Significance
Myotubular myopathy is a fatal muscle disease due to deficiency in a phosphoinositide 3-phosphatase called myotubularin. We identify critical alterations involved in the pathophysiology of the disease, and we reveal the beneficial effect of a pharmacological treatment. Specifically, we demonstrate that the disease-associated dysfunction of Ca2+ signaling is strongly heterogeneous at the subcellular level, affects the amplitude and activation kinetics of sarcoplasmic reticulum Ca2+ release, and promotes a Ca2+-gated opening mode of the calcium release channels. Pharmacological inhibition of phosphoinositide 3-kinase activity substantially alleviates these functional defects and prolongs survival of myotubularin-deficient mice, suggesting a crucial role of this kinase activity in the dysfunction of Ca2+ homeostasis and a potential benefit of this therapeutic approach for the disease.
Keywords: skeletal muscle, excitation–contraction coupling, ryanodine receptor, sarcoplasmic reticulum Ca2+ release, myotubularin
Abstract
Mutations in the gene encoding the phosphoinositide 3-phosphatase myotubularin (MTM1) are responsible for a pediatric disease of skeletal muscle named myotubular myopathy (XLMTM). Muscle fibers from MTM1-deficient mice present defects in excitation–contraction (EC) coupling likely responsible for the disease-associated fatal muscle weakness. However, the mechanism leading to EC coupling failure remains unclear. During normal skeletal muscle EC coupling, transverse (t) tubule depolarization triggers sarcoplasmic reticulum (SR) Ca2+ release through ryanodine receptor channels gated by conformational coupling with the t-tubule voltage-sensing dihydropyridine receptors. We report that MTM1 deficiency is associated with a 60% depression of global SR Ca2+ release over the full range of voltage sensitivity of EC coupling. SR Ca2+ release in the diseased fibers is also slower than in normal fibers, or delayed following voltage activation, consistent with the contribution of Ca2+-gated ryanodine receptors to EC coupling. In addition, we found that SR Ca2+ release is spatially heterogeneous within myotubularin-deficient muscle fibers, with focally defective areas recapitulating the global alterations. Importantly, we found that pharmacological inhibition of phosphatidylinositol 3-kinase (PtdIns 3-kinase) activity rescues the Ca2+ release defects in isolated muscle fibers and increases the lifespan and mobility of XLMTM mice, providing proof of concept for the use of PtdIns 3-kinase inhibitors in myotubular myopathy and suggesting that unbalanced PtdIns 3-kinase activity plays a critical role in the pathological process.
Impaired skeletal muscle excitation–contraction (EC) coupling is believed to be a main cause of the severe muscle weakness associated with myotubular myopathy (1). EC coupling operates through interactions between the voltage-sensing CAV1.1 Ca2+ channel (also known as the dihydropyridine receptor) in the transverse (t) tubule membrane and the type 1 ryanodine receptor (RYR1) Ca2+ release channel in the junctional sarcoplasmic reticulum (SR) membrane: Opening of RYR1 channels under the control of the CAV1.1 voltage-sensing activity is responsible for the Ca2+ flux that raises cytosolic Ca2+ to trigger contraction (2, 3). Myotubular myopathy is due to genetic deficiency in the phosphoinositide (phosphatidylinositol, PtdInsP) phosphatase MTM1, which dephosphorylates PtdIns(3,5)P2 and PtdIns(3)P at the D3 position of the inositol ring (4, 5). How MTM1 deficiency is responsible for defective EC coupling remains unclear, and this issue is highly relevant to understanding the mechanisms of the disease but also to gaining insights into the interactions between phosphoinositides and Ca2+ signaling in muscle (see ref. 6). The Mtm1-KO mouse model reproduces the main symptomatic features of the human disease: Mutant mice develop a progressive myopathy that starts at about 3–4 wk of age, leading to death 3–5 wk later (7). An initial analysis of EC coupling in muscle fibers isolated from 5-wk-old Mtm1-KO mice revealed that the peak amplitude of the Ca2+ transient triggered by a maximally activating depolarization was reduced, with no concurrent change in resting Ca2+, rate of Ca2+ removal, and SR Ca2+ content (1). This highlighted a very specific alteration of the SR Ca2+ release process. Membrane structure defects and disorganization as well as decreased content in RYR1 and CAV1.1 were also identified, but whether they correspond to a primary or secondary defect during the disease progression is unclear. In the present work, we explored the properties of SR Ca2+ release over the full range of voltage sensitivity of EC coupling at the macroscopic and subcellular level, using the Ca2+ dye rhod-2 under conditions of high EGTA buffering. We reveal that the dysfunction of Ca2+ release is strongly heterogeneous at the subcellular level and not only affects the amplitude but also the activation kinetics of Ca2+ release, with the disease promoting a Ca2+-gated opening mode of the RYR1 channels that is denied to normal muscle. Moreover, we show that pharmacological inhibition of PtdIns 3-kinase substantially alleviates the Ca2+ release defects in muscle fibers and prolongs survival of Mtm1-KO mice.
Results
SR Ca2+ Release in MTM1-Deficient Muscle Fibers and Effect of PtdIns 3-Kinase Inhibitors.
Using voltage-clamp and confocal line-scan microscopy, we characterized the voltage-dependent SR Ca2+ release flux in muscle fibers isolated from WT and MTM1-deficient mice. Fig. 1A shows representative line-averaged rhod-2 F/F0 Ca2+ transients and the corresponding Ca2+ release flux (d[Ca]Tot/dt) elicited by depolarizing pulses from –80 mV to the indicated values (top traces) in a WT and in a MTM1-deficient muscle fiber (also referred to as KO fibers). The nonprocessed rhod-2 line-scan image recorded during the pulse to –10 mV in each fiber is shown below the pulse protocol. The rhod-2 transients yield a rapid phase of rise upon depolarization followed by a slower increase for low and midactivation voltages, whereas for the largest depolarization levels the signal spontaneously decays during the pulse. The underlying Ca2+ release flux exhibits an early peak phase that decays rapidly to a low, slowly declining level, as classically reported in this preparation. The maximal amplitude of the rhod-2 transients is smaller in the MTM1-deficient fiber than in the WT fiber due to reduced amplitude of the peak Ca2+ release flux. The activation kinetics of the Ca2+ release flux are also altered in the MTM1-deficient fiber: The time to peak is larger than in the WT fiber, and a delayed secondary phase of Ca2+ release takes place during the pulse to –10 mV. Fig. 1 B and C presents the voltage dependence of the mean values for peak amplitude of the Ca2+ release flux and for its time to peak, respectively, in WT (n = 20) and MTM1-deficient fibers (n = 13). Fitting a Boltzmann function to the amplitude versus voltage data points in each fiber revealed a 62% reduction in the mean maximal release flux in MTM1-deficient fibers (Fig. 1B, Inset) with no concurrent change in either the midactivation voltage or the steepness factor (Table S1).
Fig. 1.
Defects in voltage-activated global Ca2+ release in MTM1-deficient muscle fibers are alleviated by PtdIns 3-kinase inhibition. (A) Changes in rhod-2 fluorescence (F/F0) and corresponding calculated rate of Ca2+ release (d[Ca]Tot/dt) in response to 500 ms-long voltage-clamp depolarizing pulses of various amplitude (top traces) in a WT (Left) and in a MTM1-deficient muscle fiber (KO, Right). F/F0 traces correspond to the average change in fluorescence over the entire scanned line. The raw rhod-2 fluorescence image collected while applying the pulse to –10 mV is shown above each series of F/F0 traces. (B) Voltage dependence of the peak rate of Ca2+ release in WT (black symbols) and MTM1-deficient muscle fibers (red symbols) under control conditions (filled symbols) and following a 1-h exposure to the PtdIns 3-kinase inhibitors wortmannin and LY294002 (PtdIns 3-K block, open symbols). The Inset shows the mean values for the maximum rate of Ca2+ release in the different conditions: For this, a Boltzmann function was fitted to the individual sets of values in each fiber. Corresponding mean values for the other Boltzmann parameters are presented in Table S1. (C) Voltage dependence of the mean time to peak rate of Ca2+ release in the different conditions. Black and red asterisks report a significant difference between WT and KO fibers and between KO fibers not treated and treated with the PtdIns 3-kinase blockers, respectively. In C and D, mean values are from 20 and 13 WT and KO fibers under control conditions and 17 and 12 WT and KO fibers treated with the PtdIns 3-kinase inhibitors, respectively. All data are expressed as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 with unpaired t test.
