Significance
Mutations in BRAF are dominant drivers of melanoma, and hence targeting them has been a success story in treatment of cancer. However, resistance to BRAF inhibitors is rapidly acquired and is a major impediment to cancer therapy. BRAF mutations per se only lead to nevi that remain nonmalignant for decades. What then is the key rate-limiting determinant of BRAF signaling for progression to melanomas? We show that mutant TERT promoter is a key downstream target of RAS-ERK and its activation downstream of BRAF signaling is a major rate-limiting step in melanoma progression, suggesting that simultaneous disruption of BRAF signaling and active chromatin remodeling at mutant TERT promoters may be an attractive strategy to overcome BRAF-inhibitor resistance during melanoma therapy.
Keywords: TERT promoter mutations, BRAF mutations, ERK-MAPK pathway, telomerase reactivation, cancer
Abstract
Although activating BRAF/NRAS mutations are frequently seen in melanomas, they are not sufficient to drive malignant transformation and require additional events. Frequent co-occurrence of mutations in the promoter for telomerase reverse transcriptase (TERT), along with BRAF alterations, has recently been noted and correlated with poorer prognosis, implicating a functional link between BRAF signaling and telomerase reactivation in melanomas. Here, we report that RAS-ERK signaling in BRAF mutant melanomas is critical for regulating active chromatin state and recruitment of RNA polymerase II at mutant TERT promoters. Our study provides evidence that the mutant TERT promoter is a key substrate downstream of the RAS-ERK pathway. Reactivating TERT and hence reconstituting telomerase is an important step in melanoma progression from nonmalignant nevi with BRAF mutations. Hence, combined targeting of RAS-ERK and TERT promoter remodeling is a promising avenue to limit long-term survival of a majority of melanomas that harbor these two mutations.
Human melanoma is a major skin malignancy accounting for nearly 80% of deaths linked to skin cancers worldwide (1). Due to the high metastatic potential of melanomas and the increased resistance of malignant melanomas to conventional therapies, melanoma is well known for its aggressiveness and poor survival rates (2, 3).
The RAS-RAF-MEK-ERK signaling pathway is one of the key oncogenic pathways that drives melanoma progression and is also known to play a central role in normal melanocyte differentiation and expansion (4). Pathological mutations in the genes encoding protein kinases that participate in the RAS-RAF-MEK-ERK pathway were identified more than a decade ago (5–7). In particular, oncogenic mutations in the B-Raf proto-oncogene, serine/threonine kinase (BRAF), which signals upstream of MEK, as well as the GTPase protein NRAS (neuroblastoma RAS viral oncogene homolog) have been widely documented in various stages of human melanoma (8, 9). The BRAF V600E mutation, a substitution of valine to glutamic acid at codon 600, is one of the most frequent BRAF mutations in melanoma that drives constitutive MEK (mitogen-activated protein kinase kinases) activation, thereby leading to aberrant stimulation of extracellular signal-regulated kinase (ERK) activity (10).
BRAF mutations are known to be dominant drivers of melanoma, and hence targeting these mutations has been one of the success stories in treatment of cancer (11). However, resistance to BRAF inhibitors is rapidly acquired and is a major impediment to cancer therapy (12). Indeed, effectively blocking the key determinants of BRAF signaling could allow for a more sustained response. It is well known that mutations in BRAF per se lead to nevi that remain nonmalignant for many years or decades (13). What, then, are the key rate-limiting determinants of BRAF signaling for progression to melanomas?
Mutations in telomerase reverse transcriptase (TERT) promoter have recently been described as predominant genetic alterations in over 70% of human melanomas (14, 15). Although the oncogenic role of BRAF V600E mutations as a major driver of melanoma progression have been well studied since its discovery in 2002, the significant association of BRAF and TERT promoter mutations was only recently reported in a high percentage of metastatic melanoma cases following the discovery of TERT promoter mutations in 2013 (16, 17). This co-occurrence of BRAF and TERT promoter alterations was also found in other cancer types such as papillary thyroid cancers and was highly correlated with worse prognosis of these cancers (17–19). TERT mutations occur in the core promoter region of the telomerase reverse transcriptase gene encoding the catalytic subunit of human telomerase and are known to generate a new motif for the E-twenty-six (ETS) family of transcription factors. Although several groups have characterized the major ETS factor(s) that bind mutant TERT promoters (20–23), it is not known if BRAF V600E-induced ERK signaling plays a key role in the transcriptional regulation of TERT reactivation at mutant TERT promoters in human melanoma.
In this study, we sought to investigate the mechanistic relevance of RAS-ERK activation in BRAF/NRAS mutant cells with mutant TERT promoters. Our findings demonstrate a major role of RAS-ERK signaling in the maintenance of an active chromatin state at mutant TERT promoters, which facilitates the recruitment of RNA polymerase II that activates TERT transcription in BRAF-mutant melanoma cells. ERK2's (mitogen-activated protein kinase 1) association at mutant TERT promoters promoted the dissociation of the histone deacetylase 1 (HDAC1) repressor complex from Sp1 sites, thereby leading to increased acetylation of histone 3 lysine 9 (H3K9ac). Hence, we provide evidence of the molecular reason for the significant coassociation of BRAF mutations and TERT promoter mutations in melanoma progression.
Results
BRAF/NRAS Mutant Melanoma Cell Lines Frequently Display TERT Promoter Mutations.
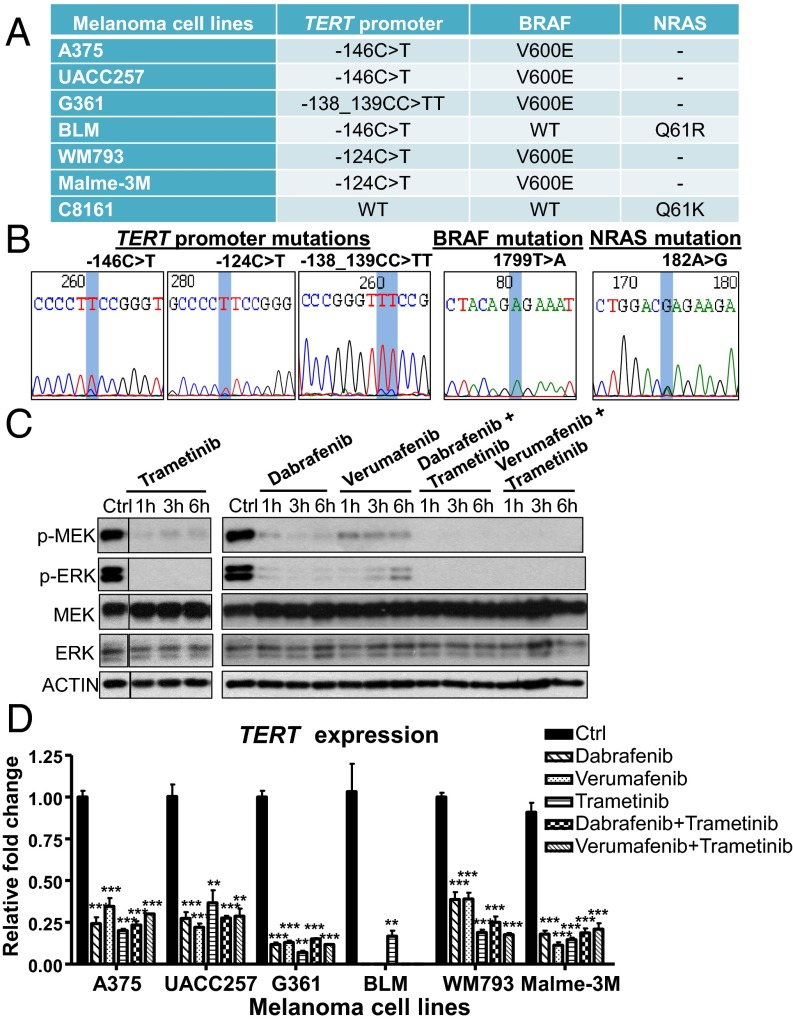
To investigate a functional coassociation, if any, between BRAF/NRAS and TERT promoter mutations, we sequenced a variety of human melanoma cell lines for BRAF, NRAS, and TERT promoter mutations. From our analysis, we found seven melanoma cell lines harboring either BRAF V600E or NRAS mutations: A375, UACC257, G361, BLM, WM793, Malme-3M, and C8161 (Fig. 1 A and B). Six BRAF/NRAS-mutant cell lines were found to contain TERT promoter mutations at positions −146C > T, −124C > T, or -138_139CC > TT (Fig. 1 A and B), which result in the de novo creation of an ETS binding motif and are associated with increased transcriptional activity at the TERT promoter (14, 15). These observations are consistent with current literature reporting the frequent co-occurrence of BRAF/NRAS and TERT promoter mutations in human melanoma tumors (16, 17, 24, 25).
