Significance
The generation of different blood lineages is regulated by hematopoietic cytokines, either in an instructive or in a permissive manner. The cytokines Interleukin-7 and fms-like tyrosine kinase-3 (Flt3) ligand are required for B-cell development but their precise mode of action remains controversial. Our study has addressed the role of these cytokines in B-cell commitment by analyzing the progenitor stage where B-cell commitment occurs in mice overexpressing one of the two cytokines in the absence of the other. Our results demonstrate a permissive role for both cytokines in B-cell commitment. Interleukin-7 promotes survival of progenitors instead of up-regulation of B-cell commitment factors early B-cell factor 1 (Ebf1) and paired box 5 (Pax5), as previously hypothesized, whereas Flt3 ligand facilitates progenitor expansion by inducing their proliferation.
Keywords: hematopoiesis, cytokines, commitment, immunology
Abstract
Hematopoietic cells are continuously generated throughout life from hematopoietic stem cells, thus making hematopoiesis a favorable system to study developmental cell lineage commitment. The main factors incorporating environmental signals to developing hematopoietic cells are cytokines, which regulate commitment of hematopoietic progenitors to the different blood lineages by acting either in an instructive or a permissive manner. Fms-like tyrosine kinase-3 (Flt3) ligand (FL) and Interleukin-7 (IL-7) are cytokines pivotal for B-cell development, as manifested by the severely compromised B-cell development in their absence. However, their precise role in regulating B-cell commitment has been the subject of debate. In the present study we assessed the rescue of B-cell commitment in mice lacking IL-7 but simultaneously overexpressing FL. Results obtained demonstrate that FL overexpression in IL-7–deficient mice rescues B-cell commitment, resulting in significant Ebf1 and Pax5 expression in Ly6D+CD135+CD127+CD19− precursors and subsequent generation of normal numbers of CD19+ B-cell progenitors, therefore indicating that IL-7 can be dispensable for commitment to the B-cell lineage. Further analysis of Ly6D+CD135+CD127+CD19− progenitors in IL-7– or FL-deficient mice overexpressing Bcl2, as well as in IL-7 transgenic mice suggests that both FL and IL-7 regulate B-cell commitment in a permissive manner: FL by inducing proliferation of Ly6D+CD135+CD127+CD19− progenitors and IL-7 by providing survival signals to these progenitors.
Hematopoiesis, the generation of all blood cells from hematopoietic stem cells (HSCs), takes place continuously in the adult bone marrow. Accumulating evidence suggests that HSCs generate the different hematopoietic lineages via oligopotent progenitors having limited self-renewal capacity and restricted developmental potentials. Activation of lineage-specific gene transcription in these progenitors eventually leads to their commitment to a particular lineage. Cytokines are the most prominent environmental factors regulating hematopoietic lineage commitment, doing so by acting either in an instructive or a permissive manner (1). In the instructive model, cytokines induce a signaling cascade in progenitors leading to the initiation of a lineage-specific gene program, typically through up-regulation and/or activation of transcription factors, eventually resulting in commitment to a particular lineage. In contrast, the permissive model advocates that commitment of progenitors to different lineages occurs in a cell-autonomous, stochastic manner, with cytokines acting as a selection rather than a commitment factor, promoting the survival and/or proliferation of a specific lineage at the expense of other lineages originating from the same progenitor. Elucidating the precise mode of action of cytokines is technically challenging and therefore the instructive versus permissive role of cytokines is hotly debated (2–4). Although the permissive model was favored in the past, recent data provide solid evidence for the instructive action of several cytokines including M-CSF, G-CSF, EPO, and fms-like tyrosine kinase-3 (Flt3) ligand (5–8). However, our understanding of how cytokines regulate hematopoiesis remains elusive, as different cytokines can act in various ways and their function might be cell-context dependent (9). Moreover, most studies to date have addressed cytokine-regulated myeloid differentiation with relatively little information on lymphoid lineage commitment.
That Interleukin-7 (IL-7) is a crucial cytokine for B-cell development is demonstrated by the dramatic defect in B-cell generation in mice lacking either the cytokine (10) or its receptor (11). Interestingly, whereas human B-cell progenitors are also responsive to IL-7 (12), disruption of IL-7 signaling caused by mutations does not ablate B-cell development in man (13, 14). IL-7 was initially identified as a growth factor for B-cell progenitors (15) and early studies demonstrated that in vivo overexpression of the prosurvival gene Bcl2 did not rescue B-cell development in the absence of IL-7 signaling, suggesting that IL-7 acts in an instructive manner in B-cell commitment (16, 17). The subsequent findings that uncommitted common lymphoid progenitors (CLPs) from Il7−/− mice lacked expression of the transcription factor early B-cell factor 1 (Ebf1) (18) and that Ebf1 overexpression partially restored B-cell generation from these CLPs (19), led to the hypothesis that IL-7, through Stat5 activation, instructs commitment to the B-cell lineage by initiating Ebf1 expression in uncommitted progenitors. Supporting this hypothesis, a putative Stat5 binding site was later identified in one of the Ebf1 promoters (20). However, a more recent study has shown that Bcl2 can rescue B-cell generation in a Stat5 conditional knockout mouse (21). Furthermore, the Ebf1-expressing fraction of CLP (Ly6D+ CLP) is dramatically reduced in Il7−/− mice (22), therefore providing an alternative possibility for the reduced Ebf1 expression observed in Il7−/− CLPs. Interestingly, B-cell lineage commitment is initiated at the molecular level in Ly6D+CD19− progenitors (23). Hence, whereas the importance of IL-7 as a growth factor for committed B-cell progenitors has been well established, it remains unclear whether it instructs oligopotent progenitors to commit to the B-cell lineage through Ebf1 and Pax5 up-regulation.
Ftl3 ligand (FL), the only known ligand for the Flt3 receptor (CD135), is a cytokine important for the generation of many hematopoietic lineages and its function has gained much attention as mutations in FL signaling are commonly found in acute myeloid leukemias (AMLs) (24). Committed B-cell progenitors do not express CD135, because expression of the B-cell commitment factor Pax5 (paired box 5) leads to its down-regulation (25). However, upon transplantation, bone marrow progenitors from Flt3−/− and Flt3l−/− mice reconstitute the B-cell compartment poorly (26, 27), and FL was found to be essential for maintaining normal numbers of uncommitted B-cell progenitors (28).
Recently, we described a FL-transgenic mouse model (hereafter Flt3ltg) expressing high levels of FL in vivo, which has enabled us to suggest an instructive role for FL in early stages of hematopoiesis (8). By breeding these mice with Il7−/− mice, we herein show that increased FL levels can rescue B-cell commitment in CD135+CD127+CD19− progenitors and restore early CD19+ B-cell progenitor numbers in the absence of IL-7 signaling, suggesting a permissive role for IL-7 in B-cell commitment. Further analyses of a combination of mouse genotypes overexpressing or lacking FL and IL-7, as well as the prosurvival gene Bcl2, have enabled us to identify a permissive role for both IL-7 and FL in B-cell commitment.
Results
Increased in Vivo Levels of FL Rescue B-Cell Commitment in Il7−/− Ly6D+CD19− Progenitors.
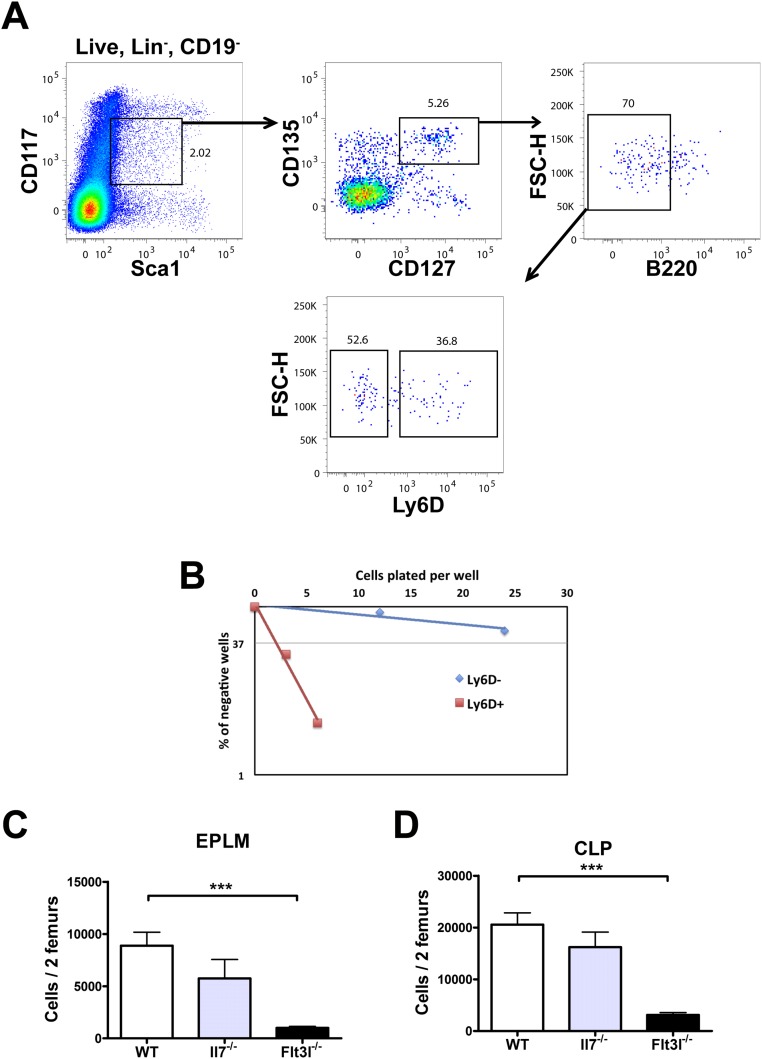
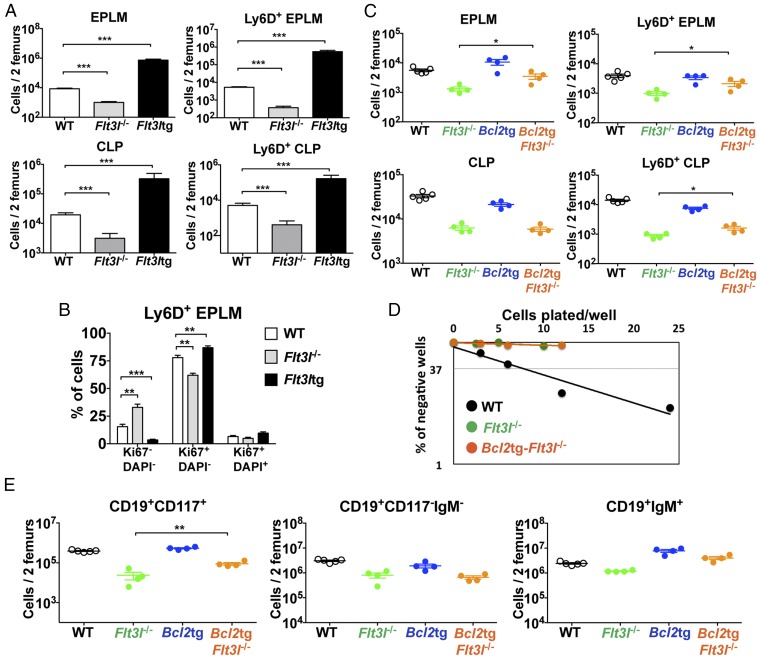
We have previously characterized an uncommitted B-cell progenitor with combined lymphoid and myeloid potential [early progenitor with lymphoid and myeloid potential (EPLM)] (29). EPLM can be further subdivided by SiglecH, CD11c, CD115, and Ly6D expression enabling us to identify the Ly6D+SiglecH−CD11c−CD115− fraction of EPLM (hereafter Ly6D+ EPLM) as the population containing most B-cell potential, while being devoid of myeloid potential (Fig. S1B). This EPLM subpopulation is identified as Lin−CD19−CD117intB220intLy6D+CD135+CD127+ (Fig. 1A), therefore partially overlapping phenotypically with Ly6D+ CLPs (Fig. S1A) and pre/pro-B cells (30, 31). Ly6D+ EPLM numbers in Il7−/− and Flt3l−/− mice are significantly decreased compared with WT: 7-fold for Il7−/− and 13-fold for Flt3l−/−, respectively, and a similar dramatic decrease was observed in Ly6D+ CLPs from both mutant mice (Fig. 1 B and C). FL deficiency also affected the numbers of Ly6D− EPLM and CLP, whereas IL-7 did not (Fig. S1 C and D). Therefore, Ly6D+ EPLM/CLP represent the earliest developmental stage of the B-cell pathway affected by the absence of IL-7.
