Significance
The Frank–Starling law of the heart describes the heart’s ability to enhance contractility in response to increased cardiac filling. This property is fundamental to how humans maintain cardiovascular function in response to changes in circulating blood volume, and is regulated by enhanced calcium sensitivity of myofilaments with biomechanical stretch. The mechanism of how biomechanical stretch leads to changes in the myofilament calcium sensitivity remains poorly understood. Using genetic and pharmacologic approaches, we show that β-arrestin and the angiotensin II type I receptor act as crucial molecular regulators of the Frank–Starling law of the heart. This work identifies β-arrestins as important regulators of this fundamental principle of cardiac contractility.
Keywords: cardiac function, hemodynamics, mechanotransduction, angiotensin II type I receptor, β-arrestin
Abstract
The Frank–Starling law of the heart is a physiological phenomenon that describes an intrinsic property of heart muscle in which increased cardiac filling leads to enhanced cardiac contractility. Identified more than a century ago, the Frank–Starling relationship is currently known to involve length-dependent enhancement of cardiac myofilament Ca2+ sensitivity. However, the upstream molecular events that link cellular stretch to the length-dependent myofilament Ca2+ sensitivity are poorly understood. Because the angiotensin II type 1 receptor (AT1R) and the multifunctional transducer protein β-arrestin have been shown to mediate mechanosensitive cellular signaling, we tested the hypothesis that these two proteins are involved in the Frank–Starling mechanism of the heart. Using invasive hemodynamics, we found that mice lacking β-arrestin 1, β-arrestin 2, or AT1R were unable to generate a Frank–Starling force in response to changes in cardiac volume. Although wild-type mice pretreated with the conventional AT1R blocker losartan were unable to enhance cardiac contractility with volume loading, treatment with a β-arrestin–biased AT1R ligand to selectively activate β-arrestin signaling preserved the Frank–Starling relationship. Importantly, in skinned muscle fiber preparations, we found markedly impaired length-dependent myofilament Ca2+ sensitivity in β-arrestin 1, β-arrestin 2, and AT1R knockout mice. Our data reveal β-arrestin 1, β-arrestin 2, and AT1R as key regulatory molecules in the Frank–Starling mechanism, which potentially can be targeted therapeutically with β-arrestin–biased AT1R ligands.
The relationship between cardiac filling and contractility, described by Otto Frank and Ernest Starling in the late 19th and early 20th century, is fundamental to how humans maintain cardiovascular function in response to changes in circulating blood volume (1). It is generally accepted that the Frank–Starling relationship is due to an immediate increase in cardiac contractility that results from lengthening of the sarcomere and is related to enhanced sensitivity of the myofilaments to calcium, rather than to alterations in intracellular calcium, a phenomenon known as length-dependent force activation (2). Recent work suggests that sarcomere lengthening affects myofilament calcium sensitivity through mechanisms involving an interplay among myofilament proteins to affect interfilament spacing and increase the number of cross bridges available for interaction with the thin filament during contraction (3). Additional mechanisms are mediated by isoform shifts and posttranslational modification of myofilament proteins, such as phosphorylation of cardiac troponin I (4–8), myosin-binding protein C (9, 10), and myosin regulatory light chain (11), to enhance the sensitivity of myofilaments to calcium. The precise upstream molecular mechanisms that link cellular stretch and length-dependent force activation remain poorly understood, however (12).
Several of the critical myofilament modifications that affect length-dependent force activation occur downstream of activated G protein-coupled receptors (GPCRs). Canonical GPCR-mediated activation of protein kinase A (PKA) and protein kinase C (PKC) are known links between adrenergic and angiotensin II neurohormonal signaling and length-dependent force activation (13). In parallel to canonical G protein-mediated signaling, activated GPCRs can activate a distinct set of molecular signals through recruitment of the adaptor protein β-arrestin (14), which has been shown to promote survival in cardiac tissue (15, 16) and to enhance cardiac contractility through the angiotensin II type 1 receptor (AT1R) without an increase in intracellular calcium level (17). Moreover, the in vivo administration of β-arrestin–biased AT1R ligands augments cardiac performance (16, 18), likely mediated in part by the mechanoactivation of AT1R-mediated β-arrestin–biased signaling (19, 20). Although these data implicate β-arrestin in the modulation of cardiac inotropy, whether β-arrestins play a role in the Frank–Starling mechanism and length-dependent activation is unclear.
In the present study, using in vivo hemodynamics, ex vivo cardiac stretch, and isolated myofilament preparations, we show that the Frank–Starling relationship is dependent on the AT1R, β-arrestin 1, and β-arrestin 2, which is lost in the presence of a conventional AT1R blocker (ARB) but restored with a β-arrestin–biased AT1R blocker. These data show that the AT1R and β-arrestins function as transducers of length-dependent force activation and the Frank–Starling mechanism of cardiac contractility.
Results
The Frank–Starling Relationship Is β-Arrestin 1 and -2 Dependent.
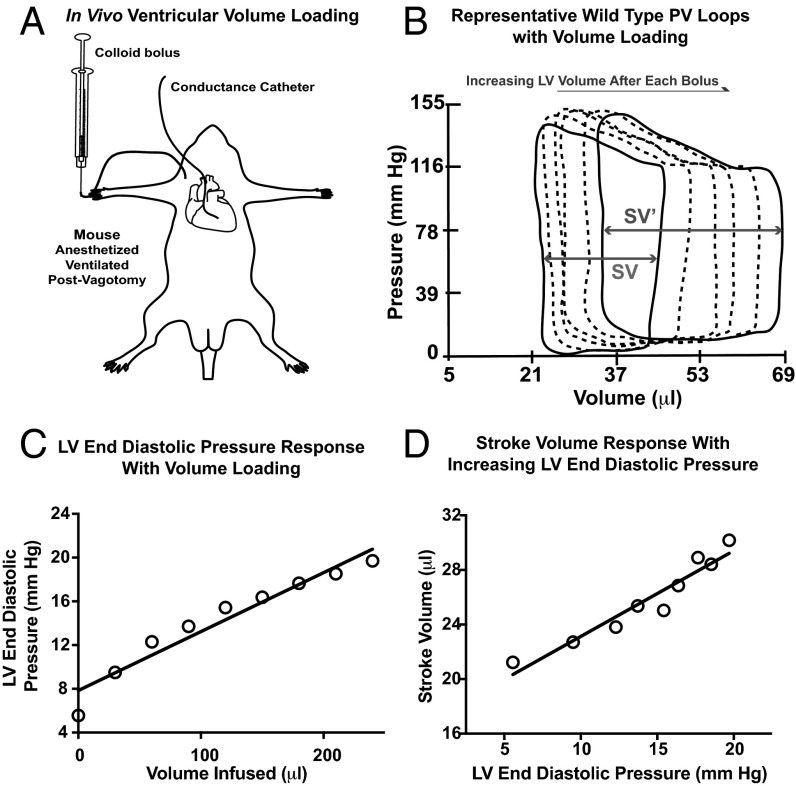
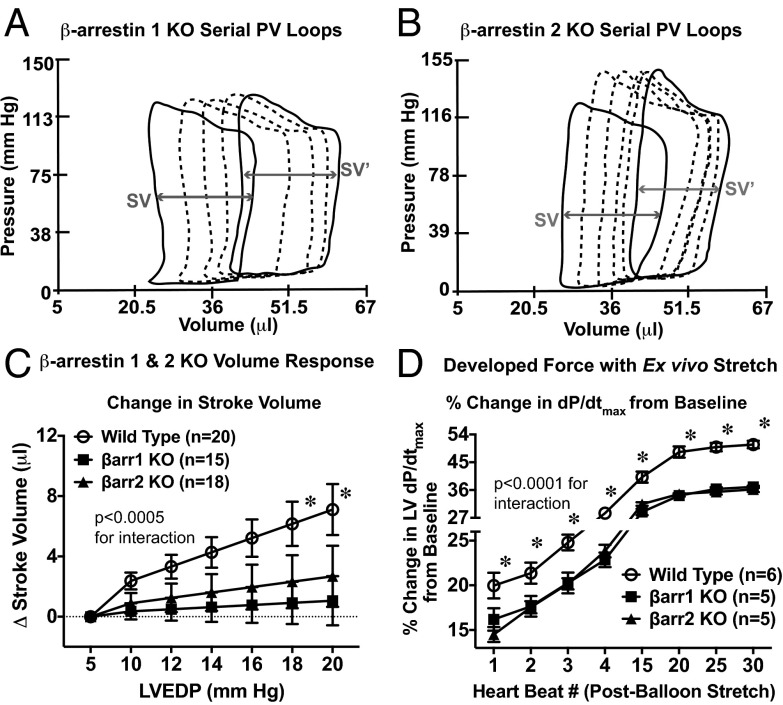
To test whether β-arrestins are involved in Frank–Starling force generation, we developed an in vivo protocol to assess changes in contractility with volume loading. Ventricular volume loading was accomplished with bolus doses of a colloid [5% albumin (12.5 g human albumin/250 mL aqueous diluent) (Grifols Pharmaceuticals)] solution administered to anesthetized mice that were cannulated with a conductance catheter (Millar) to continuously record cardiac pressure and volume (Fig. 1A). Pressure-volume (PV) loop data were analyzed at 1 min after each bolus dose (Fig. 1B) to generate linear regression models relating cardiac filling pressures (Fig. 1C and Fig. S1) or left ventricular (LV) volumes (Fig. S2) to the amount of volume infused. Mice with adequate ventricular loading, defined as an increase in LV end-diastolic pressure (LVEDP) >5 mmHg or an LV end-diastolic volume (LVEDV) >10 μL, underwent further analyses in which standard curves were generated relating stroke volume (SV) to cardiac filling pressure (Fig. 1D and Fig. S3). The following mice were excluded because of inadequate volume loading: 5 of 32 wild-type (WT) mice, 1 of 19 β-arrestin-2 KO mice, 0 of 15 β-arrestin-1 KO mice, and 1 of 7 AT1R mice. We used LVEDP as an index of cardiac loading because cardiac compliance, defined by the end-diastolic PV relationship (EDPVR), was not significantly different among the genotypes (Table S1). Averaged data from the SV vs. LVEDP curves show an increase in SV with increasing cardiac filling pressures in WT mice, thus demonstrating the Frank–Starling mechanism of cardiac contractility (Fig. 1D and Fig. S3).
Fig. 1.
In vivo testing of the Frank–Starling relationship. (A) Schematic of the volume infusion protocol. Anesthetized mice underwent vagotomy, followed by retroaortic cannulation of a pressure conductance catheter into the left ventricle. (B) Representative serial pressure-volume (PV) loops measured by conductance catheter placed in a WT mouse left ventricle. Continuous PV loop data were recorded. The basal PV loop recording is shown as a solid black line. At 1 min after each colloid bolus, the PV loop is analyzed, denoted here by a dashed line. With each successive bolus, incremental increases in both LV volume and LVEDP are seen, confirming successful ventricular loading. SV in both basal loops (SV) and loops recorded after completion of volume loading (SV′) is denoted by gray arrows. (C) Example of the relationship between volume infusion and LVEDP in WT mice. (D) Example of the relationship between SV and LVEDP in WT mice.