Fig. 1.
Disruption of the RAS-ERK pathway inhibits the transcriptional activation of TERT at mutant TERT promoters in BRAF/NRAS mutant melanoma cells. (A) Table shows the profiles of BRAF, NRAS, and TERT promoter mutations in human melanoma cell lines. (B) Representative DNA chromatogram sequences of BRAF, NRAS, and TERT promoter mutations of melanoma cell lines analyzed. (C) A375 cells were treated with 0.4 µM verumafenib, 0.1 µM dabrafenib, and 20 nM trametinib alone or in combination with trametinib for 1, 3, and 6 h. Cell lysates of control and treated samples were analyzed for changes in MEK1/2 and ERK1/2 phosphorylation by Western blotting. (D) BRAF/NRAS-mutant, TERT promoter mutant melanoma cells were treated with 0.1 µM dabrafenib, 0.4 µM verumafenib, and 20 nM trametinib alone or in combination with trametinib for 1 d and analyzed for TERT expression by quantitative real-time PCR (qPCR). Data from three experiments are represented as the relative fold change in mRNA expression (mean ± SEM). Student’s t test (two-tailed) was used for all statistical analyses: **P < 0.01; ***P < 0.001.
BRAF and MEK Inhibitors Reduce TERT Expression in BRAF/NRAS-Mutant Melanoma Cells.
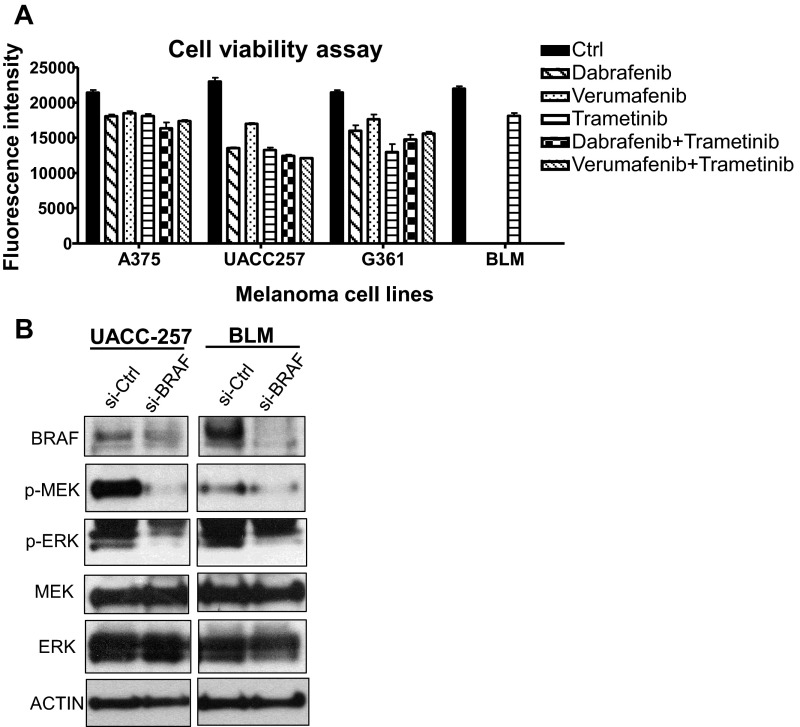
The C8161 cell line was recently reported to contain WT TERT promoter, thereby rendering it unresponsive to TERT inhibition when treated with MEK inhibitor (26). We thus analyzed the effect of RAS-ERK inhibition on TERT expression in other BRAF/NRAS-mutant cell lines carrying TERT promoter mutations. Short-term treatment of BRAF-mutant melanoma cell lines with the BRAF inhibitors verumafenib and dabrafenib alone or in combination with the MEK inhibitor trametinib, as well as treatment of NRAS-mutant BLM cells with trametinib, resulted in the corresponding downregulation of MEK1/2 and ERK1/2 phosphorylation (Fig. 1C). Downstream reduction of ERK phosphorylation led to a rapid decline in TERT expression in all TERT promoter mutant melanoma cell lines following 1 d of treatment of BRAF and/or MEK inhibitors (Fig. 1D). This reduction in TERT expression corresponded with their decreased proliferation (Fig. S1A), suggesting that RAS-ERK–mediated regulation of TERT transcription is important for the survival of melanoma cells.
Fig. S1.
Downregulation of RAS-ERK signaling reduces the proliferation of BRAF/NRAS-mutant melanoma cells carrying the TERT promoter mutation. (A) Cell viability assay of A375, UACC257, G361, and BLM cells following 3 d treatment of 0.1 µM dabrafenib, 0.4 µM verumafenib, and 20 nM trametinib alone or in combination with trametinib. Data from two experiments are represented as average fluorescence intensity (mean ± SEM). (B) si-Ctrl or si-BRAF–treated UACC257 and BLM cells were analyzed for levels of phosphorylated or total MEK1/2 and ERK1/2 by Western blotting.
Downregulation of RAS-ERK Signaling Inhibits the Transcriptional Activation of TERT at Mutant TERT Promoters in BRAF/NRAS-Mutant Melanoma Cells.
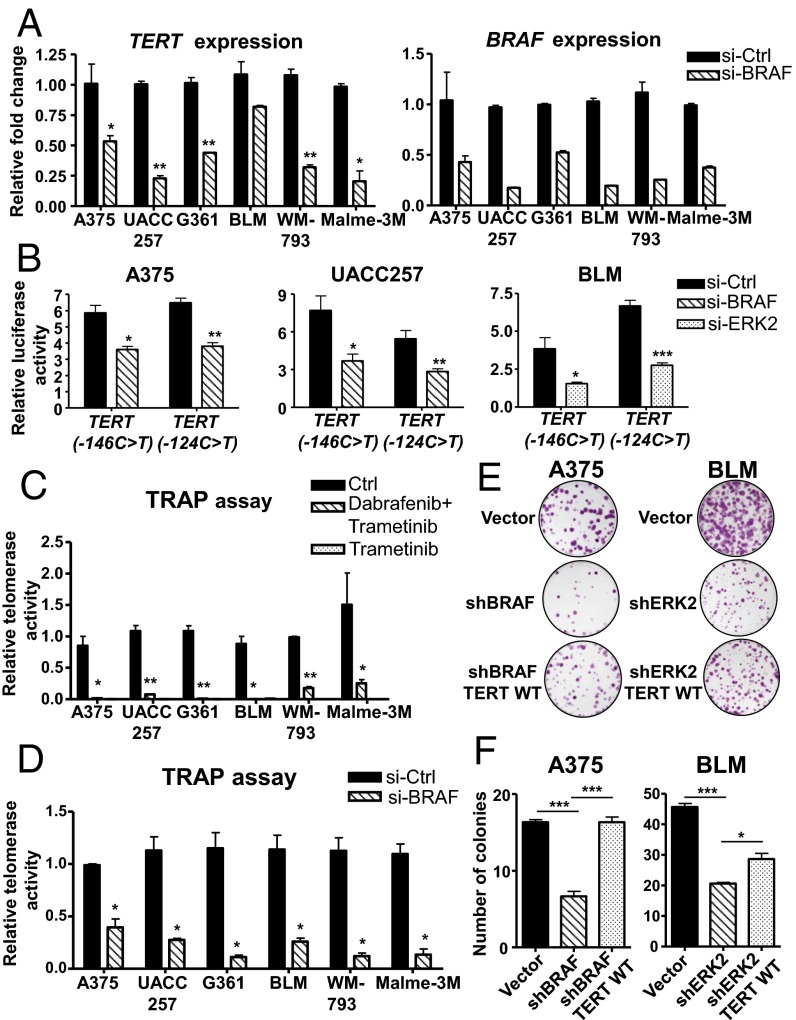
Consistent with our findings using BRAF and MEK inhibitors, downregulation of RAS-ERK signaling via siRNA-mediated knockdown of BRAF in BRAF-mutant, TERT promoter mutant melanoma cells resulted in a similar decrease in TERT expression (Fig. 2A). These observations were further corroborated by luciferase reporter assays using reporter constructs for TERT promoters carrying either of the two hotspot mutations, −146C > T and −124C > T (14, 15), in three melanoma cell lines (Fig. 2B). Downregulation of ERK1/2 phosphorylation in BRAF-mutant A375 and UACC257 cells using siRNA targeting BRAF or in NRAS-mutant BLM cells via siRNA knockdown of ERK2 resulted in reduced transcriptional activation of both mutant TERT promoter reporters (Fig. 2B), demonstrating that the RAS-ERK pathway regulates transcriptional activation of TERT at TERT promoter mutations.
