Significance
Here we present an exception that supports the rule that the 20 human tRNA synthetases acquired new architectures to expand their functions during evolution. The new features are associated with novel, appended domains that are absent in prokaryotes and retained by their many splice variants. Alanyl-tRNA synthetase (AlaRS) is the single example that has a prototypical appended domain—C-Ala—even in prokaryotes, which is spliced out in humans. X-ray structural, small-angle X-ray scattering, and functional analysis showed that human C-Ala lost its prokaryotic tRNA functional role and instead was reshaped into a nuclear DNA-binding protein. Thus, we report another paradigm for tRNA synthetase acquisition of a novel function, namely, repurposing a preexisting domain rather than addition of a new one.
Keywords: appended domain, evolution, structural plasticity, DNA binding, splice variant
Abstract
The 20 aminoacyl tRNA synthetases (aaRSs) couple each amino acid to their cognate tRNAs. During evolution, 19 aaRSs expanded by acquiring novel noncatalytic appended domains, which are absent from bacteria and many lower eukaryotes but confer extracellular and nuclear functions in higher organisms. AlaRS is the single exception, with an appended C-terminal domain (C-Ala) that is conserved from prokaryotes to humans but with a wide sequence divergence. In human cells, C-Ala is also a splice variant of AlaRS. Crystal structures of two forms of human C-Ala, and small-angle X-ray scattering of AlaRS, showed that the large sequence divergence of human C-Ala reshaped C-Ala in a way that changed the global architecture of AlaRS. This reshaping removes the role of C-Ala in prokaryotes for docking tRNA and instead repurposes it to form a dimer interface presenting a DNA-binding groove. This groove cannot form with the bacterial ortholog. Direct DNA binding by human C-Ala, but not by bacterial C-Ala, was demonstrated. Thus, instead of acquiring a novel appended domain like other human aaRSs, which engendered novel functions, a new AlaRS architecture was created by diversifying a preexisting appended domain.
Mammalian aminoacyl tRNA synthetases (aaRSs) have diverse ex-translational functions that include extracellular and nuclear roles manifested in, among other functions, proangiogenesis and antiangiogenesis, immunoregulation, neurogenesis, and stress responses (1–7). These functions are considered to link aaRSs to heritable diseases (3, 4, 8). Although absent with one exception in bacteria, novel appended domains were gained in a progressive and accretive way during the evolution of eukaryotes (1, 9). These domains are dispensable for the catalytic function, but required for new nontranslational functions (1, 9). A total of 13 different appended domains have been annotated (9–11). Some, such as the WHEP [TrpRS(W), HisRS(H), GluProRS(EP)] domain, are joined to more than one tRNA synthetase, but differentiated by wide sequence divergences that are idiosyncratic to the aaRSs (1, 11–15). Most of the more than 250 recorded splice variants of human aaRSs ablate the catalytic domain but retain the noncatalytic addition (9). Whereas 19 tRNA synthetases acquired new domains during evolution, AlaRS is an exception, with a noncatalytic C-terminal domain (C-Ala) that is also present in prokaryotes (1, 10, 16).
Although C-Ala is not essential for sustaining AlaRS-dependent cell growth in bacteria, it enhances aminoacylation by providing contacts with the outside corner of the L-shaped tRNA substrate (16). It is also produced as a splice variant of human AlaRS (9). Here we used functional analysis of two crystal structures to show that human C-Ala is reshaped from docking tRNA in prokaryotes into a DNA-binding domain in humans. Thus, instead of acquiring a special appended domain, a new AlaRS architecture was created by diversifying a preexisting domain.
Results and Discussion
Human C-Ala Has No Effect on Charging Activity.
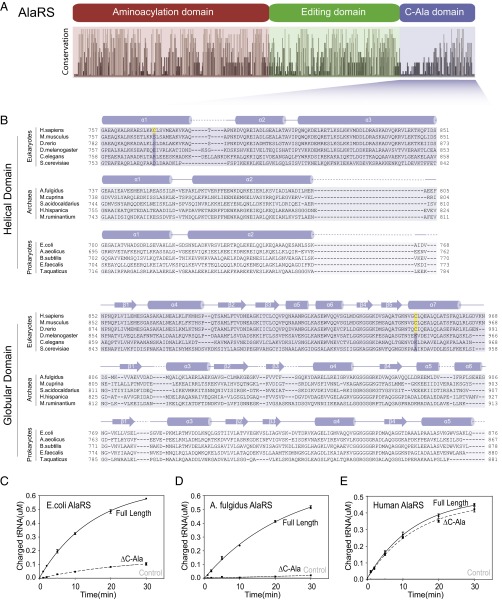
The sequence of C-Ala diverged widely in the evolutionary progression to humans, and this divergence raises the possibility that C-Ala may have developed to play a different role in higher organisms and, in that respect, to be akin to the appended domains of the 19 other aaRSs. To investigate this possibility, we aligned 410 AlaRS sequences from all three kingdoms of life: eukaryotes, archae, and prokaryotes (16) (Fig. 1A). The alignment clearly showed the three well-characterized aminoacylation, editing, and C-Ala domains, and demonstrated that although the aminoacylation and editing domains are well conserved, C-Ala diverges widely (16) (Fig. 1A). Based on the crystal structure of Archaeoglobus fulgidus C-Ala, C-Ala consists of a helical region followed by a globular domain (17). For the various diverged C-Ala domains, we used Predictprotein (18) to predict that the helical subdomains of archaeal and bacterial C-Alas have two α-helices, whereas eukaryotes have a third, long α-helix. In contrast, the globular domains are similar across the three kingdoms (Fig. 1B).
Fig. 1.
Human C-Ala has no effect on the charging activity. (A) Conservation analysis of AlaRS sequences across bacteria, archaea, and eukaryotes showing the relative sequence identity of the 410 aligned AlaRS sequences (16). (B) Alignment generated using the online Clustal Omega server (28). Secondary structural elements of C-Ala are indicated above the sequences. The two cysteines (disulfide bond) are colored in yellow. (C, D, and E) In vitro aminoacylation assay showing that human AlaRS-ΔC-Ala has similar activity relative to human full-length AlaRS (E), whereas E. coli (C) or A. fulgidus (D) AlaRS-ΔC-Ala reduces the charging activity toward tRNAAla compared with the corresponding full-length AlaRS. Error bars indicate SDs.
The helical domain of C-Ala provides contacts for dimerization of A. fulgidus AlaRS (17) and for docking the outside corner of the L-shaped tRNA to the enzyme (19). Consistently, although the C-Ala segment is not essential for aminoacylation, it enhances catalytic efficiency (16, 20–22). Given the low similarity of human and bacterial or archaeal C-Ala, we compared the aminoacylation activity of full-length and C-Ala–truncated human AlaRS (Hs AlaRS and Hs AlaRS-ΔC-Ala) with their Escherichia coli and A. fulgidus orthologs. Consistent with previous studies (16, 20, 21), Ec or Af AlaRS-ΔC-Ala exhibited sharply reduced activity relative to full-length AlaRS, but in contrast, deletion of C-Ala did not significantly affect the activity of Hs AlaRS (Fig. 1 C–E). Thus, Hs C-Ala is completely dispensable for aminoacylation.