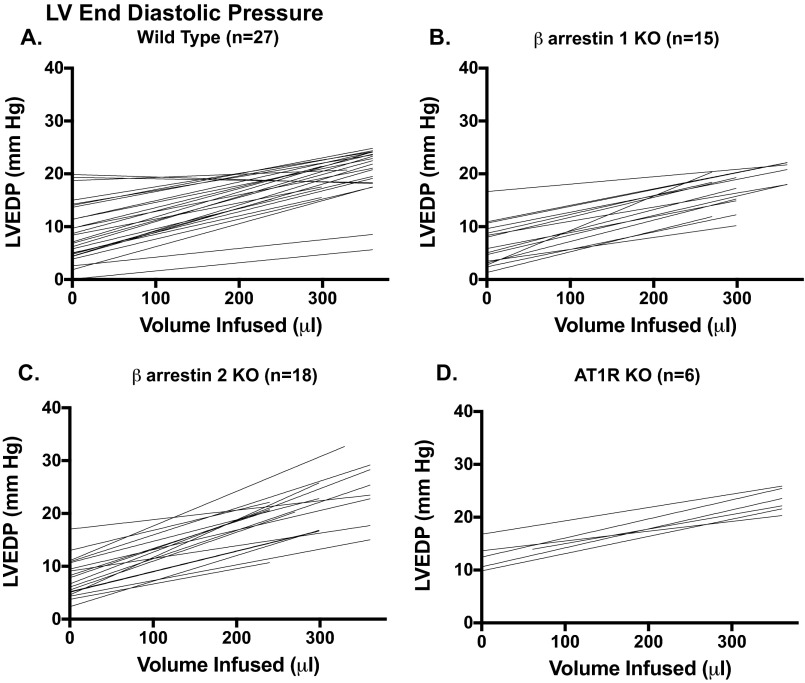
Fig. S1.
Individual responses of LVEDP to 30-μL bolus infusions of 5% albumin (12.5 g human albumin/250 mL aqueous diluent) (Grifols Pharmaceuticals) in WT (A), β-arrestin 1 KO (B), β-arrestin 2 KO (C), and AT1R KO (D) mice. Mice that did not develop an increase in LVEDP of >5 mm Hg were excluded from the contractility analyses.
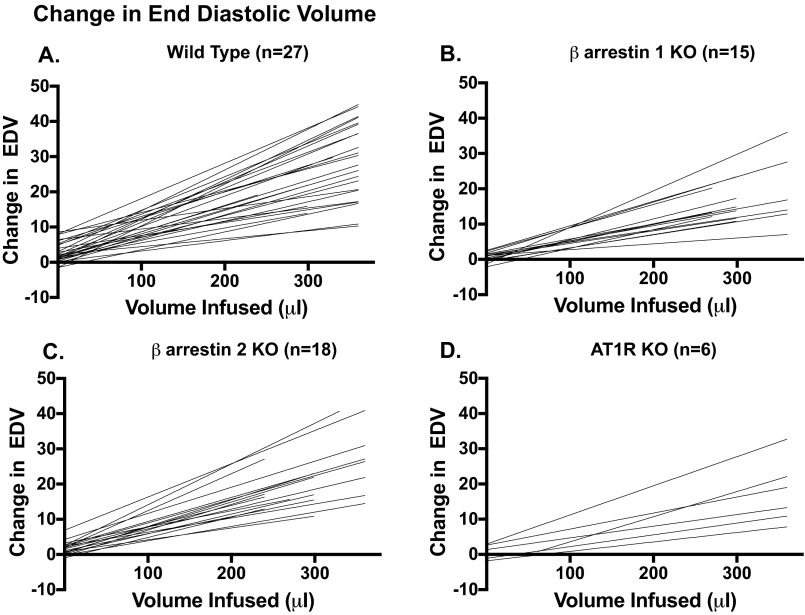
Fig. S2.
Individual responses of LVEDV to 30-μL bolus infusions of 5% albumin (12.5 g human albumin/250 mL aqueous diluent) (Grifols Pharmaceuticals) in WT (A), β-arrestin 1 KO (B), β-arrestin 2 KO (C), and AT1R KO (D) mice. Mice that did not develop an increase in LVEDV of >10 μL were excluded from the contractility analyses.
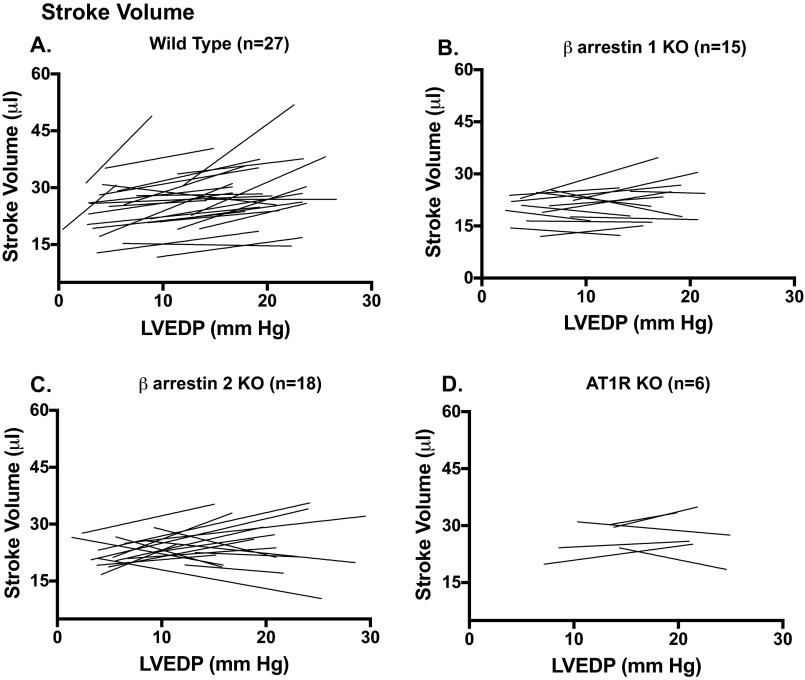
Fig. S3.
Individual SV response vs. LVEDP in mice included in the contractility analyses: (A) WT mice; (B) β-arrestin 1 KO mice; (C) β-arrestin 2 KO mice; (D) AT1R KO mice.
Table S1.
Load-independent hemodynamic measures
| Parameter | WT (n = 11) | β-arrestin 1 KO (n = 4) | β-arrestin 2 KO (n = 6) | AT1R KO (n = 13) |
| Compliance parameters | ||||
| EDPVR (linear) slope | 0.26 ± 0.05 | 0.17 ± 0.01 | 0.21 ± 0.02 | 0.35 ± 0.04 |
| EDPVR (linear) intercept | −3.06 ± 1.34 | −1.46 ± 1.72 | −2.45 ± 1.26 | −7.74 ± 2.67 |
| EDPVR (quadratic) β coefficient | 0.083 ± 0.015 | 0.038 ± 0.010 | 0.115 ± 0.041 | 0.047 ± 0.008 |
| EDPVR (quadratic) α coefficient | 1.164 ± 0.445 | 1.974 ± 0.992 | 1.070 ± 0.563 | 1.260 ± 0.554 |
| Contractility parameters | ||||
| ESPVR (linear) slope | 4.02 ± 0.68 | 3.19 ± 0.29 | 3.48 ± 0.91 | 2.68 ± 0.26 |
| ESPVR (linear) intercept | −4.53 ± 7.39 | −6.60 ± 7.59 | −7.19 ± 10.55 | −12.12 ± 6.71 |
| ESPVR (quadratic) a | −1.82 ± 1.14 | −0.61 ± 0.25 | −0.34 ± 0.12 | −0.04 ± 0.01* |
| ESPVR (quadratic) V0 | 9.85 ± 5.80 | 4.70 ± 7.00 | 9.47 ± 4.95 | −0.90 ± 5.68 |
| ESPVR (quadratic) E′max | 16.09 ± 3.84 | 11.59 ± 2.24 | 9.93 ± 1.93 | 5.02 ± 0.55* |
| PRSW slope | 59.69 ± 4.11 | 59.26 ± 4.91 | 42.63 ± 7.86 | 44.46 ± 4.83 |
| PRSW intercept | 8.10 ± 6.94 | 9.88 ± 4.53 | 1.87 ± 8.70 | −0.92 ± 3.68 |
| dP/dtmax vs. EDV slope | 154.50 ± 22.74 | 103.50 ± 9.78 | 88.09 ± 10.21 | 113.40 ± 17.71 |
| dP/dtmax vs. EDV intercept | −14.59 ± 8.39 | −19.63 ± 5.16 | −32.39 ± 11.67 | −30.26 ± 13.79 |
| Emax | 7.79 ± 1.29 | 8.29 ± 1.11 | 6.44 ± 1.41 | 4.80 ± 0.47 |
Average load-independent parameters obtained by inferior vena cava constriction performed on a separate cohort of WT (n = 11), β-arrestin 1 KO (n = 4), β-arrestin 2 KO (n = 6), and AT1R KO (n = 7) mice. Parameters of LV compliance (linear- and quadratic-derived EDPVR) and LV contractility (linear- and quadratic-derived ESPVR, PRSW, dP/dtmax vs. EDV and Emax) are listed separately for each genotype. Errors reflect SEM. ESPVR, end-systolic PV relationship; a, coefficient of curvilinearity; Vo, volume intercept; E′max, maximum slope of quadratic ESPVR; PRSW, preload recruitable stroke work; Emax, maximal elastance.
P < 0.05 vs. WT, one-way ANOVA with Bonferroni’s multiple comparison test.