Fig. S1.
(A) CLP FACS staining in WT mice. FACS plots showing the gating strategy used for the identification of Ly6D+ CLP. Lineage staining was as follows: SiglecH, CD115, CD11c, NK1.1, Gr-1. (B) In vitro limiting dilution analysis of Ly6D+ and Ly6D− EPLM B-cell potential. Cells were sorted as shown in Fig. 1A and plated at the indicated concentrations on OP9 stromal cells together with IL-7. A representative of three independent experiments is shown. (C and D) Numbers of EPLM (C) and CLP (D) progenitors in WT (n = 13), Il7−/− (n = 5), and Flt3l−/− (n = 10) mice. EPLM were stained as shown in Fig. 1A and CLP as shown in A. Student’s t test. ***P ≤ 0.001. Bars show mean ± SEM.
Fig. 1.
IL-7 and FL are necessary for the generation of a normal Ly6D+CD135+CD127+CD19− compartment. (A) FACS plots showing the gating strategy used for identification of Ly6D+ EPLM and their percentage of CD135 and CD127 expression. Lineage staining was as follows: SiglecH, CD115, CD11c, NK1.1, Gr-1. (B) Representative FACS plots of EPLM (Upper row) and CLP (Lower row) from the bone marrow of WT, Il7−/−, and Flt3l−/− mice. (C) Absolute numbers of Ly6D+ EPLM (Upper graph) and CLP (Lower graph) from the bone marrow of WT (n = 13), Il7−/− (n = 5), and Flt3l−/− (n = 10) mice. (D) Representative FACS plots of EPLM and CLP from WT and Flt3ltg mice. (E) Absolute numbers of total EPLM and CLP (Left graphs) and Ly6D+ EPLM and CLP (Right graphs) from WT and Flt3ltg mice. ***P ≤ 0.001.
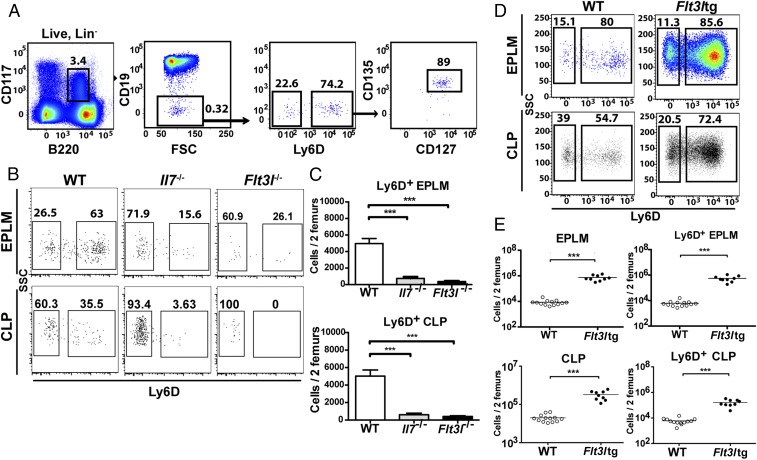
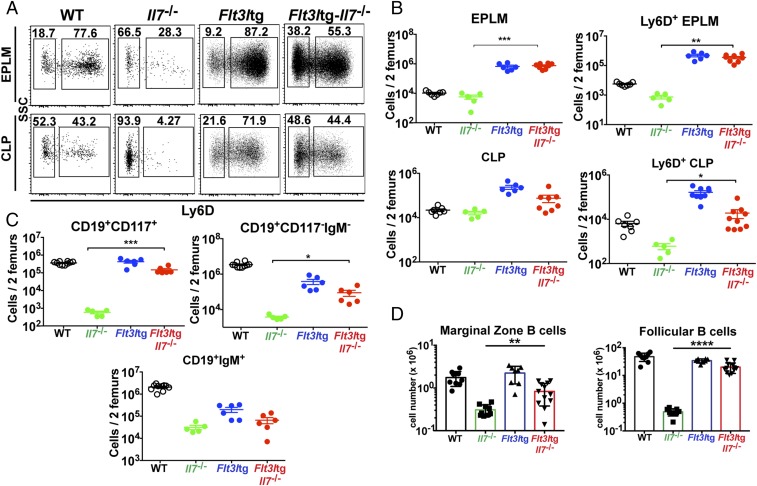
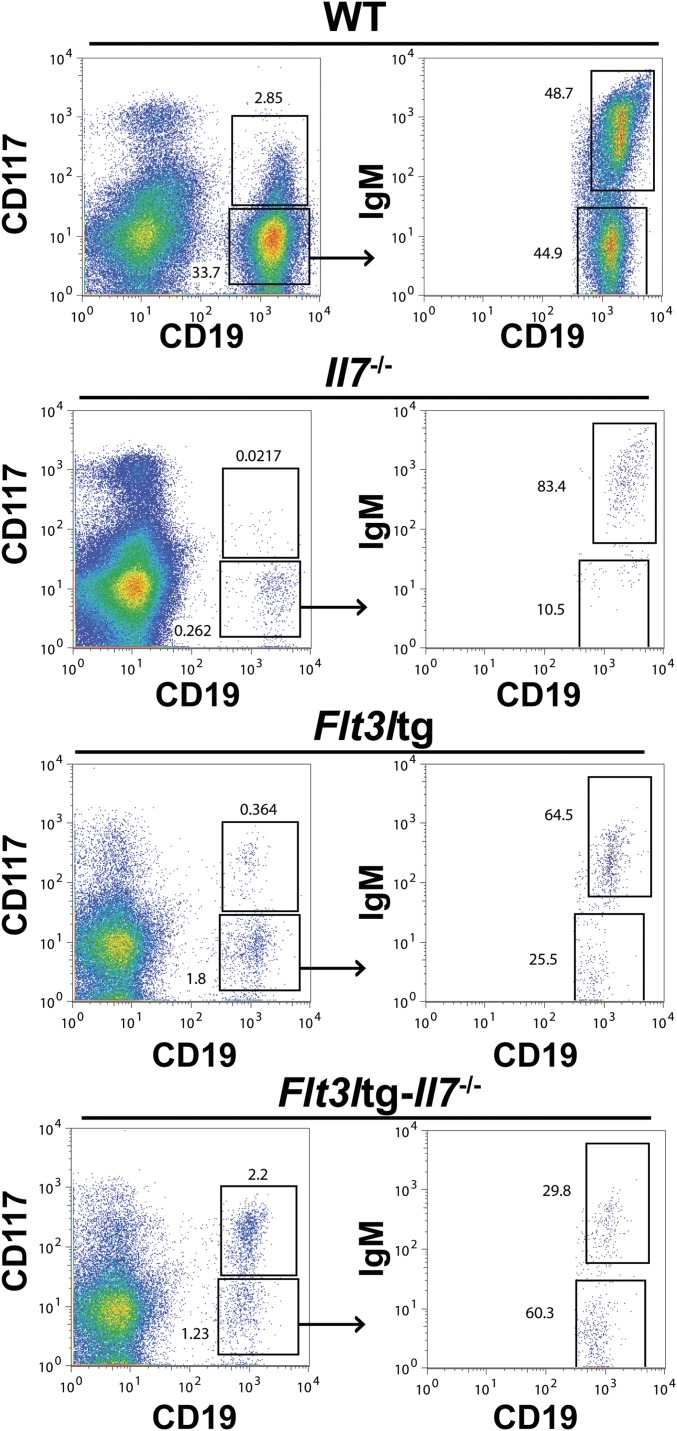
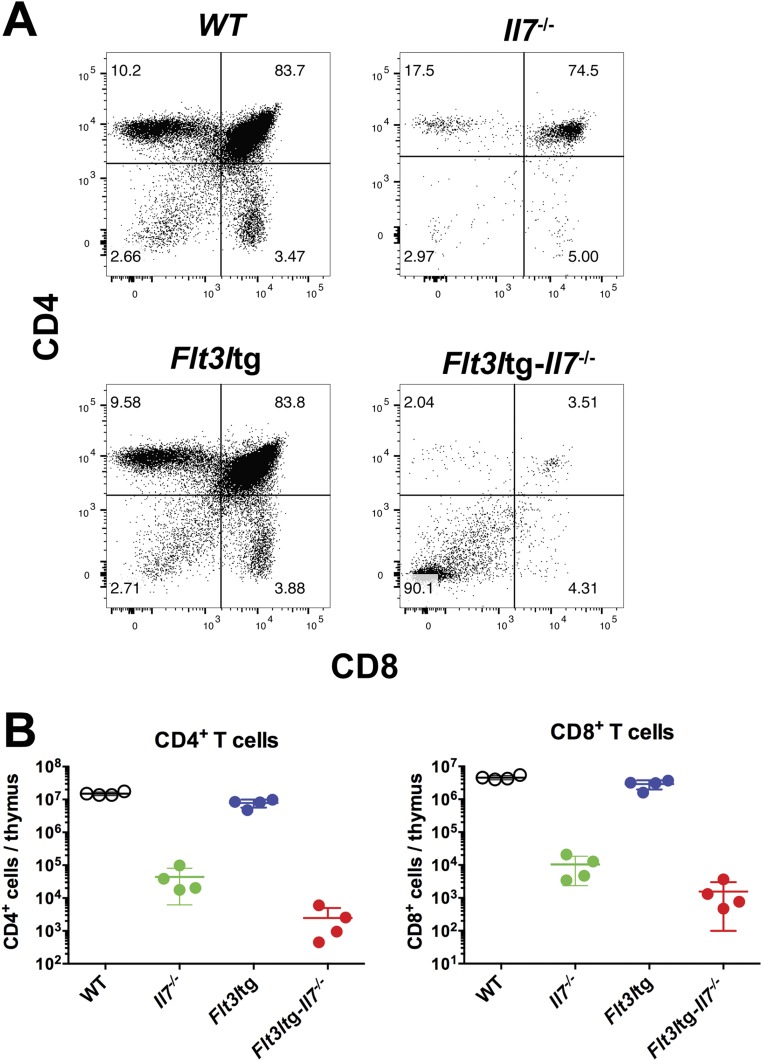
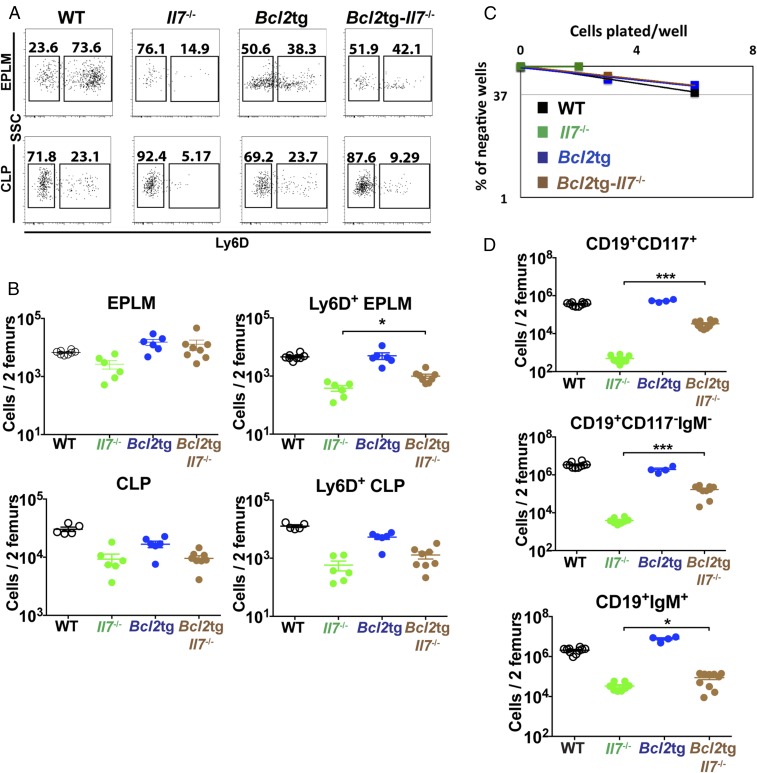
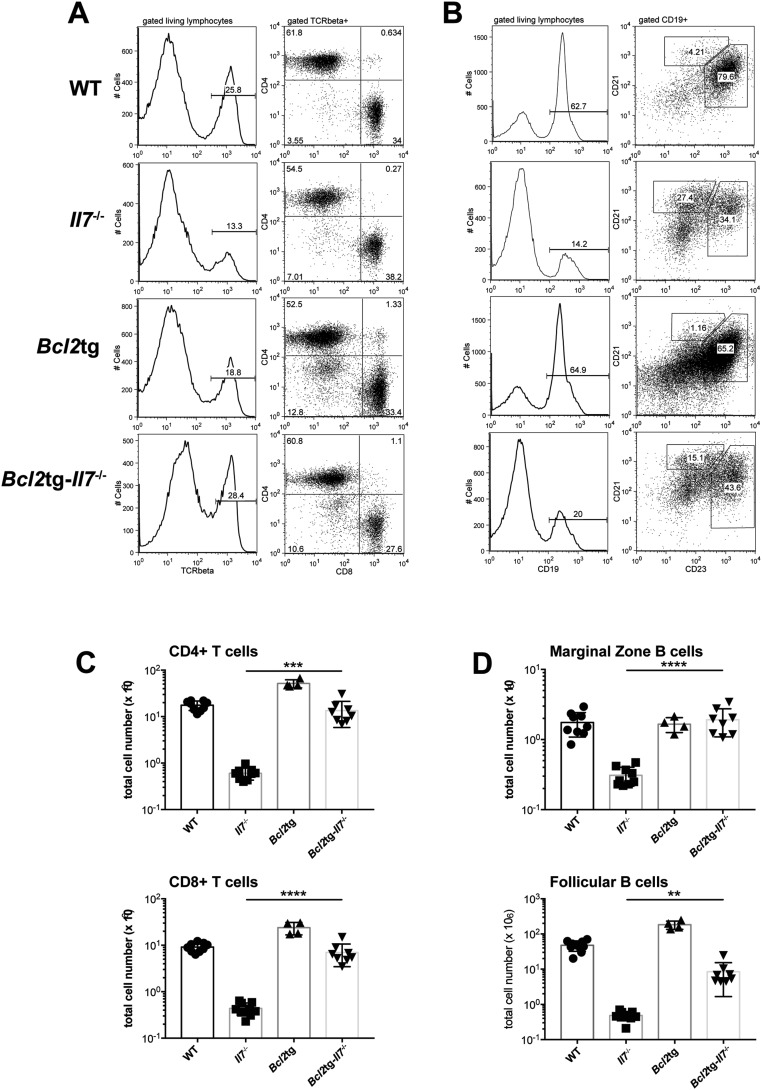
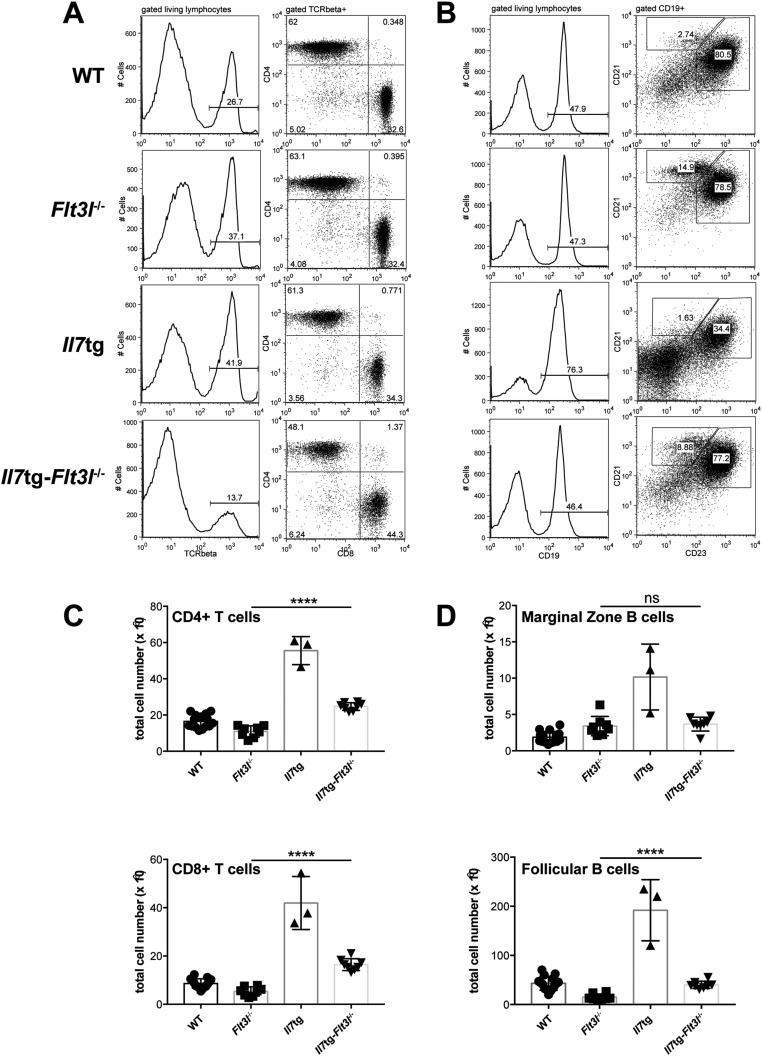
We have recently generated a mouse model expressing high in vivo levels of FL (8). The progenitor compartment of these mice showed a dramatic increase in EPLM and CLP numbers, with their Ly6D+ fractions increased 90-fold and 28-fold, respectively, relative to WT (Fig. 1 D and E). We crossed Flt3ltg with Il7−/− mice to assess the extent to which increased FL levels could potentially rescue the loss of Ly6D+CD19− progenitors in Il7−/− mice. As shown in Fig. 2 A and B, in vivo overexpression of FL leads to a significant increase in Flt3ltg-Il7−/− EPLM and CLP numbers, reaching levels of those in