Crystal Structure of Human C-Ala.
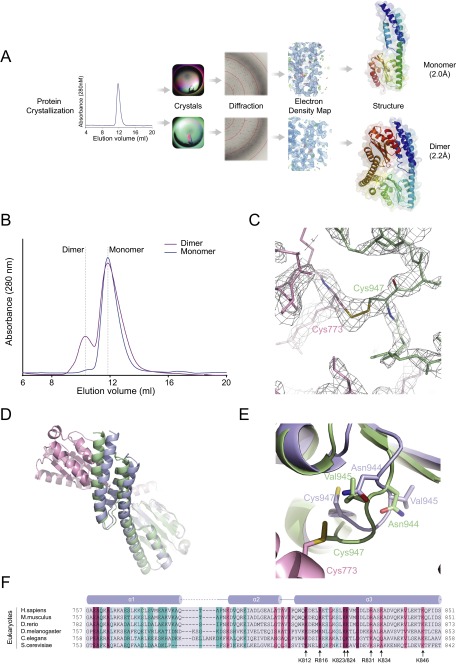
Given Hs C-Ala’s wide sequence divergence and lack of role in aminoacylation, we speculated that its structure is distinct from its prokaryote ortholog. Based on its dispensability for aminoacylation, we hypothesized that Hs C-Ala lacks the contact between C-Ala and the tRNA elbow region that is seen in the bacterial enzyme. Because C-Ala also contributes part of the α2 dimerization interface of bacterial AlaRS, and given the previously noted α2 dimeric quaternary structure of Hs AlaRS, efforts were made to understand whether Hs C-Ala formed a dimer. Based on secondary structure predictions and the previously solved archaeal C-Ala structure (17), we made a C-Ala construct consisting of the C-terminal 757–968 amino acids of human AlaRS. We purified the recombinant protein from the soluble fraction of the bacterial lysate and, during gel filtration, observed only a monomeric form of the recombinant human C-Ala protein in buffer containing 1 mM DTT (Fig. S1B). However, in addition to the monomer, a dimeric form appeared with buffer containing oxidative agents, such as 1 mM glutathione disulfide (GSSG) (Fig. S1B). After screening through a variety of conditions, we obtained two different crystal forms, each of which was specific to a particular condition (Fig. S1A). One of these crystal forms harbored the monomer and was obtained using 0.1 M Tris pH 8.5 and 25% (wt/vol) polyethylene glycol 3350, whereas the other captured a dimer using 0.2 M ammonium acetate, 0.1 M Tris pH 8.5, and 25% (wt/vol) polyethylene glycol 3350.
Fig. S1.
The monomer and dimer of human C-Ala. (A) The flowchart for crystal structure determination. The resolution for human monomer C-Ala is 2.0Å, while for the dimer is 2.2Å. (B) Gel filtration analysis (Superdex 75 10/300 GL, GE Healthcare) of bacterially purified human C-Ala. Traces are shown of human C-Ala in buffer containing 1mM DTT (blue) and buffer containing 1mM GSSG (purple). The elution positions are indicated by dashed lines. (C) The 2Fo - Fc electron density map at the disulfide bond region (contoured at 1.5 σ), which was formed between Cys773 of one molecule and Cys947 of the other. (D) Superimposition of the monomer and dimer structures of human C-Ala. The structures are colored as indicated above. (E) Asn944 and Val945 in the dimer form pushed Cys947 out of the globular subdomain to contact Cys773 from the helical domain of the other molecule. (F) Sequence alignment colored by Consurf score according to the color key shown. The secondary structure for the helical domain is shown along the top of the alignment. The positively charged residues (lysine or arginine), which is highly conserved from the helical domain, is labeled.
The structure of the monomer was determined from a selenomethionine-substituted crystal (Fig. 2A). This crystal (space group P21, with unit-cell parameters a = 41.645, b = 38.449, and c = 62.471Å) diffracted up to 2.0 Å with an asymmetric unit containing one molecule of C-Ala, and a refined model Rwork factor of 21.46% and Rfree factor of 25.76%. The dimer-containing crystal (space group C2221, with unit-cell parameters a = 90.829, b = 136.141, and c = 59.077Å) had one molecule of C-Ala in the asymmetric unit, with a refined model Rwork factor of 20.78% and and Rfree factor of 25.45% (Fig. 2B). Details of the structure determination are provided in SI Methods and Table S1.
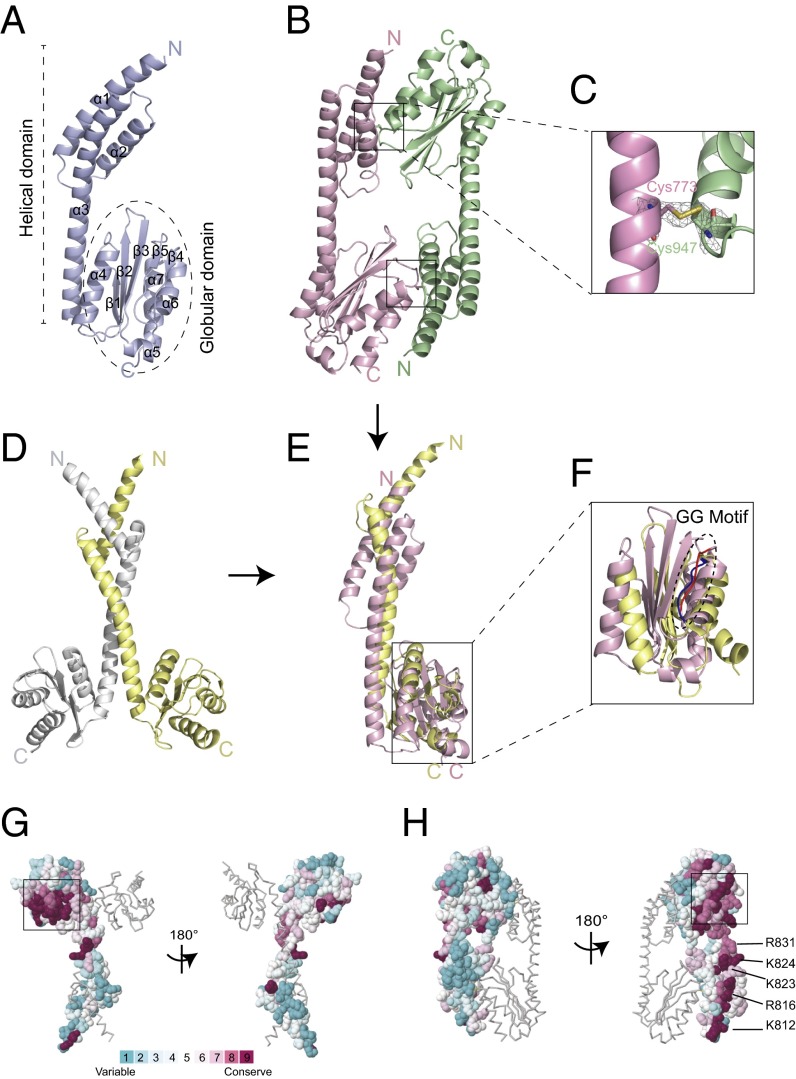
Fig. 2.