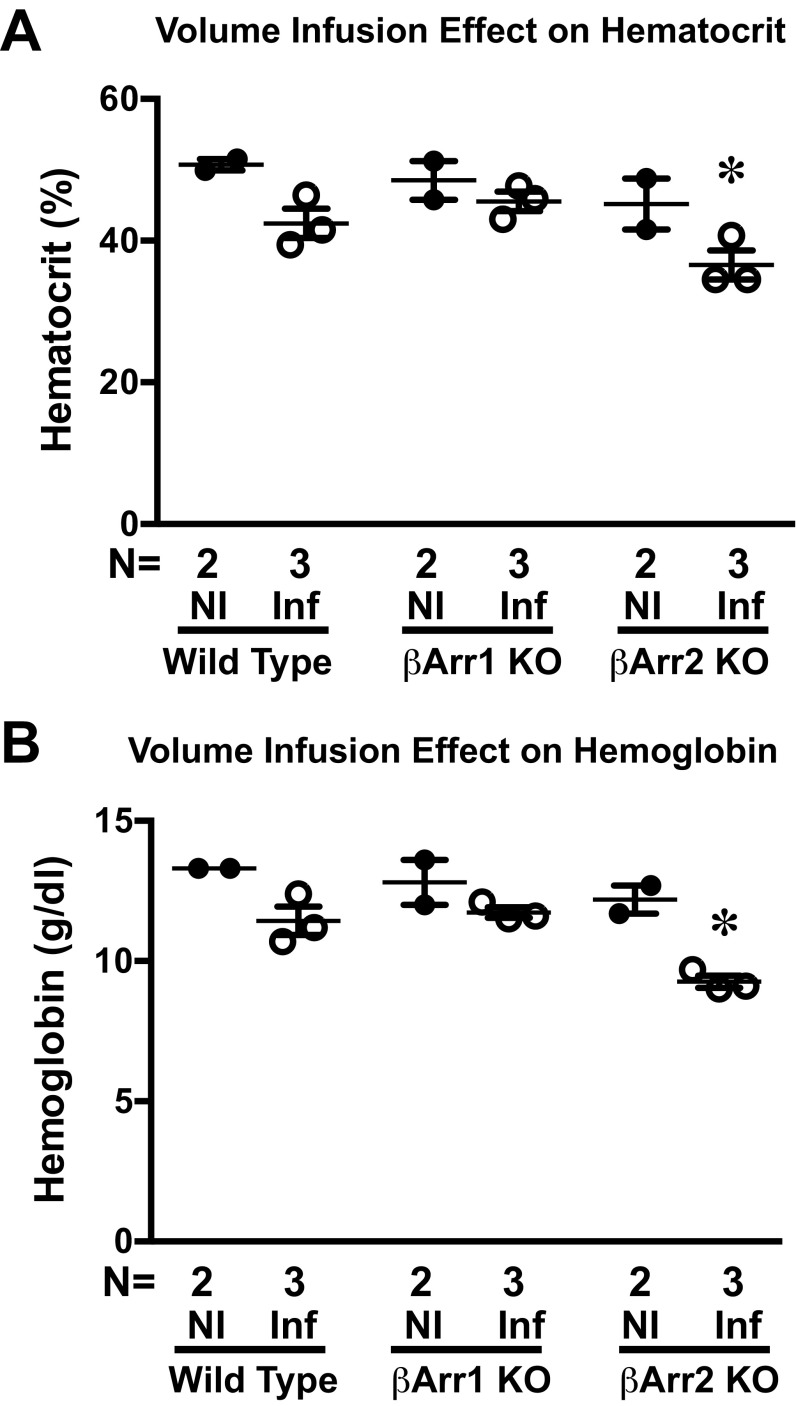
In response to in vivo volume loading, cardiac SV was augmented in the WT mice (Fig. 2C and Fig. S3), but was abrogated in the β-arrestin 1 KO and β-arrestin 2 KO mice (Fig. 2 A–C and Table S2). The β-arrestin 1 KO mice did not differ from the WT mice in baseline contractility parameters, whereas β-arrestin 2 KO mice showed a small but significant reduction in basal dP/dtmax, although other measures of cardiac function, such as ejection fraction and cardiac output, were not different from those of β-arrestin 1 KO and WT mice (Tables S1 and S3). Given that volume administration can affect conductance and artifactually increase volume through hemodilution (21), we measured both hemoglobin and hematocrit after volume infusion. Although hemodilution was more pronounced in the β-arrestin 2 KO mice than in the WT and β-arrestin 1 KO mice (Fig. S4), the rise in conductance catheter-determined LVEDV was largely similar across all genotypes (Tables S2 and S4), suggesting that hemodilution did not account for the measured difference in SV.
Fig. 2.
Effect of β-arrestin 1 and -2 on the cardiac response to volume loading. (A and B) Representative serial PV loops from a β-arrestin 1 KO mouse (A) and a β-arrestin 2 KO (B) mouse during volume infusion protocol. SV in both basal loops (SV) and in loops recorded after completion of volume loading (SV′) is denoted by the gray arrows. (C) Effect of volume infusion on absolute change in SV from baseline in β-arrestin 1 KO and β-arrestin 2 KO mice compared with WT mice. P values for the interaction between LVEDP and change in SV for data comparison were obtained by two-way repeated-measures ANOVA. *P < 0.005 for comparison between genotypes at a given LVEDP value using Bonferroni’s multiple comparison test. (D) Effect of balloon stretch on developed force in an ex vivo hanging heart preparation. Shown is the average percentage increase in LV dP/dtmax from baseline shown for WT, β-arrestin 1 KO, and β-arrestin 2 KO mice. P values for the interaction between heart beat number and genotype for data comparison were obtained by two-way repeated-measures ANOVA. *P < 0.05 for comparison between genotypes at a given heart rate using Bonferroni’s multiple comparison test. Error bars reflect SEM.
Table S2.
β-arrestin KO vs. WT response to LV loading
| LVEDP, mmHg | LVEDV, μL | LVESV, μL | Stroke volume, μL | Stroke work, μL⋅ mmHg | dP/dtmax, mmHg/s | Arterial elastance, mmHg/μL | Systolic pressure, mmHg |
| WT (n = 20) | |||||||
| 5 | 42.00 ± 2.17 | 19.49 ± 1.20 | 22.51 ± 1.55 | 2,548.4 ± 221.3 | 11,686 ± 663 | 6.99 ± 0.50 | 157.3 ± 3.9 |
| 10 | 50.30 ± 1.82* | 25.42 ± 1.08* | 24.88 ± 1.42* | 2,794.5 ± 199.7* | 11,799 ± 634 | 6.64 ± 0.48 | 159.7 ± 3.8 |
| 12 | 53.62 ± 1.75* | 27.79 ± 1.13* | 25.83 ± 1.43* | 2,892.9 ± 196.0* | 11,844 ± 628 | 6.49 ± 0.47 | 160.6 ± 3.8 |
| 14 | 56.94 ± 1.73* | 30.16 ± 1.23* | 26.77 ± 1.47* | 2,991.3 ± 195.4* | 11,889 ± 626 | 6.35 ± 0.47 | 161.5 ± 3.9 |
| 16 | 60.25 ± 1.77* | 32.53 ± 1.37* | 27.72 ± 1.55* | 3,089.7 ± 197.9* | 11,934 ± 628 | 6.21 ± 0.48* | 162.5 ± 4.0* |
| 18 | 63.57 ± 1.86* | 34.90 ± 1.54* | 28.67 ± 1.65* | 3,188.1 ± 203.4* | 11,979 ± 634 | 6.06 ± 0.49* | 163.4 ± 4.2* |
| 20 | 66.89 ± 2.00* | 37.27 ± 1.73* | 29.62 ± 1.78* | 3,286.5 ± 211.8* | 12,025 ± 644 | 5.92 ± 0.50* | 164.3 ± 4.4* |
| β-arrestin 1 KO (n = 15) | |||||||
| 5 | 39.86 ± 3.88 | 21.32 ± 3.07 | 20.41 ± 1.12 | 2,120.5 ± 179.5 | 9,047 ± 534† | 7.20 ± 0.55 | 144.6 ± 4.6 |
| 10 | 46.39 ± 3.46* | 27.62 ± 2.91* | 20.76 ± 1.19 | 2,099.8 ± 161.3† | 8,875 ± 561† | 7.21 ± 0.56 | 143.2 ± 4.6† |
| 12 | 49.00 ± 3.34* | 30.14 ± 2.88* | 20.90 ± 1.28† | 2,091.5 ± 160.9† | 8,806 ± 596† | 7.21 ± 0.60 | 142.7 ± 4.8† |
| 14 | 51.62 ± 3.24* | 32.66 ± 2.86* | 21.04 ± 1.40† | 2,083.2 ± 164.5† | 8,737 ± 641† | 7.22 ± 0.64 | 142.1 ± 5.0† |
| 16 | 54.23 ± 3.18* | 35.18 ± 2.85* | 21.18 ± 1.55† | 2,074.9 ± 171.9† | 8,668 ± 696† | 7.22 ± 0.70 | 141.6 ± 5.4† |
| 18 | 56.84 ± 3.16* | 37.70 ± 2.87* | 21.32 ± 1.70† | 2,066.6 ± 182.6† | 8,600 ± 757† | 7.23 ± 0.76 | 141.0 ± 5.8† |
| 20 | 59.46 ± 3.17* | 40.22 ± 2.89* | 21.46 ± 1.87† | 2,058.3 ± 196.2† | 8,531 ± 823† | 7.23 ± 0.83 | 140.4 ± 6.2† |
| β-arrestin 2 KO (n = 18) | |||||||
| 5 | 38.58 ± 2.71 | 16.61 ± 2.26 | 22.92 ± 0.91 | 2,437.5 ± 135.9 | 8,910 ± 461† | 6.19 ± 0.33 | 145.5 ± 5.0 |
| 10 | 45.23 ± 2.69* | 21.41 ± 2.40* | 23.82 ± 0.80 | 2,565.4 ± 139.0 | 9,027 ± 536† | 6.26 ± 0.24 | 149.0 ± 4.8 |
| 12 | 47.88 ± 2.75* | 23.71 ± 2.34* | 24.17 ± 0.90 | 2,616.6 ± 156.3 | 9,074 ± 574† | 6.29 ± 0.25 | 150.4 ± 4.8 |
| 14 | 50.54 ± 2.83* | 26.01 ± 2.30* | 24.53 ± 1.07 | 2,667.7 ± 179.6 | 9,121 ± 615† | 6.32 ± 0.29 | 151.8 ± 4.9* |
| 16 | 53.20 ± 2.95* | 28.31 ± 2.27* | 24.89 ± 1.27 | 2,718.9 ± 206.8 | 9,168 ± 659† | 6.35 ± 0.35 | 153.2 ± 5.1* |
| 18 | 55.86 ± 3.09* | 30.61 ± 2.27* | 25.25 ± 1.49* | 2,770.1 ± 236.7* | 9,215 ± 706† | 6.37 ± 0.42 | 154.5 ± 5.3* |
| 20 | 58.52 ± 3.25*† | 32.92 ± 2.29* | 25.60 ± 1.73* | 2,821.2 ± 268.3* | 9,262 ± 754† | 6.40 ± 0.50 | 155.9 ± 5.6* |
P < 0.05 vs. LVEDP 5 mm Hg of same genotype; †P < 0.05 vs. corresponding measure/LVEDP WT mice, one-way repeated-measures ANOVA with Bonferroni’s multiple comparison test. Errors reflect SEM.
Table S3.
Baseline hemodynamic parameters of mice undergoing the volume infusion protocol
| Parameter | WT (n = 27) | β-arrestin 1 KO (n = 15) | β-arrestin 2 KO (n = 18) | AT1R KO (n = 13) |
| Heart rate, bpm | 452.7 ± 10.6 | 442.4 ± 12.3 | 416.4 ± 14.2 | 429.8 ± 15.9 |
| LVESP, mm Hg | 142.5 ± 5.0 | 136.2 ± 4.8 | 136.7 ± 4.5 | 135.7 ± 4.9 |
| LVEDP, mm Hg | 7.17 ± 0.79 | 5.81 ± 0.81 | 6.06 ± 0.77 | 17.17 ± 2.32*† |
| Arterial elastance, mmHg/μL | 6.78 ± 0.59 | 6.90 ± 0.46 | 6.31 ± 0.27 | 5.31 ± 0.47 |
| Systolic function parameters | ||||
| Stroke volume, μL | 23.18 ± 1.25 | 20.50 ± 1.01 | 22.02 ± 0.76 | 27.21 ± 2.01 |
| Ejection fraction, % | 51.17 ± 2.96 | 54.09 ± 5.67 | 57.77 ± 3.26 | 40.03 ± 2.74 |
| Cardiac output, μL/min | 10,475 ± 593.9 | 9,075 ± 536.7 | 9,195 ± 472.3 | 11,667 ± 985.0 |
| Stroke work, mmHg⋅μL | 2,418 ± 169.6 | 2,118 ± 157.1 | 2,260 ± 114.7 | 2,569 ± 225.7 |
| dP/dtmax, mmHg/s−1 | 10,818 ± 493.3 | 9,158 ± 536.1 | 8,786 ± 505.2* | 9,070 ± 359.2 |
| Diastolic function parameters | ||||
| dP/dtmin, mmHg/s−1 | −9,351 ± 314.3 | −7,827 ± 379.2* | −8,017 ± 391.5* | −7,322 ± 249.7* |
| Tau, ms (Weiss) | 7.43 ± 0.15 | 7.65 ± 0.27 | 7.64 ± 0.29 | 9.29 ± 0.29*† |
| Tau, ms (Glantz) | 12.76 ± 0.41 | 12.06 ± 0.35 | 12.52 ± 0.45 | 16.45 ± 0.94*† |
P < 0.05 vs. WT; †P < 0.05 vs. β-arrestin 1 KO and β-arrestin 2 KO, one-way ANOVA.
