Significance
The uppermost superficial zone of articular cartilage plays multifaceted roles in maintaining cartilage structure, function, and mechanical properties and in preventing cartilage degeneration during osteoarthritis initiation. However, its regulation by growth factors and hormones is still largely unknown. Here we report that EGFR signaling is an important growth factor pathway that maintains superficial chondrocyte number, promotes boundary lubricant secretion and cartilage surface lubrication, and stimulates mechanical strength of articular cartilage. Reduction in EGFR activity leads to structurally, functionally, and mechanically compromised articular cartilage during development and drastically accelerates cartilage degeneration under normal and surgically induced osteoarthritis conditions. Thus, our studies strongly suggest that targeting cartilage surface EGFR signaling should be considered as a novel direction for osteoarthritis treatment.
Keywords: EGFR, articular cartilage, chondrocyte, lubrication, osteoarthritis
Abstract
Osteoarthritis (OA) is the most common joint disease, characterized by progressive destruction of the articular cartilage. The surface of joint cartilage is the first defensive and affected site of OA, but our knowledge of genesis and homeostasis of this superficial zone is scarce. EGFR signaling is important for tissue homeostasis. Immunostaining revealed that its activity is mostly dominant in the superficial layer of healthy cartilage but greatly diminished when OA initiates. To evaluate the role of EGFR signaling in the articular cartilage, we studied a cartilage-specific Egfr-deficient (CKO) mouse model (Col2-Cre EgfrWa5/flox). These mice developed early cartilage degeneration at 6 mo of age. By 2 mo of age, although their gross cartilage morphology appears normal, CKO mice had a drastically reduced number of superficial chondrocytes and decreased lubricant secretion at the surface. Using superficial chondrocyte and cartilage explant cultures, we demonstrated that EGFR signaling is critical for maintaining the number and properties of superficial chondrocytes, promoting chondrogenic proteoglycan 4 (Prg4) expression, and stimulating the lubrication function of the cartilage surface. In addition, EGFR deficiency greatly disorganized collagen fibrils in articular cartilage and strikingly reduced cartilage surface modulus. After surgical induction of OA at 3 mo of age, CKO mice quickly developed the most severe OA phenotype, including a complete loss of cartilage, extremely high surface modulus, subchondral bone plate thickening, and elevated joint pain. Taken together, our studies establish EGFR signaling as an important regulator of the superficial layer during articular cartilage development and OA initiation.
Osteoarthritis (OA) is the most common chronic condition of the joints, affecting ∼27 million adults in the United States alone. Mature articular cartilage consists of four zones: superficial, transitional, middle, and calcified zones (1). The combination of the first three zones are also termed uncalcified zone. Among them, the superficial layer is the first line of defense against OA initiation because of its surface location, unique composition, and functions. It has 2–4 layers of flat cells expressing unique molecules, such as proteoglycan 4 (Prg4, lubricin) and tenascin C, and contains a fine network of collagen fibrils that are oriented horizontally and parallel to the articular surface (2). Its multifaceted roles include, but are not limited to, producing lubricant proteins, harboring chondroprogenitors, resisting shear stresses, serving as a gliding surface, and isolating deeper layers from synovial fluid (2). In OA, degenerative changes initiate with cellular disorganization, gradual stiffening, and irregular surface of this layer (3–5). Despite the critical role of superficial chondrocytes in maintaining articular cartilage and in blocking OA, its regulation by growth factors and hormones is still largely unknown.
Epidermal growth factor receptor (EGFR) signaling is important for tissue homeostasis. Multiple ligands bind to and activate the EGFR, including epidermal growth factor (EGF), transforming growth factor-α (TGFα), amphiregulin, heparin-binding EGF (HB-EGF), epiregulin, and betacellulin. Once activated, it stimulates an intracellular signal transduction cascade to markedly influence cell behavior (6). Our current knowledge of EGFR function in articular cartilage mostly came from previous studies of its ligands and its intracellular inhibitor, mitogen-inducible gene 6 (Mig-6). Large-scale gene profiling experiments have identified that the expression of TGFα (7) and HB-EGF (8) was increased in articular chondrocytes in both rodent surgical OA models and OA patients. Mig-6 null mice develop a severe OA-like joint degeneration phenotype at an early adult stage (9). However, mice with limb- (Prx1-Cre) or cartilage- (Col2-Cre) specific deletion of Mig-6 showed a much milder OA phenotype (10–12). Indeed, a common phenotype in these two conditional knockout models is an expansion of knee articular cartilage at a young age. In addition to EGFR, Mig-6 also suppresses the signaling of several other growth factor receptors, such as HGF/MET (13), implicating that the cartilage phenotypes observed in Mig-6 null mice might not be solely due to EGFR overactivation.
In the past, we established a chondrocyte-specific Egfr knockout mouse model to demonstrate a pivotal role of EGFR signaling in growth plate development and secondary ossification center (SOC) formation (14, 15). In this study, we used this model to investigate the function of EGFR in articular cartilage development. A surgery model by destabilization of the medial meniscus (DMM) was applied to elucidate the action of EGFR in OA initiation. Together with human cartilage data, our results clearly demonstrated that chondrogenic EGFR signaling is an essential regulator of the superficial layer of articular cartilage by maintaining chondrocyte number, its mechanical properties, and lubrication function. Furthermore, it plays a critical role in protecting cartilage from OA initiation.
Results
EGFR Signaling in Healthy and Diseased Articular Cartilage.
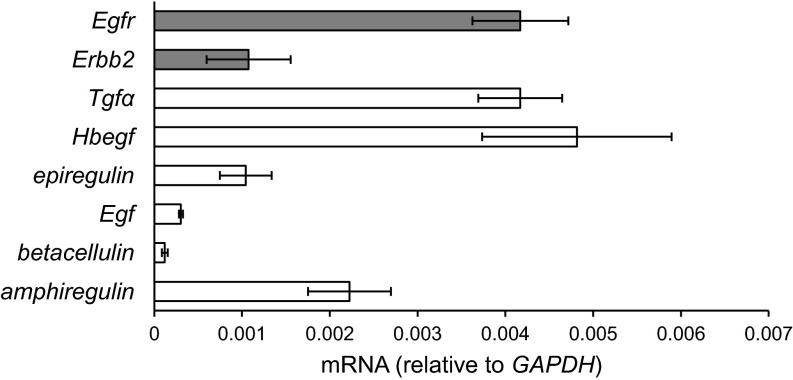
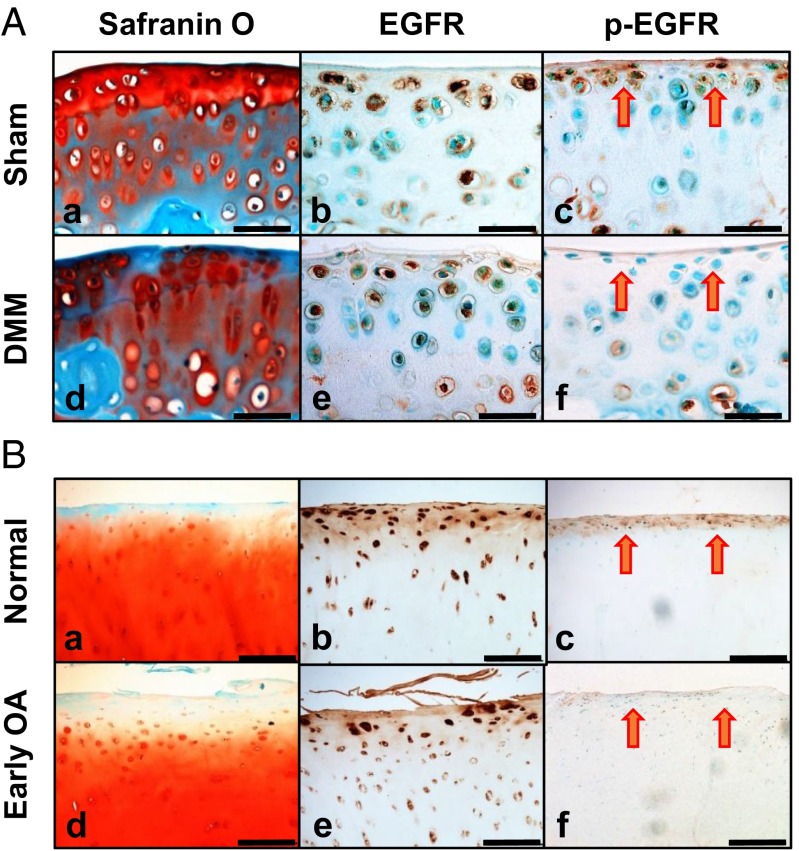
To study EGFR signaling in the articular cartilage, we first analyzed the gene expression of EGFR family members and its cognate ligands in mouse femoral head cartilage. Real-time RT-PCR revealed high levels of Egfr, its coreceptor Erbb2, and its ligands Tgfα, Hb-egf, amphiregulin, and epiregulin mRNA and relatively lower levels of other ligands, Egf and betacellulin (Fig. S1). Although all chondrocytes within articular cartilage were positive for EGFR staining (Figs. 1 A, b and 2 A, a), EGFR activity, indicated by phospho (p)-EGFR, was located mostly in surface chondrocytes and infrequently in calcified chondrocytes (Figs. 1 A, c and 2 A, b). DMM surgery induces OA-like phenotypes in mouse knees. At 1 mo after DMM, when articular cartilage had yet to show any morphological changes, EGFR staining remained throughout the cartilage, but p-EGFR staining had already been diminished at the surface (Fig. 1 A, d–f). In line with these data, healthy human articular cartilage exhibited strong EGFR staining throughout the cartilage (Fig. 1 B, b) and p-EGFR staining only at the superficial layer (Fig. 1 B, c). At an early OA stage, when the superficial zone (SZ) was still intact but proteoglycan staining was partially lost (Fig. 1 B, d), p-EGFR staining was remarkably reduced (Fig. 1 B, f). These expression and activity profiles strongly implicate a potential role of EGFR signaling in the SZ of healthy and diseased articular cartilage.