Crystal structure of human C-Ala. (A) The crystal structure of monomeric human C-Ala. (B) The crystal structure of dimeric human C-Ala. Two disulfide bonds are shown in the black boxes. One molecule is shown in light purple, and the other is in pale green. (C) A 2Fo-Fc electron density map contoured at 1.5 σ. A disulfide bond was formed between Cys773 of one molecule and Cys947 of the other. (D) A dimeric form of A. fulgidus C-Ala. One molecule is shown in light yellow, and the other is in gray. (E) Superimposition of the monomers of human and A. fulgidus C-Ala. (F) A zoom-in view showing the superimposition of the globular domains of human and A. fulgidus C-Ala. Human C-Ala GG motif is colored in red, and A. fulgidus C-Ala GG motif is shown in blue. (G) Structure of A. fulgidus C-Ala with surface residues colored in accordance with evolutionary conservation (high, magenta; low, cyan) among amino acid sequences from different 150 C-Ala sequences. The boxed area shows the highly conserved GG motif. These figures were prepared using ConSurf server. (H) Structure of human C-Ala with surface residues colored in accordance with evolutionary conservation among amino acid sequences from different 150 C-Ala sequences. The positively charged residues (lysine or arginine), which is highly conserved from the helical domain, is labeled.
Table S1.
Data collection and refinement statistics
| Parameter* | SeMet monomer C-Ala | Native dimer C-Ala |
| Data collection | ||
| Space group | P21 | C2221 |
| Resolution, Å | 50.0–2.0 (2.07–2.00) | 30.0–2.20 (2.28–2.20) |
| Cell dimensions | ||
| a, b, c, Å | 41.65 38.45 62.47 | 90.83 136.14 59.08 |
| α, β, γ, ° | 90, 90.5, 90 | 90, 90, 90 |
| Unique reflections | 13,269 (1,305) | 18,685 (1,858) |
| Multiplicity | 6.4 (6.4) | 7.1 (7.3) |
| Completeness, % | 99.5 (100) | 98.6 (99.9) |
| Mean I/sigma, I | 16.7 (4.9) | 11.5 (4.2) |
| Wilson B-factor | 25.33 | 28.57 |
| Rmerge, %† | 9.1 (40.7) | 12.3 (53.2) |
| Refinement | ||
| Rwork/Rfree, % | 21.46/25.76 | 20.78/25.45 |
| Nonhydrogen protein atoms | 1,740 | 1,712 |
| Macromolecules | 1,583 | 1,577 |
| Water | 157 | 135 |
| Protein residues | 208 | 207 |
| rmsd | ||
| Bond length, Å | 0.011 | 0.003 |
| Bond angles, ° | 1.31 | 0.62 |
| Ramachandran favored, % | 99 | 99 |
| Ramachandran outliers, % | 0 | 0 |
| Average B-factor | 30.90 | 46.90 |
| Macromolecules | 30.40 | 47.10 |
| Solvent | 35.70 | 44.40 |
Values in parentheses are for the highest-resolution shell.
Rmerge = Σhkl|I − <I>|/ΣhklI, where I is the intensity of unique relfection hkl and <I> is the average over symmetry-related observations of unique reflection hkl.
As expected, the monomer of Hs C-Ala consists of a helical subdomain and a separate globular subdomain, similar to the A. fulgidus C-Ala. The helical subdomain contains three α-helices, consistent with the secondary structure predictions, whereas the globular subdomain comprises a five-stranded β-sheet and four α-helices (Figs. 1B and 2A). Interestingly, in the dimeric form, a disulfide bridge was formed between the helical subdomain of one molecule and the globular subdomain of the other molecule (Fig. 2 B and C and Fig. S1C).
The root-mean-squared deviation (rmsd) of the Cα positions between the monomer and dimer is ∼1.5 Å for the superimposed helical subdomains and 0.6 Å for the globular subdomains (Fig. S1D). When the two structures were superimposed, we observed that Asn944 and Val945 in the dimer forced Cys947 out of the globular subdomain to contact Cys773 from the other molecule. In contrast, in the monomer, Cys773 and Cys947 extend in opposite directions and cannot make a disulfide bond (Fig. S1E).
Comparison of Human C-Ala with A. fulgidus C-Ala.
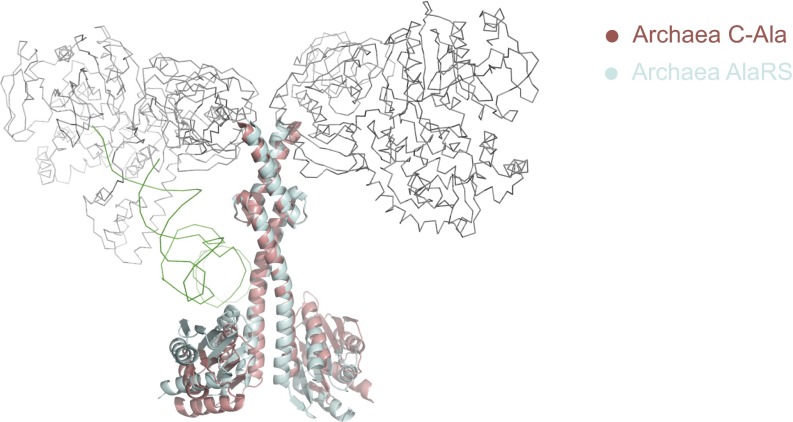
As noted above, dimeric A. fulgidus C-Ala contains a long helical domain and a separate globular domain (Figs. 1B and 2D). The A. fulgidus C-Ala dimer is formed through a helix-loop-helix zipper (HLHZ) between the helical domains of the two partners (17). The structure of this dimer is unchanged in the context of the full-length dimeric A. fulgidus AlaRS (17, 19) (Fig. S2). However, after binding to one tRNA molecule, the globular domain of the full-length A. fulgidus AlaRS exhibits a conformational shift toward tRNA to contact the elbow region (Fig. S2). A comparison of human and archaeal C-Ala dimers shows that whereas archaeal C-Ala has a parallel dimer organization relying on HLHZ interactions, the Hs C-Ala has a “head-to-tail” or antiparallel organization, with the globular domain of one monomer interacting with the helical domain of the other monomer and vice versa (Fig. 2 B and D).