Fig. S4.
Hemodilution effect of infusion. Average hematocrit (A) and average hemoglobin (B) in WT, β-arrestin 1 KO, and β-arrestin 2 KO mice that were not infused (NI) or had completed the volume infusion protocol (Inf). *P < 0.05 vs. noninfused of the same genotype using one-way ANOVA with Bonferroni’s multiple comparison test. Error bars reflect SEM.
Table S4.
AT1R KO vs. WT response to LV loading
| LVEDP, mmHg | LVEDV, μL | LVESV, μL | Stroke volume, μL | Stroke work, μL⋅mmHg | dP/dtmax, mmHg/s | Arterial elastance, mmHg/μL | Systolic pressure, mmHg |
| WT (n = 7) | |||||||
| 5 | 40.69 ± 7.04 | 16.86 ± 5.06 | 23.82 ± 3.33 | 2,239.8 ± 320.7 | 11,238 ± 1,230 | 6.28 ± 1.16 | 133.1 ± 7.2* |
| 10 | 58.38 ± 11.44* | 29.29 ± 7.46* | 29.07 ± 4.99 | 2,837.0 ± 485.7 | 11,795 ± 1,254 | 5.65 ± 1.15 | 141.8 ± 7.8* |
| 12 | 65.45 ± 13.24* | 34.26 ± 8.48* | 31.17 ± 5.73* | 3,075.9 ± 563.3* | 12,017 ± 1,275* | 5.40 ± 1.17 | 145.3 ± 8.1* |
| 14 | 72.53 ± 15.05* | 39.23 ± 9.53* | 33.26 ± 6.48* | 3,314.8 ± 644.0* | 12,240 ± 1,302* | 5.15 ± 1.19* | 148.8 ± 8.5* |
| 16 | 79.61 ± 16.87* | 44.20 ± 10.59* | 35.36 ± 7.25* | 3,553.7 ± 726.9* | 12,462 ± 1,336* | 4.90 ± 1.23* | 152.2 ± 9.0* |
| 18 | 86.68 ± 18.69* | 49.17 ± 11.66* | 37.46 ± 8.02* | 3,792.6 ± 811.2* | 12,685 ± 1,374* | 4.65 ± 1.27* | 155.7 ± 9.5* |
| 20 | 93.76 ± 20.52* | 54.14 ± 12.74* | 39.56 ± 8.80* | 4,031.5 ± 896.5* | 12,908 ± 1,417* | 4.40 ± 1.32* | 159.2 ± 10.1* |
| AT1R KO (n = 6) | |||||||
| 5 | 48.27 ± 7.08 | 22.56 ± 6.97 | 24.88 ± 1.81 | 2,162.7 ± 281.9 | 8,800 ± 463 | 4.26 ± 0.514 | 112.4 ± 9.1* |
| 10 | 56.08 ± 6.80 | 29.65 ± 6.70 | 25.78 ± 1.37 | 2,263.4 ± 181.5 | 9,076 ± 321 | 4.56 ± 0.452 | 121.6 ± 7.1* |
| 12 | 59.20 ± 6.77 | 32.48 ± 6.60* | 26.14 ± 1.30 | 2,303.6 ± 147.7 | 9,187 ± 269 | 4.68 ± 0.444 | 125.3 ± 6.4* |
| 14 | 62.32 ± 6.78 | 35.32 ± 6.50* | 26.50 ± 1.32 | 2,343.9 ± 122.3 | 9,297 ± 222 | 4.81 ± 0.446 | 129.0 ± 5.8* |
| 16 | 65.44 ± 6.85* | 38.15 ± 6.41* | 26.86 ± 1.41 | 2,384.1 ± 111.2 | 9,407 ± 186 | 4.93 ± 0.459 | 132.7 ± 5.3* |
| 18 | 68.57 ± 6.95* | 40.99 ± 6.33* | 27.22 ± 1.56 | 2,424.4 ± 118.6 | 9,518 ± 166 | 5.05 ± 0.481 | 136.4 ± 4.9* |
| 20 | 71.69 ± 7.10* | 43.82 ± 6.26* | 27.59 ± 1.77 | 2,464.6 ± 141.5 | 9,628 ± 168 | 5.17 ± 0.512 | 140.1 ± 4.7* |
P < 0.05 vs. LVEDP 5 mmHg of same genotype; †P < 0.05 vs. corresponding measure/LVEDP in WT mice, one-way repeated-measures ANOVA with Bonferroni’s multiple comparison test. Errors reflect SEM.
To investigate whether the attenuation of volume-induced cardiac contractility in β-arrestin KO mice is due to extracardiac causes (i.e., neural or vascular), and to determine the kinetics of developed force with acute stretch, we used a Langendorff perfused model of ex vivo cardiac stretch as described previously (19). Acute cardiac stretch in isolated left ventricles induced a 20% rise in dP/dtmax from baseline in WT mice, whereas a significantly blunted rise in dP/dtmax was seen in both β-arrestin 1 and β-arrestin 2 KO mice after the first cardiac cycle (Fig. 2D). A marked attenuation of developed force was seen in the β-arrestin KO mice up to 30 cardiac cycles after imposition of an acute stretch (Fig. 2D). These in vivo and ex vivo data suggest that cardiac β-arrestin 1 and β-arrestin 2 are necessary for the Frank–Starling mechanism of force generation.
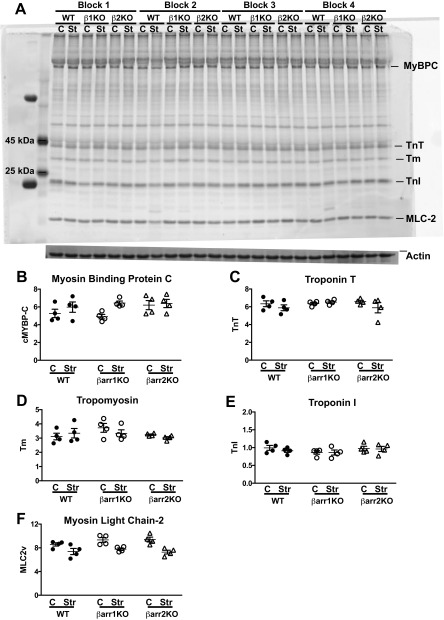
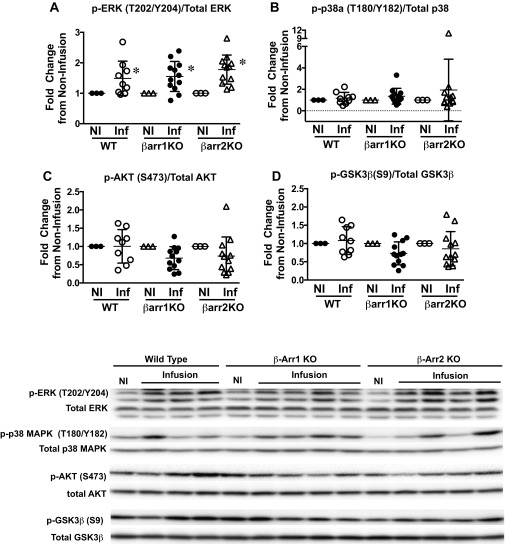
Given that the Frank–Starling relationship is mediated by length-dependent force activation, we tested whether myofilaments isolated from β-arrestin 1 and -2 KO mice lacked this mechanism. In fiber preparations from WT and β-arrestin 1 and -2 KO mice, length-dependent activation was defined as the difference in pCa50 (ΔpCa50) at two different sarcomere lengths. Increasing the sarcomere length from 1.9 μm to 2.3 μm resulted in a significant leftward shift in the force–pCa2+ curve, indicating increased myofilament Ca2+ sensitivity in WT muscle fibers, consistent with robust myofilament length dependency on force generation in WT heart muscle (Fig. 3A). In sharp contrast, ventricular muscle fibers from β-arrestin 1 KO and β-arrestin 2 KO hearts showed a lack of leftward shift in the force–Ca2+ curve, indicating significantly blunted length-dependent force activation (Figs. 3 B and C). These data reveal that length-dependent force activation is dependent on the presence of both β-arrestin 1 and β-arrestin 2 (Fig. 3D and Table S5). Whereas length-dependent force activation was markedly blunted in β-arrestin KO mice, these mice did not exhibit significant differences in the overall phosphorylation of myofilament proteins (Figs. S6 and S7), titin isoforms (Fig. S8), or upstream kinases (Fig. S9) compared with WT mice. There was, however, a significant reduction in passive tension in β-arrestin 1 KO and β-arrestin 2 KO mice compared with WT controls (Table S5). The reduction in passive tension is likely due to alterations in titin, which in turn could modify the effect of length on force generation. Such titin alterations will require more extensive analysis.
Fig. 3.
Effect of β-arrestin 1 and -2 on the force–Ca2+ relationship. (A) Force–Ca2+ relationship in myofilaments harvested from left ventricles of WT mice at 1.9 μm and 2.3 μm. The change in calcium concentration producing one-half maximal tension (ΔpCa50) at 1.9 μm vs. 2.3 μm was significantly different. *P < 0.05 for WT mice (n = 10 mice) at 1.9 μm vs. 2.3 μm by one-way ANOVA with Bonferroni’s multiple comparison test. (B) Force–Ca2+ relationship in β-arrestin 1 KO mice (n = 10 mice) at 1.9 μm and 2.3 μm. (C) Force–Ca2+ relationship in β-arrestin 2 KO mice (n = 11 mice) at 1.9 μm and 2.3 μm. (D) Average change in calcium concentration producing one-half maximal tension (ΔpCa50) at 1.9 μm vs. 2.3 μm. *P < 0.05 vs. WT average ΔpCa50 by one-way ANOVA with Bonferroni’s multiple comparison test. Error bars reflect SEM.
Table S5.
