Abstract
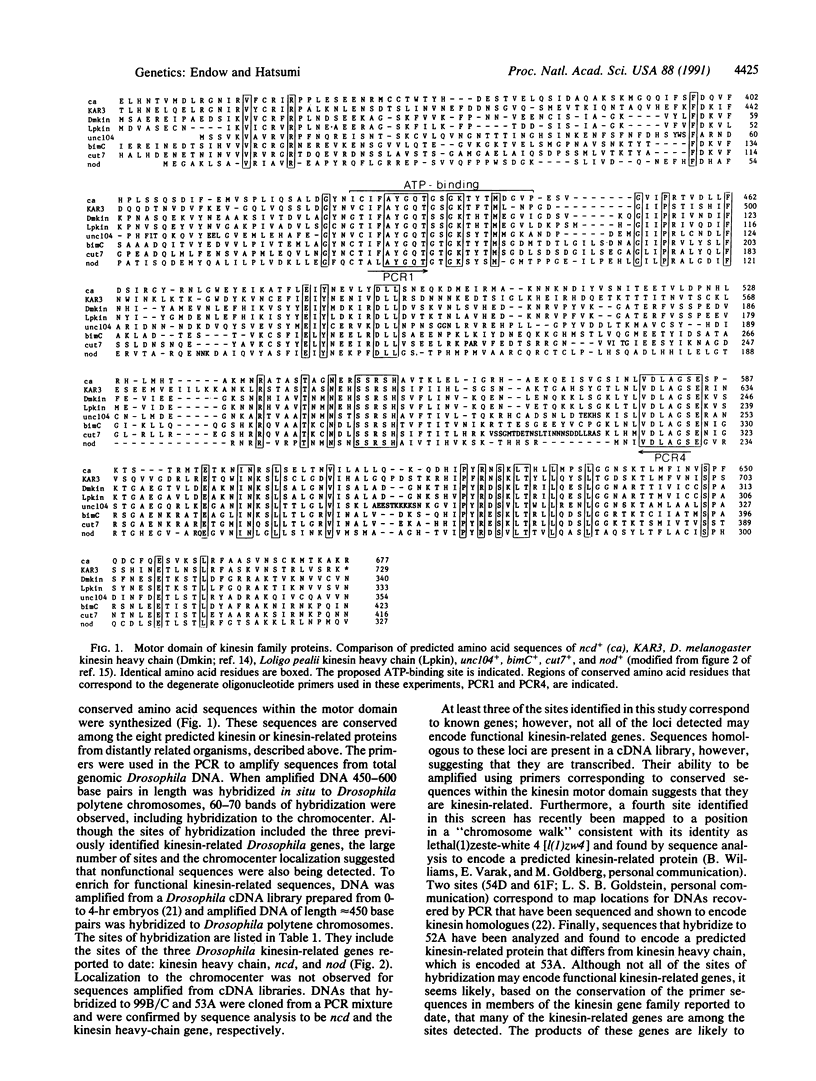
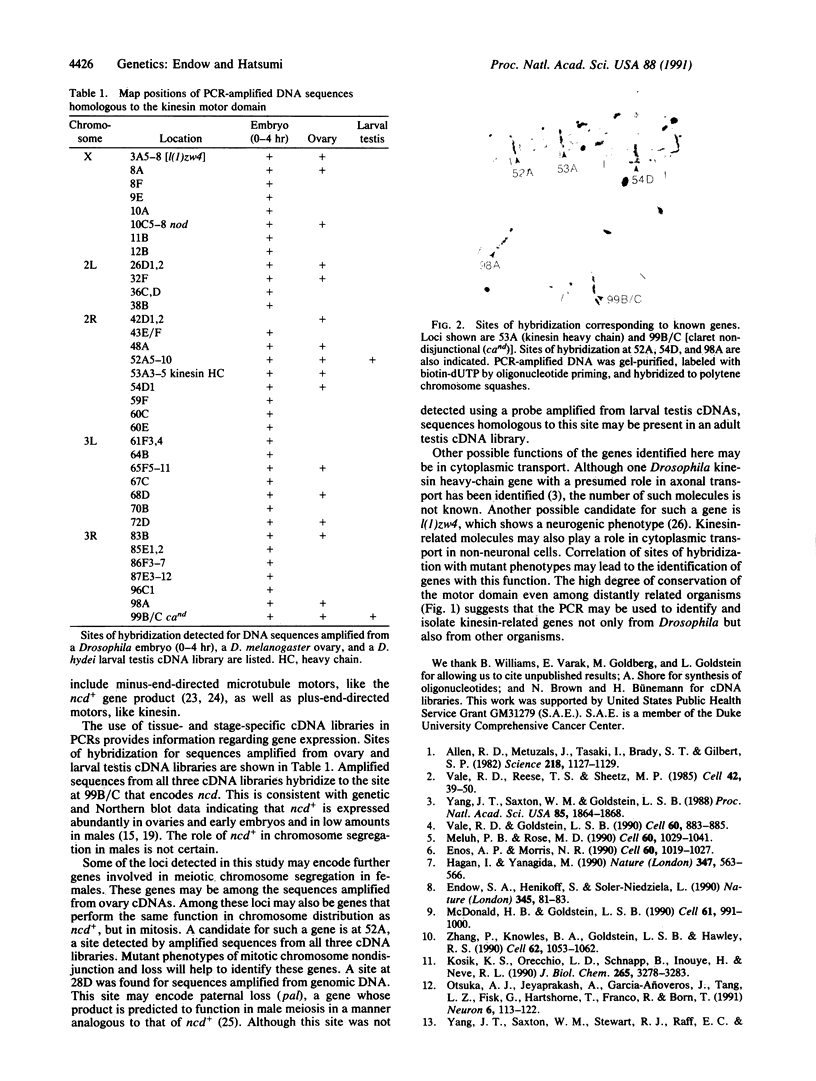
Degenerate primers to the kinesin motor domain were used in the polymerase chain reaction to amplify DNA sequences from Drosophila genomic DNA and cDNA libraries. The amplified DNA sequences were hybridized to polytene chromosomes and the map positions of the hybridizing sites were determined. More than 30 sites of hybridization were detected, indicating that the kinesin gene family may be much larger than previously thought. One new family member has already been identified as a result of this screen. The map positions should aid in the identification of further kinesin family members. Some of these kinesin-related genes are anticipated to function in previously undiscovered roles in the cell.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. D., Metuzals J., Tasaki I., Brady S. T., Gilbert S. P. Fast axonal transport in squid giant axon. Science. 1982 Dec 10;218(4577):1127–1129. doi: 10.1126/science.6183744. [DOI] [PubMed] [Google Scholar]
- Baker B. S. Paternal loss (pal): a meiotic mutant in Drosophila melanogaster causing loss of paternal chromosomes. Genetics. 1975 Jun;80(2):267–296. doi: 10.1093/genetics/80.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. H., Kafatos F. C. Functional cDNA libraries from Drosophila embryos. J Mol Biol. 1988 Sep 20;203(2):425–437. doi: 10.1016/0022-2836(88)90010-1. [DOI] [PubMed] [Google Scholar]
- Endow S. A., Henikoff S., Soler-Niedziela L. Mediation of meiotic and early mitotic chromosome segregation in Drosophila by a protein related to kinesin. Nature. 1990 May 3;345(6270):81–83. doi: 10.1038/345081a0. [DOI] [PubMed] [Google Scholar]
- Enos A. P., Morris N. R. Mutation of a gene that encodes a kinesin-like protein blocks nuclear division in A. nidulans. Cell. 1990 Mar 23;60(6):1019–1027. doi: 10.1016/0092-8674(90)90350-n. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Hagan I., Yanagida M. Novel potential mitotic motor protein encoded by the fission yeast cut7+ gene. Nature. 1990 Oct 11;347(6293):563–566. doi: 10.1038/347563a0. [DOI] [PubMed] [Google Scholar]
