‐blinded, placebo‐controlled crossover trial. J Neurotrauma 18: 757–771 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Review Process File