Fig. S1.
The gene expression of Egfr and its ligands in the femoral head articular cartilage of 2-mo-old WT mice analyzed by real-time RT-PCR. n = 6 mice.
Fig. 1.
EGFR signaling in healthy and diseased articular cartilage. (A) Safranin O staining (a and d) and immunohistochemistry of EGFR (b and e) and p-EGFR (c and f) in tibial articular cartilage of WT mice at 1 mo postsham (a–c) or post-DMM (d–f) surgery. (Scale bars, 50 μm.) (B) Safranin O staining (a and d) and immunohistochemistry of EGFR (b and e) and p-EGFR (c and f) in human joints with healthy (a–c) and early OA (d–f) cartilage. (Scale bars, 200 μm.) Red arrows point to superficial cells.
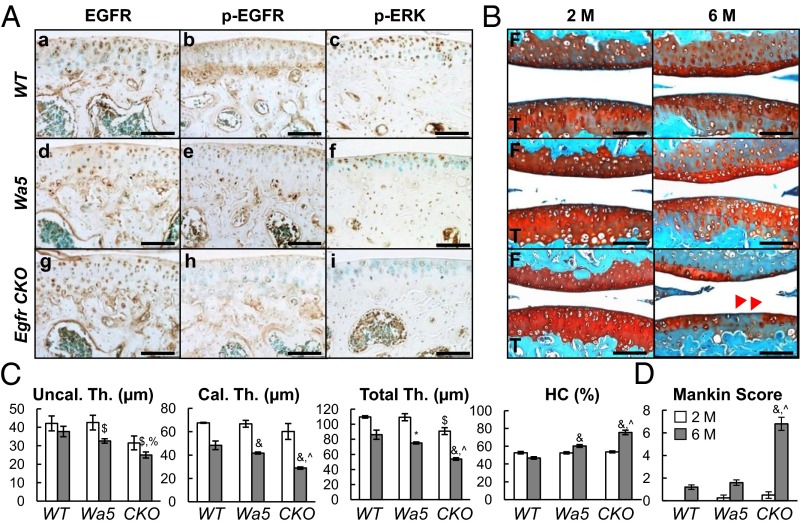
Fig. 2.
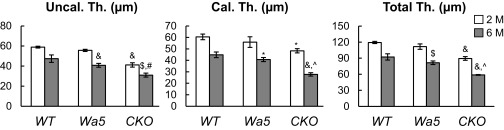
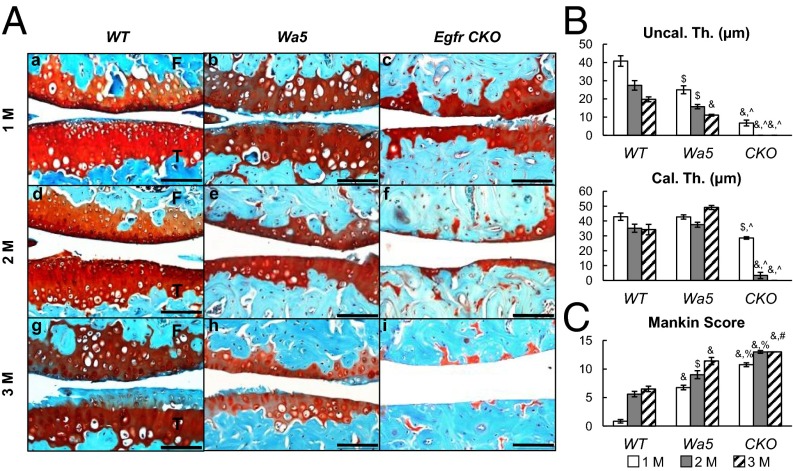
EGFR signaling is important for articular cartilage structure. (A) Immunostaining of EGFR (a, d, and g), p-EGFR (b, e, and h), and p-ERK (c, f, and i) in tibial articular cartilage of 2-mo-old WT (a–c), Wa5 (d–f), and Egfr CKO (g–i) mice. (Scale bars, 100 μm.) (B) Safranin O staining of mouse joints at 2 and 6 mo of age. Red arrows point to reduced cartilage thickness and staining intensity in CKO mice. F, femur; T, tibia. (Scale bars, 100 μm.) (C) Average thicknesses of uncalcified (Uncal. Th.), calcified (Cal. Th.), and total (Total. Th.) articular cartilage in femoral condyle were quantified. The percentage of hypertrophic chondrocyte (HC) in both femoral condyle and tibial plateau was quantified together. (D) Mankin score confirms that CKO mice develop early OA symptoms at 6 mo of age. n = 6 per age per genotype. *P < 0.05, $P < 0.01, &P < 0.001 vs. WT; %P < 0.01, ^P < 0.001 vs. Wa5.
Deficiency in Chondrogenic EGFR Signaling Leads to Abnormal Articular Cartilage Development.
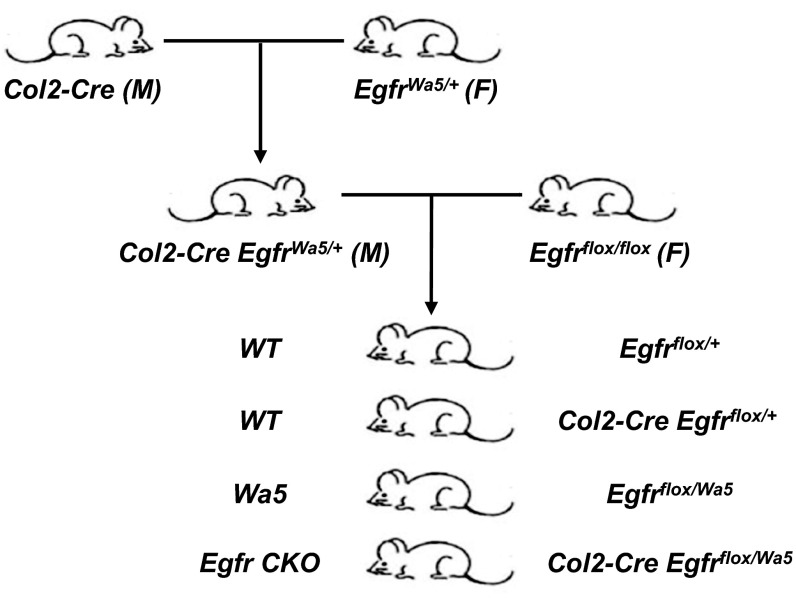
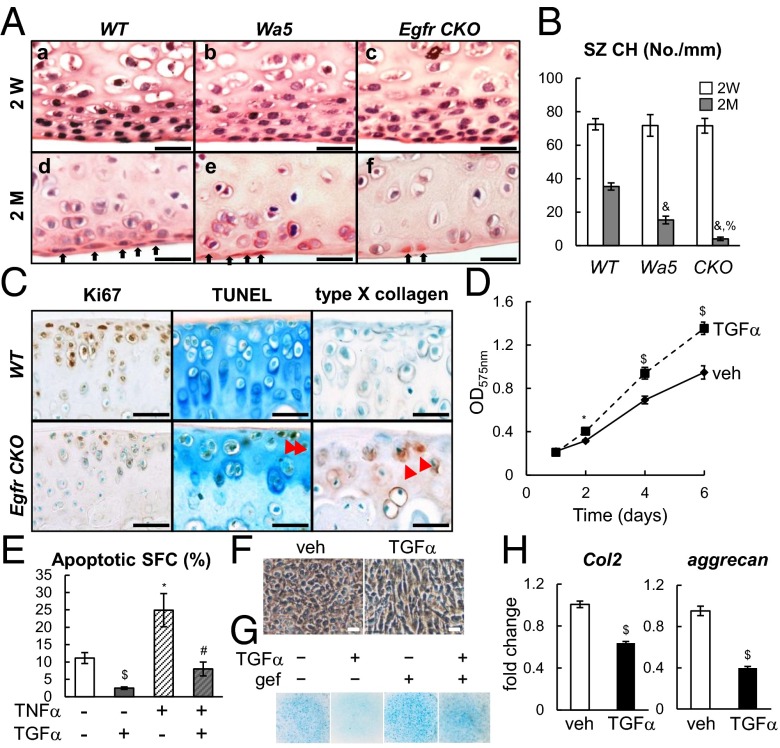
Next we used a chondrogenic Egfr CKO (Col2-Cre EgfrWa5/flox) mouse model (Fig. S2) to investigate the action of EGFR in the articular cartilage. We previously demonstrated that EGFR activity in chondrocytes isolated from CKO mice and their sibling controls follows this sequence: WT > Wa5 >> CKO (15). Although CKO mice expressed EGFR ubiquitously in the articular cartilage (Fig. 2 A, g) due to the presence of a Wa5 allele, the amounts of p-EGFR and p-ERK, both EGFR signaling targets, were indeed drastically reduced compared with WT (Fig. 2 A, h and i). Consistent with cell culture results, the staining patterns of p-EGFR and p-ERK in Wa5 cartilage were more similar to WT (Fig. 2 A, e and f).