Table S1.
Mean values for the parameters obtained from fitting a Boltzmann function to the peak Ca2+ release flux versus voltage data
| Boltzmann parameters | WT, n = 20 | KO, n = 13 | WT PtdIns 3-K block, n = 17 | KO WT PtdIns 3-K block, n = 12 |
| Maximal release flux, µM·ms−1 | 50.0 ± 5.4 | 19.0 ± 2.9 | 51.5 ± 4.9 | 34.1 ± 3.5 |
| Midactivation voltage, mV | −5.9 ± 1.7 | −5.9 ± 2.1 | −8.2 ± 2.6 | −0.1 ± 2.1 |
| Steepness factor, mV | 7.6 ± 0.4 | 6.5 ± 0.5 | 7.4 ± 0.5 | 9.6 ± 0.8 |
Data are mean ± SEM.
The decreased peak amplitude of Ca2+ release in the MTM1-deficient fibers was associated with an increase of its time to peak (Fig. 1C), with the largest difference between WT and MTM1-deficient fibers being observed for pulses to values between –20 and 0 mV. This is also the voltage range where presence of a secondary onset phase of Ca2+ release during the pulses was most routinely observed (Fig. S1). Such a secondary phase was never observed in WT fibers, and there is, to our knowledge, no other report of such behavior of Ca2+ release in any model of differentiated muscle fibers under voltage-clamp control. Thus, this late phase of Ca2+ release activation is certainly the consequence of a mode of RYR1 channels opening that is different from the standard gating process controlled by t-tubule CAV1.1 channels. Because RYR1 channels are also known to be potentially activated by a rise in cytosolic Ca2+ (see ref. 8), Ca2+-induced Ca2+ release could be the responsible mechanism. Fig. S1B presents quantitative features of the secondary delayed onset of Ca2+ release as inferred from 19 MTM1-deficient muscle fibers where such delayed onset was distinctly identified during a voltage-clamp depolarization (as illustrated in Fig. 1A and Fig. S1A): The Ca2+ concentration level immediately preceding the onset (threshold Ca2+) yielded a mean value of 0.24 ± 0.01 µM, whereas the mean peak amplitude of the delayed phase was 3.0 ± 0.4 µM·ms−1.
Fig. S1.
Evidence for Ca2+-induced Ca2+ release in MTM1-deficient muscle fibers. (A) Change in cytosolic [Ca2+] (Δ[Ca2+]) and corresponding rate of Ca2+ release (d[Ca]Tot/dt) calculated from line-averaged rhod-2 fluorescence signals collected while applying a 500 ms-long depolarizing pulse to –10 mV in a WT fiber (Left) and in a MTM1-deficient fiber (Right). The MTM1-deficient fiber yields a delayed onset phase of Ca2+ release during the pulse: Its amplitude and the corresponding threshold [Ca2+] level were determined as indicated. (B) Peak amplitude of the delayed onset phase of Ca2+ release as a function of the threshold [Ca2+] level. Each data point is from a separate MTM1-deficient muscle fiber.
MTM1 is a PtdIns 3-phosphatase, and thus, accumulation of its enzymatic substrates may play a role in the disease. We therefore tested whether inhibition of PtdIns 3-kinase activity would ameliorate the EC coupling defects. The open symbols in Fig. 1 B and C correspond to the values for the peak and time to peak Ca2+ release measured in WT (n = 17) and MTM1-deficient fibers (n = 12) exposed for 1 h to the PtdIns 3-kinase blockers wortmannin (0.1 µM) and LY294002 (50 µM) before taking the measurements. PtdIns 3-kinase inhibition had no effect in WT fibers, but it significantly enhanced the maximal Ca2+ release flux in MTM1-deficient fibers (Table S1). In addition, mean values for the time to peak Ca2+ release were reduced in treated compared with untreated MTM1-deficient fibers, although the difference was not significant for all values of membrane potential (red asterisks in Fig. 1C). These results show that PtdIns 3-kinase inhibition substantially improves the diseased Ca2+ release. MTM1 deficiency is also associated with a decreased activity of the CAV1.1 Ca2+ channel activity (1), but this effect was not alleviated by treatment with PtdIns 3-kinase blockers (Fig. S2).
Fig. S2.
CAV1.1 Ca2+ current in WT and MTM1-deficient muscle fibers untreated and treated with PtdIns 3-kinase blockers. (A) Ca2+ current traces in response to depolarizing steps to values ranging between –30 and +30 mV in a WT (Left) and in a MTM1-deficient fiber (Right). (B) Mean voltage dependence of peak Ca2+ current in WT (black symbols) and MTM1-deficient muscle fibers (red symbols) under control conditions (filled symbols, n = 19 WT fibers and 12 KO fibers, respectively) and following a 1-h exposure to the PtdIns 3-kinase inhibitors wortmannin and LY294002 (PtdIns 3-K block, open symbols; n = 16 and 12 fibers, respectively). (C) Mean values for the maximal conductance (Gmax), reversal potential (Vrev), midactivation voltage (V0.5), and steepness (k) obtained from fitting the appropriate function to the individual series of peak Ca2+ current values versus voltage in each fiber under the different conditions. All data are expressed as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 with unpaired t test.
To test whether a more prolonged exposure to PtdIns 3-kinase blockers would further correct the Ca2+ release defects, a separate series of measurements was performed on muscle fibers kept overnight in the presence of the PtdIns 3-kinase blockers. This did not further improve the Ca2+ release parameters in MTM1-deficient fibers (Fig. S3). Thus, the recovery of Ca2+ release defects by PtdIns 3-kinase inhibition is a rapid and acute process that likely does not involve profound molecular and/or structural remodeling of the muscle fibers. Wortmannin and LY294002 are broad inhibitors of PtdIns 3-kinase activity with no isoform specificity. Therefore, we also tested the effect of 3-methyladenine (3-MA), which is reported to have some preference for class III PtdIns 3-kinase (9). Results show that 3-MA did not improve Ca2+ release in MTM1-deficient fibers (Fig. S4), suggesting that class III enzymatic activity is not involved in the pathological mechanism.
Fig. S3.
Defects in voltage-activated global Ca2+ release in MTM1-deficient muscle fibers are alleviated by a prolonged exposure to PtdIns 3-kinase inhibitors. (A) Voltage dependence of the peak rate of Ca2+ release in WT (black symbols) and MTM1-deficient muscle fibers (red symbols) under control conditions (filled symbols, n = 7 and 8 WT and KO fibers, respectively) and following overnight exposure to the PtdIns 3-kinase inhibitors wortmannin and LY294002 (PtdIns 3-K block, open symbols, n = 8 and 9 WT and KO fibers, respectively). The Inset shows the mean values for the maximum rate of Ca2+ release in the different conditions: For this, a Boltzmann function was fitted to the individual sets of values in each fiber. (B) Voltage dependence of the mean time to peak rate of Ca2+ release in the different conditions. Black and red asterisks report a significant difference between WT and KO fibers and between KO fibers untreated and treated with the PtdIns 3-kinase blockers, respectively. All data are expressed as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 with unpaired t test.