Flt3ltg mice. Crucially, a full rescue of Ly6D+ EPLM and CLP can be seen in these mice, with a striking 470-fold and 31-fold increase in numbers compared with their Il7−/− counterparts (Fig. 2 A and B). Furthermore, the numbers of the earliest committed CD19+CD117+ pro-B cells were fully restored in Flt3ltg-Il7−/− mice, showing a 251-fold increase compared with Il7−/− (Fig. 2C and Fig. S2). However, this rescue was less pronounced in downstream CD19+CD117−IgM− and CD19+IgM+ B-cell stages, because these cells require IL-7 to expand. As a consequence of this rescue in bone marrow B-cell development, numbers of splenic marginal zone and follicular B cells were significantly increased in Flt3ltg-Il7−/− mice compared with Il7−/− (Fig. 2D). Whereas thymic T-cell development was not rescued in Flt3ltg-Il7−/− mice (Fig. S3), a significant increase in splenic T-cell numbers was observed (Fig. S4) as a result of their expansion upon FL overexpression (32).
Fig. 2.
Increased in vivo FL levels rescue B-cell generation in Il7−/− mice. (A) Representative FACS plots of EPLM (Upper) and CLP (Lower) from WT, Il7−/−, Flt3ltg, and Flt3ltg-Il7−/− mice. (B) Numbers of EPLM (Upper Left), CLP (Lower Left), Ly6D+ EPLM (Upper Right), and Ly6D+ CLP (Lower Right) from the mouse genotypes indicated on the x axes. For each mouse genotype, mean ± SEM is shown. (C) Numbers of CD19+CD117+ (Upper Left), CD19+CD117−IgM− (Upper Right), and CD19+IgM+ (Lower) bone marrow cells from the mice indicated on the x axes. For each mouse genotype, mean ± SEM is shown. (D) Numbers of CD19+CD21highCD23low marginal zone (Left) and CD19+CD21+CD23+ follicular (Right) B cells in the spleens of WT or mutant mice, as indicated on the x axes. For each mouse genotype, mean ± SD is shown. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
Fig. S2.
Rescue of CD19+ bone marrow B-cell progenitors in Flt3ltg-Il7−/− mice. Representative FACS plots for the identification of CD19+CD117+, CD19+CD117−IgM−, and CD19+IgM+ bone marrow cells are shown.
Fig. S3.
Thymic T-cell development in Flt3ltg-Il7−/− mice. (A) Representative FACS plots showing CD4/CD8 thymocyte staining from 6- to 8-wk-old WT, Il7−/−, Flt3ltg, and Flt3ltg-Il7−/− mice (n = 4 per group). (B) Total numbers of CD4+ (Left) and CD8+ (Right) single-positive thymocytes from the mouse genotypes indicated on the x axes.
Fig. S4.