- Komma D. J., Horne A. S., Endow S. A. Separation of meiotic and mitotic effects of claret non-disjunctional on chromosome segregation in Drosophila. EMBO J. 1991 Feb;10(2):419–424. doi: 10.1002/j.1460-2075.1991.tb07963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik K. S., Orecchio L. D., Schnapp B., Inouye H., Neve R. L. The primary structure and analysis of the squid kinesin heavy chain. J Biol Chem. 1990 Feb 25;265(6):3278–3283. [PubMed] [Google Scholar]
- McDonald H. B., Goldstein L. S. Identification and characterization of a gene encoding a kinesin-like protein in Drosophila. Cell. 1990 Jun 15;61(6):991–1000. doi: 10.1016/0092-8674(90)90064-l. [DOI] [PubMed] [Google Scholar]
- McDonald H. B., Stewart R. J., Goldstein L. S. The kinesin-like ncd protein of Drosophila is a minus end-directed microtubule motor. Cell. 1990 Dec 21;63(6):1159–1165. doi: 10.1016/0092-8674(90)90412-8. [DOI] [PubMed] [Google Scholar]
- Meluh P. B., Rose M. D. KAR3, a kinesin-related gene required for yeast nuclear fusion. Cell. 1990 Mar 23;60(6):1029–1041. doi: 10.1016/0092-8674(90)90351-e. [DOI] [PubMed] [Google Scholar]
- Otsuka A. J., Jeyaprakash A., García-Añoveros J., Tang L. Z., Fisk G., Hartshorne T., Franco R., Born T. The C. elegans unc-104 gene encodes a putative kinesin heavy chain-like protein. Neuron. 1991 Jan;6(1):113–122. doi: 10.1016/0896-6273(91)90126-k. [DOI] [PubMed] [Google Scholar]
- Pardue M. L., Gall J. G. Nucleic acid hybridization to the DNA of cytological preparations. Methods Cell Biol. 1975;10:1–16. doi: 10.1016/s0091-679x(08)60727-x. [DOI] [PubMed] [Google Scholar]
- Perrimon N., Engstrom L., Mahowald A. P. Zygotic lethals with specific maternal effect phenotypes in Drosophila melanogaster. I. Loci on the X chromosome. Genetics. 1989 Feb;121(2):333–352. doi: 10.1093/genetics/121.