Fig. S4.
3-MA does not rescue the defects in voltage-activated global Ca2+ release in MTM1-deficient muscle fibers. (A) Voltage dependence of the peak rate of Ca2+ release in WT (black symbols) and MTM1-deficient muscle fibers (red symbols) under control conditions (filled symbols, n = 7 and 7 WT and KO fibers, respectively) and following 1 h exposure to 1 mM of the PtdIns 3-kinase inhibitor 3-MA (open symbols, n = 9 and 6 WT and KO fibers, respectively). The Inset shows the mean values for the maximum rate of Ca2+ release in the different conditions: For this, a Boltzmann function was fitted to the individual sets of values in each fiber. (B) Voltage dependence of the mean time to peak rate of Ca2+ release in the different conditions. Asterisks report a significant difference between WT and KO fibers. All data are expressed as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 with unpaired t test.
Spatially Localized Disruptions of SR Ca2+ Release in MTM1-Deficient Muscle Fibers.
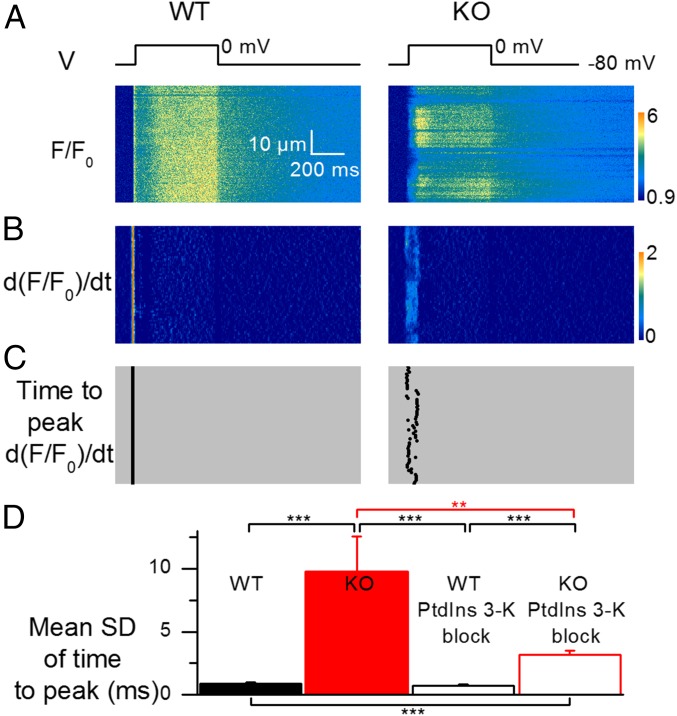
Examination of the spatial profile of the line-scan rhod-2 F/F0 signals triggered by depolarizing pulses revealed another hallmark of the altered Ca2+ release in MTM1-deficient fibers: Whereas Ca2+ transients were homogeneous along the scanned line in WT fibers, images from MTM1-deficient fibers exhibited localized alterations, demonstrating that the EC coupling defect is spatially discontinuous at the subcellular level. This is exemplified in Fig. 2 and Fig. S5, which show rhod-2 images from a WT and MTM1-deficient fiber taken while a pulse to –10, 0, or +10 mV was applied. Qualitative features of the local Ca2+ release defects were diverse: Compared with adjacent regions of the same line, locally deficient areas exhibited either reduced peak amplitude, delayed time to peak, retarded onset, or a combination thereof. This is illustrated in Fig. 3, which shows three examples of rhod-2 line-scan images taken from separate MTM1-deficient fibers depolarized to –10 or 0 mV. For each image, the time course of change in F/F0 fluorescence and the corresponding rate of Ca2+ release are shown at three different positions along the line (color-coded arrows and corresponding traces).
Fig. 2.
Spatial heterogeneity of Ca2+ release in MTM1-deficient muscle fibers. (A) Line-scan rhod-2 F/F0 images collected in a WT fiber (Left) and in a MTM1-deficient fiber (Right) while a 500 ms-long depolarizing pulse to 0 mV was applied. (B) Corresponding images of the rate of change in F/F0 (dF/F0/dt); these images were resampled from 512 to 64 pixels (0.1–0.8 µm per pixel) after linear averaging in the space domain, and the time derivative was calculated with a dt of 4 pixels (4.6 ms). (C) Corresponding distribution of the time to peak rate of change in F/F0 along the scanned line. (D) Mean SD of the time to peak rate of change in F/F0 in WT and KO fibers (n = 30 and 26, respectively) and in WT and KO fibers treated with the PtdIns 3-kinase blockers (n = 26 and 24, respectively). All data are expressed as mean ± SEM. **P < 0.01; ***P < 0.001 with unpaired t test.
Fig. S5.
Line-scan rhod-2 fluorescence images taken from a WT (Left) and from a MTM1-deficient muscle fiber (Right) while a 500 ms-long pulse at –10 or +10 mV was applied.
Fig. 3.
Local alterations in voltage-activated SR Ca2+ release in MTM1-deficient muscle fibers. (A–C) Rhod-2 F/F0 line-scan images collected in MTM1-deficient fibers while a 500 ms-long depolarizing pulse to the indicated value was applied. In each panel, bottom traces show the changes in rhod-2 fluorescence (F/F0) and the corresponding calculated rate of Ca2+ release (d[Ca]Tot/dt) at three different positions of the line (colored arrows).
Because MTM1-deficient fibers display alterations in membranous structures and triad disorganization (1), we attempted to determine whether the locally defective Ca2+ release areas were associated with disrupted t-tubules. For this purpose, voltage-activated rhod-2 Ca2+ transients were recorded in fibers stained with di-8-anepps. Overall we could not achieve a reproducible strict correlation between local alterations in t-tubule pattern and in Ca2+ release, as illustrated in Fig. S6: It shows three examples of rhod-2 line-scan images taken in MTM1-deficient fibers depolarized from –80 to 0 mV, together with the corresponding t-tubule profile along the scanned line. Fig. S6A shows an example where local defects in the t-tubule pattern do correlate with a reduced peak rate of Ca2+ release (areas pointed by the black and red arrows). Conversely, in Fig. S6B, the rhod-2 line-scan image yields a large region of depressed Ca2+ release, whereas the t-tubule pattern appears very neat all along the line, with no indication of structure defects. Finally, Fig. S6C shows a line-scan image taken from a fiber yielding a very much devastated t-tubule network, which is associated with Ca2+ release yielding either slow or retarded onset. Altogether, areas with a clearly altered t-tubule pattern did yield defective (either depressed and/or delayed) Ca2+ release compared with adjacent areas with a more standard triadic pattern, but the reciprocal was not systematically true. This suggests that either some t-tubule defects remained undetectable or that defective t-tubule structure is not the primary or not the only primary mechanism responsible for altered Ca2+ release.
Fig. S6.
T-tubule defects and local alterations of voltage-activated Ca2+ release in MTM1-deficient fibers. The t-tubule network was stained with di-8-anepps. (A–C) Each panel shows a line-scan rhod-2 F/F0 Ca2+ transient (Right) triggered by a voltage-clamp depolarization to the indicated value. Traces below correspond to the rate of Ca2+ release at three positions of the scanned line (indicated by colored arrows). The green image on Left is an x,y frame of the di-8-anepps fluorescence in the same fiber. The longitudinal profile of di-8-anepps fluorescence measured within a rectangular region (white rectangle) encompassing five pixels on each side of the position of the rhod-2 scanned line is reported next to the x,y di-8-anepps image (green trace) to better view the spatial correlation or absence of spatial correlation between defects in t-tubule pattern and in Ca2+ transient. (A) Example of clear correlations between localized defects in t-tubule pattern and in Ca2+ release. (B) Example showing a large fiber region with depressed Ca2+ release but no apparent sign of related alteration in t-tubule pattern. (C) Example of slow and delayed Ca2+ release in a fiber yielding an overall very much altered t-tubule pattern.