Myofilament function
| Length, μm | WT | β-arrestin 1 KO | β-arrestin 2 KO | AT1R KO |
| pCa50, −log M | ||||
| 1.9 | 5.62 ± 0.01 | 5.70 ± 0.02‡ | 5.68 ± 0.01 | 5.68 ± 0.01 |
| 2.3 | 5.85 ± 0.02* | 5.74 ± 0.02† | 5.73 ± 0.01† | 5.74 ± 0.02† |
| Hill coefficient | ||||
| 1.9 | 2.07 ± 0.11 | 3.18 ± 0.32‡ | 3.11 ± 0.15‡ | 2.35 ± 0.12 |
| 2.3 | 1.84 ± 0.11 | 2.55 ± 0.16 | 2.68 ± 0.16† | 2.32 ± 0.11 |
| Maximal tension, mN/mm2 | ||||
| 1.9 | 32.48 ± 1.57 | 33.83 ± 1.83 | 32.86 ± 1.57 | 34.79 ± 1.57 |
| 2.3 | 41.14 ± 1.46* | 36.12 ± 1.80 | 35.00 ± 1.48 | 39.02 ± 1.49 |
| Change in passive tension, mN/mm2 | ||||
| 8.25 ± 0.17 | 6.56 ± 0.22# | 6.43 ± 0.19# | 6.49 ± 0.19# | |
Average data obtained from force–Ca2+ curves obtained from skinned fiber preparations for WT (n = 10 mice), β-arrestin 1 KO (n = 10), β-arrestin 2 KO (n = 11), and AT1R KO (n = 13) mice. *P < 0.05 vs. 1.9 μm of the same genotype; †P < 0.05 vs. WT 2.3 μm; ‡P < 0.05 vs. WT 1.9 μm; #P < 0.05 vs. WT passive tension, one-way ANOVA.
Fig. S6.
Myofilament phosphoprotein survey of ex vivo stretch left ventricles. (A) Representative phosphoprotein gel image of ex vivo stretch left ventricles. β1KO, β-arrestin 1 KO; β2KO, β-arrestin 2 KO; C, control; Str, ex vivo stretch. (B) Average phosphorylated myosin-binding protein (MyBPC). (C) Average phosphorylated troponin T (TnT). (D) Average phosphorylated tropomyosin (Tm). (E) Average phosphorylated troponin I (TnI). (F) Average phosphorylated myosin light chain 2 (MLC-2). n = 4 separate left ventricles per genotype and condition (control and stretch). Statistical comparisons are made using one-way ANOVA with Bonferroni’s multiple comparison test. No significant differences were noted between the WT and KO mice. Error bars reflect SEM.
Fig. S7.
Myofilament 2D-DIGE ex vivo stretch left ventricles. (A) Representative 2D gels of ex vivo stretch left ventricles. From top to bottom: merged, WT stretch (green), β-arrestin 1 KO stretch (blue), and β-arrestin 2 KO stretch (red). (B–H) Average ratios of phosphorylated to total tropomyosin (P-TM/T-TM) (B), P1 to myosin light chain 2 (P1-MLC-2/MLC-2) (C), P2 to myosin light chain 2 (P2-MLC-2/MLC-2) (D), total P to myosin light chain 2 (P-MLC-2/MLC-2) (E), P1 to troponin T3 (P1-TnT3/TnT3) (F), P1 to troponin T4 (P1-TnT4/TnT4) (G), and P to troponin T (P-TnT/TnT) (H). n = 5 separate left ventricles per genotype and condition (control and stretch). Statistical comparisons were made using one-way ANOVA with Bonferroni’s multiple comparison test. No significant differences were noted between the WT and KO mice. Error bars reflect SEM. βarr1KO, β-arrestin 1 KO; βarr2KO, β-arrestin 2 KO; C, control; Str, ex vivo stretch.
Fig. S8.
Titin isoform switching in ex vivo stretch left ventricles. (A) Representative gel image of ex vivo stretch left ventricles showing Titin N2BA and N2B isoforms. βarr1KO, β-arrestin 1 KO; βarr2KO, β-arrestin 2 KO; Con, control; Str, ex vivo stretch. (B) Average N2BA isoform (N2BA/total). n = 4 separate left ventricles per genotype and condition (control and stretch). Statistical comparisons were made using one-way ANOVA with Bonferroni’s multiple comparison test. No significant differences were noted between the WT and KO mice. Error bars reflect SEM.
Fig. S9.
Kinase phosphorylation with volume infusion. (A) Average phosphorylated ERK (T202/Y204)/total ERK. (B) Average phosphorylated p-38 alpha (T180/Y182)/total p-38 alpha. (C) Average phosphorylated AKT (S473)/total AKT. (D) Average GSK3β (S9)/total GSK3β in WT (noninfused, n = 3; infused, n = 9), β-arrestin 1 KO (βarr1KO) (noninfused, n = 3; infused, n = 12), and β-arrestin 2 KO (βarr1KO) (noninfused, n = 3; infused, n = 12) mice undergoing the in vivo infusion protocol. The fold change in kinase phosphorylation with volume infusion (Inf) was normalized to noninfusion controls (NI) of the same genotype. (Bottom) Representative gel image showing kinase phosphorylation. Statistical comparisons between infused vs. noninfused conditions in the same genotype were made using Wilcoxon’s rank-sum test, where *P < 0.05. Differences between genotypes were assessed using one-way ANOVA with Bonferroni’s multiple comparison test. No significant differences were noted between the WT and KO mice. Error bars reflect SEM.
The Frank–Starling Relationship Is AT1R-Dependent.
We recently showed that β-arrestin can be activated by cellular stretch through mechanosensitive AT1Rs (19, 22). To test the hypothesis that AT1Rs are also necessary for the Frank–Starling mechanism, we used a genetic and pharmacologic experimental approach. AT1R KO mice exhibited a loss of the Frank–Starling relationship with volume loading compared with WT mice (Fig. 4 A and B and Table S4). Similar to the experiments in β-arrestin KO mice, force–Ca2+ experiments in skinned fibers harvested from hearts of AT1R KO mice also showed impaired length-dependent force activation (Fig. 4C and Table S5).
Fig. 4.
Response of AT1R KO mice to volume loading and the force–Ca2+ relationship. (A) Representative serial PV loops of an AT1R KO mouse during the volume infusion protocol. Stroke volume in the both basal loops (SV) and in loops recorded after completion of volume loading (SV′) are denoted by gray arrows. (B) Effect of volume infusion on absolute change in SV in AT1R KO and WT mice reveals marked depression of Frank–Starling force generation in AT1R KO mice. P values for the interaction between LVEDP and genotype for data comparison were provided by two-way repeated-measures ANOVA. *P < 0.05 for comparison between genotypes at a given LVEDP using Bonferroni’s multiple comparison test. (C) Force–Ca2+ relationship in myofilaments harvested from global AT1R KO mice (n = 10) at 1.9 μm and 2.3 μm. The ΔpCa50 value was not significantly different at 1.9 μm vs. 2.3 μm by one-way ANOVA with Bonferroni’s multiple comparison test.
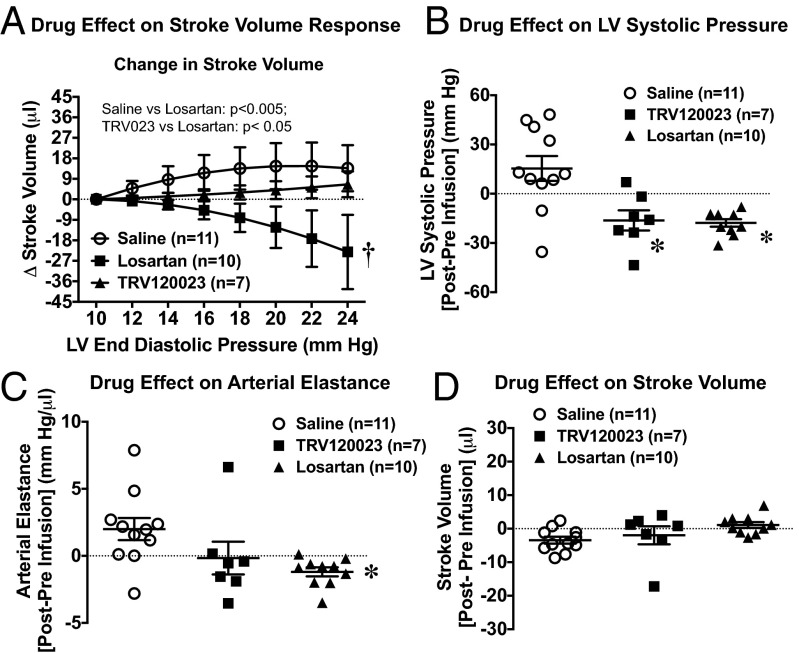
Pharmacologic inhibition of AT1Rs in WT mice pretreated with the nonselective ARB losartan markedly depressed the Frank–Starling relationship compared with saline pretreatment (Fig. 5A). In contrast, pretreatment with the β-arrestin–biased AT1R ligand TRV120023, which selectively activates β-arrestin but not Gq protein-mediated AT1R signaling (16, 18), preserved the Frank–Starling relationship in response to volume loading (Fig. 5A). Importantly, both losartan and TRV120023 similarly reduced LV systolic pressure (Fig. 5B) and arterial elastance (Fig. 5C) before volume loading, owing to their action on inhibiting AT1R-Gαq activation, but had no effect on basal SV (Fig. 5D), indicating that the two AT1R ligands have similar effects on reducing cardiac afterload. Taken together, the results of our pharmacologic and genetic experiments demonstrate that the molecular basis for force generation via length-dependent activation in vivo (i.e., the Frank–Starling mechanism) requires both β-arrestin and the AT1R.
Fig. 5.
Effect of ARB on cardiac response to volume loading. (A) Effect of volume infusion on absolute change in SV in WT mice infused for 5 min with saline, TRV120023 at 100 μg⋅kg–1⋅min–1, or losartan at 5 mg⋅kg–1⋅min–1. The losartan-infused mice had markedly depressed contractility with volume infusion compared with the saline- or TRV120023-treated mice. P values for the interaction of LVEDP and drug were provided for data comparison by two-way repeated-measures ANOVA. *P < 0.05 for saline vs. losartan; †P < 0.05 for TRV120023 vs. losartan at a given LVEDP using Bonferroni’s multiple comparison test. (B) Average change in LV systolic pressure before and immediately after infusion of saline, TRV120023, and losartan revealing similar systolic pressure responses after administration of TRV120023 or losartan. (C) Average change in arterial elastance after infusion of saline, TRV120023, and losartan. (D) Average change in SV before and immediately after infusion of saline, TRV120023, and losartan, showing similar prevolume loading SV in all groups. *P < 0.05 vs. saline using one-way ANOVA with Bonferroni’s multiple comparison test. Error bars reflect SEM.
Discussion
The cellular mechanisms that mediate the Frank–Starling relationship have been of much interest given the central importance of this phenomenon in the maintenance of cardiovascular function. Our data reveal that the fundamental mechanism for length-dependent enhancement of myofilament Ca2+ sensitivity requires β-arrestin 1, β-arrestin 2, and the AT1R. Moreover, we have shown that the conventional ARB losartan has a profound negative effect on cardiac contractility after volume infusion, which is not seen with the β-arrestin–biased AT1R ligand TRV120023.