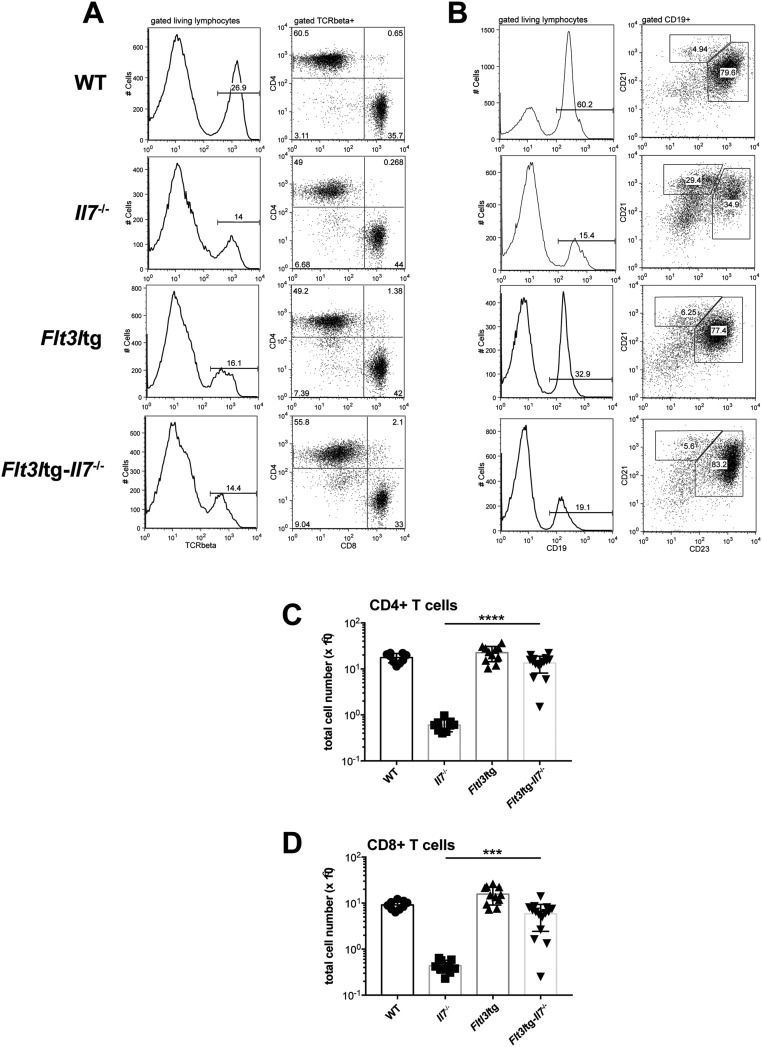
(A) Representative FACS plots illustrating T cells in the spleens of WT (first row), Il7−/− (second row), Flt3ltg (third row), and Flt3ltg-Il7−/− (fourth row) mice. After gating on living lymphocytes, TCRβ+ cells are further subgrouped in CD4+ and CD8+ T cells. (B) Representative FACS plots illustrating B cells in the spleens of WT (first row), Il7−/− (second row), Flt3ltg (third row), and Flt3ltg-Il7−/− (fourth row) mice. After gating on living lymphocytes, CD19+ cells are further subgrouped in CD21highCD23low marginal zone B cells and CD21+CD23+ follicular B cells. (C and D) Numbers of splenic CD4+ (C) and CD8+ (D) T cells, stained as shown in A, from WT and mutant mice as indicated on the x axes. ***P ≤ 0.001, ****P ≤ 0.0001. Student’s t test; n = 9–15. Data shown above are mean ± SD.
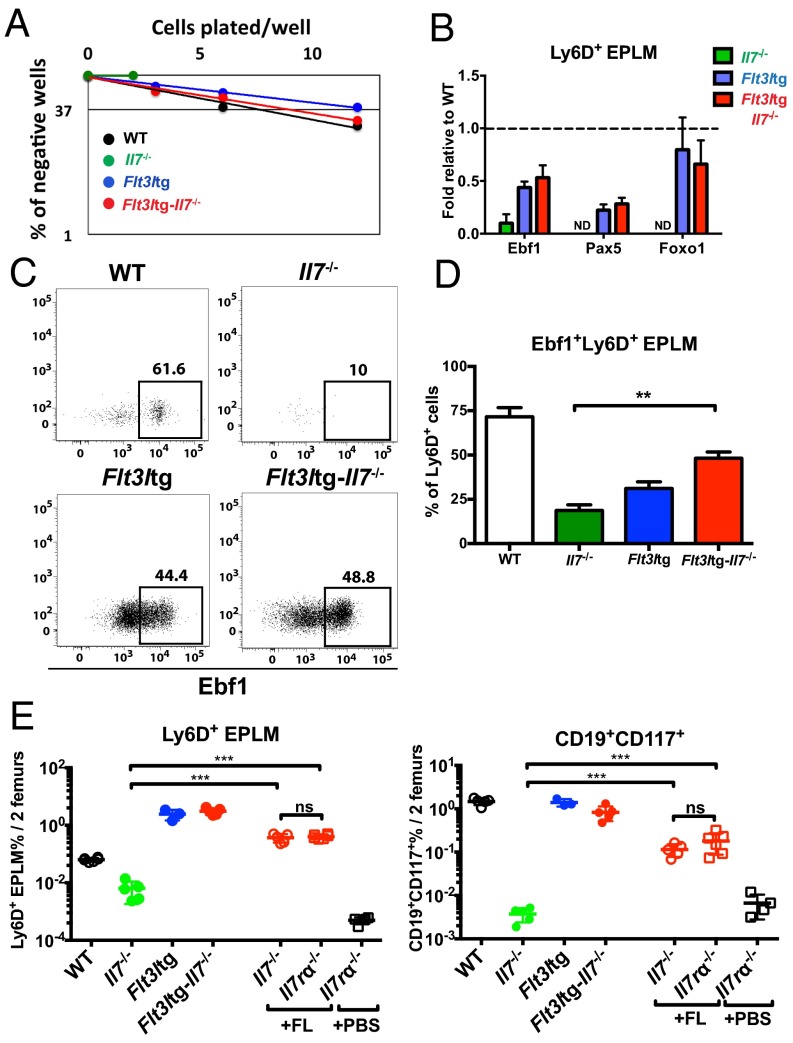
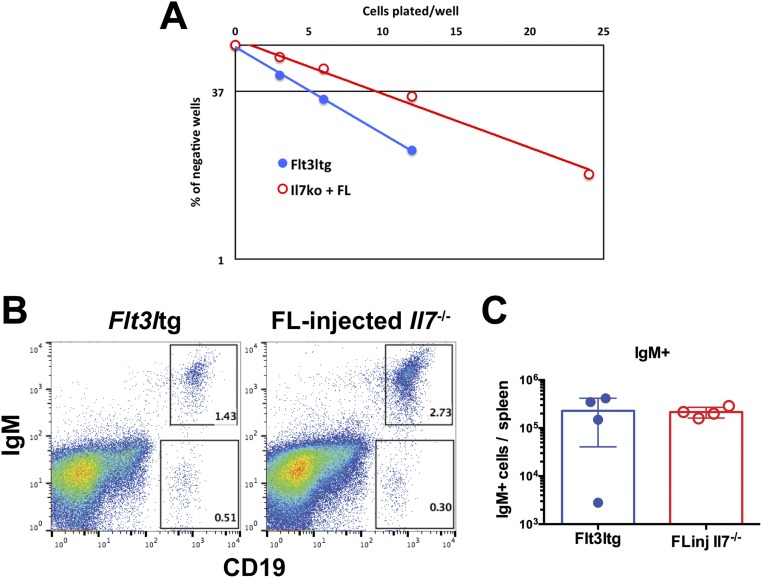
To assess whether these rescued Flt3ltg-Il7−/− Ly6D+CD19− progenitors could give rise to B cells in vitro, we sorted Flt3ltg-Il7−/− Ly6D+ EPLM and plated them at limiting dilution on OP9 stromal cells in the presence of IL-7. As shown in Fig. 3A, Flt3ltg-Il7−/− Ly6D+ EPLM could generate B cells at similar frequencies to their WT and Flt3ltg counterparts, whereas the few Il7−/− Ly6D+ EPLM isolated could not. A rescue in Ly6D+ EPLM was also observed when Il7−/− mice were injected with FL (Fig. 3E) and when plated under the same conditions these rescued Ly6D+ EPLM also showed a restored in vitro B-cell potential (Fig. S5A). Further, when transplanted into irradiated Rag2−/− mice, they were able to generate IgM+ B cells (Fig. S5 B and C). Thus, increased FL levels restore the generation of Ly6D+ progenitors, rather than merely expanding the few Ly6D+ EPLM/CLPs found in Il7−/− mice. RT quantitative PCR (RT-qPCR) analysis of Ly6D+ EPLM from Flt3ltg-Il7−/− mice revealed significant expression of Ebf1, Pax5, and Foxo1 transcription factors’ mRNA in the absence of IL-7 (Fig. 3B). Ebf1 expression at the protein level was confirmed by intracellular FACS staining (Fig. 3 C and D). Even though the percentage of Flt3ltg-Il7−/− Ebf1+Ly6D+ EPLM did not reach WT levels, it was similar to the one found in Flt3ltg mice, which produce IL-7. Therefore, Ebf1/Pax5 expression and subsequent commitment to the B-cell fate can occur in the absence of IL-7 signaling, arguing against an instructive role of this cytokine in B-cell commitment.
Fig. 3.
Increased in vivo FL rescues B-cell commitment in the absence of IL-7 and/or TSLP. (A) In vitro limiting dilution analysis of Ly6D+ EPLM B-cell potential. Ly6D+ EPLM were sorted from WT, Il7−/−, Flt3ltg, and Flt3ltg-Il7−/− mice and plated at the indicated concentrations on OP9 stromal cells together with IL-7. One representative out of four independent experiments is shown. (B) RT-qPCR analysis showing expression of Ebf1, Pax5, and Foxo1 mRNAs in Ly6D+ EPLM sorted from the indicated mouse genotypes. Bars show fold expression relative to WT (set as 1). Error bars represent the SEM from three to six independent experiments. (C) Representative FACS plots showing expression of Ebf1 protein within the Ly6D+ EPLM of the indicated WT or mutant mice. (D) Percentages of Ebf1-expressing Ly6D+ EPLM from WT (n = 7), Il7−/− (n = 3), Flt3ltg (n = 11), and Flt3ltg-Il7−/− (n = 6) mice. Bars show mean ± SEM (E) Ly6D+ EPLM (Left), and CD19+CD117+ (Right) numbers from WT (n = 5), Il7−/− (n = 5), Flt3ltg (n = 3), and Flt3ltg-Il7−/− (n = 5) mice, as well as from Il7−/− (n = 5) and Il7rα−/− (n = 6) mice injected intraperitoneally with 10 daily doses of 10 μg FL each (indicated as +FL) or PBS (+PBS, n = 4). Shown is the mean ± SEM. n.s., not significant, **P ≤ 0.01, ***P ≤ 0.001.
Fig. S5.
B-cell potential of Ly6D+ EPLM cells from Il7−/− mice injected with FL. Il7−/− mice were injected with FL (10 daily doses of 10 μg per mouse) and Ly6D+ EPLM were sorted from their bone marrows 1 d after the last injection. (A) In vitro limiting dilution analysis of the B-cell potential of FL-injected Il7−/− Ly6D+ EPLM. Cells were plated at the indicated concentrations on OP9 stromal cells plus IL-7. Flt3ltg Ly6D+ EPLM were used as positive controls. (B and C) In vivo B-cell potential of FL-injected Il7−/− Ly6D+ EPLM. Five thousand Ly6D+ EPLM from FL-injected Il7−/− or Flt3ltg mice were i.v. injected into sublethally irradiated Rag2−/− mice. Four weeks after cell transfer, spleens were analyzed for expression of CD19 and IgM. (B) Representative FACS plots of recipient spleens. (C) Numbers of CD19+IgM+ B cells harvested from the analyzed spleens (n = 4 mice per group).
CD127 (IL7Rα) is a receptor shared between IL-7 and thymic stromal lymphopoietin (TSLP), a cytokine capable of rescuing B-cell development when overexpressed in the absence of IL-7 (33). Because TSLP is produced by dendritic cells (34), which are dramatically expanded in Flt3ltg mice (8), in vivo FL overexpression could lead to increased levels of TSLP, thereby rescuing B-cell development in Flt3ltg-Il7−/− mice. To investigate this possibility, we injected Il7−/− or Il7rα−/− mice with FL as described above and assessed the rescue of Ly6D+ EPLM and downstream CD19+ progenitors. FL injections into Il7−/− mice resulted in a significant increase in Ly6D+ EPLM and CD19+CD117+ B-cell progenitors, comparable to the rescue observed in Flt3ltg-Il7−/− mice (Fig. 3E). FL-injected Il7rα−/− mice also demonstrated a significant rescue of Ly6D+ EPLM and CD19+CD117+ pro-B cells, indicating that the observed rescue of B-cell commitment in Flt3ltg-Il7−/− mice is not mediated through the action of TSLP.
IL-7 Promotes Survival, but Not Proliferation, of Ly6D+CD135+CD127+CD19− Progenitors.