Because PtdIns 3-kinase inhibition ameliorates the defects in global Ca2+ release of MTM1-deficient fibers, it was of interest to determine if it could also reduce the extent of spatially localized Ca2+ release defects. For this, we quantified the spatial heterogeneity of Ca2+ release activation by calculating the SD of the time to peak rate of rise in the rhod-2 F/F0 signal along the scanned line in each image. Analysis was performed on a space- and time-filtered version of the rhod-2 line-scan images obtained in response to a pulse from –80 to +10 mV (Materials and Methods). Fig. 2B shows the d(F/F0)/dt images calculated from the above respective rhod-2 line-scan images (Fig. 2A), and Fig. 2C shows the corresponding position along the scanned line of the time to peak rate of rise of rhod-2 F/F0. Although this time value was very well conserved along the scanned line in the WT fiber, there was much greater variability in the time to peak along the line in the MTM1-deficient fiber. Fig. 2D presents mean values for the SD of the time to peak rate of rise of F/F0 in WT and MTM1-deficient fibers treated and not treated with wortmannin and LY294002. In Fig. 2D, SD values from short-term– (data shown in Fig. 1) and longer term-treated fibers (data shown in Fig. S3) were pooled together for the analysis and so were the values from the corresponding control fibers. The mean SD was significantly elevated in nontreated diseased fibers compared with the mean SD in treated and nontreated WT fibers and also compared with treated diseased fibers. Thus, PtdIns 3-kinase inhibition not only enhanced peak Ca2+ release in MTM1-deficient fibers (Fig. 1), but it also reduced its spatial discontinuity. The result could be obtained independently if using only the batch of short-term–treated fibers or the batch of longer term-treated ones. We also checked whether there could be a relationship between spatial heterogeneity and peak amplitude of Ca2+ release that could explain the improved spatial homogeneity in treated MTM1-deficient fibers. For this, we compared the spatial SD of untreated MTM1-deficient fibers at +10 mV to the SD of untreated WT fibers at –10 mV, for which mean values of peak Ca2+ release are very similar (Fig. 1). The mean SD from WT fibers at –10 mV was somewhat elevated compared with the mean SD from WT fibers at +10 mV (1.4 ± 0.1 ms vs. 0.9 ± 0.1 ms, respectively, P = 0.011), but it remained much lower than the mean SD from MTM1-deficient fibers at +10 mV (10.0 ± 2.1 ms, P < 0.0001). This indicates that spatial heterogeneity of Ca2+ release in KO fibers is not strictly related to the reduced Ca2+ release peak amplitude.
Wortmannin Treatment Enhances Life Expectancy of Mtm1-KO Mice.
Defects in EC coupling are thought to make a critical contribution to the fatal generalized muscle weakness that characterizes myotubular myopathy. The effect of pharmacological PtdIns 3-kinase inhibition on EC coupling in MTM1-deficient fibers prompted us to assess whether this strategy could also be effective in improving the phenotype of mutant mice. For that purpose, we treated WT and MTM1-deficient mice with wortmannin. Details of the protocol were devised according to previous data showing beneficial effects of wortmannin in other disease conditions in mice with no signs of toxicity (10, 11). Under our conditions, careful routine observations of the animals and bodyweight measurements showed no sign of adverse effects of wortmannin treatment in WT mice (Fig. 4B). Whereas the body weight of treated Mtm1-KO mice did not reach the values of WT animals, administration of wortmannin increased significantly the life expectancy of mutant animals (Fig. 4A), with six treated mice exceeding the average lifetime of nontreated littermates by more than 8 wk. Importantly, the mobility of these long-term survivors was apparently normal, compared with nontreated KO mice, which develop a progressive reduction in motor activity (see Movies S1 and S2). These results show the benefit of PtdIns 3-kinase inhibition on the muscle function of mice with myotubular myopathy, in accordance with the observed in vitro effects on EC coupling.
Fig. 4.
In vivo wortmannin treatment enhances life expectancy of Mtm1-KO mice. (A) Survival curves of WT and Mtm1-KO mice treated with wortmannin (n = 10 and n = 11, respectively) and untreated (n = 9 and n = 10, respectively). The x axis corresponds to the number of days following the beginning of the treatment. An analysis with the log-rank test gave a statistically increased survival rate in treated versus nontreated KO mice (P = 0.001). (B) Absolute body weight of the WT and Mtm1-KO mice treated with wortmannin and untreated. The Inset shows the corresponding relative weight of the animals. One untreated KO mouse unexpectedly survived about 3 wk longer than all other ones; corresponding data points during that period are presented as stars.
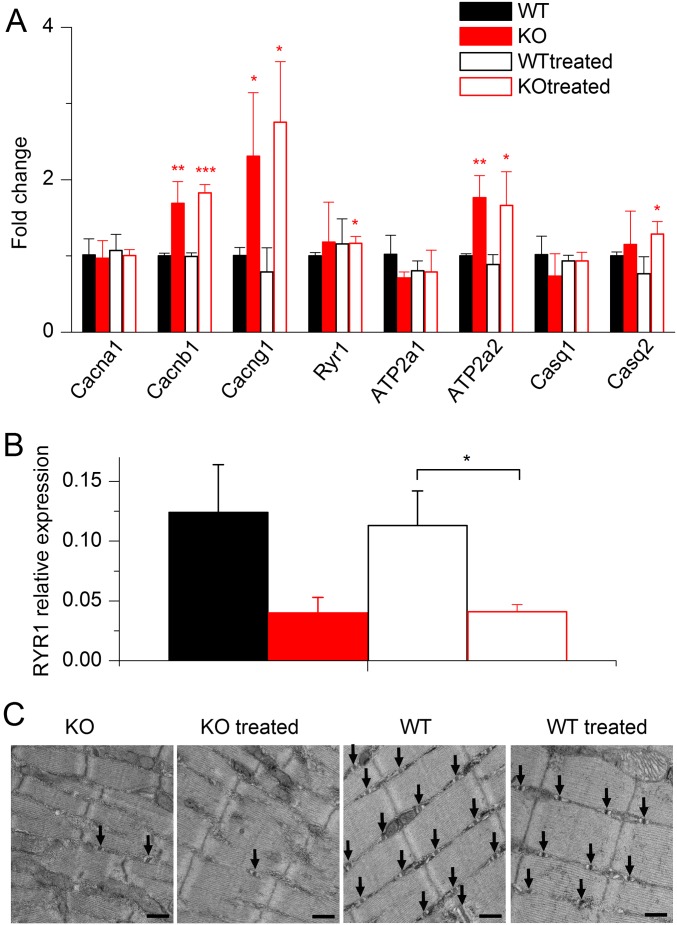
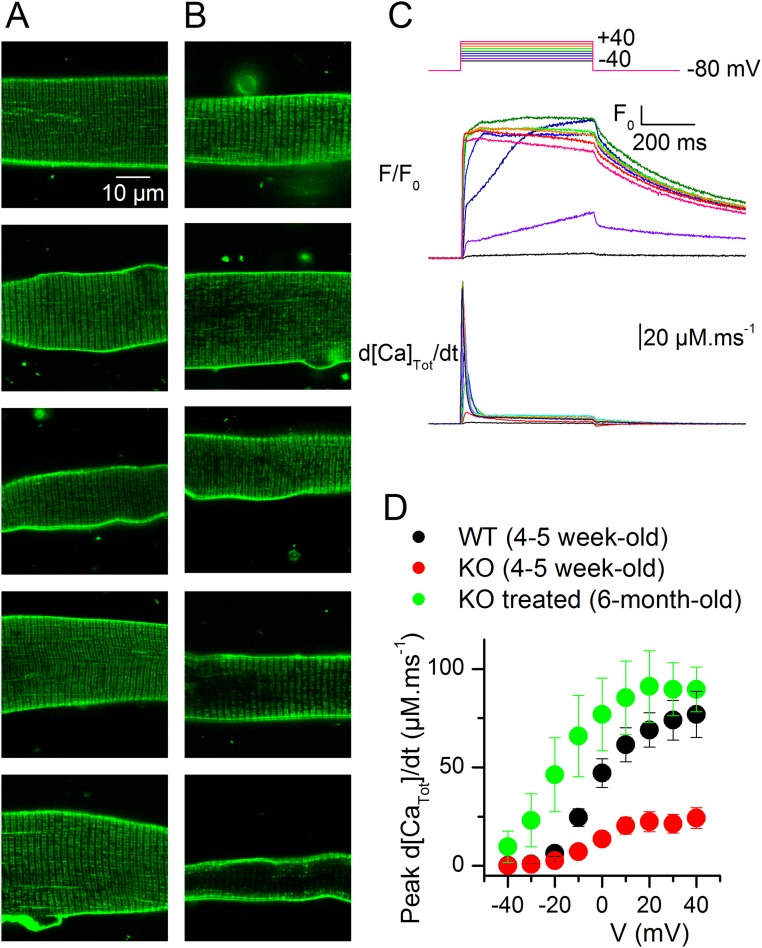
In an attempt to get further insights into how the treatment improves muscle function, the expression of EC coupling genes and the structure properties of muscle fibers were studied in animals treated during 3 wk with wortmannin, just before the period when nontreated KO animals start to die massively. In this situation, we could not detect any change between muscles of untreated and treated KO animals (Fig. S7 and Table S2). This could be taken as an indication that, at least at this stage, EC coupling was improved in the KO fibers independently from changes in structure and molecular content. However, we also cannot rule out the possibility that there was yet no substantial benefit of the treatment at this age. In any case, muscle function in treated, long-term surviving KO animals cannot be thought to be maintained if structure and molecular content are not. In this respect, Fig. S8 presents results obtained from a wortmannin-treated, 6-mo-old KO mouse, showing that muscle fibers from such animals can maintain a normal t-tubule network and strong voltage-activated Ca2+ release.