Fig. S2.
The breeding strategy for generating cartilage-specific Egfr-deficient (Egfr CKO) mice as well as their WT and Wa5 controls. F, female; M, male.
At 2 mo of age, compared with WT and Wa5, Egfr CKO mice had slightly but noticeably reduced total thickness of articular cartilage (Fig. 2 B and C and Fig. S3). At 6 mo of age, this reduction became much more evident in CKO mice, and Wa5 mice started to show similar changes. This drastic reduction of total thickness in CKO mice was accompanied by decreases in both uncalcified and calcified zones and an increase in the percentage of morphologically hypertrophied cells (Fig. 2C). In addition, CKO cartilage had less Safranin O staining and exhibited histological defects, such as minor fibrillation and clefting at the cartilage surface, resulting in an early OA-like phenotype with a Mankin score of 6.8 ± 0.6 (Fig. 2D).
Fig. S3.
EGFR signaling is important for articular cartilage structure. Average thicknesses of uncalcified (Uncal. Th.), calcified (Cal. Th.), and total (Total, Th.) articular cartilage were quantified in the tibial plateau of 2- and 6-mo-old WT, Wa5, and Egfr CKO mice. n = 6 per age per genotype. *P < 0.05, $P < 0.01, &P < 0.001 vs. WT; #P < 0.05, ^P < 0.001 vs. Wa5.
EGFR Is Required for Maintaining Superficial Chondrocytes.
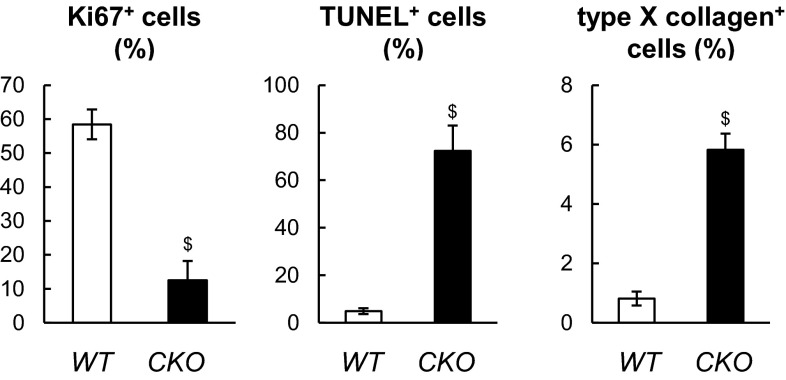
Because cartilage surface possesses a high EGFR activity, we next took a close look at the SZ in CKO mice. Interestingly, although the structure of articular cartilage appeared normal in 2-wk-old animals (Fig. 3 A, a–c and B), the superficial layer in 2-mo-old Wa5 and CKO mice had 56.6% and 88.5%, respectively, less cells than that in WT mice (Fig. 3 A, d–f and B). Decreases in chondrocyte cellularity were also noticeable in the rest of uncalcified cartilage but not as prominent as in the SZ (Fig. 3 A, f). Those superficial cells in CKO mice had less Ki67 staining but more TUNEL staining, and their deeper layer showed positive type X collagen (Fig. 3C and Fig. S4), suggesting that EGFR signaling is important for regulating the proliferative ability and cell survival of superficial chondrocytes and hypertrophy of chondrocytes. Consistently, activating EGFR by TGFα stimulated proliferation of cultured superficial chondrocytes (Fig. 3D) and increased resistance to apoptosis after serum depletion and TNFα treatment (Fig. 3E). Upon chondrogenic differentiation, TGFα-treated cells maintained a flat cell shape and a low level of proteoglycan accumulation, whereas vehicle-treated cells became polygonal in shape and accumulated alcian blue-stained proteoglycan (Fig. 3 F and G). Cotreatment of gefitinib, an EGFR inhibitor, abolished this effect of TGFα (Fig. 3G). TGFα also attenuated the expression of two cartilage matrix genes, Col2 and aggrecan (Fig. 3H). These data suggest a critical role of EGFR signaling in maintaining cell number and properties of superficial chondrocytes.
Fig. 3.
EGFR signaling maintains the number of chondrocytes in the SZ. (A) H&E staining of femoral articular cartilage in WT (a and d), Wa5 (b and e), and CKO (c and f) mice at 2 wk (a–c) and 2 mo (d–f) of age. Black arrows point to superficial chondrocytes. (Scale bars, 50 μm.) (B) The number of superficial chondrocytes was quantified. n = 5 per age per genotype. &P < 0.001 vs. WT; %P < 0.01 vs. Wa5. (C) Immunostaining of Ki67, TUNEL, and type X collagen in 2-mo-old mouse tibia. Red arrows point to positive cells. (Scale bars, 70 μm.) (D) TGFα stimulates the proliferation of superficial chondrocytes. Superficial chondrocytes received either vehicle or TGFα (100 ng/mL) treatment on day 1. Cells were harvested at indicated time points for MTT assay. *P < 0.05, $P < 0.01 vs. vehicle. (E) TGFα inhibits the apoptosis of superficial chondrocytes. Superficial chondrocytes were serum-starved overnight, pretreated with TNFα (25 ng/mL), and then treated with TGFα (100 ng/mL) for 2 d before apoptosis assay. *P < 0.05, $P < 0.01 vs. serum starvation only; #P < 0.05 vs. serum starvation plus TNFα. (F) TGFα maintains the elongated cell shape in superficial cells after cells were cultured in chondrogenic differentiation medium for 10 d. (Scale bars, 20 μm.) (G) Activating EGFR suppresses chondrogenic differentiation of superficial chondrocytes. Cells were cultured in chondrogenic differentiation medium with or without TGFα and gefitinib (gef, 10 µM). Alcian blue staining was performed after 10 d. (H) Real-time RT-PCR analysis shows that 2 d of TGFα treatment suppressed the expression of cartilage matrix genes Col2 and aggrecan in superficial chondrocytes. $P < 0.01 vs. vehicle.
Fig. S4.
Quantification of percentages of Ki67+, TUNEL+, and type X collagen+ chondroyctes in the uncalcified zone of WT and Egfr CKO tibial articular cartilage at 2 mo of age. n = 3 per genotype. $P < 0.01 vs. WT.
EGFR Signaling Promotes the Lubrication Function of Articular Cartilage.
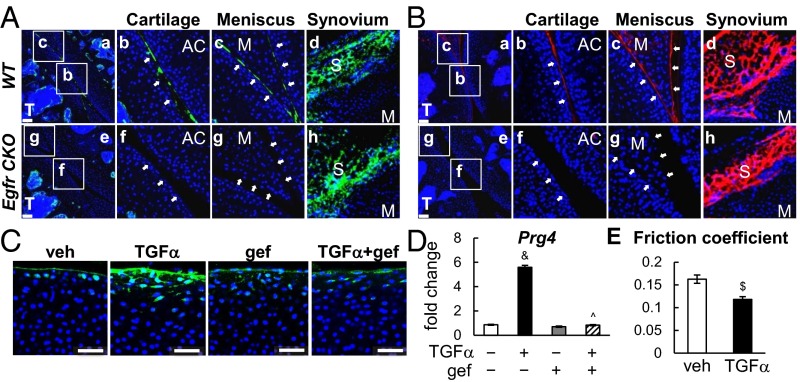
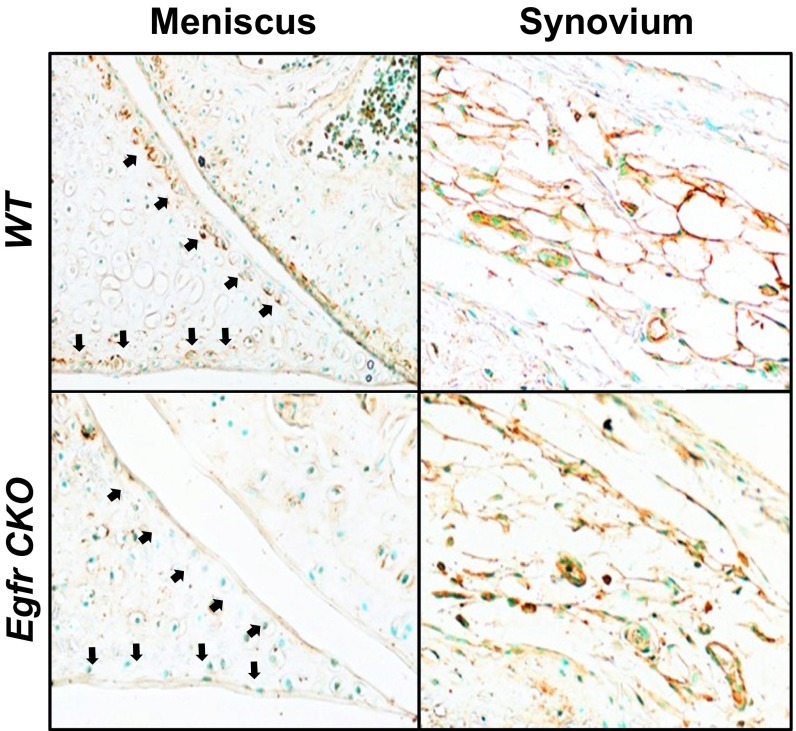
One key function of superficial chondrocytes is producing boundary lubricants Prg4 and hyaluronic acid (HA). In WT joints, most cartilage and meniscus surfaces were covered by Prg4 (Fig. 4 A, a–c) and HA (Fig. 4 B, a–c). Strikingly, Prg4 and HA signals were nearly absent at those surfaces in CKO joints (Fig. 4 A, e–g and B, e–g). Note that their amounts in synovial membrane were not affected (Fig. 4 A, d and h and B, d and h). These results are consistent with the p-EGFR staining pattern in our CKO model in which EGFR activity was also reduced at the meniscus surface but not in synovial membrane (Fig. S5).