Identifying a role for β-arrestin in length-dependent force activation advances our current understanding of the neurohormonal control of cardiac function at the level of the sarcomere. β-arrestins are multifunctional scaffolding proteins that function to desensitize ligand-stimulated GPCRs, but also can stimulate cellular signaling pathways distinct from G protein-mediated signaling (14). Although it is now recognized that G protein-mediated signaling downstream of AT1Rs can modulate force activation (4, 8, 23–27), a role for β-arrestin has not been identified previously. Recent work using β-arrestin–biased AT1R ligands, referring to ligands that preferentially activate β-arrestin signaling but do not activate G protein-mediated signaling (28), has suggested a role of AT1R-mediated β-arrestin activation in the regulation of cardiac contractility. In isolated cardiomyocytes, treatment with the β-arrestin–biased AT1R agonist [Sar1, Ile4, Ile8])-angiotensin enhanced contractility without increasing intracellular calcium concentration (17). The inotropic effects of AT1R-biased agonists also have been demonstrated in vivo using novel ligands with greater potency for β-arrestin activation, further documenting β-arrestin’s role in modulating cardiac inotropy in the intact heart (16, 18). The effects of chronic administration of a β-arrestin–biased AT1R ligand were shown to enhance myofilament calcium sensitivity and were associated with reduced TnI and MyBPC phosphorylation and enhanced tropomyosin phosphorylation.
The foregoing studies support the idea that β-arrestin–biased agonism of the AT1R are mild positive cardiac inotropes that act to enhance myofilament calcium sensitivity through posttranslational modification of myofilament proteins. Using an ex vivo preparation, we did not detect similar modifications in myofilament proteins (Figs. S6–S8), although further extensive proteomic analysis is required. Collectively, our findings further advance the current understanding by showing the importance of stretch activated β-arrestin-mediated AT1R signaling in the intact heart as the central basis for myofilament length-dependent activation and the Frank–Starling mechanism of force generation.
Although we were unable to identify protein phosphorylation as a downstream mechanism by which β-arrestin modulates myofilament length-dependent force activation, there is considerable evidence to suggest that cellular signaling alters some element of mechanosensing by the major sarcomeric proteins. In our current understanding of length-dependent activation, interactions among myofilament proteins, which promote an increase in the number of cross bridges reacting with the thin filament in response to stretch of the sarcomere, are subject to modulation by protein phosphorylation. Phosphorylation of troponin I (29–31), myosin-binding protein C (32), and titin (33, 34) has been shown to alter myofilament length-dependent activation. These findings provide important leads for a further detailed investigation of posttranslational modifications associated with β-arrestin signaling in addition to phosphorylation of myofilament proteins, as well as identification of novel proteins in the signaling network. Such a detailed investigation also should reveal site-specific phosphorylations not detected by our approach using Pro-Q staining and phos-tag methodologies. The need for this investigation is exemplified by detailed mass spectrometry proteomic data identifying subtle changes in signaling, presumably at the Z-disk, via GSK3β as responsible for an effect of cardiac resynchronization on myofilament Ca response (35) with no change in protein phosphorylation. We speculate that β-arrestin signaling alters some aspect of mechanosignaling at the Z-disk (36) or M-band (37) that modifies the way in which cross-bridges respond to stretch. Future extensive proteomic analyses are likely to uncover this signaling pathway.
It is now appreciated that the AT1R is mechanosensor that activates intracellular signaling by mechanical stretch, a process that does not require the neurohormone angiotensin II (19, 38), but does require the recruitment and activation of the transducer β-arrestin (19, 22). β-arrestin is selectively recruited to the AT1R by mechanical stretch that allosterically stabilizes distinct stretch-induced receptor conformational states of the AT1R (22), as demonstrated by enhanced binding affinity for β-arrestin–biased AT1R ligands compared with the agonist angiotensin II (22), supporting that concept that stretch induces an AT1R conformation distinct from that induced by angiotensin II (22, 38). Our present work identifies AT1Rs and β-arrestins as critical molecules for coupling ventricular stretch and force production in vivo, operating through alterations in myofilament calcium sensitivity, and supports our contention that the mechanosensitive properties of the AT1R linking the cell membrane to the myofilaments serve as the molecular basis for the century-old physiological principle of the Frank–Starling law of the heart.
Ventricular stretch induces both a rapid increase in cardiac contractility that is dependent on changes in myofilament calcium sensitivity and a later increase in contractility that is dependent on Ca2+ flux, termed “slow force” or the Anrep effect (39). The latter phenomenon was first described by Gleb von Anrep in the early 1900s in experiments in which increased cardiac contractility was observed in canine hearts after aortic constriction (40). More recently, the Anrep effect has been shown to depend on both the AT1R (41) and epidermal growth factor receptor (EGFR) function (42). Because β-arrestin has been shown to be necessary for ligand- and stretch-mediated AT1R-stimulated EGFR transactivation (19, 20), it is possible that the observed attenuation in volume infusion-induced contractility in the β-arrestin KO mice is due in part to a loss of the Anrep effect. Although we did not directly test this possibility, our ex vivo stretch experiments (Fig. 2E) suggest that both Frank–Starling force generation, notable within a single cardiac cycle, and the Anrep effect, notable after many cardiac cycles, are modulated by β-arrestins; however, further studies examining the effect of β-arrestins on myocardial stretch-induced changes in intracellular calcium are needed to adequately address the relationship between β-arrestins and the Anrep effect.
The finding that conventional AT1R blockers have a profound negative effect on the Frank–Starling relationship has potential important implications for clinical care. Conventional AT1R blockers have been shown to improve mortality and morbidity in patients with congestive heart failure who are intolerant of angiotensin II-converting enzyme (ACE) inhibitors (43). Thus, our data suggest an apparent conundrum between the simultaneous positive and negative effects ARB therapy. Clues to addressing this situation are present in the clinical literature examining the comparative efficacy of ACE inhibitors and ARBs on preventing cardiovascular events. Large clinical trials using conventional ARBs in hypertensive patients have not demonstrated a mortality benefit and have identified an increased risk for cardiovascular events (44), which is distinct from outcomes with patients treated with ACE inhibitors. The paradox between the expected benefit of ARB monotherapy and the observed neutral or negative effects have called into question the utility of this therapy (45). Our data identify β-arrestin–biased AT1R agonism as a way to enhance the Frank–Starling mechanism through length-dependent force activation. In conclusion, our work identifies β-arrestin and the AT1R as critical modulators of the Frank–Starling law of the heart, and expands our current understanding of the molecular basis for length-dependent force activation of the heart muscle.
Materials and Methods
Experimental Animals.
Eight- to 12-wk-old control C57/B6 WT, AT1R KO, β-arrestin 1 KO, and β-arrestin 2 KO mice were used for this study. The animal experiments performed for this study were conducted according to approved protocols and animal welfare regulations of the Institutional Review Board of Duke University Medical Center and the Animal Care and Use Committee of the University of Illinois at Chicago.
Volume Infusion Protocol.
After administration of ketamine/xylazine for sedation, each mouse was placed on a ventilator, and bilateral vagotomy was performed. A polyethylene-50 catheter was inserted into the right external jugular vein for drug infusion. A 1.4-F pressure-conductance catheter (Millar) was then inserted retrograde via the right carotid into the left ventricle, and baseline hemodynamic recordings were made once the mouse achieved a stable state. A continuous recording was made, during which the mouse received 9–12 boluses of a 5% (12.5 mg/250 mL) albumin solution (Grifols Pharmaceuticals) administered every 1 min by hand (30 μL for each bolus). The total duration of the protocol was ∼13 min, during which each mouse received a total volume of 270–360 μL (10.8–14.4 mL/kg).
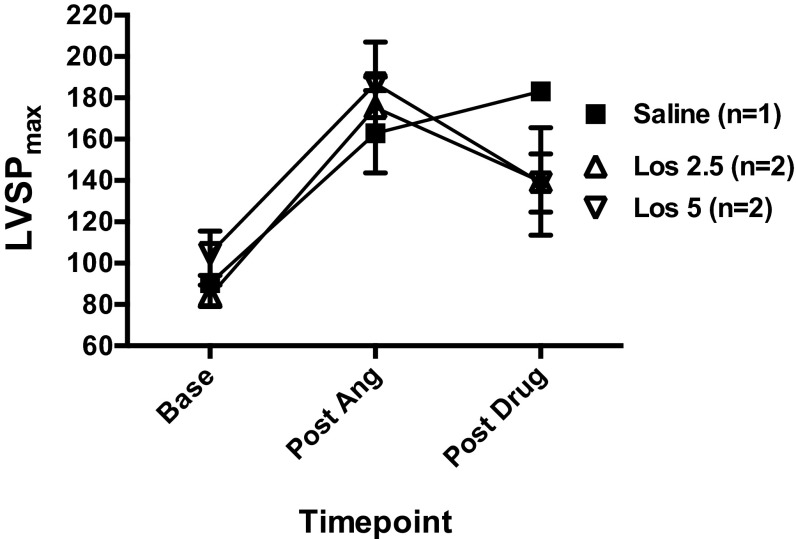
For drug infusion studies, each mouse was pretreated with a continuous i.v. drug infusion (saline, the AT1R β-arrestin-biased ligand TRV120023 100 μg⋅kg–1⋅min–1, or losartan 5 5 mg⋅kg–1⋅min–1) via the right jugular vein for 5 min before undergoing volume infusion protocol. The TRV120023 and losartan doses used in these experiments were based on previously published work (16) and new data (Fig. S5) showing a similar efficacy of these doses in blocking angiotensin II-induced blood pressure elevations. After completion of the studies, the mice were killed, and left ventricles were harvested and flash-frozen in liquid nitrogen. Trevena Inc. generously provided the TRV120023 for these experiments.
Fig. S5.
Losartan dose finding. The dose of losartan used in drug infusion experiments was based on the ability to block angiotensin II infusion-induced increases in LV systolic pressure, in a manner comparable to TRV120023 at a dose of 100 μg⋅kg–1⋅min–1. All mice were given a continuous infusion of angiotensin 100 ng⋅kg–1⋅min–1 for 8 min, during which time they were coadministered saline, losartan 2.5 mg⋅kg–1⋅min–1, or losartan 5 mg⋅kg–1⋅min–1. Error bars reflect SEM.
PV Loop Analysis.
The PV loops used for analysis were those recorded before drug infusion, after drug infusion, and approximately 1 min after a bolus (just before the next bolus) during the volume infusion protocol. Subsequently, parallel conductance (Vp) was determined by a 10-μL injection of 15% saline (15 mg NaCl/100 mL distilled H2O) into the right jugular vein to establish the parallel conductance of the blood pool. The Vp value thus derived was used to correct the PV loop data. In cases where a Vp value could not be accurately recorded, an imputed Vp value from a mouse of the same age, weight, and genotype was used to correct the PV loop data. This occurred in 26% (7 of 27) of the WT mice, in 22% (4 of 18) of the β-arrestin 2 KO mice, and in 13% (2 of 15) of the β-arrestin 1 KO mice. Animals that underwent the volume infusion protocol and did not develop an increase in LVEDP of >5 mmHg or in LVEDV of >10 μL were excluded from the analysis because of inadequate ventricular loading. The LVEDP and LVEDV responses for mice undergoing the volume infusion protocol are shown in Figs. S1 and S2. Load-independent parameters of cardiac compliance and contractility were obtained by continuous PV loop measurement during inferior vena cava constriction. Data were recorded digitally at 1,000 Hz and analyzed with PVAN 3.3 pressure volume analysis software (Millar Instruments).
LV Loading vs. Function Plots.