Fig. S7.
Absence of changes in the expression of genes of the EC coupling machinery and in muscle fiber structure after 3 wk of wortmannin treatment. (A) Relative fold changes in mRNA level between tibialis muscles from WT, WT treated, KO, and KO treated animals for DHPR α1, β1, and γ1 subunits (Cacna1s, Cacnb1, and Cacng1); type 1 ryanodine receptor (Ryr1); type 1 and 2 SERCA pumps (Atp2a1 and Atp2a2); and calsequestrin 1 and 2 (Casq1 and Casq2). Data are mean ± SD. All fold changes were calculated versus the value of the WT group. *P < 0.05; **P < 0.01; ***P < 0.001 with unpaired t test. The major changes in the diseased muscles corresponded to an increased level of DHPR β1 and γ1 subunits and in SERCA2. These changes persisted in muscles from the treated KO animals, whereas no other substantial modification was detected in muscles from both treated WT and KO animals. (B) Immunoblot analysis showing no change in the protein level of RYR1 between treated and untreated KO muscles (n = 5 in each group). GAPDH was used as the internal control. Data are expressed as mean ± SEM. *P < 0.05 with unpaired t test. (C) Ultrastructure evaluation of muscles from wortmannin-treated and untreated animals. Electron microscopy performed on longitudinal sections of skeletal muscle reveals approximately appropriate myofibrillar and mitochondrial morphology, with variable numbers of triad structures (arrows) in the intermyofibrillar space. Images shown are at 30,000× magnification. (Scale bar, 500 nm.) Upon visual inspection, the MTM and WT specimens were easily distinguishable from one another due to the presence of sarcotubular disorganization and differences in myofibril thickness and organization. A distinction between wortmannin-treated and untreated states within each genotype was not possible, and this was supported by our quantitation of t-tubules, l-tubules, and triads (Table S2).
Table S2.
Quantitation of t-tubules, l-tubules, and triads in muscles from mice treated with wortmannin and untreated during 3 wk
| Tubules and triads counts | WT, n = 50 | KO, n = 40 | WT treated, n = 50 | KO treated, n = 40) |
| t-tubules counts | 20.42 ± 0.88 | 2.55 ± 0.67 | 16.36 ± 0.92 | 2.6 ± 0.46 |
| l-tubules counts | 0.02 ± 0.02 | 0.15 ± 0.09 | 0.06 ± 0.03 | 0.38 ± 0.12 |
| Triad counts | 20.44 ± 0.87 | 2.83 ± 0.69 | 16.42 ± 0.91 | 2.98 ± 0.5 |
Ten images per muscle from 4 KO and 5 WT mice were examined in each condition. Data are mean ± SEM.
Fig. S8.
T-tubule network and voltage-activated Ca2+ release in muscle fibers from a wortmannin-treated, long-term–surviving KO mouse. (A and B) Confocal x,y frames of di-8-anepps fluorescence from 10 muscle fibers: A majority of fibers exhibited either no sign of alteration of the t-tubule network (A) or a few disrupted areas (B, top three frames). Two fibers exhibited a more severely disrupted t-tubule network (B, bottom two frames). (C) Voltage-activated rhod-2 fluorescence transients and corresponding Ca2+ release flux from a muscle fiber from the same mouse. (D) Mean voltage dependence of peak Ca2+ release from six fibers from the same mouse compared with the mean values from untreated young WT and KO animals (same values as in Fig. 1B).
Discussion
Our results reveal salient functional features of EC coupling in muscle fibers of mice suffering from myotubular myopathy and establish proof of concept of PtdIns 3-kinase inhibition to treat the disease.
We demonstrate that global SR Ca2+ release is depressed in MTM1-deficient fibers at all depolarizing voltages, providing evidence for an intrinsic deficiency of EC coupling, independent from the activation level of the process. Our results also reveal that, in addition to the reduced peak amplitude of Ca2+ release, activation kinetics are also affected. This indicates not only that the SR Ca2+ release flux due to activation of RYR1 channels is reduced under this pathological condition but also that it takes longer for the recruited RYR1 channels to open following t-tubule depolarization. In addition, we also uncovered another hallmark of the diseased EC coupling, which is spatial discontinuity of Ca2+ release, with the presence of discrete areas exhibiting the defects identified at the global level. Therefore, the disease does not affect simultaneously the entire cellular EC coupling mechanism but instead progresses through accumulation of focally defective areas.
Spatial heterogeneity, referred to as reduced synchrony or dyssynchrony of Ca2+ release, was reported in cardiac cells in several models of heart failure (12–14), and the defects were associated with reduced density or disorganization of t-tubules. The spatial heterogeneity of Ca2+ release that we report here could thus result from t-tubule defects, in accordance with previous histological observations (1). Consistent with this are the examples of colocalization between t-tubule network alterations and defective Ca2+ release (Fig. S6). This interpretation also fits with the proposed role of MTM1 and associated protein amphiphysin-2 in membrane tubulation (15) and with the fact that disrupted continuity of Ca2+ transients was observed in muscle fibers down-expressing amphiphysin 2 (16).