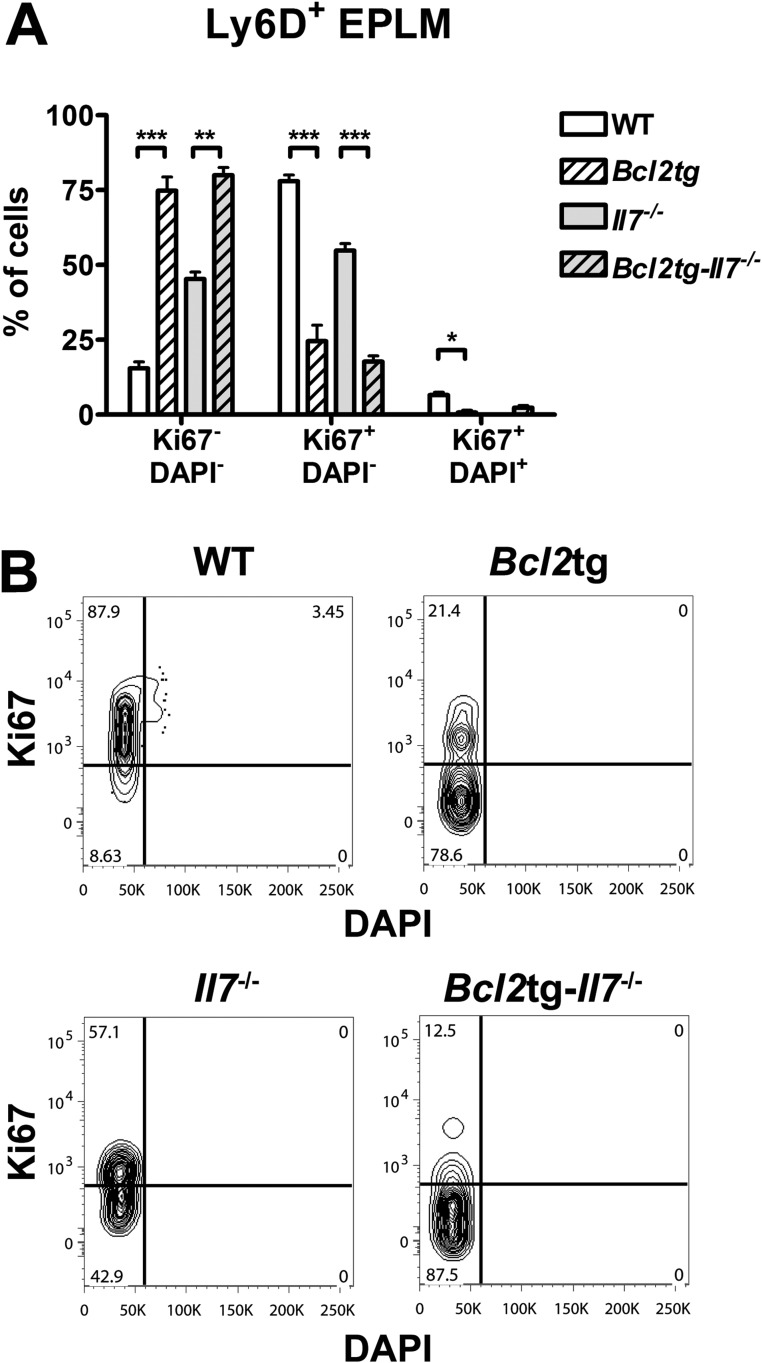
Even though our Flt3ltg-Il7−/− mouse model suggests that IL-7 is dispensable for B-cell commitment, the dramatic decrease in Il7−/− Ly6D+ EPLM/CLPs argues for a role of IL-7 in the maintenance of this population when FL levels are limiting, by promoting either their survival or their proliferation. To investigate the potential role of IL-7 as a survival factor for Ly6D+CD135+CD127+CD19− progenitors, we crossed Il7−/− mice with mice expressing the prosurvival gene Bcl2 (35). Bcl2tg-Il7−/− mice demonstrated a minor but statistically significant 2.6-fold increase in Ly6D+ EPLM and 2.2-fold increase in Ly6D+ CLP numbers compared with Il7−/− mice (Fig. 4 A and B). Cell cycle stage analysis of Ly6D+ EPLM of these mice indicated that most of the cells rescued by Bcl2 are in a quiescent state (Fig. S6) and do not proliferate in response to cytokines, thereby compromising to some extent the rescue of these progenitors’ numbers. Importantly, when plated on OP9 stromal cells plus IL-7, Bcl2tg-Il7−/− Ly6D+ EPLM generated B cells at frequencies similar to WT mice (Fig. 4C), indicating that these rescued Ly6D+ cells had B-cell potential. Indeed, when analyzing bone marrow CD19+ committed progenitors, we could see a significant 68-fold increase in the earliest CD19+CD117+ pro–B-cell compartment, compared with Il7−/− (Fig. 4D). Due to their quiescent state (Fig. S6) (36) and the IL-7 dependence of their proliferation, Bcl2tg-Il7−/− CD19+CD117+ numbers did not reach WT levels, whereas downstream CD19+ immature B cells showed a less pronounced, but significant rescue (Fig. 4D). In the spleens of these mice, marginal zone and follicular B-cell numbers were increased, whereas as previously reported (16), T-cell numbers were rescued (Fig. S7). Therefore, providing an extra Bcl2-mediated survival signal in vivo partially rescues Il7−/− Ly6D+CD19− progenitors with B-cell potential and restores significantly the generation of CD19+ progenitors. This result suggests a role for IL-7 in facilitating the survival of Ly6D+CD135+CD127+CD19− progenitors.
Fig. 4.
Bcl2 overexpression partially rescues B-cell commitment in Il7−/− mice. (A) Representative FACS plots of EPLM (Upper) and CLP (Lower) from WT, Il7−/−, Bcl2tg, and Bcl2tg-Il7−/− mice. (B) Numbers of EPLM (Upper Left), CLP (Lower Left), Ly6D+ EPLM (Upper Right), and Ly6D+ CLP (Lower Right) from WT and mutant mice, as indicated on the x axes. For each mouse genotype, mean ± SEM is shown. (C) In vitro limiting dilution analysis of Ly6D+ EPLM B-cell potential. Ly6D+ EPLM were sorted from WT, Il7−/−, Bcl2tg, and Bcl2tg-Il7−/− mice and plated at the indicated concentrations on OP9 stromal cells together with IL-7. One representative of three independent experiments is shown. (D) Numbers of CD19+CD117+ (Top), CD19+CD117−IgM− (Middle), and CD19+IgM+ (Bottom) bone marrow cells from WT and mutant mice, as indicated on the x axes. For each mouse genotype, mean ± SEM is shown. *P ≤ 0.05, ***P ≤ 0.001.
Fig. S6.
Quiescent state of Bcl2-rescued cells in vivo. (A) Cell cycle analysis of Ly6D+ EPLM from WT (n = 5), Bcl2tg (n = 2), Il7−/− (n = 2), and Bcl2tg-Il7−/− (n = 4) mice. Graph shows percentages of Ki67−DAPI−, Ki67+DAPI−, and Ki67+DAPI+ Ly6D+ EPLM. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. Student’s t test. Bars show mean ± SEM. (B) Representative Ki67/DAPI FACS plots of the Ly6D+ EPLM cell cycle analysis collectively presented in A.
Fig. S7.
Bcl2-mediated rescue of splenic T and B cells in the absence of IL-7. (A) Representative FACS plots illustrating T cells in the spleens of WT (first row), Il7−/− (second row), Bcl2tg (third row), and Bcl2tg-Il7−/− (fourth row) mice. After gating on living lymphocytes, TCRβ+ cells are further subgrouped in CD4+ and CD8+ T cells. (B) Representative FACS plots illustrating B cells in the spleens of WT (first row), Il7−/− (second row), Bcl2tg (third row), and Bcl2tg-Il7−/− (fourth row) mice. After gating on living lymphocytes CD19+ cells are further subgrouped in CD21highCD23low marginal zone B cells and CD21+CD23+ follicular B cells. (C) Numbers of splenic CD4+ (Upper) and CD8+ (Lower) T cells, stained as shown in A, from WT and mutant mice as indicated on the x axes. (D) Numbers of splenic marginal zone (Upper) and follicular (Lower) B cells, stained as shown in B, from WT and mutant mice as indicated on the x axes. **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001. Student’s t test; n = 4–9. Data shown above are mean ± SD.
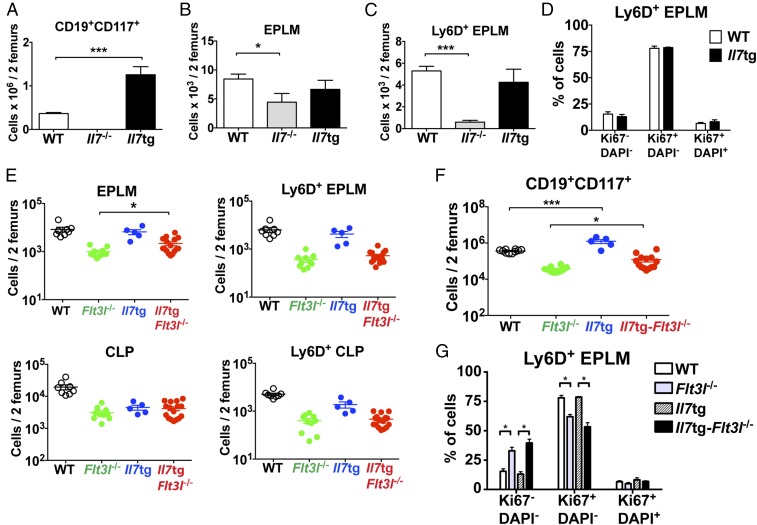
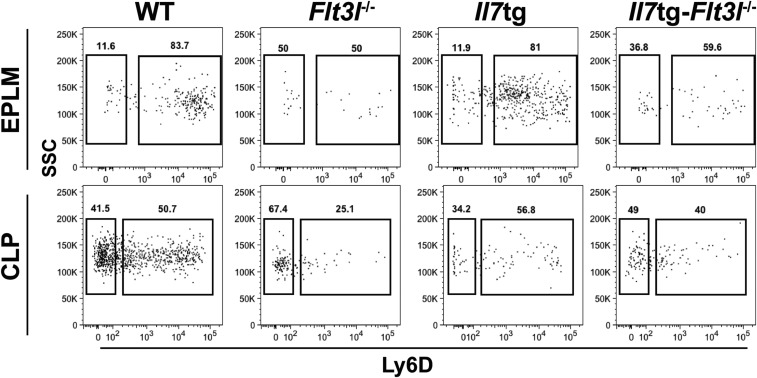
To evaluate the potential proliferative effect of IL-7 on Ly6D+CD135+CD127+CD19− progenitors, we analyzed a transgenic mouse model, in which Il7 expression is driven by an MHC class II promoter, resulting in increased in vivo levels of IL-7 (37). These mice exhibit a lymphoproliferative phenotype with increased numbers of CD19+ B cells (38). In contrast to bone marrow CD19+ cells, Ly6D+ EPLM numbers did not increase in response to elevated IL-7 (Fig. 5 A–C). In addition, the cell cycle profile of Ly6D+ EPLM remained unaltered in Il7tg mice compared with WT (Fig. 5D), arguing against a proliferative action of IL-7 on these progenitors. To exclude the possibility that a proliferative signal by FL present in these mice compromised the effect of increased IL-7 on the cell cycle status of Ly6D+ EPLM, we crossed Il7tg with Flt3l−/− mice. Overexpression of IL-7 in vivo did not result in a significant increase in Ly6D+ EPLM or CLP numbers in the absence of FL (Fig. 5E and Fig. S8). In contrast, a threefold increase in CD19+CD117+ numbers was observed (Fig. 5F), in agreement with the proliferative effect of IL-7 on CD19+ B cells. This resulted in a small, but significant, increase in splenic follicular B cells (Fig. S9). Moreover, cell cycle analysis of Il7tg-Flt3l−/− Ly6D+ EPLM showed no significant change in their cycling profile compared with their Flt3l−/− counterparts (Fig. 5G). Therefore, we conclude that, whereas IL-7 acts as a proliferative factor for CD19+ committed B cells, it does not do so for their Ly6D+CD135+CD127+CD19− precursors.