Fig. 2.
Inhibition of RAS-ERK signaling reduces the telomerase activity and proliferation of BRAF/NRAS-mutant melanoma cells carrying TERT promoter mutations. (A) A375, UACC257, G361, BLM, WM793, and Malme-3M cells were treated with si-Control (Ctrl) or si-BRAF and analyzed for TERT and BRAF expression after 3 d. Plots represent relative fold changes in mRNA expression (mean ± SEM) from two independent experiments. (B) Luciferase reporter assays were performed in A375, UACC257, and BLM cells that were transfected with pGL3 reporter vector containing the TERT promoter region (−340 to −55) with −146C > T or −124C > T mutation. Transfected cells were treated with siRNA-targeting BRAF or ERK2. Data shown are representative of two independent experiments. (C) A375, UACC257, G361, WM793, and Malme-3M cells were treated with BRAF and MEK inhibitors (0.1 µM dabrafenib and 20 nM trametinib), and BLM cells were treated with MEK inhibitor (20 nM trametinib). DMSO control (Ctrl) or BRAF/MEK inhibitor-treated cells were analyzed for telomerase activity after 2 d by a Telomere Repeat Amplification Protocol (TRAP) assay. Data shown are from two experiments and represent relative telomerase activity (mean ± SEM). (D) A375, UACC257, G361, BLM, WM793, and Malme-3M cells were transfected with si-Ctrl or si-BRAF and analyzed for telomerase activity after 3 d. Data from two experiments are shown (mean ± SEM). (E) Colony formation assay was performed in vector or shBRAF-expressing A375 and vector or shERK2 BLM cells. TERT was re-expressed in shBRAF and shERK2 cells via lentiviral transduction of Flag-TERT WT construct, and colony formation assay was performed in parallel. Representative images from two independent experiments are shown. (F) Plot depicts average number of colonies counted per field (mean ± SEM) from all experiments in the earlier assay. Student’s t test (two-tailed) was used for all statistical analyses: *P < 0.05; ***P < 0.001.
Reduction of ERK Phosphorylation Results in a Dramatic Loss of Telomerase Activity in BRAF/NRAS-Mutant Melanoma Cells.
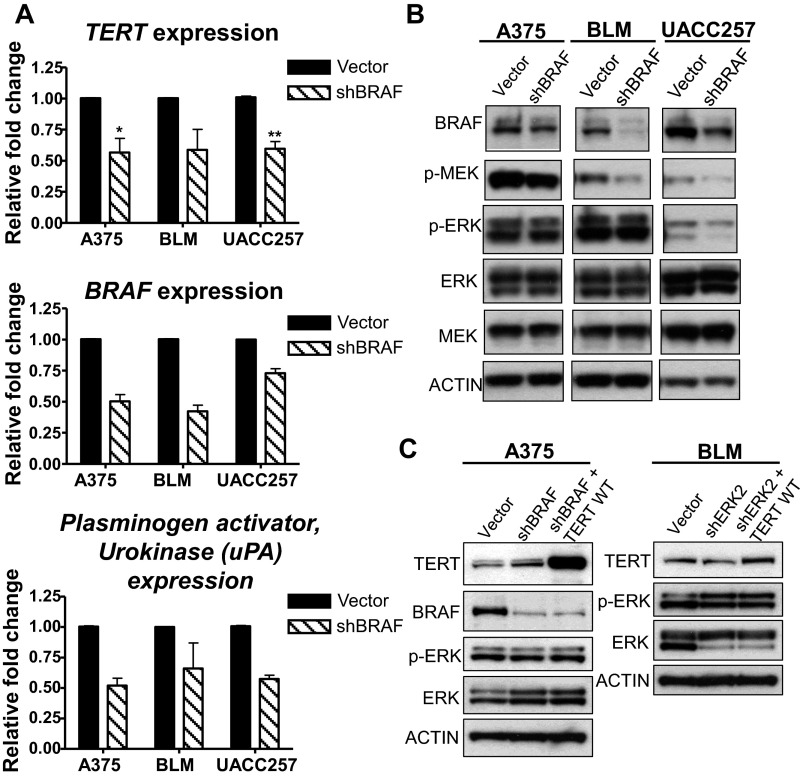
The reduction of phosphorylated ERK1/2 in BRAF/NRAS-mutant melanoma cells carrying TERT promoter mutations, using either BRAF and MEK inhibitors (Fig. 1C) or si-BRAF treatment (Fig. S1B), both resulted in a dramatic loss of telomerase activity (Fig. 2 C and D). This loss of telomerase activity correlated with the decreased long-term proliferation of shBRAF or shERK2 melanoma cells (Fig. 2 E and F), which also display reduced expression of TERT and urokinase plasminogen activator (uPA), a RAS/ERK target gene (Fig. S2A), following downregulation of BRAF (Fig. S2B). Re-expression of TERT in shBRAF or shERK2 melanoma cells (Fig. S2C) rescued the effect of RAS-ERK inhibition on cell proliferation (Fig. 2 E and F), indicating that RAS-ERK–mediated regulation of TERT transcription is essential for the survival of melanoma cells. These findings therefore demonstrate the significant involvement of RAS-ERK signaling in the maintenance of telomerase expression and function in TERT promoter mutant melanoma cells.
Fig. S2.
Inhibition of RAS-ERK signaling via BRAF downregulation reduces TERT expression of BRAF/NRAS-mutant melanoma cells with TERT promoter mutation. (A) A375, BLM, and UACC257 cells were transduced with shRNA targeting BRAF and analyzed for TERT, BRAF, and uPA expression by qPCR. Plots show relative fold changes in mRNA expression (mean ± SEM) from two experiments. *P < 0.05; **P < 0.01; Student’s t test (two-tailed). (B) Vector and shBRAF-expressing melanoma cells were analyzed for protein levels of BRAF and phosphorylated or total MEK1/2 and ERK1/2. (C) A375 and BLM were transduced with shBRAF, shERK2 alone, or in combination with the TERT WT overexpression construct and analyzed for protein levels of TERT, BRAF, and phosphorylated or total ERK1/2.
RAS-ERK Signaling Regulates the Active Chromatin State of Mutant TERT Promoters.