However, it is still not clear whether or not t-tubule and triad alterations fully underlie or not the defective EC coupling associated with MTM1 deficiency (see ref. 6). For instance, knockdown of the PtdInsP phosphatase MTMR14, which uses the same substrates as MTM1, affects EC coupling in zebrafish without structure defects (17). Some of our present results also concur with this possibility, including the nonsystematic correlations between defective Ca2+ release and apparent t-tubule disorganization but also the relieving effect of a short exposure of MTM1-deficient fibers to PtdIns 3-kinase blockers. Indeed, it is hard to conceive that t-tubule structure, triad organization, and proper arrangement of interactions between the molecular partners of EC coupling could be fixed within such a short time of treatment. Along this line, the depressed CAV1.1-mediated Ca2+ current was not corrected by PtdIns 3-kinase inhibition (Fig. S2). The pharmacologically induced enhancement of Ca2+ release in MTM1-deficient fibers rather suggests that, before—or in addition to—structure defects, unbalanced PtdIns 3-kinase activity plays a critical role in the defective Ca2+ release. Because it was reported that direct injection of MTM1 substrates in normal muscle fibers depresses Ca2+ release (18), it is possible that RYR1 channels, which are known to bind PtdInsPs (19), become refractory to activation by CAV1.1 in the presence of excess PtdIns 3-kinase products. Nevertheless, it remains uncertain whether t-tubule structure disruption occurs as a separate mechanism or is intimately linked to the deficiency of Ca2+ release. A separate mechanism could rely on the known functional interactions between MTM1, dynamin 2 (DNM2), and amphiphysin 1 (BIN1) that are believed to promote membrane tubulation (15). As recently suggested (20), MTM1 loss of activity may lead to decreased levels of PtdIns(5)P and PtdIns(4,5)P2. Because these appear necessary to recruit DNM2 and BIN1 at the plasma membrane to ensure proper membrane tubulation, this would provide an explanation as to how MTM1 phosphatase activity is necessary to maintain t-tubule integrity. One could also speculate that there is a causative link between depressed RYR1 activity due to MTM1 deficiency and altered t-tubule structure. However, there is so far no straight evidence from other models that it could be the case.
In cardiac muscle cells where SR Ca2+ release through RYR2 channels is triggered by Ca2+ entry across the t-tubule membrane, there is evidence that transverse propagation of Ca2+-induced Ca2+ release can compensate for partial loss of t-tubule (e.g., ref. 21). In mammalian skeletal muscle, EC coupling requires direct interactions between t-tubule CAV1.1 and SR RYR1 channels, and Ca2+-induced Ca2+ release is not physiologically involved under normal conditions (22). Proper t-tubule SR organization is thus compulsory for normal EC coupling function, and t-tubule disruption should annihilate the capacity of disconnected RYR1 channels to respond to t-tubule depolarization. Although, as discussed earlier, this likely contributes to the depressed Ca2+ release amplitude in MTM1-deficient fibers, it does not readily explain the slow and delayed onsets of activation observed at both the global and local levels. We believe that delayed Ca2+ release is due, at least in part, to RYR1 channels activated through Ca2+-induced Ca2+ release. Indeed, we provide evidence that this process is operant in MTM1-deficient muscle fibers, at least under the form of a delayed onset phase of Ca2+ release during a depolarizing pulse (Fig. S1). Because this phenomenon was clearly observed in regions of a strongly altered t-tubule pattern, it suggests the involvement of RYR1 channels orphaned from CAV1.1 control and activated by Ca2+ released from neighboring, still connected, RYR1s. This interpretation also fits with the fact that spontaneous elementary Ca2+ release events believed to result from Ca2+-activated RYR1 channels are observed in mammalian muscle under conditions of disrupted interactions between t-tubules and SR (see ref. 23). It should be noted that, when a delayed onset phase of Ca2+ release was clearly separated from the normal voltage-activated Ca2+ release (Fig. S1), its peak amplitude rarely exceeded 5 µM·ms−1, which remains much lower than the peak values reached in response to a large depolarizing pulse in normal conditions. Therefore, although Ca2+-induced Ca2+ release is likely to contribute to EC coupling in MTM1-deficient fibers, it remains insufficient to ensure the fast and large rise in cytosolic Ca2+ necessary for proper muscle contraction.
The contribution of Ca2+-induced Ca2+ release certainly plays a role in the slow kinetics of activation of voltage-dependent Ca2+ release in MTM1-deficient fibers, but it may not be the only underlying reason. Indeed, PtdIns 3-kinase inhibition tended to reduce the time to peak rate of global Ca2+ release (Fig. 1C) and enhanced the spatial homogeneity of the Ca2+ transients (Fig. 2D). Thus, slower activation is partially reversible on a time scale that hardly fits with improvement of structure defects that are likely the reason for Ca2+-induced Ca2+ release to operate. Again, it may be postulated that an excess of PtdIns 3-kinase products directly alters the normal EC coupling and delays the opening of RYR1 channels.
Although the diversity of cellular functions linked to PtdIns 3-kinase activity would tend to refrain any attempt to use corresponding pharmacological blockers as a therapeutic option, our results provide encouraging evidence that this approach may be beneficial in myotubular myopathy. While revising this manuscript, another study reported that genetic ablation of class II PtdIns 3-kinase corrects the myotubular myopathy phenotype in mice and that oral administration of wortmannin is able to prolong survival of Mtm1-KO mice by an average of 10 d (24). In our conditions, the striking increase in survival and maintained mobility of wortmannin-treated Mtm1-KO mice are also consistent with an effect of PtdIns 3-kinase inhibition in the whole skeletal musculature, as observed in single isolated muscle fibers. In this context, the effect of wortmannin necessarily includes proper maintenance of the EC-coupling membrane architecture and organization in addition to the acute enhancement of Ca2+ release observed in the isolated fibers.
The use of wortmannin in clinics has been traditionally limited by its poor solubility, instability, and toxicity, but interestingly, a nanoparticle formulation of the drug was shown to overcome these drawbacks (25). In any case, our results provide the proof of concept that pharmacological inhibition of PtdIns 3-kinase could represent a strategy to ameliorate the consequences of the disease. Because the search of PtdIns 3-kinase inhibitors is a very active field with a number of compounds being developed and tested in preclinical and clinical trials for cancer (e.g., ref. 26), we speculate that some of these compounds may be beneficial for the treatment of patients with myotubular myopathy.
Materials and Methods
Detailed materials and methods are presented in SI Materials and Methods.
Experiments on Isolated Muscle Fibers.
Confocal measurements of intracellular Ca2+ were performed in single isolated, voltage-clamped muscle fibers from mouse, following previously described procedures (27–29). The holding voltage was always set to –80 mV.
Treatment of Mice with Wortmannin.
All experiments and procedures were performed in accordance with the guidelines of the local animal ethics committee of the University Claude Bernard–Lyon 1, the French Ministry of Agriculture (87/848), and the European Community (86/609/EEC). Mice were manipulated and taken care of according to European recommendations on animal experimentation. The experimental protocol used for treatment of mice with wortmannin (DR2015-62) was approved by the animal care committee of the University Claude Bernard Lyon 1 and the French Ministry of Education and Research. WT and Mtm1-KO male mice, 23–25 d of age, were injected intraperitoneally with wortmannin (2 mg/kg) three times per week. Wortmannin was dissolved in DMSO diluted 1:25 in sterile saline. Control WT and Mtm1-KO mice were treated with the vehicle alone.
Statistics.
Unless otherwise specified, data values are presented as means ± SEM for n fibers. Statistical significance was determined using a Student’s t test (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001).
SI Materials and Methods
MTM1-Deficient Mice.
We used WT and MTM1-deficient mice in the 129PAS background. MTM1-deficient mice exhibit a progressive impairment of muscle function as described by Buj-Bello et al. (7).
Experiments on Isolated Muscle Fibers.