Fig. 4.
EGFR signaling promotes lubricant secretion from cartilage and meniscus surfaces. (A) Immunofluorescence staining of Prg4 (green) in 1-mo-old WT and CKO joints. The articular cartilage surface (b and f) and meniscus surface (c and g), pointed by white arrows, are magnified images from a and e. Synovium is shown in d and h. AC, articular cartilage; M, meniscus; S, synovium; T, tibia. (Scale bars, 70 μm.) (B) Detection of HA (red) in 1-mo-old WT and CKO joints. The articular cartilage surface (b and f) and meniscus surface (c and g) are magnified images from a and e. Synovium is shown in d and h. (Scale bars, 70 μm.) (C) Immunofluorescence shows that TGFα increases the Prg4 amount (green) at the bovine cartilage explant surface in an EGFR-dependent manner. Blue, DAPI. (Scale bars, 50 μm.) (D) TGFα increased Prg4 mRNA in the top 1-mm region of cartilage explant in an EGFR-dependent manner as assayed by real-time RT-PCR. &P < 0.001 vs. vehicle; ^P < 0.001 vs. TGFα. (E) TGFα treatment reduced the friction coefficient of the articular surface in cartilage explants. n = 5 per group. $P < 0.01 vs. vehicle.
Fig. S5.
EGFR activity is diminished in the meniscus surface (pointed by arrows) but not in the synovial membrane in Egfr CKO mice as detected by p-EGFR staining.
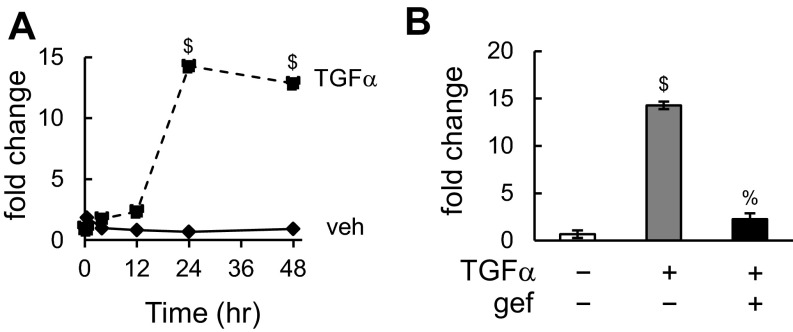
The loss of lubricants in CKO articular cartilage could be a direct regulatory effect of EGFR signaling on Prg4 expression or an indirect effect due to decreased superficial chondrocyte number. Indeed, primary culture experiments showed that TGFα stimulates Prg4 expression in chondrocytes in a time-dependent fashion (Fig. S6A) and that this up-regulation can be abolished by gefinitib (Fig. S6B). Bovine articular cartilage explant represents a superior model to study the actions of growth factors under controlled and physiologically relevant conditions in a 3D environment. Using the uppermost 1 mm of bovine cartilage surface, we confirmed that TGFα increases Prg4 expression at both mRNA and protein levels in an EGFR-dependent manner (Fig. 4 C and D), proving that EGFR autonomously regulates Pgr4 in the articular cartilage. Most importantly, tribological testing revealed that 3 d of TGFα treatment significantly reduces the equilibrium friction coefficient of articular surface by 28% (Fig. 4E), demonstrating that EGFR signaling is an important regulator of cartilage lubrication function.
Fig. S6.
TGFα (100 ng/mL) stimulates Prg4 expression in primary chondrocytes in time-dependent (A) and EGFR-dependent (B) manners as shown by real-time RT-PCR. Gef, gefitinib (10 μM). $P < 0.01 vs. vehicle; %P < 0.01 vs. TGFα.
Deficiency of EGFR Signaling Weakens the Articular Cartilage.
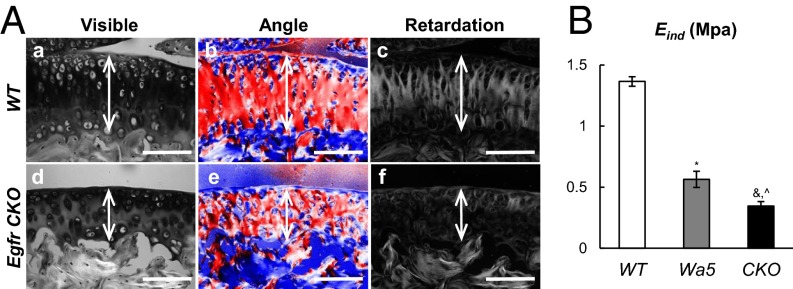
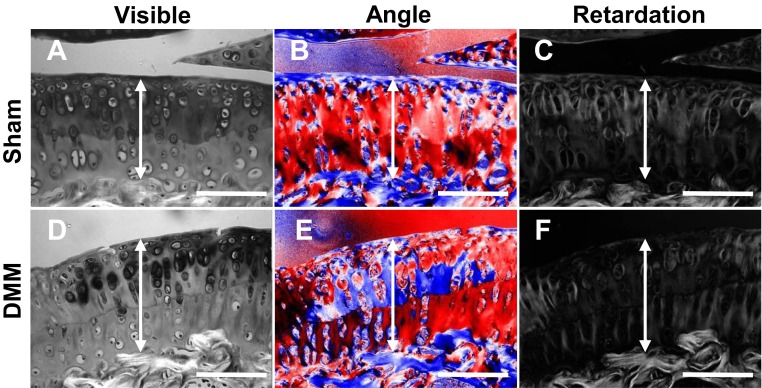
The principle function of articular cartilage is to provide load transmission and energy dissipative shock absorption during joint motion. Therefore, mechanical properties, mainly determined by cartilage extracellular matrix, are important parameters in characterizing cartilage function. Under the polarized light microscope, we observed that WT mice had mostly horizontally aligned collagen fibrils (in blue color, Fig. 5 A, b) at the cartilage surface and perpendicularly aligned fibrils (in red color, Fig. 5 A, b) with high collagen organization (high retardation values, Fig. 5 A, c) in the deep layers of cartilage. In contrast, the collagen fibrillar structure changed remarkably in the middle and calcified zones of CKO mice, resulting in random orientation of collagen fibers (mixed blue and red colors, Fig. 5 A, e) and reduced collagen organization (lower retardation values, Fig. 5 A, f). Interestingly, these changes are very similar to the disruption of the collagen fibril network in OA. As shown in Fig. S7, WT articular cartilage developed disoriented collagen fibrils and reduced collagen organization in the middle zone of degenerated cartilage at 2 mo after DMM.
Fig. 5.
EGFR signaling regulates the mechanical properties of articular cartilage. (A) Representative polarized light microscopy images of WT (a–c) and Egfr CKO (d–f) tibial articular cartilage at 5 mo of age. The blue and red colors in angle images are 90° apart in orientation. Double arrows depict the articular cartilage. (Scale bars, 100 μm.) (B) AFM-based nanoindentation shows that EGFR deficiency significantly reduces the indentation modulus (Eind) of mouse femoral cartilage surface as early as 2 mo of age. n = 6 per genotype. *P < 0.05, &P < 0.001 vs. WT; ^P < 0.001 vs. Wa5.
Fig. S7.
Collagen fibril network in articular cartilage is altered by DMM surgery. Polarized light microscopy images of sham (A–C) and DMM (D–F) tibial articular cartilage at 2 mo postsurgery. The blue and red colors in the angle images are 90° apart in orientation. Double arrows depict the articular cartilage. (Scale bars, 100 μm.)
We recently developed an atomic force microscopy (AFM)-based nanoindentation approach to accurately measure mouse cartilage surface mechanical properties. In line with the above polarized light microscopy data, we detected remarkable decreases in the effective nanoindentation modulus, Eind, in both Wa5 (58.7%) and CKO (74.7%) cartilage compared with WT (Fig. 5B). These data also match our current observation that DMM surgery rapidly reduced surface Eind of WT mouse cartilage (16). Taken together, our results clearly demonstrate that EGFR signaling regulates mechanical properties of articular cartilage.
Loss of EGFR in Chondrocytes Remarkably Accelerates Cartilage Damage After DMM Surgery.
Because the articular cartilage of Egfr CKO mice shows structural, functional, and mechanical defects, they should be prone to develop OA after joint instability. One month after DMM, while WT joints remained intact, CKO joints showed prominent cartilage damage, including surface fibrillation, clefts, and erosion (Fig. 6 A, c), as well as remarkable reductions in uncalcified and calcified cartilage thicknesses (Fig. 6B). At 2 mo postsurgery, CKO joints developed a more severe phenotype with a large part of articular cartilage eroded at the medial site (Fig. 6 A, f), resulting in a maximum Mankin score (Fig. 6C). At 3 mo postsurgery, CKO joints lost almost the entire articular cartilage, with no measurements in both uncalcified and calcified cartilage thicknesses (Fig. 6 A, i and B). Consistent with our previous report (17), Wa5 DMM joints (Fig. 6 A, b, e, and h) had modestly accelerated OA development compared with WT, but their symptoms were much milder compared with CKO.
Fig. 6.