Fig. S2.
Superimposition of the full-length and dimerization domain (C-Ala) of A. fulgidus AlaRS. The aminoacylation domain (gray), editing domain (gray), and tRNA (green) are shown in ribbon, and the C-Ala domain is shown in cyan. The C-Ala domain alone is shown in red.
Comparison of the monomers shows that whereas A. fulgidus C-Ala has two α-helices in the helical domain, C-Ala has an additional α-helix at the C-terminal end of the helical domain (Fig. 2E). Although these monomers’ overall structures are similar, they did not superimpose well. When superimposing only the globular domains, the rmsd of Cα positions between human C-Ala and the C-Ala portion of A. fulgidus AlaRS is ∼5.8 Å, which means that the two domains are not well conserved as structures; however, we found that the glycine-rich or “GG” motifs (the GKGGG segment in human C-Ala and GSGGG segment in A. fulgidus C-Ala) are highly conserved (Fig. 2F). A comparison of the human and A. fulgidus C-Ala structures clearly shows that a significant sequence divergence between the two species results in completely distinct architectures. Thus, our two crystal structures, which are the first of the eukaryotic AlaRS C-Ala domain, reveal a completely different dimerization interface that results in an antiparallel organization.
To investigate the evolutionary conservation of amino acids within C-Ala, we used ConSurf (23, 24) to determine the importance of each amino acid within the protein. We used both human and A. fulgidus structures as templates and compared them with 150 sequences of all homologous organisms displaying a sequence identity between 30% and 90%. For both structures, the region with the highest sequence conservation score is the GG motif that we previously identified in the globular domain (Fig. 2 G and H). Similarly, for both human and A. fulgidus templates, we found that all residues present at the dimer interface are highly variable and have the lowest conservation scores (1 and 2 on the scale) (Fig. 2 G and H). This low conservation explains how the HLHZ dimerization mode was lost during evolution, allowing the Hs C-Ala to form a structurally distinct dimer that may be associated with novel functions. Interestingly, within the eukaryote-specific α3 helix, a highly conserved motif occurs that contains structurally contiguous positively charged residues (K812, R816, K823, K824, R831, K834, and K846) (Fig. 2H and Fig. S1F). It is tempting to speculate that this highly conserved motif is involved specific functions in higher organisms.
Comparison of Human AlaRS with A. fulgidus AlaRS.
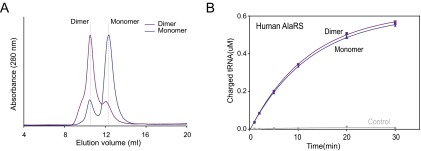
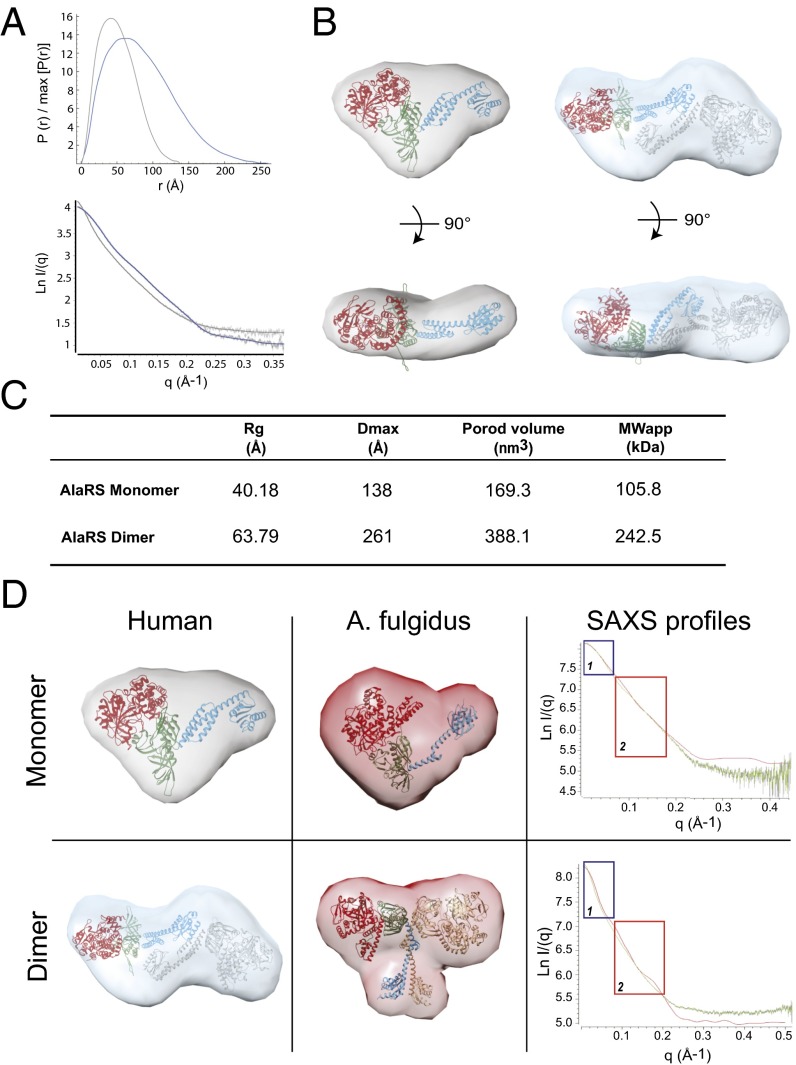
To confirm that this distinct dimerization extends to the full-length AlaRS, we performed small-angle X-ray scattering (SAXS) on both the monomeric and dimeric forms of the full-length human AlaRS that was isolated from gel filtration (Fig. S3A). The shapes of the SAXS profiles and the corresponding Guinier plots obtained for both samples are independent of protein concentration, indicating the absence of significant aggregation (Fig. 3A). Guinier analysis in the low-q region gives radius of gyration (Rg) values of 40.2 Å for the monomeric form and 63.8 Å for the dimeric form (Fig. 3 A and C). The molecular masses calculated from the Porod volume are in agreement with what we expected for the monomeric form (105.8 kDa) and the dimeric form (242.5 kDa) of the protein (Fig. 3C).
Fig. S3.
The monomer and dimer of human AlaRS. (A) Gel filtration analysis (Superdex 200 Increase 10/300 GL; GE Healthcare) of bacterially purified human AlaRS. Traces are shown of human AlaRS in buffer containing 1 mM DTT (blue) and buffer without DTT (purple). The elution positions are indicated by dashed lines. (B) In vitro aminoacylation assay showing that the monomer and dimer human AlaRS has the similar activity toward tRNAAla.
Fig. 3.