Single fibers isolated from the flexor digitorum brevis and interosseus muscles of 4–5-wk-old WT and Mtm1-KO male mice were used under voltage-clamp conditions according to previously described procedures (27–29). In brief, mice were killed by cervical dislocation before removal of the muscles. Muscles were treated with collagenase (Sigma, type 1) for 60 min at 37 °C. Single fibers were then obtained by triturating the muscles within the experimental chamber. Fibers were partially insulated with silicone grease so that only a short portion of the fiber extremity was left out of the silicone, as described previously (27). The Ca2+-sensitive fluorescent indicator rhod-2 was introduced into the myoplasm together with a high concentration of EGTA (see Solutions) by diffusion through the tip of the voltage-clamp pipette. The tip of the micropipette was inserted through the silicone within the insulated part of the fiber. Intracellular equilibration of the solution was allowed for a period of 30 min with the holding voltage held at −80 mV, before initiating measurements. To test the effect of PtdIns 3-kinase inhibition on EC coupling, isolated fibers were incubated with 0.1 µM wortmannin and 50 µM LY294002 for either 1 h (short exposure) or overnight (long exposure) before the experiments and throughout the measurements. All experiments were performed at room temperature (20–22 °C).
Electrophysiology.
An RK-400 patch-clamp amplifier (Bio-Logic) was used in whole-cell voltage-clamp configuration. Command voltage pulse generation was achieved with an analog-digital converter (Digidata 1440A, Axon Instruments) controlled by pClamp 9 software (Axon Instruments). Analog compensation was adjusted to decrease the effective series resistance. For calcium current measurements, removal of the linear leak component was achieved by using the appropriately scaled value of the steady change in membrane current elicited by a 20-mV hyperpolarization from the holding potential.
Confocal Imaging.
Confocal imaging was conducted with a Zeiss LSM 5 Exciter microscope equipped with a 63× oil immersion objective (numerical aperture, 1.4). For detection of rhod-2 fluorescence, excitation was from the 543 nm line of a HeNe laser, and fluorescence was collected above 560 nm. Rhod-2 fluorescence changes were expressed as F/F0, where F0 is the baseline fluorescence. For imaging of the t-tubule network, fibers were incubated for 30–60 min in the presence of 10 µM di-8-anepps before establishing the voltage-clamp and rhod-2 measurements conditions. Di-8-anepps fluorescence was measured above 505 nm in response to 458 nm excitation. Intracellular Ca2+-related rhod-2 fluorescence changes were imaged by using the line-scan mode (x,t) of the system with the line parallel to the longitudinal fiber axis. Images were taken with a scanning frequency of 1.15 ms per line. Analysis of the spatial heterogeneity of Ca2+ release (Fig. 2) was performed with an ImageJ (https://imagej.nih.gov/ij/) macro program, available on request.
[Ca2+] and Ca2+ Release Calculation.
The Ca2+ release flux underlying the calculated global [Ca2+] transients was estimated according to a previously described procedure (28, 29). For this, changes in [Ca2+] were calculated from the rhod-2 signals using the pseudoratio equation (30), assuming a basal [Ca2+] of 0.1 µM and a Kd of rhod-2 for Ca2+ of 1.2 µM. The SR calcium release flux was then calculated from the time derivative of the total myoplasmic Ca2+ ([Ca]Tot) obtained from the occupancy of intracellular calcium binding sites. The model included troponin C binding sites, parvalbumin binding sites, and calcium transport across the SR membrane with the same parameters as used in Lefebvre et al. (29). Ca2+ binding sites on EGTA were included with a total site concentration of 6 mM, an “on” rate constant kon, EGTA of 0.056 µM−1·ms−1, and an “off” rate constant koff, EGTA of 0.002 ms−1. In all sets of data where a comparison was made between several groups of muscle fibers, all fibers were issued from the same type of muscle (either flexor digitorum brevis or interosseous) isolated from age-matched animals (except in Fig. S8).
Quantitative RT-PCR Analysis.
Tibialis anterior muscle of 6-wk-old male mice was homogenized using MagNa Pure LC RNA Isolation Tissue Lysis Buffer (Roche), and total RNA was isolated using an automated nucleic acid extraction instrument (MagNA Pure 96 System), followed by DNase digestion to minimize genomic DNA contamination (Ambio DNA-free DNA Removal Kit, Life Technologies). RNA was reverse-transcribed using RevertAid H minus Reverse Transcriptase (Fermentas) in the presence of random primers (Fermentas). Quantitative PCR amplifications of cDNA were performed on ABI Prism 7900HT apparatus (Applied Biosystems). Primer sequences for amplification were as described previously (1).
Immunoblot Analysis.
Proteins were extracted from mouse quadriceps using a RIPA buffer that contains 50 mM Tris·HCl pH 8.0, 150 mM NaCl, 0.5% Na deoxycholate, 1% Nonidet P-40, 0.1% SDS, and a Protease Inhibitor Mixture (Roche Applied Science) and homogenized in tubes of Lysing Matrix A with a FastPrep-24 (MP Biomedicals). We loaded 50 μg of total proteins into NuPage 3–8% (wt/vol) Tris–Acetate gels in Tris Acetate SDS Running Buffer (Invitrogen) and transferred them onto Immobilon-P membranes (Millipore). Membranes were probed with a mouse monoclonal antibody raised against the RYR1 (Abcam, ab2868), and a mouse monoclonal antibody specific for GAPDH (Millipore, MAB374) was used as the internal control. Detection was performed with a secondary antibody coupled to IRDye 680 (LI-COR) using the Odyssey infrared imaging system (LI-COR Biotechnology Inc.).
Electron Microscopy.
Electron microscopy was performed for the evaluation of sarcotubular architecture. Briefly, glutaraldehyde-fixed skeletal muscle specimens were processed at the Medical College of Wisconsin EM Core Facility for the evaluation of triad morphology. After confirming appropriate tissue quality on scout sections, longitudinally oriented 30-nm sections were stained with 2% (wt/vol) uranyl acetate and Reynold’s lead citrate, and electron microscopy was performed using a Hitachi H600 transmission electron microscope. Longitudinally oriented muscle fibers were evaluated and photographed, with photography of the single best oriented region from each myofiber in the specimen at 10,000×, 20,000×, and 30,000× magnification. Ten fibers per specimen were evaluated and photographed in this manner. The number of t-tubules, longitudinal (l-) tubules, and triad structures were then manually quantified by a blinded, board-certified neuropathologist using the 20,000× magnification image. The values for all images in a given treatment condition were pooled together to generate average numbers of t-tubules, l-tubules, and triads in that condition.
Solutions.
The extracellular solution used contained (in mM) 140 TEA-methanesulfonate, 2.5 CaCl2, 2 MgCl2, 1 4-aminopyridine, 10 Hepes, and 0.002 tetrodotoxin. The intracellular solution contained (in mM) 120 K-glutamate, 5 Na2-ATP, 5 Na2-phosphocreatine, 5.5 MgCl2, 12 EGTA, 4.8 CaCl2, 0.1 rhod-2, 5 glucose, and 5 Hepes. All solutions were adjusted to pH 7.20. Wortmannin and 3-MA were purchased from Sigma. LY294002 was purchased from Echelon.
Supplementary Material
Acknowledgments
We thank Jimmy Perrot, members of Genethon’s platforms, members of the Dubois Animal Housing Facility at University Lyon 1 for expert technical assistance, and Clive Wells from the Electron Microscopy Core Facility at the Medical College of Wisconsin. This work was supported by grants from CNRS, INSERM, and the Université Claude Bernard–Lyon 1 (to the Institut NeuroMyoGène), by a grant from the Société Française de Myologie (SFM) (to C.K.), and by Grant 18648 from the Association Française contre les Myopathies (AFM–Téléthon) (to V.J.). A.B.-B. was supported by the AFM–Téléthon, the Myotubular Trust, and INSERM.