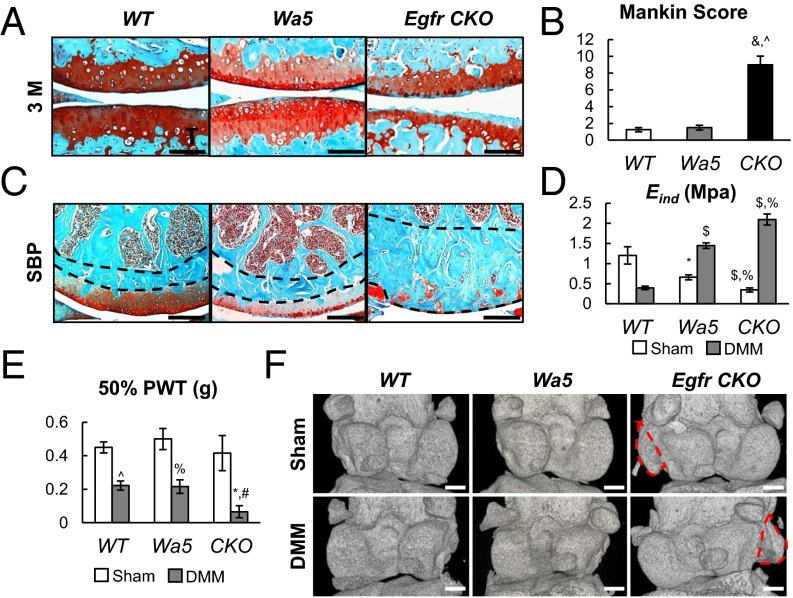
EGFR chondrogenic deficiency causes severe OA after DMM surgery. (A) Safranin O staining of WT (a, d, and g), Wa5 (b, e, and h), and CKO (c, f, and i) mouse joints at the medial site at 1 (a–c), 2 (d–f), and 3 (g–i) mo postsurgery. (Scale bars, 100 μm.) (B) Average thicknesses of uncalcified (Uncal. Th.) and calcified (Cal. Th.) cartilage were quantified. n = 6 per age per genotype. $P < 0.01, &P < 0.001 vs. WT; ^P < 0.001 vs. Wa5. (C) The OA severity was measured by Mankin score. $P < 0.01, &P < 0.001 vs. WT; #P < 0.05, %P < 0.01 vs. Wa5. n = 6 per age per group.
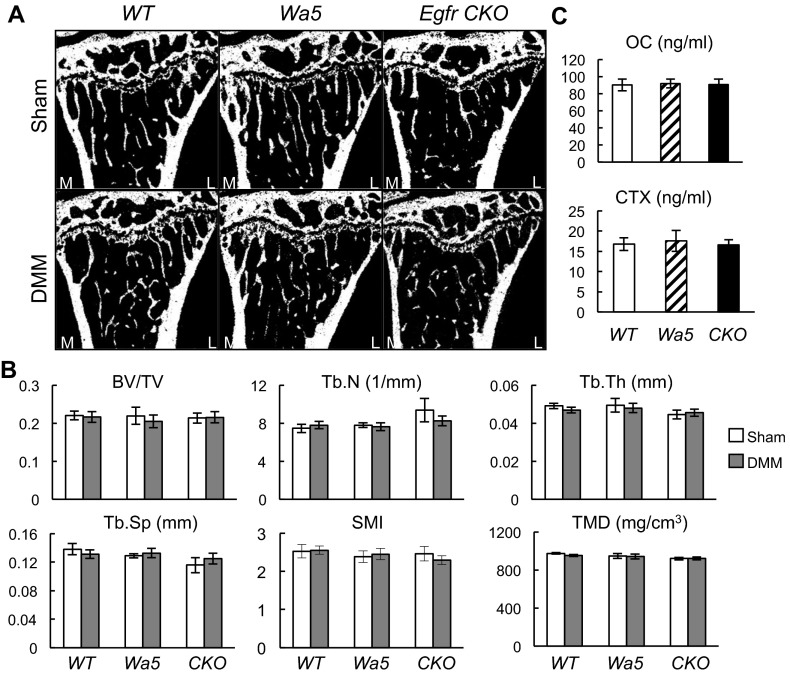
Consistent with these cartilage phenotypes, Egfr CKO mice developed several late OA phenotypes. First, although the lateral site of WT joints remained healthy, OA symptoms in CKO mice had propagated from the medial to lateral site (Fig. 7 A and B). Second, we observed a substantial increase in the thickness of the subchondral bone plate under the damaged cartilage area at the medial site in CKO mice (Fig. 7C), suggesting a subchondral sclerosis phenotype. Third, Eind of the CKO DMM site increased remarkably to 4.3-fold of WT DMM joints, even more than WT sham joints (Fig. 7D), suggesting that the indented surface on CKO DMM joints is most likely calcified cartilage or even subchondral bone. Note that at the same time, DMM resulted in a 67.2% reduction in Eind of articular cartilage surface in WT mice (Fig. 7D). As OA further develops, the DMM site of WT mice also exhibited the same or elevated Eind compared with the sham site (16). Fourth, pain is the major symptom in OA. To detect mechanical allodynia, we performed von Frey assay (18) and found that CKO DMM legs were extremely sensitive to the stimulus, with a paw withdrawal threshold (PWT) only 29.7% of that observed in WT DMM legs (Fig. 7E), implicating that they experience much more pain than others after DMM. Lastly, although we did not observe any osteophyte in sham and DMM joints of 16 WT mice and 9 Wa5 mice, 6 out of 18 sham and 8 out of 18 DMM joints of Egfr CKO had osteophytes originating from either the tibial or femoral growth plate (Fig. 7F). Combining all evidence together, we conclude that Egfr CKO mice reached the late stage of OA at 2 mo postsurgery through a pace much faster than WT mice. Additionally, we found that there are no changes in the trabecular bone structure in the metaphyseal area among WT, Wa5, and CKO mice regardless of surgery (Fig. S8 A and B). Serum levels of bone formation (osteocalcin) and resorption (CTX) markers also remained the same among these mice (Fig. S8C). These data clearly demonstrate that our CKO model has no effect on overall bone metabolism.
Fig. 7.
Egfr CKO mice develop late OA symptoms after DMM surgery. (A) Safranin O staining of WT, Wa5, and CKO mouse joints at the lateral site at 3 mo postsurgery. (Scale bars, 100 μm.) (B) OA severity of the lateral site was measured by Mankin score. n = 5 per genotype. &P < 0.001 vs. WT; ^P < 0.001 vs. Wa5. (C) Egfr CKO mice had thickened subchondral bone plates at the medial site at 2 mo after DMM surgery. Lines depict the area of the subchondral bone plate. (Scale bars, 100 μm.) (D) Both sham and DMM femurs were harvested at 2 mo postsurgery for nanoindentation to measure cartilage surface modulus. n = 6 per genotype. *P < 0.05, $P < 0.01 vs. WT; %P < 0.01 vs. Wa5. (E) von Frey assay was performed at 1 mo postsurgery. n = 8 per genotype. *P < 0.05 vs. WT; #P < 0.05, %P < 0.01, ^P < 0.001 vs. sham. (F) 3D microCT images show osteophytes were only found in Egfr CKO joints. Red circles indicate osteophytes. (Scale bars, 500 μm.)
Fig. S8.
Loss of EGFR activity does not directly affect bone. (A) Representative longitudinal microCT images of proximal tibiae in WT, Wa5, and CKO mice at 2 mo after surgery. (B) The trabecular bone structural parameters in the secondary spongiosa were quantified. n = 6 per genotype. (C) Serum bone formation (osteocalcin) and resorption (CTX) markers were quantified by ELISA. n = 8 per genotype.
Discussion
The SZ is an indispensable part of articular cartilage and plays critical roles in maintaining the integrity and function of articular cartilage and preventing their degeneration. However, little is known about how growth factors and hormones, as well as their downstream signaling pathways, regulate superficial chondrocytes and their biological and mechanical functions. Our current studies identify EGFR and its ligands as an essential growth factor pathway that regulates chondrocyte number, boundary lubrication, and mechanical properties of this superficial layer. We found several EGFR ligands, such as Tgfα, Hb-egf, amphiregulin, and epiregulin, are expressed at abundant levels in mouse articular cartilage. Previous studies revealed that both TGFα (7) and HB-EGF (8) mRNA levels are increased in OA articular chondrocytes. Because activating EGFR by those ligands has both anabolic and catabolic activities on primary chondrocytes, whether an increase of EGFR ligand amount plays a destructive or a compensatively repairing role in OA is unclear. Nevertheless, our data demonstrate that reduction in EGFR activity leads to structurally, functionally, and mechanically compromised articular cartilage during development and drastically accelerates cartilage degeneration under normal and surgically induced OA conditions.
Our data clearly showed that EGFR signaling is required for obtaining a sufficient number of superficial chondrocytes by preserving their proliferation ability, promoting their survival, and maintaining their superficial properties. Previous studies with chondrocyte-specific knockout of Mig-6 (10–12) also support our conclusion, as young mice exhibit hyperproliferation and hypercellularity in the top part of articular cartilage. A recent study identified Prg4-expressing cells located at the cartilage surface as chondrogenitors for deeper layers of the mature articular cartilage (19). We also observed a decrease in chondrocyte cellularity in an uncalcified area below the superficial layer, accompanied with an increase in the percentage of hypertrophic chondrocytes. These data indicate that EGFR might regulate chondroprogenitors in the superficial layer of articular cartilage.