Comparison of human AlaRS with A. fulgidus AlaRS. (A) The pairwise distance distribution function, P(r), of AlaRS-monomer (gray) and AlaRS-dimer (blue) (Top), and the theoretical scattering calculated from the average of 20 ab initio reconstructions (continuous lines, with AlaRS-monomer in gray and AlaRS-dimer in blue), plotted with the experimental scattering intensity curves (Bottom). The data are presented as the natural logarithm of the intensity. (B) The human full-length AlaRS model docked into the average ab initio SAXS envelope of the monomeric AlaRS (Left) and the dimeric full-length AlaRS model docked into the average ab initio SAXS envelope of the dimeric AlaRS (Right). The dimerization interface is based on the crystal structure of human dimer C-Ala. The aminoacylation domain is in red (PDB ID code 4XEM), the editing domain is in green, and C-Ala is in blue. (C) Summary of SAXS parameters. The Rg value was determined from the Guinier plot using AutoRg, and the maximum particle dimension (Dmax) and the Porod volume were calculated using GNOM. An estimate of the molecular weight was obtained by multiplying the Porod volume by 0.625. (D) Comparison of the human (Left) and A. fulgidus (Middle) envelopes for monomeric (Top) and dimeric (Bottom) full-length AlaRS. (Right) Alignment of the experimental SAXS profile for the human AlaRS (green) with the SAXS profile of the A. fulgidus AlaRS extracted from the crystal structure (red).
We next used DAMMIF to perform ab initio shape reconstruction from the SAXS data. Several series of independent runs were carried out with no forced symmetry. All models were reproducible, with an average normalized spatial discrepancy (NSD) <1.0, indicating structurally similar solutions. Then the models resulting from 20 independent DAMMIF runs were superimposed and averaged using the DAMAVER suite to obtain a final averaged and filtered model (Fig. 3B).
We also generated a model of the full-length human AlaRS using the available human crystal structures for the aminoacylation and C-Ala domains, and also for the editing domain based on its structure in the A. fulgidus enzyme (25). The dimeric model was generated using the dimer interface of the crystal structure of the human C-Ala described herein. The resulting models for both the monomeric and dimeric human C-Ala fit well within the calculated SAXS envelopes (Fig. 3B). Importantly, a dimeric model based on the archaeal enzyme’s dimerization interface cannot fit into the dimeric envelope, which clearly highlights the difference in the dimeric interface between human and archaeal orthologs.
We extracted the SAXS profile of A. fulgidus AlaRS from the previously solved crystal structure, and compared it with the experimental SAXS profile recorded for the Hs AlaRS for both monomer and dimer forms. The overall shapes of the two AlaRS monomers are quite similar (Fig. 3D), and superposition of the SAXS profile shows a good superimposition in the low- and medium-q value regions (Fig. 3D, boxes 1 and 2, respectively). The SAXS profile in the low-q region characterizes the global shape and size of the particle analyzed, whereas the medium-q region is related to local conformational differences. In the case of the dimeric proteins, the envelopes for the human AlaRS and the A. fulgidus AlaRS are clearly distinct, in agreement with a completely different dimerization interface between the AlaRSs of the two organisms. Moreover, superimposition of the SAXS profile clearly confirmed this difference, with a large deviation in both low- and medium-q value regions, thus supporting the different shapes of the proteins in solution.
Human C-Ala Binds DNA.
Our observations led us to wonder why the human AlaRS evolved to form a new dimerization mode. Charging assays with both the monomer and dimer forms of human AlaRS revealed that this new dimerization mode does not affect tRNA binding or charging activity (Fig. S3B). Kinetic analysis showed that kcat was unchanged for human AlaRS vs. AlaRS-∆C-Ala, whereas the Km for tRNA was nominally improved (an ∼30% decrease) by the presence of the C-Ala domain (Table S2). In contrast, the effect of C-Ala is dramatic for the E. coli and A. fulgidus enzymes (16, 22). Therefore, it is likely that human C-Ala has little or no contact with bound tRNA, suggesting that it evolved to gain other functions.
Table S2.
Kinetic parameters for tRNAAla of human AlaRS and AlaRS-ΔC-Ala proteins
| Enzyme | Km, μM | kcat, S−1 | kcat/Km, μM−1S−1) | Relative kcat/Km |
| AlaRS | 4.33 ± 0.39 | 4.17 ± 0.09 | 0.96 ± 0.06 | 1 |
| AlaRS-ΔC-Ala | 6.29 ± 0.61 | 4.27 ± 0.14 | 0.68 ± 0.09 | 0.7 |
The aminoacylation assays were performed at room temperature.
50 mM Hepes pH 7.5, 20 mM KCl, 5 mM MgCl2, 4 mM ATP, 2 mM DTT, 4 μg/mL pyrophosphatase, 21.3 μM alanine, 15 nM AlaRS or AlaRS-ΔC-Ala and various amounts of tRNAs.
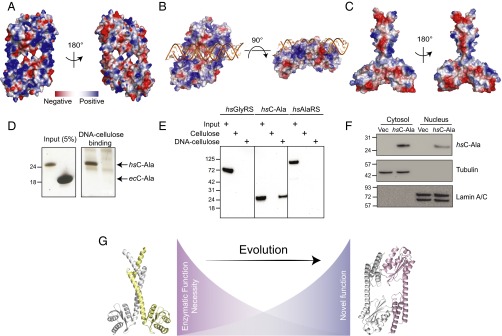
From the crystal structure of the human C-Ala dimer, a disulfide bond is formed across the dimer interface between Cys773 and Cys947 (the numbering system of AlaRS). Whereas Cys947 was acquired in evolution by zebrafish and mouse and retained by human C-Ala, only Hs C-Ala added Cys773. Thus, under oxidative conditions, the C-Ala dimer should be further stabilized and become the dominant form. To obtain clues about the function of the Hs C-Ala dimer, we analyzed the surface charge of our crystal structure. Interestingly, one face of the C-Ala dimer had major positive patches, whereas the other face was rather neutral (Fig. 4A). Residues R793, K820, K823, K824, R831, K834, K839, and K846 from the helical domain combined with K869, K876, K898, K930, and K943 from the globular domain to form a positively charged groove that closely recapitulates a DNA-binding groove (Fig. 4A). Moreover, our conservation analysis with ConSurf showed that most of these residues (K823, K824, R831, K834, K846, K869, K876, K898, and K930) had a high conservation score (8 or 9) in the eukaryotic-specific helical motif (Fig. 2H and Fig. S1F).
Fig. 4.
Human C-Ala binds DNA. (A) Electrostatic surface views of human C-Ala dimer structure. (B) A DNA-binding model created by ZDOCK program showing that DNA binds to the human C-Ala dimer through the positive charges on surface of the dimer interface. (C) Electrostatic surface views of A. fulgidus C-Ala dimer structure. (D) DNA cellulose-binding assay of Hs C-Ala and Ec C-Ala. (E) DNA cellulose-binding assay of Hs GlyRS, C-Ala and AlaRS. (F) The nuclear distribution of Hs C-Ala. Lamin A/C and tubulin were used as nuclear (N) and cytoplasmic (C) markers, respectively. (G) The distinct dimerization mode for C-Ala during evolution.