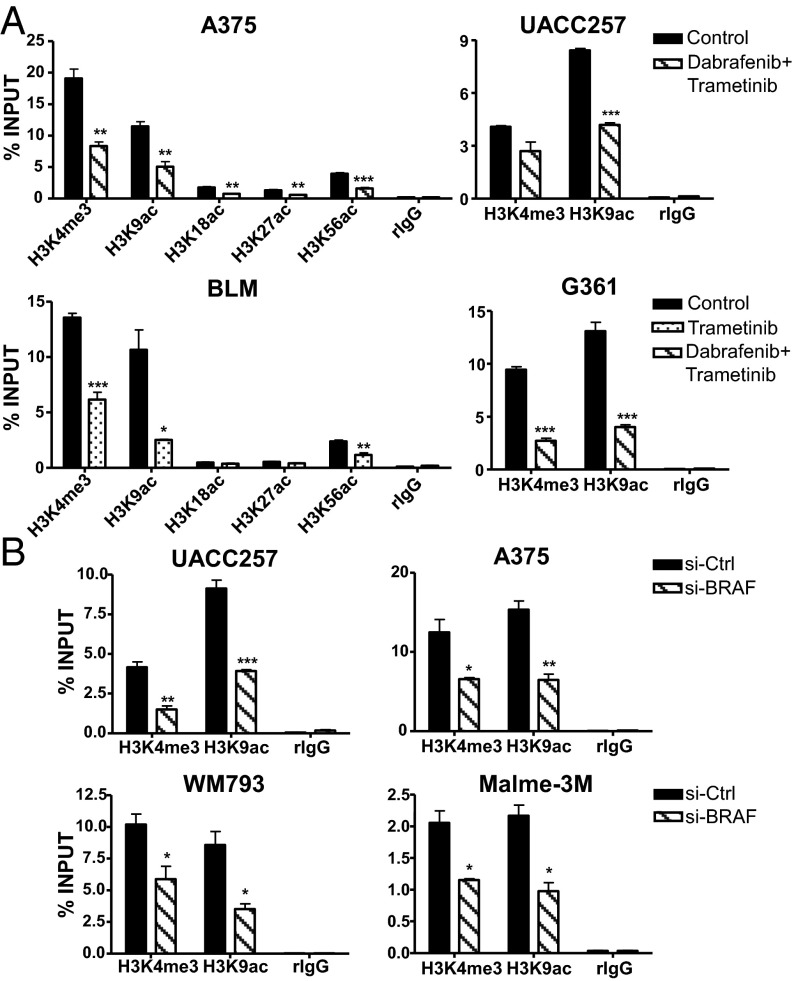
Because a dramatic epigenetic switch has been reported to underscore the activation of TERT at mutant TERT promoters (22), we sought to investigate the molecular basis of the RAS-ERK pathway in the regulation of telomerase reactivation at mutant TERT promoters by performing chromatin immunoprecipitation (ChIP) assays using antibodies targeting various histone marks that are associated with active chromatin. Consistent with our earlier observations, downregulation of RAS-ERK signaling using BRAF and MEK inhibitors resulted in the reduction of trimethylation of histone H3 at lysine 4 (H3K4me3), a histone mark that is associated with transcriptional activation, at the mutant TERT promoters of four melanoma cell lines (Fig. 3A). Moreover, various acetylated histone marks associated with active enhancers and transcriptional elongation, of which the H3K9ac is most prominently enriched, were substantially depleted at mutant TERT promoters following ERK inhibition (Fig. 3A). The reduction of H3K4me3 and H3K9ac levels at mutant TERT promoters was similarly observed in BRAF-mutant, TERT (−146C > T) or TERT (−124C > T) melanoma cell lines, UACC257, A375, WM793, and Malme-3M, following si-BRAF treatment (Fig. 3B). These data collectively demonstrate the critical role of RAS-ERK signaling in regulating the active chromatin state of mutant TERT promoters in BRAF/NRAS-mutant human melanoma.
Fig. 3.
RAS-ERK signaling regulates the active chromatin state of mutant TERT promoters. (A) ChIP was performed in control or BRAF/MEK inhibitor-treated A375, UACC257, BLM, and G361 cells using antibodies specific for different histone marks and IgG as negative control. Enrichment of TERT promoter DNA fragments in ChIP DNA was normalized to DNA input. (B) ChIP was performed in si-Ctrl or si-BRAF–transfected UACC257, A375, WM793, and Malme-3M cells using antibodies specific for H3K4me3 and H3K9ac and IgG as negative control. Data shown represent at least two independent ChIP assays for all cell lines. Student’s t test (two-tailed) was used for all statistical analyses of control versus treated samples for each ChIP: *P < 0.05; **P < 0.01; ***P < 0.001.
Inhibition of the RAS-ERK Pathway Reduces the Recruitment of RNA Polymerase II at Mutant TERT Promoters in Human Melanoma Cells.
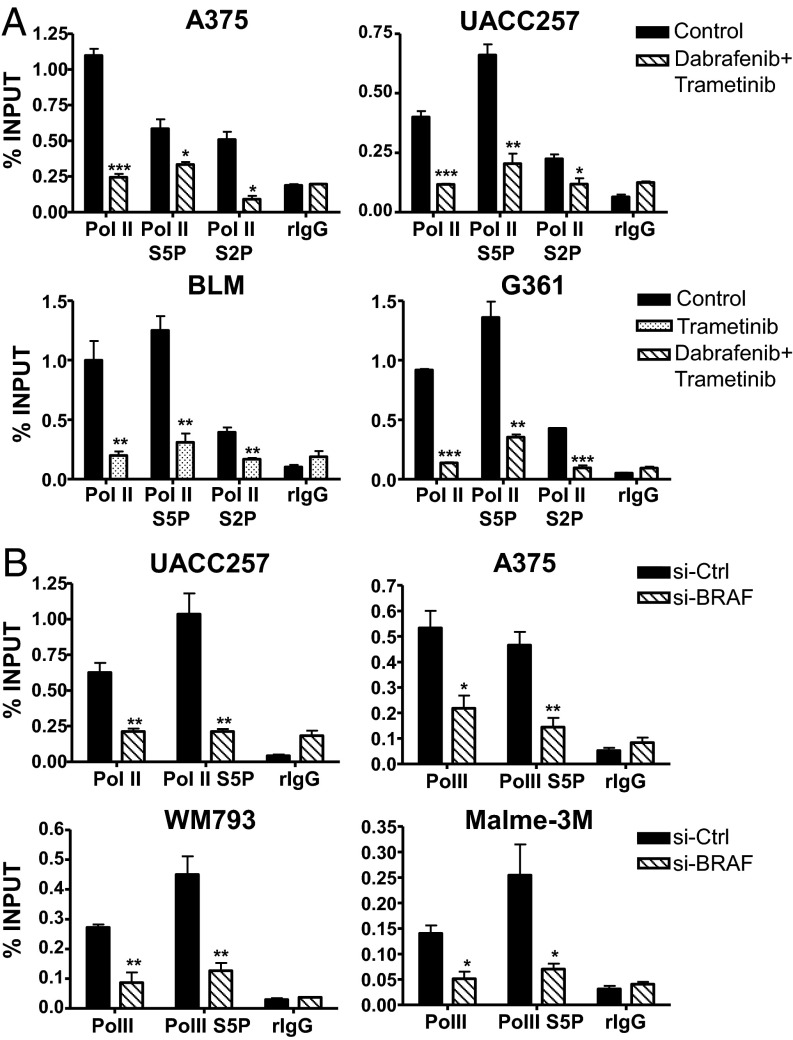
Consistent with the downregulation of an epigenetic signature for active chromatin, disruption of the RAS-ERK pathway by BRAF and MEK inhibitors led to the substantial reduction of RNA polymerase II (Pol II) recruitment at the mutant TERT promoters of BRAF/NRAS-mutant melanoma cell lines (Fig. 4A). Notably, specific enrichment of serine 5 phosphorylated Pol II (Pol II S5P), which is most highly enriched and associated with transcriptional activation at gene promoters, was substantially depleted at the mutant TERT promoters of BRAF/MEK inhibitor-treated melanoma cells (Fig. 4A). The decline in Pol II recruitment was also found in si-BRAF–treated UACC257, A375, WM793, and Malme-3M cells (Fig. 4B), providing further support for the crucial role of RAS-ERK pathway in mediating the recruitment of Pol II during telomerase reactivation at mutant TERT promoters.
Fig. 4.
The RAS-RAF-MEK-ERK pathway regulates the recruitment of RNA polymerase II at mutant TERT promoters in human melanoma cells. (A) ChIP was performed in control or BRAF/MEK inhibitor-treated A375, UACC257, BLM, and G361 cells using antibodies specific for RNA polymerase II (Pol II), Pol II phosphorylated on Serine 5 (Pol II S5P) and Serine 2 (Pol II S2P), and IgG as negative control. Enrichment of TERT promoter DNA fragments in ChIP DNA was normalized to DNA input. (B) ChIP was performed in si-Ctrl or si-BRAF–transfected UACC257, A375, WM793, and Malme-3M cells using antibodies specific for Pol II, Pol II S5P, and IgG as negative control. Plots shown represent at least two independent ChIP assays for all cell lines. *P < 0.05; **P < 0.01; ***P < 0.001; Student’s t test (two-tailed).
Conversion of TERT Promoter Mutation to Wild-Type Promoter Abolishes the Regulation of the RAS-ERK Pathway on Pol II Recruitment.