Fig. 5.
IL-7 does not induce proliferation of Ly6D+CD135+CD127+CD19− progenitors. (A) CD19+CD117+ numbers in bone marrow of WT (n = 10), Il7−/− (n = 5), and Il7tg (n = 8) mice. (B) EPLM numbers in bone marrow of WT (n = 14), Il7−/− (n = 7), and Il7tg (n = 5) mice. (C) Ly6D+ EPLM numbers in bone marrow of WT (n = 14), Il7−/− (n = 7), and Il7tg (n = 5) mice. (D) Cell cycle analysis of Ly6D+ EPLM from WT (n = 5) and Il7tg (n = 2) mice. Graph shows percentages of Ki67−DAPI−, Ki67+DAPI−, and Ki67+DAPI+ Ly6D+ EPLM. Bars in A–D show mean ± SEM. (E) Numbers of EPLM (Upper Left), CLP (Lower Left), Ly6D+ EPLM (Upper Right), and Ly6D+ CLP (Lower Right) from WT and mutant mice, as indicated on the x axes. For each mouse genotype, mean ± SEM is shown. (F) Numbers of CD19+CD117+ bone marrow cells from WT and mutant mice, as indicated on the x axis. For each mouse genotype, mean ± SEM is shown. (G) Cell cycle analysis of Ly6D+ EPLM from WT (n = 5), Flt3l−/− (n = 3), Il7tg (n = 2), and Il7tg-Flt3l−/− (n = 3) mice. Graph shows percentages of Ki67−DAPI−, Ki67+DAPI−, and Ki67+DAPI+ Ly6D+ EPLM. Bars show mean ± SEM. *P ≤ 0.05, ***P ≤ 0.001.
Fig. S8.
Representative FACS plots of EPLM (Upper) and CLP (Lower) from WT, Flt3l−/−, Il7tg, and Il7tg-Flt3l−/− mice.
Fig. S9.
Effect of IL-7 overexpression on WT and Flt3l−/− splenic T and B cells. (A) Representative FACS plots illustrating T cells in the spleens of WT (first row), Flt3l−/− (second row), Il7tg (third row), and Il7tg-Flt3l−/− (fourth row) mice. After gating on living lymphocytes, TCRβ+ cells are further subgrouped in CD4+ and CD8+ T cells. (B) Representative FACS plots illustrating B cells in the spleens of WT (first row), Flt3l−/− (second row), Il7tg (third row), and Il7tg-Flt3l−/− (fourth row) mice. After gating on living lymphocytes, CD19+ cells are further subgrouped in CD21highCD23low marginal zone B cells and CD21+CD23+ follicular B cells. (C) Numbers of splenic CD4+ (Upper) and CD8+ (Lower) T cells, stained as shown in A, from WT and mutant mice as indicated on the x axes. (D) Numbers of splenic marginal zone (Upper) and follicular (Lower) B cells, stained as shown in B, from WT and mutant mice as indicated on the x axes. ns, not significant or P > 0.05, ****P ≤ 0.0001. Student’s t test; n = 3–15. Data shown above are mean ± SD.
FL Induces Proliferation of Ly6D+CD135+CD127+CD19− Progenitors.
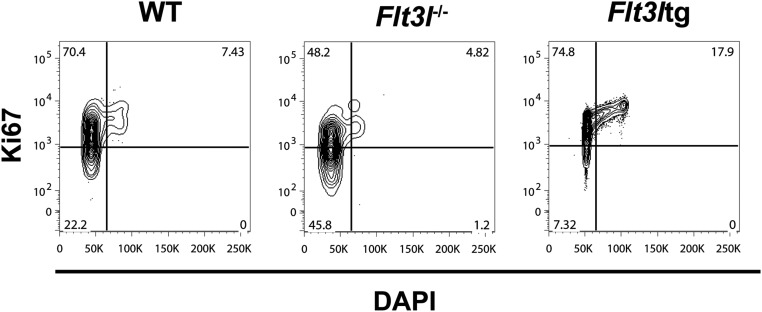
As evident in Fig. 5G, loss of in vivo FL signaling affected the proliferative status of Ly6D+ EPLM. Comparison of Ly6D+ EPLM numbers in mice either lacking or overexpressing FL showed a 14-fold reduction in Flt3l−/− Ly6D+ EPLM numbers compared with WT, whereas Flt3ltg Ly6D+ EPLM increased 105-fold (Fig. 6A). A similar response to FL levels was observed for Ly6D+ CLPs (Fig. 6A). Cell cycle analysis of Ly6D+ EPLM from these mice showed a significant increase in the percentage of Ki67−DAPI− cells and a decrease in the percentage of Ki67+ cells when FL signaling was absent, whereas Flt3ltg Ly6D+ EPLM showed the reverse (Fig. 6B and Fig. S10). Thus, our data indicate that FL promotes the proliferation of Ly6D+CD135+CD127+CD19− progenitors.
Fig. 6.
FL promotes proliferation but not survival of Ly6D+CD135+CD127+CD19− progenitors. (A) Numbers of EPLM (Upper Left), CLP (Lower Left), Ly6D+ EPLM (Upper Right), and Ly6D+ CLP (Lower Right) from WT (n = 14), Flt3l−/− (n = 10) and Flt3ltg (n = 9) mice. Bars show mean ± SEM. (B) Cell cycle analysis of Ly6D+ EPLM from WT (n = 5), Flt3l−/− (n = 3), and Flt3ltg (n = 9) mice. Graph shows percentages of Ki67−DAPI−, Ki67+DAPI−, and Ki67+DAPI+ Ly6D+ EPLM. Bars show mean ± SEM. (C) Numbers of EPLM (Upper Left), CLP (Lower Left), Ly6D+ EPLM (Upper Right), and Ly6D+ CLP (Lower Right) from WT and mutant mice, as indicated on the x axes. For each mouse genotype, mean ± SEM is shown. (D) In vitro limiting dilution analysis of Ly6D+ EPLM B-cell potential. Ly6D+ EPLM were sorted from WT, Flt3l−/−, and Bcl2tg-Flt3l−/− mice and plated at the indicated concentrations on OP9 stromal cells together with IL-7. (E) Numbers of CD19+CD117+ (Left), CD19+CD117−IgM− (Middle), and CD19+IgM+ (Right) bone marrow cells from WT and mutant mice, as indicated on the x axes. For each mouse genotype, mean ± SEM is shown. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Fig. S10.
Effect of in vivo FL levels on Ly6D+ EPLM cell cycle. Representative Ki67/DAPI FACS plots of the Ly6D+ EPLM cell cycle analysis collectively presented in Fig. 6B.
To evaluate whether FL also regulates the survival of Ly6D+CD135+CD127+CD19− progenitors, we crossed Flt3l−/− mice with Bcl2tg mice. Thus, Bcl2tg-Flt3l−/− mice showed a minor twofold increase in Ly6D+ EPLM numbers compared with their Flt3l−/− counterparts (1.8-fold for Ly6D+ CLPs) (Fig. 6C). Nevertheless, the in vitro B-cell potential of Flt3l−/− Ly6D+ EPLM progenitors was not improved by Bcl2 overexpression (Fig. 6D). Downstream CD19+ progenitors also demonstrated a partial, but significant, rescue (Fig. 6E). Our analysis of Bcl2tg-Flt3l−/− mice suggests that the reduction in Ly6D+CD135+CD127+CD19− progenitors observed in Flt3l−/− mice can only be partially explained by a survival role of FL. In contrast, the clear change in the numbers and cycling profile of these progenitors in response to the absence or overabundance of FL in vivo, as well as the inability of Bcl2 to rescue their in vitro B-cell potential, points toward proliferation as being the main effector function of FL at this developmental stage.
FL Does Not Instruct Commitment to the B-Cell Lineage.
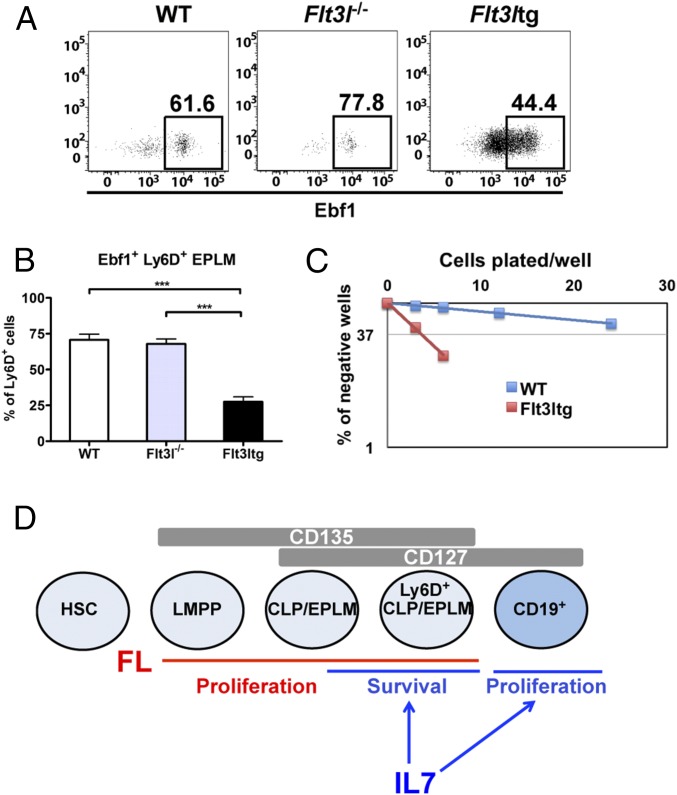
The striking rescue in B-cell commitment observed in our Flt3ltg-Il7−/− mice could be explained by a potential instructive role of FL when present at high levels in vivo. However, increased FL did not result in Ebf1 or Pax5 up-regulation (Fig. 3 B–D). Moreover, analysis of Flt3l−/− Ly6D+ EPLM showed that, whereas absence of FL in vivo leads to a reduction in the numbers of Ly6D+ EPLM (Fig. 1C), it does not significantly reduce the percentage of Ebf1+ cells within the population (Fig. 7 A and B), consistent with a permissive rather than instructive role of FL. Finally, the decrease in the Ebf1+ fraction of Ly6D+ EPLM upon exposure to high levels of FL was reflected in the increased ability of these progenitors to give rise to T cells in vitro, as manifested by the high frequency of T-cell clone generation when Flt3ltg Ly6D+ EPLM were plated on OP9-DL1 stromal cells in the presence of IL-7 (Fig. 7C). The above data suggest that FL does not instruct commitment to the B-cell lineage through up-regulation of Ebf1 and Pax5 expression.
Fig. 7.