Prg4, an O-linked glycosylated protein, and HA, a polymer of disaccharides, coat the surface of articular cartilage, meniscus, and synovium and serve as two major boundary lubricants in the knee joints (20). Our studies clearly identify EGFR signaling as an important pathway that promotes the production of Prg4 at the cartilage surface. Many studies suggest a synergistic interaction between Prg4 and HA to promote more effective boundary lubrication (21–23). Interestingly, our data reveal that both lubricants are remarkably decreased in EGFR-deficient joints. How EGFR signaling regulates HA on the cartilage surface is not known. Because Prg4 is required to concentrate HA to the tissue surface (21), an intriguing possibility is that EGFR indirectly regulates surface HA attachment by maintaining Prg4. Most importantly, our cartilage explant study explicitly demonstrated that EGFR signaling stimulates cartilage lubrication function. The reduced friction coefficient on the articular surface after TGFα treatment could result from the increased secretion of either PRG4 or both PRG4 and HA. In summary, future studies on the interactions among the superficial chondroprogenitor pool, lubricant secretion, and mechanical strength of cartilage surface using our Egfr CKO mouse model will likely provide more mechanistic insights into articular cartilage homeostasis.
Defective cartilage surface in CKO mice inevitably results in cartilage degeneration. At 2–3 mo after DMM, although WT and Wa5 mice only developed mild to moderate OA, CKO mice had already exhibited many late OA symptoms, such as a complete depletion of articular cartilage at the medial site, subchondral bone plate thickening underneath the damaged cartilage, moderate OA development at the lateral site, osteophyte formation, and a high level of joint pain. One potential concern of using our Egfr CKO mice to study OA progression, a disease in which subchondral bone also plays an important role, is that this model might not be chondrocyte-specific. A recent study implicated that Col2-Cre is capable of inducing fluorescence reporter expression in bone marrow mesenchymal progenitors (24). Because our previous studies have demonstrated an important regulatory role of EGFR signaling on bone marrow mesenchymal progenitors (25–28), it is possible that OA symptoms observed in Egfr CKO mice might partially result from changes in bone remodeling. However, we found that trabecular bone in the secondary spongiosa and serum bone markers are not altered in CKO mice, suggesting that Col2 promoter-driven Cre does not efficiently delete the EGFR gene in bone marrow mesenchymal progenitors and thus does not affect bone formation directly. Our data indicate that Rosa-Tomato used in lineage tracing experiments seems to be a much more sensitive target for Cre than floxed Egfr allele in this Col2-Cre model.
Previous studies of EGFR signaling in articular cartilage development and degeneration focused on an indirect approach analyzing mice lacking Mig-6, an EGFR inhibitor (9–12). Because Mig-6 is not specific to EGFR, those results are too complicated to clearly interpret EGFR function. To our knowledge, this is the first study unambiguously demonstrating the important roles of EGFR signaling in maintaining articular cartilage homeostasis. Our results illustrate that EGFR mainly promotes anabolic activity in articular cartilage, which is opposite from its catabolic activity during growth plate development and SOC formation discovered in our previous reports (14, 15). Because articular chondrocytes arise from a distinct progenitor population from growth plate and epiphyseal chondrocytes (29), the EGFR function we identified in endochondral ossification may not be the same in articular cartilage. Furthermore, the severe OA progression observed in Egfr CKO mice after DMM clearly indicates a protective function of EGFR signaling on articular cartilage against OA degeneration, which is consistent with our previous observation that EGFR inhibitor (gefitinib) treatment via oral gavage in mice enhances cartilage destruction after DMM (17). However, continuous infusion of another EGFR inhibitor, AG1478, in rats was shown to have opposite effects because it modestly delays OA progression after anterior cruciate ligament transection and partial medial meniscectomy (30). Because Egfr CKO mice already have defects in the superficial layer of articular cartilage before injury, it is difficult to dissociate the level of importance of EGFR signaling in this region from its function in other regions during OA development. Interestingly, we observed a loss of EGFR activity in superficial chondrocytes in both human and mouse samples at the OA initiation stage. The underlying mechanism is not known, but it provides substantial evidence that EGFR signaling must play an important role in OA disease. Currently we believe that EGFR signaling has context-, stage-, and dose-dependent roles in OA progression. Future studies using inducible Egfr CKO mice with aggrecan-CreER (31) and superficial layer-specific knockout Prg4-CreER (19) are required to further elucidate spatiotemporal target cells and actions of this pathway and its effects on articular cartilage degeneration. Nevertheless, according to our studies, therapeutic agents specifically targeting cartilage surface EGFR should be considered as a novel direction for OA treatment.
Materials and Methods
All animal work performed in this report was approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania. Details on animal studies, surgery, histology, human articular cartilage sample, AFM-nanoindentation analysis, chondrocyte culture, bovine cartilage explant culture and friction test, real-time RT-PCR analysis, polarized light microscopy analysis, OA pain analysis, microcomputed tomorgraphy (microCT) analysis, and statistical analysis are presented in SI Materials and Methods and real-time PCR primer sequences are given in Table S1.
Table S1.
Real-time PCR primer sequences used in this study
| Gene | Forward primer | Reverse primer |
| β-actin | 5′-TCCTCCTGAGCGCAAGTACTCT-3′ | 5′-CGGACTCATCGTACTCCTGCTT-3′ |
| Col2a1 | 5′-GCTCCCAGAACATCACCTACCA-3′ | 5′-TCATTGGAGCCCTGGATG-3′ |
| Aggrecan | 5′-CCTTGTCACCATAGCAACCT-3′ | 5′-CTACAGAACAGCGCCATCA-3′ |
| Prg4 | 5′-TCCGAGAACCATGGGGTGG-3′ | 5′-TCTCCCTGCACAGCTTGATA-3′ |
| Ggpdh | 5′-GCAAAGTGGAGATTGTTGCCAT-3′ | 5′-CCTTGACTGTGCCGTTGAATTT-3′ |
| Egfr | 5′-GGAAACCGAAATTTGTGCTACG-3′ | 5′-GCCTTGCAGTCTTTCTCAGCTC-3′ |
| Erb2 | 5′-CAGTGAGGATCCCACATTAC-3′ | 5′-CTCTGGCTGGTTCACATAC-3′ |
| Tgfα | 5′-TGTGCTGATCCACTGCTGTCA-3′ | 5′-AGCAAGCAGTCCTTCCCTTCA-3′ |
| Hbegf | 5′-ACAAGGACTACTGCATCCACGG-3′ | 5′-AGAGTCAGCCCATGACACCTGT-3′ |
| Epiregulin | 5′-TTCAGATGGAAGACGATCCCC-3′ | 5′-TGTCCACCAGGTAGATGCACTG-3′ |
| Egf | 5′-CATCCATTGGCAAAACCAGGT-3′ | 5′-TCACGACACAAACACCGAGCT-3′ |
| Betacellulin | 5′-GCCCCAAGCAGTACAAGCATT-3′ | 5′-AAACAGGTCCACTCGCTCACA-3′ |
| Amphiregulin | 5′-GCATCGGCATCGTTATCACAG-3′ | 5′-GCGTGCACAGTCCCATTTTC-3′ |
| β-actin (bovine) | 5′-TGGCGCTTGACTCAGGATTT-3′ | 5′-AATCAAGTCCTCGGCCACACT-3′ |
| Prg4 (bovine) | 5′-TGAACCGACTCCTACCTCTTTA-3′ | 5′-CAGCATCTCCATCCTCATTCTC-3′ |
All primers are designed for mouse genes, except the last two pairs, which are designed for bovine genes.
SI Materials and Methods
Animals.
Egfr CKO (Col2-Cre EgfrWa5/flox) mice were generated as previously described (14). We first bred Col2a1-Cre with EgfrWa5/+ (Wa5 is a dominant negative allele of Egfr) to obtain Col2-Cre EgfrWa5/+, which was then crossed with Egfrflox/flox to generate Egfr CKO mice and their Wa5 (EgfrWa5/flox) and WT (Col2-Cre Egfrflox/+ and Egfrflox/+) siblings (Fig. S2). As reported in that study, Col2-Cre Egfrflox/flox mice had incomplete deletion of Egfr alleles with no cartilage phenotype. Similarly, we have demonstrated that pre/osteoblast-specific Col1-Cre Egfrflox/flox mice did not have any bone phenotype, but Col1-Cre EgfrWa5/flox mice had significant bone loss in both trabecular and cortical bone due to defect bone formation (32). The requirement of a dominant negative Wa5 allele for detecting cartilage phenotypes is likely due to the dosage effect of active EGFR protein in maintaining normal cell functions. Egfr null mice normally die around birth, but Egfr+/− mice are indistinguishable from WT (33, 34). EgfrWa5/+ mice do not have any bone phenotype (32, 35) and relatively minor growth plate (14) and articular cartilage (17) phenotypes. The kinase activities of Wa5 and null alleles and their corresponding phenotypes follow this sequence: +/+ (normal) > +/− (normal) > +/Wa5 (very minor) > Wa5/Wa5 = −/− (lethal). Therefore, to maximize the inactivation of EGFR signaling, we constructed Col2-Cre EgfrWa5/flox mice instead of Col2-Cre Egfr–/flox mice for our study.