To predict how DNA would bind to Hs C-Ala, we used the Discovery Studio ZDOCK program (26), a rigid-body protein-docking algorithm that uses a fast Fourier transform algorithm to speed search in translational space, to create a DNA-binding model (Fig. 4B). In this model, DNA binds to the human C-Ala dimer through the positive charges on surface of the dimer interface, with an unobstructed fit into the DNA-binding groove.
Although the A. fulgidus C-Ala dimer showed some positive patches on the surface, it did not exhibit any region that would likely form a DNA-binding groove (Fig. 4C). Given the knowledge that E. coli AlaRS can bind to and regulate expression of its own gene (27), we tested the DNA-binding activities of both Hs C-Ala and Ec C-Ala (Fig. 4D) through a DNA cellulose-binding assay. We incubated 2 μg of Hs C-Ala and 80 μg of Ec C-Ala with DNA cellulose and evaluated whether the proteins could be pulled down with the DNA cellulose. The proteins that bound to DNA cellulose were eluted in SDS sample buffer and run on an SDS/PAGE gel. The amount of protein bound to DNA was determined by silver staining. Although higher concentrations of Ec C-Ala were used, Ec C-Ala did not show any DNA binding, whereas Hs C-Ala showed robust DNA binding.
We also compared the DNA binding of Hs AlaRS (1 μg) and Hs C-Ala (1 μg), with Hs GlyRS (1 μg) used as a control (Fig. 4E). We determined the amount of protein bound to DNA through Western blot analysis with a His tag antibody. Whereas Hs AlaRS showed no binding to either cellulose (control) or DNA cellulose, Hs C-Ala showed strong binding to DNA cellulose, but not to cellulose alone. This result demonstrates that Hs C-Ala may have to be separated from full-length protein to have strong DNA-binding activities. The sequence-specificity of this DNA binding activity is under investigation. Because of its small size (23 kDa), we expected Hs C-Ala to enter the nucleus. This expectation was confirmed (Fig. 4F).
When we initiated the present study, AlaRS appeared to be an isolated example in which an appended domain was not introduced during eukaryote evolution to change the overall synthetase architecture and thereby introduce a new function. Here we show that AlaRS followed a different evolutionary path, by exploiting what appears to be a highly adaptable structure of a preexisting appended domain (C-Ala). This evolution resulted in a distinct dimerization mode for C-Ala, which in turn also changed the quaternary shape of Hs AlaRS (Fig. 4G). We raise the possibility that the change in architecture of both C-Ala and AlaRS resulted from the same evolutionary pressures that introduced one or more novel functions, as occurred by fusing new domains to the other 19 tRNA synthetases.
SI Methods
Protein Expression and Purification.
Human full-length AlaRS was cloned into pET-21a and the truncation AlaRS-ΔC-Ala (residues 1–756) and C-Ala (residues 757–968) were cloned into pET-28a vector. E. coli AlaRS was cloned into the pET-20b vector, and the truncation AlaRS-ΔC-Ala (residues 1–699) and C-Ala (residues 700–876) were cloned into the pET-28a vector. All vectors are from Novagen. Constructs were transformed into BL21(DE3) cells. Human AlaRS, human AlaRS-ΔC-Ala, E. coli AlaRS, E. coli AlaRS-ΔC-Ala, and E. coli C-Ala were sequentially purified using Ni-NTA beads (Qiagen), a HiTrap Q HP column (GE Healthcare), and a HiLoad 16/60 Superdex 200 prep grade column (GE Healthcare). The final purified proteins were stocked in the 20 mM Tris pH 8.0, 200 mM NaCl, and 1 mM DTT. The human C-Ala was purified using Ni-NTA beads (Qiagen), a phenyl column (GE Healthcare), and a HiLoad 16/60 Superdex 200 prep grade column (GE Healthcare). The purified human C-Ala was collected and concentrated to 20 mg/mL for crystallization. Mutant proteins were constructed by site-directed mutagenesis and purified using the same methods.
Selenyl-methionine (SeMet) substituted human C-Ala was expressed using a defined medium supplemented with 25 mg/L SeMet. SeMet C-Ala was purified in a manner identical to that for the wild-type protein.
Crystallization and Data Collection.
A high-throughput crystallization screen was performed using a Mosquito liquid transfer robot (TTP Labtech). Human C-Ala proteins were crystallized with the sitting-drop vapor diffusion method. Each drop contained 100 nL of 20 mg/mL protein and 100 nL of reservoir solution, and equilibrated against 70 μL of reservoir solution. The SeMet monomer crystals were grown with the reservoir solution [0.1 M Tris pH 8.5, 25% (wt/vol) polyethylene glycol 3350], whereas the native dimer was captured in a condition with 0.2 M ammonium acetate, 0.1 M Tris pH 8.5, and 25% (wt/vol) polyethylene glycol 3350 at 16 °C. Crystals were cryoprotected with 15% (vol/vol) glycerol added to the reservoir solution and then flash-frozen with liquid nitrogen. Diffraction data were collected at 100 K on beamline 11–1 at the Stanford Synchrotron Radiation Lightsource (SSRL), and processed with HKL2000 (29).
Structure Determination and Refinement.
The crystal structure of human C-Ala was determined by single-wavelength anomalous dispersion (SAD). The SeMet crystal belongs to the P21 space group, and there is one C-Ala molecule per asymmetric unit. Se sites were found using SHELXD (30), and phasing and generation of the initial model were done using PHENIX (31). Iterative cycles of model building and refinement were carried out using Coot (32) and PHENIX. The final monomer structure was refined to 2.0-Å resolution with Rwork = 21.5% and Rfree = 25.8%. The dimer structure was determined by molecular replacement method using the monomer structure as the model and MOLREP (33). The final dimer structure was refined to 2.2-Å resolution with Rwork = 20.8% and Rfree = 25.5%. The coordinates for the refined models have been deposited in the PDB. Detailed statistics of data collection and refinement are shown in Table S1.
SAXS Measurements and ab initio 3D Shape Reconstructions.