FL does not instruct Ebf1 expression and B-cell commitment. (A) Representative FACS plots showing expression of Ebf1 protein within the Ly6D+ EPLM of WT, Flt3l−/−, and Flt3ltg mice. (B) Percentages of Ebf1-expressing Ly6D+ EPLM from WT (n = 7), Flt3l−/− (n = 5), and Flt3ltg (n = 12) mice. Bars show mean ± SEM. (C) In vitro limiting dilution analysis of Ly6D+ EPLM T-cell potential. Ly6D+ EPLM were sorted from WT and Flt3ltg mice and plated at the indicated concentrations on OP9-DL1 stromal cells together with IL-7. One representative of four independent experiments is shown. (D) Schematic model for the permissive role of IL-7 and FL acting on hematopoietic progenitors and CD19+ committed B-cell precursors. LMPP, lymphoid-primed multipotent progenitor. ***P ≤ 0.001.
Discussion
Commitment to the B-cell lineage is mediated by the expression of Ebf1 and Pax5 transcription factors and it is initiated in Ly6D+CD135+CD127+ progenitors before CD19 expression (30, 31). In Il7−/− mice, this Ly6D+ CLP compartment is significantly reduced (22), a finding confirmed in the present study for both CLP and EPLM, a B220int/+ population partly overlapping with CLP and pre/pro-B cells (Fig. 1 B and C). The proliferative effect of IL-7 on committed CD19+ B-cell progenitors (38) makes the investigation of its role in B-cell commitment challenging when using CD19+ cells as readout. Hence, we assessed the role of IL-7 in B-cell commitment by analyzing the Ly6D+ CLP/EPLM compartment in different mouse models. Our analysis of Flt3ltg-Il7−/− mice showed a complete rescue of Ly6D+ CLP/EPLM numbers in vivo and their B-cell potential in vitro and in vivo, whereas Ebf1 and Pax5 were expressed at similar levels to Flt3ltg mice, thereby indicating that IL-7 signaling is not required for their up-regulation at the Ly6D+CD19− stage (Figs. 2 and 3). These results suggest that IL-7 is not acting as an instructive cytokine in B-cell commitment by initiating Ebf1 and Pax5 expression at the CD135+CD127+CD19− stage, as previously hypothesized (18–20), but rather as a permissive one.
Early investigations had shown that Bcl2 overexpression in the absence of IL-7 signaling could rescue T-cell (39, 40) but not B-cell development (16, 17). However, a more recent study demonstrated a Bcl2-mediated rescue of CD19+ progenitors in conditional Stat5−/− mice, as well as a strong activation of the prosurvival gene Mcl1 expression by Stat5 (21), therefore suggesting a survival role for IL-7 in B-cell development. Our use of Il7−/− mice instead of Il7rα−/−, which allows the assessment of progenitor in vitro B-cell potential, and our focus on Ly6D+CD135+CD127+CD19− progenitors, has enabled us to confirm the latter findings and extend them to the CD19− stage where B-cell commitment events are initiated at the molecular level. Interestingly, Il7tg mice analysis showed that IL-7 indeed acts as a proliferative factor for committed CD19+ cells, but not for their CD19− precursors. Even in the absence of FL, excess IL-7 was unable to significantly increase Ly6D+ CLP/EPLM numbers, whereas it did so for CD19+ B-cell progenitors (Fig. 5). Hence, we propose that the main role of IL-7 at the CD135+CD127+CD19− stage is to provide survival signals to the progenitors until they commit to the B-cell lineage upon Pax5 and CD19 expression, after which it additionally induces their proliferation (Fig. 7D). This survival role becomes particularly critical when FL levels are limiting, thereby explaining the reduction in Ly6D+ CLP/EPLM seen in Il7−/− mice. Our study, in agreement with previous data (21), identifies a common, permissive rather than instructive role for IL-7 in both B- and T-cell development (39, 40).
The rescue in B-cell commitment without active IL-7 signaling occurs when FL is expressed above physiological levels. Even though a minor role for FL as a survival factor for Ly6D+CD135+CD127+CD19− progenitors cannot be excluded, the main effect of FL on these progenitors seems to be the induction of their proliferation, as suggested by their expansion and their increased cycling upon FL overexpression, with the reverse phenotype observed upon loss of FL signaling (Fig. 6). Moreover, increased FL leads to expansion of Lin−CD117+Sca1+ cells (LSK) (8), thereby increasing the developmental input into the Ly6D+CD135+CD127+CD19− progenitor stage. None of the mouse models analyzed in the present study gave any evidence for an instructive role of FL in B-cell commitment. In contrast, excess FL resulted in a proportional reduction of Ebf- and Pax5-expressing Ly6D+CD19− progenitors (Figs. 3 and 7). One explanation for this reduction could be the increased percentage of cycling Flt3ltg Ly6D+CD19− progenitors, resulting in a decreased fraction initiating the B-cell developmental program. Alternatively, another environmental factor, responsible for initiation of Ebf1/Pax5 expression and B-cell commitment, could be the limiting factor in Flt3ltg mice, thus leading to a smaller fraction of the expanded Ly6D+CD19− compartment entering the B-cell pathway. Our conclusion is that FL is mainly responsible for generating enough Ly6D+CD135+CD127+CD19− progenitors, both by inducing their proliferation and by increasing their developmental input from the LSK compartment (Fig. 7D) (41, 42). As a result, increased levels of FL in Flt3ltg-Il7−/− mice lead to a dramatic increase in Ly6D+CD135+CD127+CD19− progenitor numbers, therefore surpassing the need for the survival role of IL-7 at this stage and resulting in a sufficient fraction of them committing to the B-cell lineage.
The generation of B-cell progenitors in Flt3ltg-Il7−/− mice is reminiscent of the apparent IL-7 independency of human B lymphopoiesis, where relatively normal numbers of B cells are seen in patients with mutations in components of the IL-7 signaling pathway (13, 14). However, all patients with such mutations are neonates, and in neonatal Il7−/− mice, B-cell development also takes place (43). Therefore, the apparent difference in the IL-7 dependency of B-cell development between man and mouse could actually reflect the corresponding difference between fetal/neonatal and adult lymphopoiesis. Our data showing that increased FL signaling can rescue B-cell commitment in the absence of IL-7 could provide a potential explanation for this difference. Fetal/neonatal CD135+CD127+CD19− progenitors might be exposed to higher levels of FL and/or show higher sensitivity to FL signaling than adult CD135+CD127+CD19− progenitors. Indeed, previous studies showed that despite a preferable response of fetal B-cell progenitors to TSLP, FL signaling remains an absolute requirement for fetal B lymphopoiesis (44, 45).
The instructive or permissive progenitor regulation of lineage commitment by cytokines is a complex process, in which cytokines can initiate developmental transcription programs in progenitors. However, the reverse is also true, because the particular epigenetic, transcriptional, and signaling landscape of a cell can affect its response to a cytokine (9). Indeed, whereas previous analysis of Flt3ltg mice indicated an instructive role for FL in promoting differentiation of multipotent progenitors toward lymphomyeloid and away from erythroid fate (8), our present data show that FL acts in a permissive manner for B-cell commitment of CD135+CD127+CD19− progenitors. In addition, whereas IL-7 induces proliferation of committed CD19+ B-cell progenitors, it does not do so on CD127+CD19− progenitors, suggesting that upon commitment to the B-cell lineage, changes in the transcription factor and intracellular signaling landscape influence the effector function of IL-7. Therefore, our present data further support the notion of a cell context-dependent cytokine action.
The Ebf1/Pax5 up-regulation and subsequent B-cell commitment in Flt3ltg-Il7−/− mice shown herein raises the issue of the potential extracellular regulation of B-cell commitment. One possibility could be that another environmental signal from the bone marrow microenvironment—other than IL-7, TSLP, and FL—initiates Ebf1 expression in Ly6D+CD135+CD127+CD19− progenitors, resulting in Pax5/CD19 expression and B-cell commitment. Alternatively, as yet uncommitted Ly6D+CD135+CD127+CD19− progenitors could express Ebf1 in a cell-autonomous, stochastic, manner with some obtaining sufficient Ebf1 to initiate the B-cell gene program and eventually commit to the B-cell lineage. The intricate transcription factor network sustaining B-cell commitment through a series of positive feedback regulatory loops (46) provides conceptual support for the latter hypothesis.
Materials and Methods
Mice.
For breeding and analysis, age- and sex-matched C57BL/6 Flt3l−/− (27), Flt3ltg (8), Il7−/− (10), Il7rα−/− (11), Il7tg (38), and (C57BL/6 × C3H) Bcl2tg (35) mice backcrossed with C57BL/6 for at least five generations were used at 6–11 wk of age. All mice were bred and maintained in our animal facility under specific pathogen-free conditions. Animal experiments were carried out within institutional guidelines (authorization number 1888 from the cantonal veterinarian office, Basel).
Antibodies, Flow Cytometry, and Sorting.
For analysis, cells were flushed from femurs of the two hind legs of mice. The procedure was performed in PBS containing 0.5% BSA and 5 mM EDTA. For detection of Ebf1 and cell cycle analysis, cells were fixed and permeabilized after cell-surface staining using the Foxp3 Fix/Perm buffer set (eBioscience), and subsequently stained with PE-conjugated anti-Ebf1 (T26-818) or FITC-conjugated anti-Ki67 (B56) and DAPI, according to the supplier’s protocol. Flow cytometry was done using a BD LSRFortessa (BD Biosciences) and data were analyzed using FlowJo software (Treestar). For cell sorting, a FACSAria IIu (BD Biosciences) was used (>98% purity).
In Vitro Limiting Dilution Assays.
Experiments have been performed as previously described (47). Briefly, OP9 or OP9-DL1 stromal cells were plated on flat-bottom 96-well plates 1 d before the initiation of cocultures, at a concentration of 3,000 cells per well. The following day, stromal cells were γ-irradiated (3,000 rad) and the sorted progenitor cells were added at different concentrations. Cultures were maintained in Iscove's modified Dulbecco's medium (IMDM) supplemented with 5 × 10−5 M β-mercaptoethanol, 1 mM glutamine, 0.03% (wt/vol) primatone, 100 units/mL penicillin, 100 μg/mL streptomycin, 5% (vol/vol) FBS, and 10% (vol/vol) IL-7–conditioned medium. After 14 d in culture, all wells were inspected under an inverted microscope, and wells containing colonies of more than 50 cells were scored as positive.
RT-qPCR.
RNA extraction was performed using TRI Reagent (Life Technologies) followed by cDNA synthesis using GoScript Reverse Transcriptase (Promega). RT-qPCR was performed using SYBR Green PCR Master Mix (Applied Biosystems).
Statistical Analysis.
Statistical analysis was performed with Prism 6.0g software (GraphPad software). Two-tailed unpaired Student t tests were used for statistical comparisons. If not differently indicated, data are presented as mean values ± SD or SEM. n.s., not significant or P > 0.05, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
SI Materials and Methods
Antibodies.
The following antibodies were used for flow cytometry (from BD Pharmingen, eBioscience, BioLegend, or produced in house): anti-B220 (RA3-6B2), anti-CD117 (2B8), anti-CD19 (1D3), anti-NK1.1 (PK136), anti-SiglecH (551), anti-CD11c (HL3), anti-CD115 (AFS98), anti-Ly6D (49-H4), anti-CD127 (SB/199), anti-CD135 (A2F10), anti-Sca1 (D7), anti-IgM (M41), anti-CD21 (7G6), anti-CD23 (B3B4), anti-CD4 (GK1.5), anti-CD8 (53.6.7), and anti-TCRβ (H57).
RT-qPCR.
The primers used were as follows: Ebf1, Ebf1-F: 5′-CAGGAAACCCACGTGACAT-3′ and Ebf1-R: 5′-CCACGTTGACTGTGGTAGACA-3′; Pax5, Pax5-F: 5′-ACGCTGACAGGGATGGTG-3′ and Pax5-R: 5′-GGGGAACCTCCAAGAATCAT-3′; Foxo1, Foxo1-F: 5′-AGTGGATGGTGAAGAGCGT-3′ and Foxo1-R: 5′-GAAGGGACAGATTGTGGCG-3′; Actin, Actin-F: 5′-CTGTCGAGTCGCGTCCACC-3′ and Actin-R: 5′-CGCAGCGATATCGTCATCCA-3′.