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauer W. R., Walsh R. C., Kalfayan L. J. Sequence and structure of the Drosophila melanogaster ovarian tumor gene and generation of an antibody specific for the ovarian tumor protein. Mol Cell Biol. 1989 Dec;9(12):5726–5732. doi: 10.1128/mcb.9.12.5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D., Goldstein L. S. One motor, many tails: an expanding repertoire of force-generating enzymes. Cell. 1990 Mar 23;60(6):883–885. doi: 10.1016/0092-8674(90)90334-b. [DOI] [PubMed] [Google Scholar]
- Vale R. D., Reese T. S., Sheetz M. P. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985 Aug;42(1):39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. A., Salmon E. D., Endow S. A. The Drosophila claret segregation protein is a minus-end directed motor molecule. Nature. 1990 Oct 25;347(6295):780–782. doi: 10.1038/347780a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto A. H., Komma D. J., Shaffer C. D., Pirrotta V., Endow S. A. The claret locus in Drosophila encodes products required for eyecolor and for meiotic chromosome segregation. EMBO J. 1989 Dec 1;8(12):3543–3552. doi: 10.1002/j.1460-2075.1989.tb08526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. T., Laymon R. A., Goldstein L. S. A three-domain structure of kinesin heavy chain revealed by DNA sequence and microtubule binding analyses. Cell. 1989 Mar 10;56(5):879–889. doi: 10.1016/0092-8674(89)90692-2. [DOI] [PubMed] [Google Scholar]
- Yang J. T., Saxton W. M., Goldstein L. S. Isolation and characterization of the gene encoding the heavy chain of Drosophila kinesin. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1864–1868. doi: 10.1073/pnas.85.6.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. T., Saxton W. M., Stewart R. J., Raff E. C., Goldstein L. S. Evidence that the head of kinesin is sufficient for force generation and motility in vitro. Science. 1990 Jul 6;249(4964):42–47. doi: 10.1126/science.2142332. [DOI] [PubMed] [Google Scholar]
- Zhang P., Knowles B. A., Goldstein L. S., Hawley R. S. A kinesin-like protein required for distributive chromosome segregation in Drosophila. Cell. 1990 Sep 21;62(6):1053–1062. doi: 10.1016/0092-8674(90)90383-p. [DOI] [PubMed] [Google Scholar]