All SAXS measurements were carried out at the SSRL on beamline BL4-2 at a working energy of 12.5 KeV. The sample-to-detector distance of the X-rays was 2.847 m, leading to scattering vector q value ranging from 0.028 to 4.525 nm−1. The exposure time was optimized to reduce radiation damage. SAXS data were collected at 20 °C using 50 μL of protein solution at 0.5, 1.1, 2.5, 3.2, and 5.2 mg/mL for the monomeric AlaRS form and 0.5, 1, 2, 6.2, 9.6, and 17.2 mg/mL for the dimeric AlaRS form. Both proteins were purified in 10 mM Tris⋅HCl (pH 8.5) buffer, and loaded into a fully automated sample charger at SSRL. Ten exposures of 10 s each were made for each protein concentration and were combined to give the average scattering curve for each measurement. Any data points affected by aggregation, possibly induced by radiation damage, were excluded. The profiles obtained at five different protein concentrations had the same shape and were flat at low q values, indicating the absence of significant aggregation. We then used the higher concentration for each sample to have the maximum information at high resolution.
Data reduction was performed using the established protocol at beamline BL4-2, and buffer background runs were subtracted from the sample runs. The Rg and forward intensity at zero angle, I(0), were determined using PRIMUS (34) according to the Guinier approximation at low q values. The Rg and P(r) values were calculated using GNOM. The maximum dimension (Dmax) value was adjusted such that the Rg value obtained from GNOM agreed with that obtained from the Guinier analysis.
The 3D bead models were built by fitting the scattering data with DAMMIF (35). Twenty independent models were generated with DAMMIF without any imposed symmetry. The models resulting from independent runs were superimposed using the DAMAVER suite (36). This yielded an initial alignment of structures based on their axes of inertia, followed by minimization of the NSD, which is 0 for identical objects and >1 for systematically different objects. The aligned structures were then averaged, giving an effective occupancy to each voxel in the model, and filtered at half-maximal occupancy to produce models of the appropriate volume that were used for all subsequent analyses.
In Vitro Transcription of tRNAs.
DNA templates containing a T7 promoter and a tRNAAla gene was synthesized by PCR of overlapping oligonucleotides. The transcription reaction was performed in 40 mM Tris⋅Cl pH 8.0, 25 mM NaCl, 20 mM MgCl2, 2 µg/mL pyrophosphatase, 0.1 mg/mL BSA, 5 mM DTT, 20 mM NTPs with T7 polymerase, and the DNA template at 37 °C for 2 h. The tRNA transcript was purified by phenol-chloroform extraction. The purified tRNA was annealed by heating to 95 °C for 3 min and then slowly cooled to room temperature with the addition of 1 mM MgCl2 at 55 °C.
Active Site Titration Assay.
Active site titration assay was performed as described previously (37) in 100 mM Hepes pH 7.5, 20 mM KCl, 10 mM MgCl2, 50 mM ATP, 22 nM [γ-32P]-ATP, 20 mM l-alanine, 2 mg/mL pyrophosphatase, and 2 mM DTT to determine the concentration of active enzymes.
In Vitro Aminoacylation Assays.
As described previously (37), the aminoacylation assays were performed at room temperature with 50 mM Hepes pH 7.5, 20 mM KCl, 5 mM MgCl2, 4 mM ATP, 2 mM DTT, 4 μg/mL pyrophosphatase, 20 μM cold l-alanine, and 1.34 μM [3H]-alanine (1 mCi/mL) as the assay solution. Various amounts of tRNAs were initially mixed with the assay solution, and the reaction was initiated by adding 200 nM AlaRS protein into the mixture. At varying time intervals, 5-μL aliquots were applied to a MultiScreen 96-well filter plate (0.45 μm pore size hydrophobic, low-protein-binding membrane; Merck Millipore), which is prewetted with quench solution containing 0.5 mg/mL DNA and 100 mM EDTA in 300 mM NaOAc (pH 3.0). After all time points were collected, 100 μL of 20% (wt/vol) trichloroacetic acid (TCA) was added to precipitate the nucleic acids. The plate was then washed four times with 200 μL of 5% TCA containing 100 mM cold alanine, followed once with 95% ethanol. On drying after completion of the washing steps, 70 μL of 100 mM NaOH was added to elute the tRNAs, which was then centrifuged into a 96-well flexible PET microplate (PerkinElmer) with 150 μL of Supermix scintillation mixture (PerkinElmer). After mixing, the radioactivity in each well of the plate was measured in a PerkinElmer 1450 Liquid Scintillation Counter and Luminescence Counter.
DNA Cellulose-Binding Assay.
For this assay, 100 mg of cellulose/DNA cellulose (Sigma-Aldrich) was resuspended in 10 mL of Basic Dilution Buffer (BDB) composed of 20 mM Tris pH 7.5, 100 mM KCl, 10% (vol/vol) glycerol, 1 mM EDTA, and 1 mg/mL BSA, and washed four times. Cellulose/DNA cellulose was rotated for 1 h in BDB with 1% BSA, and later resuspended in BDB with 1 mM DTT and 0.1% Triton X-100. Then 150 or 300 μL of cellulose/DNA cellulose was incubated with hC-Ala and eC-Ala or hAlaRS for 30–60 min at 4 °C, and washed in 20 mM Tris pH 7.5, 110 mM KOAc, 10% glycerol, 1 mM EDTA, 1 mg/mL BSA, and 0.2% Triton X-100 buffer three times. Cellulose/DNA cellulose was resuspended in SDS buffer and the sample was boiled for 10 min, loaded on an SDS/PAGE gel, and then silver-stained or Western blotted with a His tag antibody.
Cell Fractionation.
Human C-Ala was cloned into a pcDNA6 vector and transfected into C2C12 cells. The cytoplasmic and nuclear fractions were separated using the NE-PER Nuclear and Cytoplasmic Extraction Reagent Kit (Thermo Fisher Scientific) according to the manufacturer’s protocol. Human C-Ala was detected by Western blot analysis using the V5 antibody (Invitrogen).
Methods
The crystal diffraction data were collected at 100 K on beamline 11-1 of the Stanford Synchrotron Radiation Lightsource (SSRL). All SAXS measurements were carried out at the SSRL on beamline BL4-2 at a working energy of 12.5 KeV. Detailed protocols for these experiments and additional procedures are described in SI Methods.
Acknowledgments
We thank the staff at beamline 11-1 and BL4-2 of Stanford Synchrotron Radiation Lightsource (SSRL) for assistance in the data collection. We thank Dr. Shigeyuki Yokoyama for providing the A. fulgidus AlaRS gene plasmid. This work was supported by the National Cancer Institute (Grant CA92577), the National Foundation for Cancer Research, and US National Institutes of Health Grant R01 NS085092.
Footnotes
The authors declare no conflict of interest.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 5T76 and 5T5S).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1617316113/-/DCSupplemental.