Average data from three to five cardiac cycles were obtained at baseline and at 1 min after infusion of each volume bolus and recorded. These data were used to generate a series of curves relating the LV loading parameters (LVEDP and LVEDV) with the volume infused (Figs. S1 and S2) and ventricular function (SV) (Fig. S3). Standard curves were generated using a linear regression model in KO mice (Figs. 1 C and D, 2C, and 4B) and a nonlinear second-order polynomial model in drug studies (Fig. 5A) with a least squares (ordinary fit), which provided the closest fit of the raw data; unknown values were interpolated from the standard curve. GraphPad Prism version 6 was used to generate standard curves and interpolate unknown values.
Myofilament Tension-Calcium Assessment.
Measurements of the force–Ca2+ relationship were performed in detergent-extracted fiber bundles isolated from papillary muscles of WT (10 fibers, 10 mice), β-arrestin 2 KO (11 fibers, 11 mice), β-arrestin 1 KO (10 fibers, 10 mice), and AT1R (13 fibers, 13 mice) mice as described previously (46). Adult male mice were anesthetized by i.p. injection of pentobarbital sodium (50 mg/kg). Hearts were quickly excised and LV papillary muscles were dissected into fiber bundles (4–5 mm long and 150–250 µm in diameter) in cold high-relaxing (HR) buffer (10 mmol/L EGTA, 6.5 mmol/L MgCl2, 42 mmol/L KCl, 6.2 mmol/L ATP, 10 mmol/L creatine phosphate, and 100 mmol/L BES, pH 7.0; ionic strength, 180 mmol/L) plus protease inhibitors (2.5 mg/L pepstatin A, 1 mg/L leupeptin, and 50 mmol/L PMSF). Fiber bundles were skinned in HR solution containing 1% Triton X-100 for 30 min and then mounted between a force transducer and a micromanipulator. Sarcomere lengths were set at 1.9 μm and 2.3 μm (in the same fiber) as determined by laser diffraction patterns. The cross-sectional areas of the fibers were calculated assuming an elliptical geometry. The fibers were bathed sequentially in a series of solutions containing increasing Ca2+ concentrations (pCa 7–4.5). Isometric tension was recorded on a chart recorder.
Isometric tension measurements were plotted as a function of pCa and fit by a nonlinear least squares regression analysis to the Hill equation using GraphPad Prism version 6. From this fitted curve, we derived the pCa50 (i.e., the pCa value required to produce 50% of the maximal tension obtained), maximal tension, and the Hill coefficient. Only fibers that maintained >85% maximal tension were included for analysis. If multiple fibers were mounted per animal, an average value was determined and used for final analysis. All experiments were carried out at 22 °C.
Ex Vivo Cardiac Stretch.
Ex vivo cardiac stretch was performed following previously published methods (19). In brief, cardiac stretch was accomplished by creating an LV balloon using a polypropylene membrane inserted into the LV through the mitral valve and inflated with water to yield an LVEDP of 30–50 mmHg. The water-filled balloon was secured to a polyethylene-50 tube and connected to a Statham P23Db pressure transducer (Gould Instruments). LV hemodynamics were continuously recorded with a Mac Lab pressure-recording system (Millar Instruments).
Myofilament Protein Analysis.
Liquid nitrogen-frozen LV tissue was homogenized and myofibrils were prepared as described previously (47), except that the myofibrils were solubilized in UTC buffer [8 M urea, 2 M thiourea, and 4% CHAPS (4 mg CHAPS/100 mL UTC buffer)]. The UTC buffer containing samples was diluted by one-half with 2× Laemmli sample buffer (Bio-Rad) to run a 12% SDS/PAGE (2.4 mL polyacrylamide and 8 mg sodium dodecyl sulfate in 8 mL total volume) gel, after which the gel was stained with Pro-Q Diamond phosphoprotein stain (Thermo Fisher Scientific), which specifically stains phosphorylated proteins, following the manufacturer’s recommendations as described previously (47). Image analysis of Pro-Q–stained gels was performed with ImageQuant TL (GE Healthcare) to obtain the band intensities used for statistical analysis. The UTC buffer containing samples was used for 2D difference gel electrophoresis (2D-DIGE) separation as described previously (48) but using a pH 4–7, 24-cm Immobiline pH gradient strip (GE Healthcare). The 2D-DIGE analysis was performed with PDQuest Advanced version 7.0 (Bio-Rad) to obtain the spot intensities used for statistical analysis. To determine titin isoform expression levels, an SDS-vertical agarose gel electrophoresis (SDS-VAGE) gel was run as described previously (49), and images were analyzed with Image Lab version 5.0 (Bio-Rad) for band intensities. Samples in UTC buffer were diluted by one-half with Laemmli 2× sample buffer to run in the titin gel. All images were acquired with either a Chemidoc XRS+ (Bio-Rad) or a Typhoon 9410 (GE Healthcare) imaging system.
Immunoblotting.
Isolated LV tissue samples isolated from the volume infusion protocol were homogenized in Nonidet P-40 lysis buffer containing 20 mM Tris, pH 7.4, 137 mM NaCl, 1% Nonidet P-40, 20% glycerol (10 mL 100% glycerol in 40 mL distilled H2O), 10 mM PMSF, 1 mM Na3VO4, 10 mM NaF, 2.5 mg/mL aprotinin, and 2.5 mg/mL leupeptin. Protein concentrations were assayed with Bio-Rad protein assay reagent, and 100 μg of protein was denatured by heating at 95 °C for 5 min before resolution by SDS/PAGE. The primary antibodies used were purchased from Cell Signaling Technology and used at a dilution of 1:1,000. Detection was carried out by an enhanced chemiluminescence detection system (Amersham Biosciences). Densitometric analysis was performed with Bio-Rad Fluor-S MultiImager software.
Statistical Analyses.
Average data are expressed as mean ± SEM. Statistical significance for ventricular loading vs. function plots was determined using two-way repeated-measures ANOVA. Comparisons of baseline hemodynamics and post-drug infusion hemodynamics were done using one-way ANOVA, with Tukey’s test for multiple comparisons. Comparisons of passive stress, pCa50, and Hill coefficients were determined using one-way ANOVA with Bonferroni’s correction for multiple comparisons. The D’Agostino–Pearson omnibus normality test was used to assess whether the values were from a Gaussian distribution. All statistical analyses were performed using GraphPad Prism version 6. A P value < 0.05 was considered statistically significant.
SI Materials and Methods
Myofilament Protein Analysis.
Liquid nitrogen-frozen LV tissue was homogenized, and myofibrils were prepared as described previously (47), except that the myofibrils were solubilized in UTC [8 M urea, 2 M thiourea, and 4% CHAPS (4 mg CHAPS/100 mL UTC buffer)] buffer. The UTC buffer containing samples was diluted by one-half with 2× Laemmli sample buffer (Bio-Rad) to run a 12% SDS/PAGE (2.4 mL polyacrylamide and 8 mg sodium dodecyl sulfate in 8 mL total volume) gel. Subsequently, the gel was stained with Pro-Q Diamond phosphoprotein stain (Thermo Fisher Scientific), which specifically stains phosphorylated proteins, following the manufacturer’s recommendations, as described previously (47). Image analysis of Pro-Q– stained gels was performed with ImageQuant TL (GE Healthcare) to obtain the band intensities used for statistical analysis. The UTC buffer containing samples were used for 2D-DIGE separation as described previously (48), but with a pH 4–7, 24 cm Immobiline gradient strip (GE Healthcare). 2D-DIGE analysis was performed with PDQuest Advanced v7.0 (BioRad) to get spot intensities used for statistical analysis. To determine titin isoform expression levels an SDS-VAGE (SDS-vertical agarose gel electrophoresis) gel was run as previously described (49) and images were analyzed with Image Lab v 5.0 (BioRad) for band intensities. Samples in UTC buffer were used and diluted by one-half with Laemmli 2X sample buffer to run in the titin gel. All images were either acquired with a Chemidoc XRS+ (BioRad) or a Typhoon 9410 (GE Healthcare).
Immunoblotting.
Isolated LV tissue samples isolated from volume infusion protocol were homogenized in Nonidet P-40 (Nonidet P-40) lysis buffer containing 20 mM Tris, pH 7.4, 137 mM NaCl, 1% Nonidet P-40, 20% glycerol (10 mL 100% glycerol in 40 mL distilled H2O), 10 mM PMSF, 1 mM Na3VO4, 10 mM NaF, aprotinin (2.5 mg/mL), and leupeptin (2.5 mg/mL). Protein concentrations were assayed with Bio-Rad (Hercules, CA) protein assay reagent, and 100μg protein was denatured by heating at 95 °C for 5 min before resolving by SDS/PAGE. The primary antibodies used were purchased from Cell Signaling Technology and used at a dilution of 1:1,000. Detection was carried out by an enhanced chemiluminescence detection system (Amersham Biosciences, Piscataway, NJ). Densitometric analysis was performed with Bio-Rad Fluor-S MultiImager software.
Acknowledgments
We thank Dr. Thomas M. Coffman for providing the AT1R KO mice and Dr. Robert J. Lefkowitz for providing the β-arrestin 1 KO and β-arrestin 2 KO mice. Requests for these mice should be addressed to Drs. Coffman or Lefkowitz. We also thank Trevena, Inc., for generously providing the TRV120023. This work was supported by National Institutes of Health Grants HL56687 (to H.A.R.), HL75443 (to H.A.R.), HL62426 (to R.J.S.), K08 HL125905 (to D.M.A.), and T32 007692 (to R.T.D.).
Footnotes
Conflict of interest statement: H.A.R. is a scientific cofounder of Trevena, Inc., a company developing G protein-coupled receptor targeted drugs.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1609308113/-/DCSupplemental.