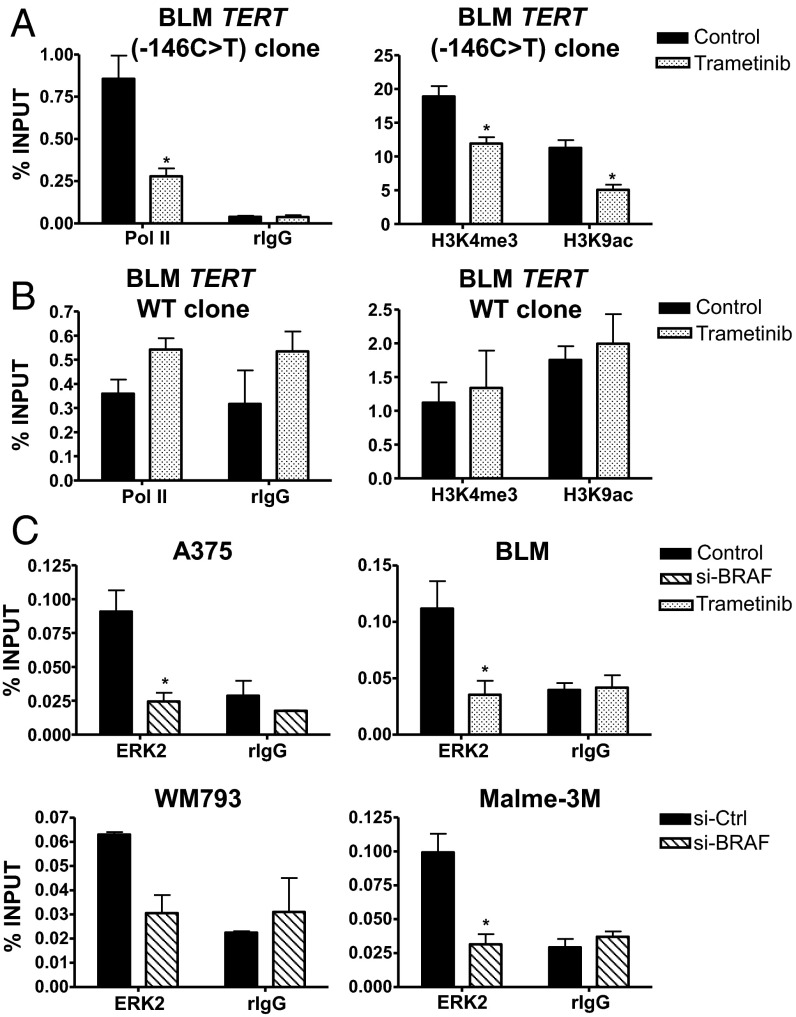
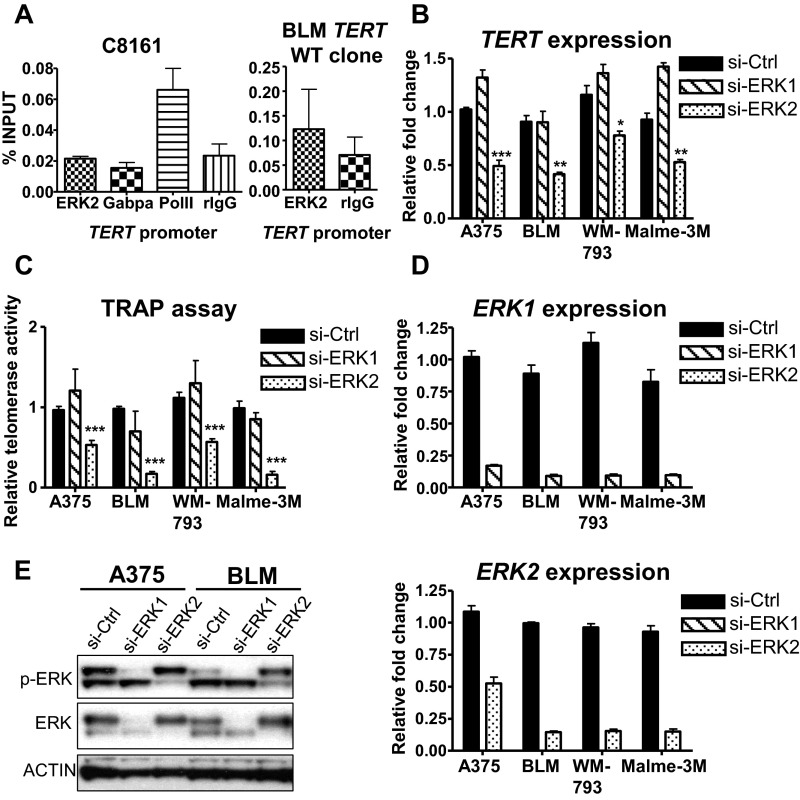
To verify that the regulation of RAS-ERK signaling is specific to mutant TERT promoters, we analyzed isogenic BLM TERT (−146C > T) and TERT WT cells that were previously created using the CRISPR/Cas9 genome editing tool (27). Although TERT (−146C > T) CRISPR cells displayed a similar reduction in active histone marks and Pol II recruitment during MEK inhibitor treatment (Fig. 5A), BLM cells carrying WT TERT promoter did not show any changes when treated similarly with MEK inhibitor (Fig. 5B). In further support of the role of mutant TERT promoter as a major target for RAS-ERK activation, ERK2 was found to be recruited to the mutant TERT promoters (−146C > T and −124C > T) of BRAF/NRAS-mutant melanoma cells (Fig. 5C). Disruption of ERK activation via si-BRAF or MEK inhibitor treatment resulted in the loss of ERK2 binding to mutant TERT promoters (Fig. 5C). Furthermore, in C8161 cells carrying WT TERT promoter, neither ERK2 nor GABPA was recruited to the TERT promoter although Pol II was enriched and BLM TERT WT cells also displayed a loss of ERK2 binding (Fig. S3A). Consistent with the essential role of ERK2-mediated activation of TERT transcription, siRNA inhibition of ERK2 but not ERK1 (mitogen-activated protein kinase 3) resulted in the reduction of TERT expression and loss of telomerase activity in BRAF/NRAS-mutant, TERT-mutant melanoma cells (Fig. S3 B–E).
Fig. 5.
Conversion of TERT promoter mutation to WT promoter abolishes the regulation of the RAS-ERK pathway on TERT reactivation. ChIP was performed in control or trametinib-treated BLM mutant TERT (−146C > T) promoter CRISPR cells (A) or BLM WT TERT promoter CRISPR cells (B) using antibodies specific for RNA polymerase II (Pol II), H3K4me3, and H3K9ac and IgG as negative control. Enrichment of TERT promoter DNA fragments in ChIP DNA was normalized to DNA input. (C) ERK2 is bound to mutant TERT promoters of BRAF/NRAS-mutant melanoma cells. ChIP was performed in si-Ctrl or si-BRAF–treated A375, WM793, and Malme-3M cells and control or trametinib-treated BLM cells using ERK-2–specific antibody and IgG as negative control. Data shown represent two independent experiments for all cell lines. *P < 0.05, Student’s t test (two-tailed).
Fig. S3.
Knockdown of ERK2 reduces telomerase function of TERT promoter mutant melanoma cells. (A) ChIP was performed in BRAF-mutant, WT TERT promoter C8161 cells using antibodies specific for ERK2, GABPA, and RNA polymerase II (Pol II) and IgG as negative control. ERK2 ChIP was performed in BLM TERT WT CRISPR cells. Data shown represent two independent experiments. (B–E) TERT promoter mutant melanoma cells were transfected with si-Ctrl, si-ERK1, or si-ERK2 and analyzed for TERT expression (B), telomerase activity (C), ERK1 and ERK2 expression (D), and protein levels of phosphorylated and total ERK1/2 (E). Expression and TRAP assay data shown are from three experiments. *P < 0.05; **P < 0.01; ***P < 0.001; Student’s t test.