Acknowledgments
A.R. is holder of the chair in immunology (University of Basel) endowed by L. Hoffmann, La Roche Ltd, Basel. This study was supported by the Swiss National Science Foundation and by the People Programme (Marie Curie Actions) of the European Union’s Seventh Framework Programme FP7/2007-2013 under Research Executive Agency Grant 315902. R.C. was supported by Science Foundation Ireland Grants SFI09/SRC/B1794 and SFI07/SK/B1233b.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1613316113/-/DCSupplemental.
References
- 1.Metcalf D. Hematopoietic cytokines. Blood. 2008;111(2):485–491. doi: 10.1182/blood-2007-03-079681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Endele M, Etzrodt M, Schroeder T. Instruction of hematopoietic lineage choice by cytokine signaling. Exp Cell Res. 2014;329(2):207–213. doi: 10.1016/j.yexcr.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Enver T, Heyworth CM, Dexter TM. Do stem cells play dice? Blood. 1998;92(2):348–351, discussion 352. [PubMed] [Google Scholar]
- 4.Metcalf D. Lineage commitment and maturation in hematopoietic cells: The case for extrinsic regulation. Blood. 1998;92(2):345–347, discussion 352. [PubMed] [Google Scholar]
- 5.Grover A, et al. Erythropoietin guides multipotent hematopoietic progenitor cells toward an erythroid fate. J Exp Med. 2014;211(2):181–188. doi: 10.1084/jem.20131189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mossadegh-Keller N, et al. M-CSF instructs myeloid lineage fate in single haematopoietic stem cells. Nature. 2013;497(7448):239–243. doi: 10.1038/nature12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rieger MA, Hoppe PS, Smejkal BM, Eitelhuber AC, Schroeder T. Hematopoietic cytokines can instruct lineage choice. Science. 2009;325(5937):217–218. doi: 10.1126/science.1171461. [DOI] [PubMed] [Google Scholar]
- 8.Tsapogas P, et al. In vivo evidence for an instructive role of fms-like tyrosine kinase-3 (FLT3) ligand in hematopoietic development. Haematologica. 2014;99(4):638–646. doi: 10.3324/haematol.2013.089482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarrazin S, Sieweke M. Integration of cytokine and transcription factor signals in hematopoietic stem cell commitment. Semin Immunol. 2011;23(5):326–334. doi: 10.1016/j.smim.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 10.von Freeden-Jeffry U, et al. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181(4):1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peschon JJ, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180(5):1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parrish YK, et al. IL-7 Dependence in human B lymphopoiesis increases during progression of ontogeny from cord blood to bone marrow. J Immunol. 2009;182(7):4255–4266. doi: 10.4049/jimmunol.0800489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noguchi M, et al. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73(1):147–157. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- 14.Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T(-)B(+)NK(+) severe combined immunodeficiency. Nat Genet. 1998;20(4):394–397. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- 15.Namen AE, et al. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988;333(6173):571–573. doi: 10.1038/333571a0. [DOI] [PubMed] [Google Scholar]
- 16.Kondo M, Akashi K, Domen J, Sugamura K, Weissman IL. Bcl-2 rescues T lymphopoiesis, but not B or NK cell development, in common gamma chain-deficient mice. Immunity. 1997;7(1):155–162. doi: 10.1016/s1074-7613(00)80518-x. [DOI] [PubMed] [Google Scholar]
- 17.Maraskovsky E, Peschon JJ, McKenna H, Teepe M, Strasser A. Overexpression of Bcl-2 does not rescue impaired B lymphopoiesis in IL-7 receptor-deficient mice but can enhance survival of mature B cells. Int Immunol. 1998;10(9):1367–1375. doi: 10.1093/intimm/10.9.1367. [DOI] [PubMed] [Google Scholar]
- 18.Dias S, Silva H, Jr, Cumano A, Vieira P. Interleukin-7 is necessary to maintain the B cell potential in common lymphoid progenitors. J Exp Med. 2005;201(6):971–979. doi: 10.1084/jem.20042393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kikuchi K, Lai AY, Hsu CL, Kondo M. IL-7 receptor signaling is necessary for stage transition in adult B cell development through up-regulation of EBF. J Exp Med. 2005;201(8):1197–1203. doi: 10.1084/jem.20050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roessler S, et al. Distinct promoters mediate the regulation of Ebf1 gene expression by interleukin-7 and Pax5. Mol Cell Biol. 2007;27(2):579–594. doi: 10.1128/MCB.01192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malin S, et al. Role of STAT5 in controlling cell survival and immunoglobulin gene recombination during pro-B cell development. Nat Immunol. 2010;11(2):171–179. doi: 10.1038/ni.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsapogas P, et al. IL-7 mediates Ebf-1-dependent lineage restriction in early lymphoid progenitors. Blood. 2011;118(5):1283–1290. doi: 10.1182/blood-2011-01-332189. [DOI] [PubMed] [Google Scholar]
- 23.Mansson R, et al. B-lineage commitment prior to surface expression of B220 and CD19 on hematopoietic progenitor cells. Blood. 2008;112(4):1048–1055. doi: 10.1182/blood-2007-11-125385. [DOI] [PubMed] [Google Scholar]
- 24.Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100(5):1532–1542. doi: 10.1182/blood-2002-02-0492. [DOI] [PubMed] [Google Scholar]
- 25.Holmes ML, Carotta S, Corcoran LM, Nutt SL. Repression of Flt3 by Pax5 is crucial for B-cell lineage commitment. Genes Dev. 2006;20(8):933–938. doi: 10.1101/gad.1396206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mackarehtschian K, et al. Targeted disruption of the flk2/flt3 gene leads to deficiencies in primitive hematopoietic progenitors. Immunity. 1995;3(1):147–161. doi: 10.1016/1074-7613(95)90167-1. [DOI] [PubMed] [Google Scholar]
- 27.McKenna HJ, et al. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95(11):3489–3497. [PubMed] [Google Scholar]
- 28.Sitnicka E, et al. Key role of flt3 ligand in regulation of the common lymphoid progenitor but not in maintenance of the hematopoietic stem cell pool. Immunity. 2002;17(4):463–472. doi: 10.1016/s1074-7613(02)00419-3. [DOI] [PubMed] [Google Scholar]
- 29.Balciunaite G, Ceredig R, Massa S, Rolink AG. A B220+ CD117+ CD19− hematopoietic progenitor with potent lymphoid and myeloid developmental potential. Eur J Immunol. 2005;35(7):2019–2030. doi: 10.1002/eji.200526318. [DOI] [PubMed] [Google Scholar]
- 30.Inlay MA, et al. Ly6d marks the earliest stage of B-cell specification and identifies the branchpoint between B-cell and T-cell development. Genes Dev. 2009;23(20):2376–2381. doi: 10.1101/gad.1836009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mansson R, et al. Single-cell analysis of the common lymphoid progenitor compartment reveals functional and molecular heterogeneity. Blood. 2010;115(13):2601–2609. doi: 10.1182/blood-2009-08-236398. [DOI] [PubMed] [Google Scholar]
- 32.Swee LK, Bosco N, Malissen B, Ceredig R, Rolink A. Expansion of peripheral naturally occurring T regulatory cells by Fms-like tyrosine kinase 3 ligand treatment. Blood. 2009;113(25):6277–6287. doi: 10.1182/blood-2008-06-161026. [DOI] [PubMed] [Google Scholar]
- 33.Chappaz S, Flueck L, Farr AG, Rolink AG, Finke D. Increased TSLP availability restores T- and B-cell compartments in adult IL-7 deficient mice. Blood. 2007;110(12):3862–3870. doi: 10.1182/blood-2007-02-074245. [DOI] [PubMed] [Google Scholar]
- 34.Kashyap M, Rochman Y, Spolski R, Samsel L, Leonard WJ. Thymic stromal lymphopoietin is produced by dendritic cells. J Immunol. 2011;187(3):1207–1211. doi: 10.4049/jimmunol.1100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Domen J, Gandy KL, Weissman IL. Systemic overexpression of BCL-2 in the hematopoietic system protects transgenic mice from the consequences of lethal irradiation. Blood. 1998;91(7):2272–2282. [PubMed] [Google Scholar]
- 36.O’Reilly LA, Huang DC, Strasser A. The cell death inhibitor Bcl-2 and its homologues influence control of cell cycle entry. EMBO J. 1996;15(24):6979–6990. [PMC free article] [PubMed] [Google Scholar]
- 37.Fisher AG, et al. Lymphoproliferative disorders in an IL-7 transgenic mouse line. Leukemia. 1993;7(Suppl 2):S66–S68. [PubMed] [Google Scholar]
- 38.Mertsching E, Grawunder U, Meyer V, Rolink T, Ceredig R. Phenotypic and functional analysis of B lymphopoiesis in interleukin-7-transgenic mice: expansion of pro/pre-B cell number and persistence of B lymphocyte development in lymph nodes and spleen. Eur J Immunol. 1996;26(1):28–33. doi: 10.1002/eji.1830260105. [DOI] [PubMed] [Google Scholar]
- 39.Akashi K, Kondo M, von Freeden-Jeffry U, Murray R, Weissman IL. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell. 1997;89(7):1033–1041. doi: 10.1016/s0092-8674(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 40.Maraskovsky E, et al. Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor-deficient mice but not in mutant rag-1-/- mice. Cell. 1997;89(7):1011–1019. doi: 10.1016/s0092-8674(00)80289-5. [DOI] [PubMed] [Google Scholar]
- 41.Beaudin AE, Boyer SW, Forsberg EC. Flk2/Flt3 promotes both myeloid and lymphoid development by expanding non-self-renewing multipotent hematopoietic progenitor cells. Exp Hematol. 2014;42(3):218–229.e4. doi: 10.1016/j.exphem.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dolence JJ, Gwin KA, Shapiro MB, Medina KL. Flt3 signaling regulates the proliferation, survival, and maintenance of multipotent hematopoietic progenitors that generate B cell precursors. Exp Hematol. 2014;42(5):380–393.e3. doi: 10.1016/j.exphem.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carvalho TL, Mota-Santos T, Cumano A, Demengeot J, Vieira P. Arrested B lymphopoiesis and persistence of activated B cells in adult interleukin 7(-/)- mice. J Exp Med. 2001;194(8):1141–1150. doi: 10.1084/jem.194.8.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jensen CT, et al. FLT3 ligand and not TSLP is the key regulator of IL-7-independent B-1 and B-2 B lymphopoiesis. Blood. 2008;112(6):2297–2304. doi: 10.1182/blood-2008-04-150508. [DOI] [PubMed] [Google Scholar]
- 45.Vosshenrich CA, Cumano A, Müller W, Di Santo JP, Vieira P. Thymic stromal-derived lymphopoietin distinguishes fetal from adult B cell development. Nat Immunol. 2003;4(8):773–779. doi: 10.1038/ni956. [DOI] [PubMed] [Google Scholar]
- 46.Rothenberg EV. Transcriptional control of early T and B cell developmental choices. Annu Rev Immunol. 2014;32:283–321. doi: 10.1146/annurev-immunol-032712-100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ceredig R, Rauch M, Balciunaite G, Rolink AG. Increasing Flt3L availability alters composition of a novel bone marrow lymphoid progenitor compartment. Blood. 2006;108(4):1216–1222. doi: 10.1182/blood-2005-10-006643. [DOI] [PubMed] [Google Scholar]