Footnotes
Conflict of interest statement: M.W.L. receives research funding support from Audentes Therapeutics, Solid GT, and Demeter Therapeutics; is a member of the Scientific Advisory Board of Audentes Therapeutics; and was recently a consultant for Sarepta Therapeutics. A.B.-B. is a scientific advisor of Audentes Therapeutics.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1604099113/-/DCSupplemental.
References
- 1.Al-Qusairi L, et al. T-tubule disorganization and defective excitation-contraction coupling in muscle fibers lacking myotubularin lipid phosphatase. Proc Natl Acad Sci USA. 2009;106(44):18763–18768. doi: 10.1073/pnas.0900705106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ríos E, Pizarro G, Stefani E. Charge movement and the nature of signal transduction in skeletal muscle excitation-contraction coupling. Annu Rev Physiol. 1992;54:109–133. doi: 10.1146/annurev.ph.54.030192.000545. [DOI] [PubMed] [Google Scholar]
- 3.Schneider M-F. Control of calcium release in functioning skeletal muscle fibers. Annu Rev Physiol. 1994;56:463–484. doi: 10.1146/annurev.ph.56.030194.002335. [DOI] [PubMed] [Google Scholar]
- 4.Taylor G-S, Maehama T, Dixon J-E. Myotubularin, a protein tyrosine phosphatase mutated in myotubular myopathy, dephosphorylates the lipid second messenger, phosphatidylinositol 3-phosphate. Proc Natl Acad Sci USA. 2000;97(16):8910–8915. doi: 10.1073/pnas.160255697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tronchère H, et al. Production of phosphatidylinositol 5-phosphate by the phosphoinositide 3-phosphatase myotubularin in mammalian cells. J Biol Chem. 2004;279(8):7304–7312. doi: 10.1074/jbc.M311071200. [DOI] [PubMed] [Google Scholar]
- 6.Csernoch L, Jacquemond V. Phosphoinositides in Ca(2+) signaling and excitation-contraction coupling in skeletal muscle: An old player and newcomers. J Muscle Res Cell Motil. 2015;36(6):491–499. doi: 10.1007/s10974-015-9422-4. [DOI] [PubMed] [Google Scholar]
- 7.Buj-Bello A, et al. The lipid phosphatase myotubularin is essential for skeletal muscle maintenance but not for myogenesis in mice. Proc Natl Acad Sci USA. 2002;99(23):15060–15065. doi: 10.1073/pnas.212498399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endo M. Calcium-induced calcium release in skeletal muscle. Physiol Rev. 2009;89(4):1153–1176. doi: 10.1152/physrev.00040.2008. [DOI] [PubMed] [Google Scholar]
- 9.Miller S, et al. Shaping development of autophagy inhibitors with the structure of the lipid kinase Vps34. Science. 2010;327(5973):1638–1642. doi: 10.1126/science.1184429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohta T, et al. Inhibition of phosphatidylinositol 3-kinase increases efficacy of cisplatin in in vivo ovarian cancer models. Endocrinology. 2006;147(4):1761–1769. doi: 10.1210/en.2005-1450. [DOI] [PubMed] [Google Scholar]
- 11.Teranishi F, et al. Phosphoinositide 3-kinase inhibitor (wortmannin) inhibits pancreatic cancer cell motility and migration induced by hyaluronan in vitro and peritoneal metastasis in vivo. Cancer Sci. 2009;100(4):770–777. doi: 10.1111/j.1349-7006.2009.01084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Louch W-E, et al. T-tubule disorganization and reduced synchrony of Ca2+ release in murine cardiomyocytes following myocardial infarction. J Physiol. 2006;574(Pt 2):519–533. doi: 10.1113/jphysiol.2006.107227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song L-S, et al. Orphaned ryanodine receptors in the failing heart. Proc Natl Acad Sci USA. 2006;103(11):4305–4310. doi: 10.1073/pnas.0509324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heinzel F-R, et al. Remodeling of T-tubules and reduced synchrony of Ca2+ release in myocytes from chronically ischemic myocardium. Circ Res. 2008;102(3):338–346. doi: 10.1161/CIRCRESAHA.107.160085. [DOI] [PubMed] [Google Scholar]
- 15.Royer B, et al. The myotubularin-amphiphysin 2 complex in membrane tubulation and centronuclear myopathies. EMBO Rep. 2013;14(10):907–915. doi: 10.1038/embor.2013.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tjondrokoesoemo A, et al. Disrupted membrane structure and intracellular Ca²⁺ signaling in adult skeletal muscle with acute knockdown of Bin1. PLoS One. 2011;6(9):e25740. doi: 10.1371/journal.pone.0025740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dowling J-J, Low S-E, Busta A-S, Feldman E-L. Zebrafish MTMR14 is required for excitation-contraction coupling, developmental motor function and the regulation of autophagy. Hum Mol Genet. 2010;19(13):2668–2681. doi: 10.1093/hmg/ddq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodríguez E-G, et al. Phosphoinositide substrates of myotubularin affect voltage-activated Ca²⁺ release in skeletal muscle. Pflugers Arch. 2014;466(5):973–985. doi: 10.1007/s00424-013-1346-5. [DOI] [PubMed] [Google Scholar]
- 19.Shen J, et al. Deficiency of MIP/MTMR14 phosphatase induces a muscle disorder by disrupting Ca(2+) homeostasis. Nat Cell Biol. 2009;11(6):769–776. doi: 10.1038/ncb1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hohendahl A, Roux A, Galli V. Structural insights into the centronuclear myopathy-associated functions of BIN1 and dynamin 2. J Struct Biol. 2016;196(1):37–47. doi: 10.1016/j.jsb.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrantini C, et al. Impact of detubulation on force and kinetics of cardiac muscle contraction. J Gen Physiol. 2014;143(6):783–797. doi: 10.1085/jgp.201311125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Figueroa L, et al. Synthetic localized calcium transients directly probe signalling mechanisms in skeletal muscle. J Physiol. 2012;590(6):1389–1411. doi: 10.1113/jphysiol.2011.225854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Csernoch L. Sparks and embers of skeletal muscle: The exciting events of contractile activation. Pflugers Arch. 2007;454(6):869–878. doi: 10.1007/s00424-007-0244-0. [DOI] [PubMed] [Google Scholar]
- 24.Sabha N, et al. PIK3C2B inhibition improves function and prolongs survival in myotubular myopathy animal models. J Clin Invest. 2016;126(9):3613–3625. doi: 10.1172/JCI86841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karve S, et al. Revival of the abandoned therapeutic wortmannin by nanoparticle drug delivery. Proc Natl Acad Sci USA. 2012;109(21):8230–8235. doi: 10.1073/pnas.1120508109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cleary J-M, Shapiro G-I. Development of phosphoinositide-3 kinase pathway inhibitors for advanced cancer. Curr Oncol Rep. 2010;12(2):87–94. doi: 10.1007/s11912-010-0091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacquemond V. Indo-1 fluorescence signals elicited by membrane depolarization in enzymatically isolated mouse skeletal muscle fibers. Biophys J. 1997;73(2):920–928. doi: 10.1016/S0006-3495(97)78124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pouvreau S, et al. Transient loss of voltage control of Ca2+ release in the presence of maurocalcine in skeletal muscle. Biophys J. 2006;91(6):2206–2215. doi: 10.1529/biophysj.105.078089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lefebvre R, et al. Defects in Ca2+ release associated with local expression of pathological ryanodine receptors in mouse muscle fibres. J Physiol. 2011;589(Pt 22):5361–5382. doi: 10.1113/jphysiol.2011.216408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng H, Lederer WJ, Cannell M-B. Calcium sparks: Elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262(5134):740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.