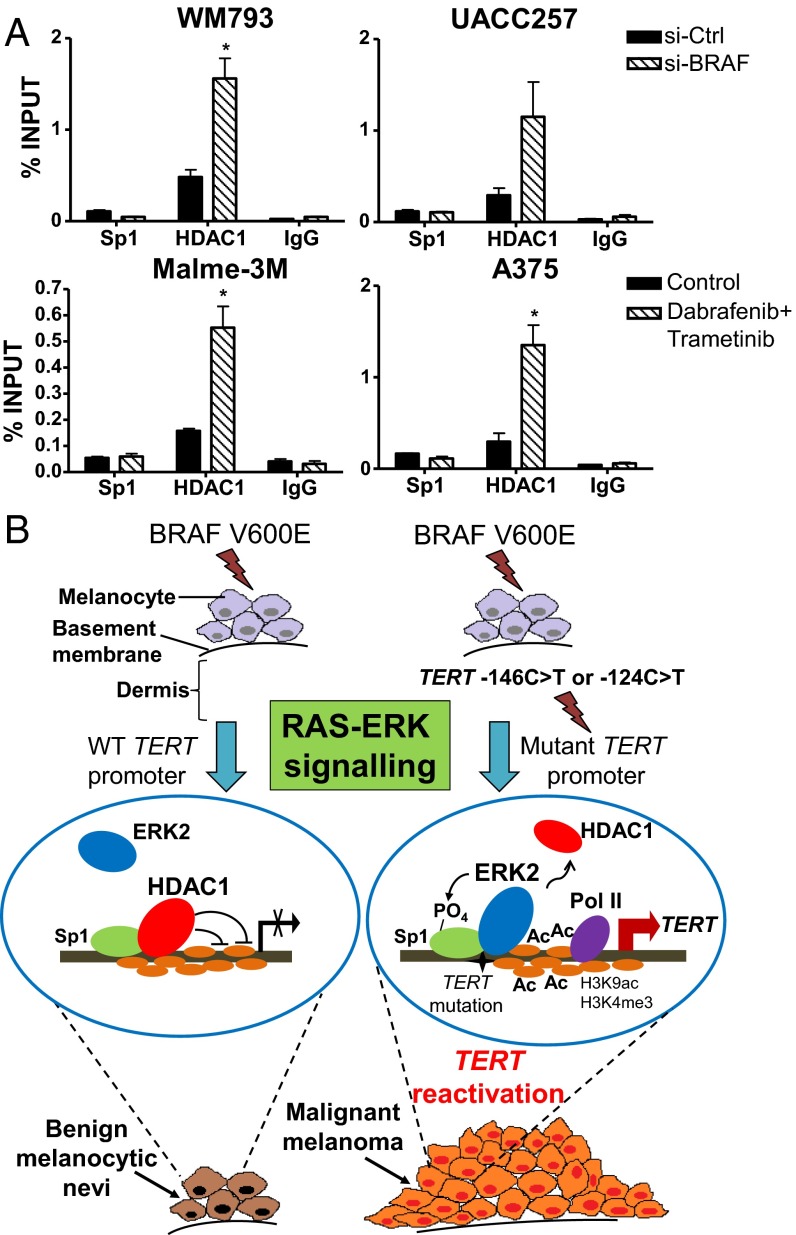
The transcriptional repression of human TERT gene in normal somatic cells is known to be mediated by histone deacetylases, which can be recruited to the TERT promoter by the Sp1 family of transcription factors (28, 29). Because Sp1 has been documented as a direct target of activated ERK and because Sp1 phosphorylation can facilitate the dissociation of the HDAC1 repressor complex from gene promoters in cancer cells (30), we analyzed whether BRAF/MEK inhibition affected HDAC1 recruitment on the mutant TERT promoters of melanoma cells. Consistent with the loss of H3K9 acetylation, disruption of RAS-ERK signaling via si-BRAF or BRAF/MEK inhibitor treatment resulted in enhanced HDAC1 recruitment to the mutant TERT promoters of melanoma cells while Sp1 was similarly enriched (Fig. 6A). These observations therefore reaffirm the crucial role of the RAS-ERK pathway on transcriptional activation at mutant TERT promoters.
Fig. 6.
Inhibition of the RAS-ERK pathway promotes the recruitment of the HDAC1 repressor complex to mutant TERT promoters of melanoma cells. (A) HDAC1 and Sp1 ChIP was performed in the BRAF-mutant, TERT promoter mutant melanoma cells WM793, UACC257, Malme-3M, and A375, which were treated either with si-BRAF or BRAF and MEK inhibitors to disrupt ERK activation. Enrichment of TERT promoter DNA fragments in ChIP DNA was normalized to DNA input. Data shown are representative of two independent experiments. *P < 0.05; Student’s t test (two-tailed). (B) A model for TERT reactivation at mutant TERT promoter during constitutive RAS-ERK signaling in BRAF-mutant melanoma tumors is shown.
Discussion
The RAS-RAF-MEK-ERK signaling pathway plays important biological roles in the regulation of cell proliferation, differentiation, and apoptosis (4). Under normal physiological conditions, transient or controlled activation of ERK by growth factors such as fibroblast growth factor or hepatocyte growth factor is essential for the proliferation and differentiation of melanocytes (4). However, activating mutations in protein kinases that mediate this pathway have been well established to play a central pathological role in the development of melanomas (6, 7). Here we report that aberrant activation of RAS-ERK signaling during BRAF V600E or NRAS mutations maintains an active chromatin state at the mutant TERT promoters of human melanoma, which facilitates the recruitment of RNA polymerase II thereby leading to Pol II (S5P)-mediated transcriptional activation of TERT. We demonstrate that inhibition of ERK phosphorylation via chemical inhibitors or siRNA targeting BRAF activation results in the distinct downregulation of H3K4me3 and H3K9ac—histone marks that are typically associated with gene activation and transcriptional elongation—at the mutant TERT promoters of BRAF/NRAS mutant melanoma cells. This epigenetic repression leads to a corresponding decline in the recruitment of RNA Pol II, particularly the serine 5 phosphorylated form, at the mutant TERT promoter.
Activated ERKs have been documented to bind the promoters of genes associated with development, cell cycle, and pluripotency and to regulate transcriptional activities through phosphorylation of various substrates including transcription factors, histone modifying proteins, and RNA Pol II (31–33). Although a recent study has identified the ETS1 transcription factor as a target of MAPK pathway activation in melanoma cells carrying TERT promoter mutation (26), our data show that RAS-ERK–mediated transcriptional regulation of mutant TERT promoters involves the remodeling of a chromatin environment that is permissive for TERT reactivation. In particular, we find that ERK2 is specifically recruited to the mutant TERT promoters of BRAF/NRAS-mutant melanoma cells and that RAS-ERK inhibition promotes the association of the HDAC1 repressor complex to the TERT core promoter, leading to transcriptional TERT silencing. Our study therefore demonstrates that mutant TERT promoter is a direct target of RAS-ERK signaling.
Significant coassociation of BRAF V600E and TERT promoter mutations has been reported in both malignant melanomas and papillary thyroid cancers. In particular, the co-occurrence of both genetic alterations was concordant with increased aggressiveness and poorer prognosis of disease (16, 17, 19), which suggests the major relevance of constitutive RAS-ERK signaling in TERT reactivation and sustained telomerase activity in malignant tumors. During the development of human melanoma, which displays a high percentage of BRAF (∼60%) and NRAS (∼20%) mutations (4, 5), both genetic alterations are initiated in the early stages of melanoma progression and result in constitutive stimulation of the RAS-ERK-MAPK pathway. In contrast to NRAS mutations that occur in intermediate lesions, BRAF V600E mutations arise exclusively from precursor lesions residing at melanocytic nevi (9). Subsequent to BRAF V600E mutations, TERT promoter mutations begin to emerge as these benign nevi turn malignant (9), indicating that TERT promoter mutations are key driver events necessary for the progression of benign BRAF-mutant lesions to metastatic melanomas.
Although several studies have cataloged the acquisition of BRAF and TERT promoter mutations during melanoma progression, the transcriptional regulation of BRAF V600E-induced ERK signaling on TERT reactivation has not been completely characterized. In this study, we found that RAS-ERK hyperactivation is critical for the maintenance of a permissive chromatin state necessary for the transcriptional activation of mutant TERT promoters in melanoma cells. In particular, short-term exposure of TERT promoter mutant melanoma cells to BRAF and MEK inhibitors resulted in a dramatic decline in TERT transcription and telomerase activity. These observations are in turn recapitulated by the reduced proliferation of these cells when BRAF is downregulated, demonstrating that RAS-ERK signaling is crucial for the maintenance of telomerase function and survival in TERT promoter mutant human melanomas. We therefore present a model for the molecular link between ERK-MAPK signaling and TERT reactivation in BRAF-mutant melanoma tumors that acquire TERT promoter mutations (Fig. 6B). In BRAF V600E-mutant melanocytic lesions, ERK2 does not bind WT TERT promoter, which is transcriptionally repressed due to the recruitment of the HDAC1 complex by Sp1 (29). Hence, RAS-ERK signaling does not activate TERT expression in the absence of TERT promoter mutations, and these precursor lesions remain as benign melanocytic nevi (Fig. 6B). However, when BRAF mutant tumors attain TERT promoter mutations, RAS-ERK hyperactivation leads to the association of ERK2 to mutant TERT promoter, potentially through interaction with GABPA or ETS1 (34, 35), and its subsequent phosphorylation of Sp1 stabilizes it on the mutant promoter. Stabilized ERK on mutant TERT promoter facilitates the dissociation of HDAC1 from mutant TERT promoters, thereby promoting histone 3 lysine 9 acetylation, which generates an active chromatin state that mediates TERT reactivation (Fig. 6B). This results in the sustained telomerase activity of tumor cells that promotes their development to malignant melanomas through canonical and noncanonical functions of TERT (Fig. 6B) (23, 36–39).