To induce OA, male mice at 3 mo of age were subjected to DMM surgery in the right knees and sham surgery in the left knees as described previously (17). Briefly, in DMM surgery, the joint capsule was opened immediately after anesthesia, and the medial meniscotibial ligament was cut to destabilize the meniscus without damaging other tissues. In Sham surgery, the joint capsule was opened in the same fashion but without any further damage. Serum was collected at euthanization to determine osteocalcin (Mouse Osteocalcin Enzyme Immunoassay Kit, Alfa Aesar) and CTX-I (RatLaps EIA, Immunodiagnostic Systems Inc.) levels.
Histology.
After euthanasia, mouse knee joints were harvested and fixed in 4% (wt/vol) paraformaldehyde overnight followed by decalcification in 0.5 M EDTA (pH 7.4) for 4 wk before paraffin embedding. A serial of 6 μm-thick sagittal sections (about 100) were cut across the entire medial compartment of the joint until anterior cruciate ligament junction. To measure the thicknesses of articular cartilage and chondrocyte numbers, three sections from each knee, corresponding to 1/4 (sections 20–30), 2/4 (sections 45–55), and 3/4 (sections 70–80) regions of the entire section set, were stained with Safranin O/Fast green and quantified using BIOQUANT software. The final measurement is an average of these three sections. We defined uncalcified cartilage as the area from articular surface to tide mark and calcified cartilage as the area from tide mark to cement line. The numbers of total cartilage chondrocytes and hypertrophic chondrocytes (those with enlarged chondrocyte lacunae and diminished Safranin-O staining) were also counted. A similar approach was used with H&E-stained sections to count the number of superficial chondrocytes (flat cells at the cartilage surface). The method to measure Mankin score was described previously (36). Briefly, two sections within every consecutive six sections in the entire section set for each knee were stained with Safranin O/Fast green and scored by two blinded observers (H.J. and W.T.). Each knee received a single score representing the maximal score of its sections.
Paraffin sections were used for immunohistochemistry and TUNEL assay. For mouse samples, after appropriate antigen retrieval, slides were incubated with primary antibodies, such as rabbit anti-EGFR (Abcam, ab2430), rabbit anti–p-EGFR (Abcam, ab40815), mouse anti–p-ERK (Santa Cruz, sc-7383), rabbit anti-Ki67 (Abcam, ab15580), and rabbit anti-type X collagen (Cosmo Bio, LSL-LB-0092), at 4 °C overnight, followed by binding with biotinylated secondary antibodies and DAB color development. The TUNEL assay was carried out according to the manufacturer’s instructions (Millipore, s7101). For human samples, rabbit anti-EGFR (Abcam, ab2430) and rabbit anti–p-EGFR (Abcam, ab40815) antibodies were used.
Frozen sections were used for immunofluorescence staining. For mouse samples, 100 µm-thick sections were incubated with rabbit anti-Prg4 antibody (Santa Cruz, sc-98454) and HA Binding Protein (Millipore, 385911) at 4 °C overnight, followed by Alexa Fluor 488-conjugated donkey anti-Rabbit IgG secondary antibody (Thermo Scientific, A-21206) and DyLight 633-conjugated Streptavidin Protein (Thermo Scientific, 21844), respectively. For bovine samples, rabbit anti-Prg4 antibody (Santa Cruz, sc-98454) was used. The images were scanned by confocal microscope LSM710 and processed and analyzed by Volocity software.
Human Articular Cartilage Samples.
The OA articular cartilage samples were prepared from the de-identified specimens obtained at the total arthroplasty of the knee joints and used for immunohistochemical examination for EGFR and p-EGFR. The serial sections were stained by Safranin O and Fast green staining to evaluate the OA phenotype.
AFM Nanoindentation.
Nanoindentation was performed on femoral articular cartilage surface as we previously described (37). Freshly dissected femoral condyle cartilage was indented at more than 10 locations by a borosilicate colloidal spherical tip (R = 5 μm, nominal spring constant k = 7.4 N/m) with maximum indentation depth of ∼1 μm at a 10 μm/s indentation rate using a Dimension Icon AFM (BrukerNano) in PBS with protease inhibitors. The effective indentation modulus, Eind (MPa), was calculated by fitting the whole loading portion of each indentation force–depth curve using the Hertz model.
Superficial Chondrocyte Culture, Primary Chondrocyte Culture, RNA Isolation, and Real-Time RT-PCR Analysis.
Superficial chondrocytes were isolated from the distal femoral and proximal tibial epiphysis of 3-d-old mice via transient enzymatic digestion as described previously (38). Briefly, tissues were incubated with 0.25% trypsin (Invitrogen) for 1 h, followed by 2 h digestion with 173 U/mL type I collagenase (Worthington Biochemical). Dissociated cells were seeded on culture dishes, and attached cells were considered superficial chondrocytes, as demonstrated in our previous report (38). They were cultured in DMEM containing 15% FBS, 100 μg/mL streptomycin, and 100 U/mL penicillin. For proliferation assay, cells were seeded in 7.5% DMEM and on the next day treated with recombinant human TGFα (R&D Systems, 100 ng/mL). MTT assay was performed on the indicated days. For apoptosis assay, cells at 40–60% confluency were serum-starved overnight and then pretreated with either vehicle or 25 ng/mL tumor necrosis factor α (TNFα) (Pepro-Tech) before the addition of TGFα. Two days later, apoptotic cells were quantified using ethidium bromide (5 mg/mL) and acridine orange (5 mg/mL) staining as described previously (25). For chondrogenic differentiation assay, confluent superficial chondrocytes were cultured in differentiation medium (growth medium with 50 μg/mL l-ascorbic acid) with or without 100 ng/mL TGFα and 10 μM gefitinib (LC Laboratories). Media were changed twice a week with fresh TGFα and gefitinib. Primary chondrocytes were isolated from the same epiphyseal cartilage tissue via thorough enzymatic digestion (86 U/mL type I collagenase for 12 h) as described previously (14) and cultured in DMEM/F12 medium containing 10% FBS, 50 μg/mL l-ascorbic acid, 1% glutamine, 100 μg/mL streptomycin, and 100 U/mL penicillin.
Total RNA was harvested from the femoral head articular cartilage of 2-mo-old mice and chondrocyte cultures using Tri Reagent (Sigma). Taqman Reverse Transcription kit (Applied BioSystems) was used to reverse-transcribe mRNA into cDNA. Following this, PCR was performed using a Power SYBR Green PCR Master Mix kit (Applied BioSystems). The primer sequences for the genes used in this study are listed in Table S1.
Bovine Cartilage Explant Culture and Friction Test.
Cartilage explants (3 mm in diameter and 2 mm in thickness) were harvested from the central region of the femoral condyle head from calf knee joints and cultured in chemically defined medium (39) in the presence and absence of 50 ng/mL TGFα and 5 μm gefitinib for 3 d. For immunofluorescent staining, explants were fixed, processed for paraffin sections, and stained by rabbit anti-Prg4 antibody (Abcam, ab28484). For RNA assay, the top 1-mm part of explants was cut for mRNA purification by Tri reagent followed by real-time RT-PCR. For friction assay, a custom-built microtribometer was used to determine the friction coefficients under a 1 N normal load at a sliding speed of 2 mm/s as we previously reported (40). The sample bathed in PBS was fixed on the microtribometer during testing. A glass slide was compressed onto the articular surface with a specified normal load, sliding over a 2-mm track achieved by the reciprocation of a linear stage at the bottom. The reciprocation of the cartilage explant against a glass slide provided a stationary contact area modality, in which the contact area remains static, as boundary lubrication is best evaluated at conditions in which the interstitial fluid lubrication has subsided. Orthogonal capacitance sensors were used to measure the normal contact force and shear force on the sliding surface. The friction coefficient was calculated by dividing the mean absolute value of the forward and reverse friction forces by the mean normal force. The test was performed for 3 h or until the friction force reached equilibrium.
Polarized Light Microscopy Analysis.
Polarized light microscopy was used to determine the collagen structure in articular cartilage as described previously (41). Briefly, histological sections were imaged under a 40× objective, from which quantitative maps of the retardation and angle were calculated at a 0.25 μm/pixel resolution. The colors in the angle image represent the averaged fibril orientation within any single imaging voxel. A 90° difference in the fiber orientation is represented by the blue and red colors. The brightness of the retardation image represents the averaged fibril organization within any single imaging voxel. A high retardation value implies a better organization among the collagen fibers.
OA Pain Analysis.
The knee joint pain after DMM surgery was evaluated in mice at 1 mo after surgery using von Frey filaments as described previously (18). An individual mouse was placed on a wire-mesh platform (Excellent Technology Co.) under a 4 × 3 × 7 cm cage to restrict movement. Mice were trained to be accustomed to this condition every day starting from 7 d before the test. During the test, a set of von Frey fibers (Stoelting Touch Test Sensory Evaluator Kit 2–9; ranging from 0.015 to 1.3 g force) were applied to the plantar surface of the hind paw until the fibers bowed and then held for 3 s. The threshold force required to elicit withdrawal of the paw (median 50% withdrawal) was determined five times on each hind paw with sequential measurements separated by at least 5 min.
MicroCT Analysis.
Both femurs and tibiae were scanned from the epiphyseal end at a 6-µm isotropic voxel size by microCT 35 (Scanco Medical AG). The 3D images of the femoral distal end were reconstructed to visualize osteophytes. To analyze the trabecular bone structural parameters in the secondary spongiosa, images (0.4–1.2 mm below the lowest point of the growth plate) from the proximal end of tibiae were smoothed by a Gaussian filter (sigma, 1.2; support, 2.0) with a threshold corresponding to 30% of the maximum available range of image gray scale values and then contoured on the transverse plane. Bone volume fraction (BV/TV), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), trabecular number (Tb.N), structure model index (SMI), and tissue mineral density (TMD) were calculated by 3D standard microstructural analysis provided by the manufacturer.