References
- 1.Guo M, Yang XL, Schimmel P. New functions of aminoacyl-tRNA synthetases beyond translation. Nat Rev Mol Cell Biol. 2010;11(9):668–674. doi: 10.1038/nrm2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim S, You S, Hwang D. Aminoacyl-tRNA synthetases and tumorigenesis: More than housekeeping. Nat Rev Cancer. 2011;11(10):708–718. doi: 10.1038/nrc3124. [DOI] [PubMed] [Google Scholar]
- 3.Park SG, Schimmel P, Kim S. Aminoacyl tRNA synthetases and their connections to disease. Proc Natl Acad Sci USA. 2008;105(32):11043–11049. doi: 10.1073/pnas.0802862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He W, et al. CMT2D neuropathy is linked to the neomorphic binding activity of glycyl-tRNA synthetase. Nature. 2015;526(7575):710–714. doi: 10.1038/nature15510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sampath P, et al. Noncanonical function of glutamyl-prolyl-tRNA synthetase: Gene-specific silencing of translation. Cell. 2004;119(2):195–208. doi: 10.1016/j.cell.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 6.Han JM, et al. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell. 2012;149(2):410–424. doi: 10.1016/j.cell.2012.02.044. [DOI] [PubMed] [Google Scholar]
- 7.Bonfils G, et al. Leucyl-tRNA synthetase controls TORC1 via the EGO complex. Mol Cell. 2012;46(1):105–110. doi: 10.1016/j.molcel.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Yao P, Fox PL. Aminoacyl-tRNA synthetases in medicine and disease. EMBO Mol Med. 2013;5(3):332–343. doi: 10.1002/emmm.201100626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lo WS, et al. Human tRNA synthetase catalytic nulls with diverse functions. Science. 2014;345(6194):328–332. doi: 10.1126/science.1252943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo M, Schimmel P. Essential nontranslational functions of tRNA synthetases. Nat Chem Biol. 2013;9(3):145–153. doi: 10.1038/nchembio.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia J, Arif A, Ray PS, Fox PL. WHEP domains direct noncanonical function of glutamyl-Prolyl tRNA synthetase in translational control of gene expression. Mol Cell. 2008;29(6):679–690. doi: 10.1016/j.molcel.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rho SB, et al. Genetic dissection of protein–protein interactions in multi-tRNA synthetase complex. Proc Natl Acad Sci USA. 1999;96(8):4488–4493. doi: 10.1073/pnas.96.8.4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ko YG, Park H, Kim S. Novel regulatory interactions and activities of mammalian tRNA synthetases. Proteomics. 2002;2(9):1304–1310. doi: 10.1002/1615-9861(200209)2:9<1304::AID-PROT1304>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 14.Chang CY, Chien CI, Chang CP, Lin BC, Wang CC. A WHEP domain regulates the dynamic structure and activity of Caenorhabditis elegans glycyl-tRNA synthetase. J Biol Chem. 2016;291(32):16567–16575. doi: 10.1074/jbc.M116.730812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grice SJ, et al. Dominant, toxic gain-of-function mutations in gars lead to non-cell autonomous neuropathology. Hum Mol Genet. 2015;24(15):4397–4406. doi: 10.1093/hmg/ddv176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo M, et al. The C-Ala domain brings together editing and aminoacylation functions on one tRNA. Science. 2009;325(5941):744–747. doi: 10.1126/science.1174343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naganuma M, Sekine S, Fukunaga R, Yokoyama S. Unique protein architecture of alanyl-tRNA synthetase for aminoacylation, editing, and dimerization. Proc Natl Acad Sci USA. 2009;106(21):8489–8494. doi: 10.1073/pnas.0901572106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yachdav G, et al. PredictProtein—an open resource for online prediction of protein structural and functional features. Nucleic Acids Res. 2014;42(Web Server issue):W337–343. doi: 10.1093/nar/gku366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naganuma M, et al. The selective tRNA aminoacylation mechanism based on a single G•U pair. Nature. 2014;510(7506):507–511. doi: 10.1038/nature13440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jasin M, Regan L, Schimmel P. Modular arrangement of functional domains along the sequence of an aminoacyl tRNA synthetase. Nature. 1983;306(5942):441–447. doi: 10.1038/306441a0. [DOI] [PubMed] [Google Scholar]
- 21.Jasin M, Regan L, Schimmel P. Dispensable pieces of an aminoacyl tRNA synthetase which activate the catalytic site. Cell. 1984;36(4):1089–1095. doi: 10.1016/0092-8674(84)90059-x. [DOI] [PubMed] [Google Scholar]
- 22.Fukunaga R, Yokoyama S. Crystallization and preliminary X-ray crystallographic study of alanyl-tRNA synthetase from the archaeon Archaeoglobus fulgidus. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2007;63(Pt 3):224–228. doi: 10.1107/S1744309107006264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Celniker G, et al. ConSurf: Using evolutionary data to raise testable hypotheses about protein function. Isr J Chem. 2013;53(3-4):199–206. [Google Scholar]
- 24.Ashkenazy H, Erez E, Martz E, Pupko T, Ben-Tal N. ConSurf 2010: Calculating evolutionary conservation in sequence and structure of proteins and nucleic acids. Nucleic Acids Res. 2010;38(Web Server issue):W529–533. doi: 10.1093/nar/gkq399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun L, et al. Evolutionary gain of alanine mischarging to non-cognate tRNAs with a G4:U69 base pair. J Am Chem Soc. 2016;138(39):12948–12955. doi: 10.1021/jacs.6b07121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pierce BG, et al. ZDOCK server: Interactive docking prediction of protein-protein complexes and symmetric multimers. Bioinformatics. 2014;30(12):1771–1773. doi: 10.1093/bioinformatics/btu097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Putney SD, Schimmel P. An aminoacyl tRNA synthetase binds to a specific DNA sequence and regulates its gene transcription. Nature. 1981;291(5817):632–635. doi: 10.1038/291632a0. [DOI] [PubMed] [Google Scholar]
- 28.Sievers F, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 30.Sheldrick GM. A short history of SHELX. Acta Crystallogr A. 2008;64(Pt 1):112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 31.Adams PD, et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 2):213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60(Pt 12 Pt 1):2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 33.Vagin A, Teplyakov A. MOLREP: An automated program for molecular replacement. J Appl Cryst. 1997;30:1022–1025. [Google Scholar]
- 34.Konarev PV, Volkov VV, Sokolova AV, Koch MHJ, Svergun DI. PRIMUS: A Windows PC-based system for small-angle scattering data analysis. J Appl Cryst. 2003;36:1277–1282. [Google Scholar]
- 35.Franke D, Svergun DI. DAMMIF, a program for rapid ab-initio shape determination in small-angle scattering. J Appl Cryst. 2009;42(Pt 2):342–346. doi: 10.1107/S0021889809000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Volkov VV, Svergun DI. Uniqueness of ab initio shape determination in small-angle scattering. J Appl Cryst. 2003;36:860–864. doi: 10.1107/S0021889809000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beebe K, Waas W, Druzina Z, Guo M, Schimmel P. A universal plate format for increased throughput of assays that monitor multiple aminoacyl transfer RNA synthetase activities. Anal Biochem. 2007;368(1):111–121. doi: 10.1016/j.ab.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]