One of the major challenges faced in the effective therapy and management of metastatic melanomas is their acquired resistance to BRAF and MEK inhibitors (40). Our findings reveal that the mutant TERT promoter is a major substrate for the RAS-ERK pathway and is a key determinant for the progression of BRAF mutant lesions to malignant melanomas. Hence, combination therapy involving BRAF/MEK inhibitors and blockers of chromatin remodeling of mutant TERT promoters may be an attractive strategy to inhibit telomerase reactivation in melanoma cancers with BRAF/NRAS and TERT promoter mutations. It will also be important to further define the transcription factors and epigenetic regulators that maintain the active chromatin state of mutant TERT promoters downstream of ERK signaling in cancers.
Materials and Methods
Human melanoma G361 and BLM cell lines were kindly provided by Birgit Lane (Institute of Medical Biology, Singapore). A375, UACC257, and Malme-3M cell lines were obtained from Ernesto Guccione (Institute of Molecular and Cell Biology, Singapore). The WM793 cell line was a gift from Jean-Pierre Abastado (Singapore Immunology Network, Agency for Science Technology and Research, Singapore). C8161 melanoma cells were given by Nicolas Dumaz (INSERM, U976, Skin Research Center, Hôpital Saint-Louis, Paris) (26). Details of the reagents and experimental procedures of various assays used for these cell lines are provided in SI Materials and Methods. Institutional review board approval or informed consent was not necessary for this study as research did not involve humans, human embryonic stem cells, or vertebrate animals.
SI Materials and Methods
Cell Culture and Reagents.
UACC257 and WM793 cells were maintained in RPMI medium supplemented with 10% FBS (HyClone), and all remaining cell lines were cultured in Dulbecco’s Modified Eagle’s Medium containing 10% FBS. The mutant TERT (−146C > T) promoter was converted to WT TERT promoter in BLM cells using the CRISPR/Cas9 method as reported previously (21, 27). The BRAF inhibitors vemurafenib (Selleckchem, S1267) and dabrafenib (Selleckchem, S2807) and the MEK inhibitor trametinib (Selleckchem, S2673) were reconstituted with DMSO according to the manufacturer’s recommendations. For BRAF (Dharmacon smartpool siRNA, L-003460), ERK1 (Dharmacon smartpool MAPK3 siRNA, L-003592), and ERK2 (Dharmacon smartpool MAPK1 siRNA, l-003555) siRNA transfection, melanoma cells were plated overnight to 60% density in 12-well plates before treatment with RNA-lipid complexes after 24 h using the X-tremeGENE siRNA transfection reagent (Roche). Lentiviral shRNA plasmids targeting BRAF and ERK2 were cloned into the pLKO.1-puro vector (Addgene) using the following target sequences: BRAF—GCTACAGAGAAATCTCGAT; ERK2—GACATTATTCGAGCACCAACC. TERT WT overexpression vector was generated in our previous study (39).
Genomic DNA Extraction and Sequencing for BRAF/NRAS and TERT Promoter Mutations.
Genomic DNA of melanoma cell lines was isolated and sequenced for TERT promoter mutations as described previously (21). Sequencing for BRAF and NRAS mutations was performed using the following primers: BRAF forward—5′-CCTAAACTCTTCATAATGCTTGCT and BRAF reverse—5′-AGTAACTCAGCAGCATCTCAGG; NRAS forward—5′-CCAGATAGGCAGAAATGGGCT-3′ and NRAS reverse—5′-GCTCTATCTTCCCTAGTGTGGT-3′.
RNA Extraction and qPCR.
Total RNA was isolated using NucleoSpin RNA kit (Machery-Nagel) according to the manufacturer’s recommendations. Superscript Vilo reverse transcriptase (Life Technologies) was used to synthesize cDNA. qPCR was performed with SsoAdvanced Universal SYBR Green Supermix (Bio-Rad) using the following primers: TERT forward—5′-CCAAGTTCCTGCACTGGCTGA and TERT reverse—5′-TTCCCGATGCTGCCTGAC; BRAF forward—5′-TGGCTTACTGGAGAAGAATTGCA and BRAF reverse—5′-ACCACATGTTTGACAGCGGA; plasminogen activator uPA forward—5′-GCCACACACTGCTTCATTGATT and uPA reverse—5′-GTCAGCGCTGTAGTCCTTGT; ERK1 forward—5′- CAGGAGACAGCACGCTTCCAG and ERK1 reverse—5′- TCTAACAGTCTGGCGGGAGAGG; ERK2 forward—5′- GCTGTTCCCAAATGCTGACT and ERK2 reverse—5′- CTCGTCACTCGGGTCGTAAT; and Actin forward—5′-GCCAACCGCGAGAAGATGA and Actin reverse—5′-CCATCACGATGCCAGTGGTA.
Western Blotting.
Western blot analysis was performed according to standard procedures using the following antibodies: anti–Raf-B (Santa Cruz, sc-166), anti-phosphorylated ERK1/2 (Thr202/Tyr204) (Cell Signaling, 9101), anti-phosphorylated MEK1/2 (Ser217/221) (Cell Signaling, 9154), anti-ERK1/2 (p44/42 MAPK) (Cell Signaling, 9102), anti-MEK1/2 (Cell Signaling, 9122), anti-TERT (Epitomics, 1531–1) and anti-actin (Santa Cruz, sc-1616).
Cell Viability Assay and Colony Formation Assay.
Melanoma cells were plated in 96-well plates at a density of 3,000 cells per well and treated with BRAF/MEK inhibitors 2 d postseeding. Alamar Blue cell viability assay was performed using Resazurin sodium salt (Sigma, R7017) according to standard methods. Colony formation assay was performed by seeding 600 cells per treatment type in six-well plates in triplicates and culturing for 12 d before crystal violet staining of viable colonies.
Luciferase Reporter Assay.
Melanoma cells were plated to 70% confluency the following day in 12-well plates. Cells were transfected 24 h later with 2 µg of pGL3-TERT (−146C > T) or pGL3-TERT (−124C > T) promoter luciferase constructs and 200 ng of renilla control plasmid using X-tremeGENE HP DNA transfection reagent (Roche). Forty-eight hours posttransfection, cells were treated with 0.1 µM dabrafenib and 20 nM trametinib for 1 d before assaying for luciferase activity. For siRNA treatment, cells were transfected with si-Ctrl or si-BRAF before transfection with luciferase plasmids. For measurement of firefly and renilla luciferase activity, Dual Luciferase Reporter Kit (Promega) was used. Three replicate wells per sample were measured and firefly luciferase activity was normalized against renilla luciferase activity or protein concentration.
TRAP Assay.
A total of 50,000 viable cells per sample were collected, and TRAP assay was performed as described previously (21, 41).
ChIP.
Melanoma cells were cultured in 15-cm dishes to 90% confluency before fixation with 1% formaldehyde. Diagenode Biorupter was used to sonicate chromatin to a size of 200–500 bp. ChIP was then performed using the following antibodies: anti-ERK2 (Millipore, 05–157), anti-Pol II (Santa Cruz, sc-899), anti-Sp1 (Santa Cruz, sc-420), anti-HDAC1 (Abcam, ab7028), anti-GABPA (Santa Cruz, sc-22810), anti-H3K4me3 (Millipore, 04–745), anti-H3K9ac (RevMAb Biosciences, 31–1054), H3K18ac (RevMAb Biosciences, 31–1055), H3K27ac (RevMAb Biosciences, 31–1056), H3K56ac (RevMAb Biosciences, 31–1078), and nonspecific IgG (Santa Cruz). Pol II ChIP on Malme-3M and C8161 cell lines was performed using anti-Pol II (Santa Cruz, sc-47701). qPCR of ChIP DNA was performed with SsoAdvanced Universal SYBR Green Supermix (Bio-Rad) using the following primers: TERT promoter (−13 to +121) forward—5′-GCGGCGCGAGTTTCAG-3′ and TERT promoter (−13 to +121) reverse—5′- AGCACCTCGCGGTAGTGG.
Acknowledgments
We thank the Agency for Science Technology and Research (Singapore) and the Institute of Molecular and Cell Biology for their support and funding of this work.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1611106113/-/DCSupplemental.