References
- 1.Katz AM. Ernest Henry Starling, his predecessors, and the “Law of the Heart.”. Circulation. 2002;106(23):2986–2992. doi: 10.1161/01.cir.0000040594.96123.55. [DOI] [PubMed] [Google Scholar]
- 2.Adhikari BB, Regnier M, Rivera AJ, Kreutziger KL, Martyn DA. Cardiac length dependence of force and force redevelopment kinetics with altered cross-bridge cycling. Biophys J. 2004;87(3):1784–1794. doi: 10.1529/biophysj.103.039131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearson JT, et al. Effects of sustained length-dependent activation on in situ cross-bridge dynamics in rat hearts. Biophys J. 2007;93(12):4319–4329. doi: 10.1529/biophysj.107.111740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H, et al. PKC-betaII sensitizes cardiac myofilaments to Ca2+ by phosphorylating troponin I on threonine-144. J Mol Cell Cardiol. 2006;41(5):823–833. doi: 10.1016/j.yjmcc.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 5.Pi Y, Zhang D, Kemnitz KR, Wang H, Walker JW. Protein kinase C and A sites on troponin I regulate myofilament Ca2+ sensitivity and ATPase activity in the mouse myocardium. J Physiol. 2003;552(Pt 3):845–857. doi: 10.1113/jphysiol.2003.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirk JA, et al. Left ventricular and myocardial function in mice expressing constitutively pseudophosphorylated cardiac troponin I. Circ Res. 2009;105(12):1232–1239. doi: 10.1161/CIRCRESAHA.109.205427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buscemi N, Foster DB, Neverova I, Van Eyk JE. p21-activated kinase increases the calcium sensitivity of rat triton-skinned cardiac muscle fiber bundles via a mechanism potentially involving novel phosphorylation of troponin I. Circ Res. 2002;91(6):509–516. doi: 10.1161/01.res.0000035246.27856.53. [DOI] [PubMed] [Google Scholar]
- 8.Burkart EM, et al. Phosphorylation or glutamic acid substitution at protein kinase C sites on cardiac troponin I differentially depress myofilament tension and shortening velocity. J Biol Chem. 2003;278(13):11265–11272. doi: 10.1074/jbc.M210712200. [DOI] [PubMed] [Google Scholar]
- 9.Chen PP, Patel JR, Rybakova IN, Walker JW, Moss RL. Protein kinase A-induced myofilament desensitization to Ca(2+) as a result of phosphorylation of cardiac myosin-binding protein C. J Gen Physiol. 2010;136(6):615–627. doi: 10.1085/jgp.201010448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadayappan S, et al. A critical function for Ser-282 in cardiac myosin- binding protein-C phosphorylation and cardiac function. Circ Res. 2011;109(2):141–150. doi: 10.1161/CIRCRESAHA.111.242560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Velden J, et al. The effect of myosin light chain 2 dephosphorylation on Ca2+ sensitivity of force is enhanced in failing human hearts. Cardiovasc Res. 2003;57(2):505–514. doi: 10.1016/s0008-6363(02)00662-4. [DOI] [PubMed] [Google Scholar]
- 12.Solaro RJ. Mechanisms of the Frank–Starling law of the heart: The beat goes on. Biophys J. 2007;93(12):4095–4096. doi: 10.1529/biophysj.107.117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi T, Solaro RJ. Calcium, thin filaments, and the integrative biology of cardiac contractility. Annu Rev Physiol. 2005;67:39–67. doi: 10.1146/annurev.physiol.67.040403.114025. [DOI] [PubMed] [Google Scholar]
- 14.Reiter E, Ahn S, Shukla AK, Lefkowitz RJ. Molecular mechanism of β-arrestin–biased agonism at seven-transmembrane receptors. Annu Rev Pharmacol Toxicol. 2012;52:179–197. doi: 10.1146/annurev.pharmtox.010909.105800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noma T, et al. Beta-arrestin–mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest. 2007;117(9):2445–2458. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim KS, et al. β-Arrestin–biased AT1R stimulation promotes cell survival during acute cardiac injury. Am J Physiol Heart Circ Physiol. 2012;303(8):H1001–H1010. doi: 10.1152/ajpheart.00475.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajagopal K, et al. Beta-arrestin 2–mediated inotropic effects of the angiotensin II type 1A receptor in isolated cardiac myocytes. Proc Natl Acad Sci USA. 2006;103(44):16284–16289. doi: 10.1073/pnas.0607583103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Violin JD, et al. Selectively engaging β-arrestins at the angiotensin II type 1 receptor reduces blood pressure and increases cardiac performance. J Pharmacol Exp Ther. 2010;335(3):572–579. doi: 10.1124/jpet.110.173005. [DOI] [PubMed] [Google Scholar]
- 19.Rakesh K, et al. Beta-arrestin–biased agonism of the angiotensin receptor induced by mechanical stress. Sci Signal. 2010;3(125):ra46. doi: 10.1126/scisignal.2000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J, Ahn S, Rajagopal K, Lefkowitz RJ. Independent beta-arrestin 2 and Gq/protein kinase Czeta pathways for ERK stimulated by angiotensin type 1A receptors in vascular smooth muscle cells converge on transactivation of the epidermal growth factor receptor. J Biol Chem. 2009;284(18):11953–11962. doi: 10.1074/jbc.M808176200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baan J, et al. Continuous measurement of left ventricular volume in animals and humans by conductance catheter. Circulation. 1984;70(5):812–823. doi: 10.1161/01.cir.70.5.812. [DOI] [PubMed] [Google Scholar]
- 22.Tang W, Strachan RT, Lefkowitz RJ, Rockman HA. Allosteric modulation of β-arrestin–biased angiotensin II type 1 receptor signaling by membrane stretch. J Biol Chem. 2014;289(41):28271–28283. doi: 10.1074/jbc.M114.585067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noland TA, Jr, et al. Cardiac troponin I mutants: Phosphorylation by protein kinases C and A and regulation of Ca(2+)-stimulated MgATPase of reconstituted actomyosin S-1. J Biol Chem. 1995;270(43):25445–25454. doi: 10.1074/jbc.270.43.25445. [DOI] [PubMed] [Google Scholar]
- 24.Abedi H, Rozengurt E, Zachary I. Rapid activation of the novel serine/threonine protein kinase, protein kinase D by phorbol esters, angiotensin II and PDGF-BB in vascular smooth muscle cells. FEBS Lett. 1998;427(2):209–212. doi: 10.1016/s0014-5793(98)00427-x. [DOI] [PubMed] [Google Scholar]
- 25.Andersen GO, et al. Alpha(1)-AR–induced positive inotropic response in heart is dependent on myosin light chain phosphorylation. Am J Physiol Heart Circ Physiol. 2002;283(4):H1471–H1480. doi: 10.1152/ajpheart.00232.2002. [DOI] [PubMed] [Google Scholar]
- 26.Cuello F, et al. Protein kinase D selectively targets cardiac troponin I and regulates myofilament Ca2+ sensitivity in ventricular myocytes. Circ Res. 2007;100(6):864–873. doi: 10.1161/01.RES.0000260809.15393.fa. [DOI] [PubMed] [Google Scholar]
- 27.Hinken AC, et al. Protein kinase C depresses cardiac myocyte power output and attenuates myofilament responses induced by protein kinase A. J Muscle Res Cell Motil. 2012;33(6):439–448. doi: 10.1007/s10974-012-9294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: Biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9(5):373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arteaga GM, Palmiter KA, Leiden JM, Solaro RJ. Attenuation of length dependence of calcium activation in myofilaments of transgenic mouse hearts expressing slow skeletal troponin I. J Physiol. 2000;526(Pt 3):541–549. doi: 10.1111/j.1469-7793.2000.t01-1-00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tachampa K, Wang H, Farman GP, de Tombe PP. Cardiac troponin I threonine 144: Role in myofilament length-dependent activation. Circ Res. 2007;101(11):1081–1083. doi: 10.1161/CIRCRESAHA.107.165258. [DOI] [PubMed] [Google Scholar]
- 31.Nowak G, et al. Correlations between alterations in length-dependent Ca2+ activation of cardiac myofilaments and the end-systolic pressure-volume relation. J Muscle Res Cell Motil. 2007;28(7-8):415–419. doi: 10.1007/s10974-008-9136-y. [DOI] [PubMed] [Google Scholar]
- 32.Mamidi R, Gresham KS, Stelzer JE. Length-dependent changes in contractile dynamics are blunted due to cardiac myosin binding protein-C ablation. Front Physiol. 2014;5:461. doi: 10.3389/fphys.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukuda N, Granzier HL. Titin/connectin-based modulation of the Frank–Starling mechanism of the heart. J Muscle Res Cell Motil. 2005;26(6-8):319–323. doi: 10.1007/s10974-005-9038-1. [DOI] [PubMed] [Google Scholar]
- 34.Hanft LM, Greaser ML, McDonald KS. Titin-mediated control of cardiac myofibrillar function. Arch Biochem Biophys. 2014;552-553:83–91. doi: 10.1016/j.abb.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirk JA, et al. Cardiac resynchronization sensitizes the sarcomere to calcium by reactivating GSK-3β. J Clin Invest. 2014;124(1):129–138. doi: 10.1172/JCI69253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pyle WG, Solaro RJ. At the crossroads of myocardial signaling: The role of Z-discs in intracellular signaling and cardiac function. Circ Res. 2004;94(3):296–305. doi: 10.1161/01.RES.0000116143.74830.A9. [DOI] [PubMed] [Google Scholar]
- 37.Pernigo S, et al. Structural insight into M-band assembly and mechanics from the titin-obscurin-like-1 complex. Proc Natl Acad Sci USA. 2010;107(7):2908–2913. doi: 10.1073/pnas.0913736107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yasuda N, et al. Conformational switch of angiotensin II type 1 receptor underlying mechanical stress-induced activation. EMBO Rep. 2008;9(2):179–186. doi: 10.1038/sj.embor.7401157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cingolani HE, Pérez NG, Cingolani OH, Ennis IL. The Anrep effect: 100 years later. Am J Physiol Heart Circ Physiol. 2013;304(2):H175–H182. doi: 10.1152/ajpheart.00508.2012. [DOI] [PubMed] [Google Scholar]
- 40.von Anrep G. On the part played by the suprarenals in the normal vascular reactions of the body. J Physiol. 1912;45(5):307–317. doi: 10.1113/jphysiol.1912.sp001553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pérez NG, de Hurtado MC, Cingolani HE. Reverse mode of the Na+–Ca2+ exchange after myocardial stretch: Underlying mechanism of the slow force response. Circ Res. 2001;88(4):376–382. doi: 10.1161/01.res.88.4.376. [DOI] [PubMed] [Google Scholar]
- 42.Villa-Abrille MC, et al. The Anrep effect requires transactivation of the epidermal growth factor receptor. J Physiol. 2010;588(Pt 9):1579–1590. doi: 10.1113/jphysiol.2009.186619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Granger CB, et al. CHARM Investigators and Committees Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: The CHARM-Alternative trial. Lancet. 2003;362(9386):772–776. doi: 10.1016/S0140-6736(03)14284-5. [DOI] [PubMed] [Google Scholar]
- 44.Cheung BM, Cheung GT, Lauder IJ, Lau CP, Kumana CR. Meta-analysis of large outcome trials of angiotensin receptor blockers in hypertension. J Hum Hypertens. 2006;20(1):37–43. doi: 10.1038/sj.jhh.1001931. [DOI] [PubMed] [Google Scholar]
- 45.Strauss MH, Hall AS. Angiotensin receptor blockers may increase risk of myocardial infarction: Unraveling the ARB-MI paradox. Circulation. 2006;114(8):838–854. doi: 10.1161/CIRCULATIONAHA.105.594986. [DOI] [PubMed] [Google Scholar]
- 46.Monasky MM, et al. The β-arrestin–biased ligand TRV120023 inhibits angiotensin II-induced cardiac hypertrophy while preserving enhanced myofilament response to calcium. Am J Physiol Heart Circ Physiol. 2013;305(6):H856–H866. doi: 10.1152/ajpheart.00327.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Layland J, et al. Protection against endotoxemia-induced contractile dysfunction in mice with cardiac-specific expression of slow skeletal troponin I. FASEB J. 2005;19(9):1137–1139. doi: 10.1096/fj.04-2519fje. [DOI] [PubMed] [Google Scholar]
- 48.Warren CM, et al. Use of 2-D DIGE analysis reveals altered phosphorylation in a tropomyosin mutant (Glu54Lys) linked to dilated cardiomyopathy. Proteomics. 2008;8(1):100–105. doi: 10.1002/pmic.200700772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warren CM, Krzesinski PR, Greaser ML. Vertical agarose gel electrophoresis and electroblotting of high-molecular-weight proteins. Electrophoresis. 2003;24(11):1695–1702. doi: 10.1002/elps.200305392. [DOI] [PubMed] [Google Scholar]