References
- 1.Paluncic J, et al. Roads to melanoma: Key pathways and emerging players in melanoma progression and oncogenic signaling. Biochim Biophys Acta. 2016;1863(4):770–784. doi: 10.1016/j.bbamcr.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 2.Johannessen CM, et al. A melanocyte lineage program confers resistance to MAP kinase pathway inhibition. Nature. 2013;504(7478):138–142. doi: 10.1038/nature12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poulikakos PI, Rosen N. Mutant BRAF melanomas: Dependence and resistance. Cancer Cell. 2011;19(1):11–15. doi: 10.1016/j.ccr.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Wellbrock C, Arozarena I. The complexity of the ERK/MAP kinase pathway and the treatment of melanoma skin cancer. Front Cell Dev Biol. 2016;4:33. doi: 10.3389/fcell.2016.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies H, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 6.Garnett MJ, Marais R. Guilty as charged: B-RAF is a human oncogene. Cancer Cell. 2004;6(4):313–319. doi: 10.1016/j.ccr.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 7.Bos JL. ras oncogenes in human cancer: A review. Cancer Res. 1989;49(17):4682–4689. [PubMed] [Google Scholar]
- 8.Poynter JN, et al. BRAF and NRAS mutations in melanoma and melanocytic nevi. Melanoma Res. 2006;16(4):267–273. doi: 10.1097/01.cmr.0000222600.73179.f3. [DOI] [PubMed] [Google Scholar]
- 9.Shain AH, et al. The genetic evolution of melanoma from precursor lesions. N Engl J Med. 2015;373(20):1926–1936. doi: 10.1056/NEJMoa1502583. [DOI] [PubMed] [Google Scholar]
- 10.Wan PT, et al. Cancer Genome Project Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116(6):855–867. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 11.Flaherty KT, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367(18):1694–1703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merlino G, et al. The state of melanoma: Challenges and opportunities. Pigment Cell Melanoma Res. 2016;29(4):404–416. doi: 10.1111/pcmr.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michaloglou C, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436(7051):720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 14.Horn S, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339(6122):959–961. doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 15.Huang FW, et al. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339(6122):957–959. doi: 10.1126/science.1229259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heidenreich B, et al. Telomerase reverse transcriptase promoter mutations in primary cutaneous melanoma. Nat Commun. 2014;5:3401. doi: 10.1038/ncomms4401. [DOI] [PubMed] [Google Scholar]
- 17.Nagore E, et al. TERT promoter mutations in melanoma survival. Int J Cancer. 2016;139(1):75–84. doi: 10.1002/ijc.30042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin L, et al. BRAF and TERT promoter mutations in the aggressiveness of papillary thyroid carcinoma: A study of 653 patients. Oncotarget. 2016;7(14):18346–18355. doi: 10.18632/oncotarget.7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X, et al. TERT promoter mutations and their association with BRAF V600E mutation and aggressive clinicopathological characteristics of thyroid cancer. J Clin Endocrinol Metab. 2014;99(6):E1130–E1136. doi: 10.1210/jc.2013-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bell RJA, et al. Cancer. The transcription factor GABP selectively binds and activates the mutant TERT promoter in cancer. Science. 2015;348(6238):1036–1039. doi: 10.1126/science.aab0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, et al. Non-canonical NF-κB signalling and ETS1/2 cooperatively drive C250T mutant TERT promoter activation. Nat Cell Biol. 2015;17(10):1327–1338. doi: 10.1038/ncb3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stern JL, Theodorescu D, Vogelstein B, Papadopoulos N, Cech TR. Mutation of the TERT promoter, switch to active chromatin, and monoallelic TERT expression in multiple cancers. Genes Dev. 2015;29(21):2219–2224. doi: 10.1101/gad.269498.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Tergaonkar V. Telomerase reactivation in cancers: Mechanisms that govern transcriptional activation of the wild-type vs. mutant TERT promoters. Transcription. 2016;7(2):44–49. doi: 10.1080/21541264.2016.1160173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griewank KG, et al. TERT promoter mutation status as an independent prognostic factor in cutaneous melanoma. J Natl Cancer Inst. 2014;106(9):dju246. doi: 10.1093/jnci/dju246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macerola E, et al. Coexistence of TERT promoter and BRAF mutations in cutaneous melanoma is associated with more clinicopathological features of aggressiveness. Virchows Archiv. 2015;467(2):177–184. doi: 10.1007/s00428-015-1784-x. [DOI] [PubMed] [Google Scholar]
- 26.Vallarelli AF, et al. TERT promoter mutations in melanoma render TERT expression dependent on MAPK pathway activation. Oncotarget. 2016;7(33):53127–53136. doi: 10.18632/oncotarget.10634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akıncılar S, et al. Long-range chromatin interactions drive mutant Tert promoter activation. Cancer Discov. 2016:CD-16-0177. doi: 10.1158/2159-8290.CD-16-0177. [DOI] [PubMed] [Google Scholar]
- 28.Takakura M, et al. Telomerase activation by histone deacetylase inhibitor in normal cells. Nucleic Acids Res. 2001;29(14):3006–3011. doi: 10.1093/nar/29.14.3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Won J, Yim J, Kim TK. Sp1 and Sp3 recruit histone deacetylase to repress transcription of human telomerase reverse transcriptase (hTERT) promoter in normal human somatic cells. J Biol Chem. 2002;277(41):38230–38238. doi: 10.1074/jbc.M206064200. [DOI] [PubMed] [Google Scholar]
- 30.Liao M, Zhang Y, Dufau ML. Protein kinase Calpha-induced derepression of the human luteinizing hormone receptor gene transcription through ERK-mediated release of HDAC1/Sin3A repressor complex from Sp1 sites. Mol Endocrinol. 2008;22(6):1449–1463. doi: 10.1210/me.2008-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Göke J, Chan Y-S, Yan J, Vingron M, Ng H-H. Genome-wide kinase-chromatin interactions reveal the regulatory network of ERK signaling in human embryonic stem cells. Mol Cell. 2013;50(6):844–855. doi: 10.1016/j.molcel.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 32.Tee W-W, Shen SS, Oksuz O, Narendra V, Reinberg D. Erk1/2 activity promotes chromatin features and RNAPII phosphorylation at developmental promoters in mouse ESCs. Cell. 2014;156(4):678–690. doi: 10.1016/j.cell.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y-J, Wang Y-N, Chang W-C. ERK2-mediated C-terminal serine phosphorylation of p300 is vital to the regulation of epidermal growth factor-induced keratin 16 gene expression. J Biol Chem. 2007;282(37):27215–27228. doi: 10.1074/jbc.M700264200. [DOI] [PubMed] [Google Scholar]
- 34.Flory E, Hoffmeyer A, Smola U, Rapp UR, Bruder JT. Raf-1 kinase targets GA-binding protein in transcriptional regulation of the human immunodeficiency virus type 1 promoter. J Virol. 1996;70(4):2260–2268. doi: 10.1128/jvi.70.4.2260-2268.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selvaraj N, Kedage V, Hollenhorst PC. Comparison of MAPK specificity across the ETS transcription factor family identifies a high-affinity ERK interaction required for ERG function in prostate cells. Cell Commun Signal. 2015;13:12. doi: 10.1186/s12964-015-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akincilar SC, Unal B, Tergaonkar V. Reactivation of telomerase in cancer. Cell Mol Life Sci. 2016;73(8):1659–1670. doi: 10.1007/s00018-016-2146-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khattar E, et al. Telomerase reverse transcriptase promotes cancer cell proliferation by augmenting tRNA expression. J Clin Invest. 2016;126(10):4045–4060. doi: 10.1172/JCI86042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Tergaonkar V. Noncanonical functions of telomerase: Implications in telomerase-targeted cancer therapies. Cancer Res. 2014;74(6):1639–1644. doi: 10.1158/0008-5472.CAN-13-3568. [DOI] [PubMed] [Google Scholar]
- 39.Koh CM, et al. Telomerase regulates MYC-driven oncogenesis independent of its reverse transcriptase activity. J Clin Invest. 2015;125(5):2109–2122. doi: 10.1172/JCI79134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samatar AA, Poulikakos PI. Targeting RAS-ERK signalling in cancer: Promises and challenges. Nat Rev Drug Discov. 2014;13(12):928–942. doi: 10.1038/nrd4281. [DOI] [PubMed] [Google Scholar]
- 41.Akincilar SC, et al. Quantitative assessment of telomerase components in cancer cell lines. FEBS Lett. 2015;589(9):974–984. doi: 10.1016/j.febslet.2015.02.035. [DOI] [PubMed] [Google Scholar]