Statistical Analysis.
Data are expressed as means ± SE (SEM) and analyzed by unpaired, two-tailed Student’s t test for comparison among WT, Wa5, and Egfr CKO mice followed by Bonferroni adjustment for multiple comparisons and by paired, two-tailed Student’s t test for comparison of sham and DMM-injured knees within the same animals. For assays using primary chondrocytes and bovine cartilage explants, experiments were repeated independently at least three times and representative data were shown here. Values of P < 0.05 were considered statistically significant.
Acknowledgments
The authors thank Dr. Frank Beier at the University of Western Ontario for critical comments and discussion on this project, Dr. Hee-Jenong Im at Rush University for kind instruction on von Frey assay, and Dr. S. Wakitani at Hiroshima University for kindly providing human articular cartilage sections. This study was supported by an ASBMR Research Career Enhancement Award (L.Q.), NIH Grants AR060991 and DK095803 (to L.Q.), AR052353 and AR069047 (to Y.X.), and AR066824 (to L.H.), and Arthritis Foundation Grant IRG 6389 (to M.E.-I.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1608938113/-/DCSupplemental.
References
- 1.Buckwalter JA, Mankin HJ, Grodzinsky AJ. Articular cartilage and osteoarthritis. Instr Course Lect. 2005;54:465–480. [PubMed] [Google Scholar]
- 2.Becerra J, et al. Articular cartilage: Structure and regeneration. Tissue Eng Part B Rev. 2010;16(6):617–627. doi: 10.1089/ten.TEB.2010.0191. [DOI] [PubMed] [Google Scholar]
- 3.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18(Suppl 3):S17–S23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 4.Pritzker KP, et al. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthritis Cartilage. 2006;14(1):13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Rolauffs B, et al. Onset of preclinical osteoarthritis: The angular spatial organization permits early diagnosis. Arthritis Rheum. 2011;63(6):1637–1647. doi: 10.1002/art.30217. [DOI] [PubMed] [Google Scholar]
- 6.Citri A, Yarden Y. EGF-ERBB signalling: Towards the systems level. Nat Rev Mol Cell Biol. 2006;7(7):505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 7.Appleton CT, Usmani SE, Bernier SM, Aigner T, Beier F. Transforming growth factor alpha suppression of articular chondrocyte phenotype and Sox9 expression in a rat model of osteoarthritis. Arthritis Rheum. 2007;56(11):3693–3705. doi: 10.1002/art.22968. [DOI] [PubMed] [Google Scholar]
- 8.Long DL, Ulici V, Chubinskaya S, Loeser RF. Heparin-binding epidermal growth factor-like growth factor (HB-EGF) is increased in osteoarthritis and regulates chondrocyte catabolic and anabolic activities. Osteoarthritis Cartilage. 2015;23(9):1523–1531. doi: 10.1016/j.joca.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang YW, et al. Targeted disruption of Mig-6 in the mouse genome leads to early onset degenerative joint disease. Proc Natl Acad Sci USA. 2005;102(33):11740–11745. doi: 10.1073/pnas.0505171102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pest MA, Russell BA, Zhang YW, Jeong JW, Beier F. Disturbed cartilage and joint homeostasis resulting from a loss of mitogen-inducible gene 6 in a mouse model of joint dysfunction. Arthritis Rheumatol. 2014;66(10):2816–2827. doi: 10.1002/art.38758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shepard JB, Jeong JW, Maihle NJ, O’Brien S, Dealy CN. Transient anabolic effects accompany epidermal growth factor receptor signal activation in articular cartilage in vivo. Arthritis Res Ther. 2013;15(3):R60. doi: 10.1186/ar4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staal B, Williams BO, Beier F, Vande Woude GF, Zhang YW. Cartilage-specific deletion of Mig-6 results in osteoarthritis-like disorder with excessive articular chondrocyte proliferation. Proc Natl Acad Sci USA. 2014;111(7):2590–2595. doi: 10.1073/pnas.1400744111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pante G, et al. Mitogen-inducible gene 6 is an endogenous inhibitor of HGF/Met-induced cell migration and neurite growth. J Cell Biol. 2005;171(2):337–348. doi: 10.1083/jcb.200502013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, et al. The critical role of the epidermal growth factor receptor in endochondral ossification. J Bone Miner Res. 2011;26(11):2622–2633. doi: 10.1002/jbmr.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, et al. Epidermal growth factor receptor (EGFR) signaling regulates epiphyseal cartilage development through β-catenin-dependent and -independent pathways. J Biol Chem. 2013;288(45):32229–32240. doi: 10.1074/jbc.M113.463554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doyran B, et al. Nanoindentation modulus of murine cartilage: A sensitive indicator of the initiation and progression of post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2016;25(16):30243–30246. doi: 10.1016/j.joca.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, et al. Reduced EGFR signaling enhances cartilage destruction in a mouse osteoarthritis model. Bone Res. 2014;2:14015. doi: 10.1038/boneres.2014.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piel MJ, Kroin JS, Im HJ. Assessment of knee joint pain in experimental rodent models of osteoarthritis. Methods Mol Biol. 2015;1226:175–181. doi: 10.1007/978-1-4939-1619-1_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kozhemyakina E, et al. Identification of a Prg4-expressing articular cartilage progenitor cell population in mice. Arthritis Rheumatol. 2015;67(5):1261–1273. doi: 10.1002/art.39030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daniel M. Boundary cartilage lubrication: Review of current concepts. Wien Med Wochenschr. 2014;164(5-6):88–94. doi: 10.1007/s10354-013-0240-2. [DOI] [PubMed] [Google Scholar]
- 21.Bonnevie ED, Galesso D, Secchieri C, Cohen I, Bonassar LJ. Elastoviscous transitions of articular cartilage reveal a mechanism of synergy between lubricin and hyaluronic acid. PLoS One. 2015;10(11):e0143415. doi: 10.1371/journal.pone.0143415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das S, et al. Synergistic interactions between grafted hyaluronic acid and lubricin provide enhanced wear protection and lubrication. Biomacromolecules. 2013;14(5):1669–1677. doi: 10.1021/bm400327a. [DOI] [PubMed] [Google Scholar]
- 23.Kwiecinski JJ, et al. The effect of molecular weight on hyaluronan’s cartilage boundary lubricating ability--Alone and in combination with proteoglycan 4. Osteoarthritis Cartilage. 2011;19(11):1356–1362. doi: 10.1016/j.joca.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 24.Ono N, Ono W, Nagasawa T, Kronenberg HM. A subset of chondrogenic cells provides early mesenchymal progenitors in growing bones. Nat Cell Biol. 2014;16(12):1157–1167. doi: 10.1038/ncb3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chandra A, Lan S, Zhu J, Siclari V, Qin L. Epidermal Growth Factor Receptor (EGFR) signaling promotes proliferation and survival in osteoprogenitors by increasing Early Growth Response Protein (Egr2) expression. J Biol Chem. 2013;288(28):20488–98. doi: 10.1074/jbc.M112.447250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin L, et al. Amphiregulin is a novel growth factor involved in normal bone development and in the cellular response to parathyroid hormone stimulation. J Biol Chem. 2005;280(5):3974–3981. doi: 10.1074/jbc.M409807200. [DOI] [PubMed] [Google Scholar]
- 27.Zhu J, Shimizu E, Zhang X, Partridge NC, Qin L. EGFR signaling suppresses osteoblast differentiation and inhibits expression of master osteoblastic transcription factors Runx2 and Osterix. J Cell Biochem. 2011;112(7):1749–1760. doi: 10.1002/jcb.23094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu J, et al. Amphiregulin-EGFR signaling mediates the migration of bone marrow mesenchymal progenitors toward PTH-stimulated osteoblasts and osteocytes. PLoS One. 2012;7(12):e50099. doi: 10.1371/journal.pone.0050099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koyama E, et al. A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Dev Biol. 2008;316(1):62–73. doi: 10.1016/j.ydbio.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Appleton CT, et al. Reduction in disease progression by inhibition of transforming growth factor α-CCL2 signaling in experimental posttraumatic osteoarthritis. Arthritis Rheumatol. 2015;67(10):2691–2701. doi: 10.1002/art.39255. [DOI] [PubMed] [Google Scholar]
- 31.Henry SP, et al. Generation of aggrecan-CreERT2 knockin mice for inducible Cre activity in adult cartilage. Genesis. 2009;47(12):805–814. doi: 10.1002/dvg.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, et al. Epidermal growth factor receptor plays an anabolic role in bone metabolism in vivo. J Bone Miner Res. 2011;26(5):1022–1034. doi: 10.1002/jbmr.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miettinen PJ, et al. Epidermal growth factor receptor function is necessary for normal craniofacial development and palate closure. Nat Genet. 1999;22(1):69–73. doi: 10.1038/8773. [DOI] [PubMed] [Google Scholar]
- 34.Wang K, Yamamoto H, Chin JR, Werb Z, Vu TH. Epidermal growth factor receptor-deficient mice have delayed primary endochondral ossification because of defective osteoclast recruitment. J Biol Chem. 2004;279(51):53848–53856. doi: 10.1074/jbc.M403114200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneider MR, et al. Normal epidermal growth factor receptor signaling is dispensable for bone anabolic effects of parathyroid hormone. Bone. 2012;50(1):237–244. doi: 10.1016/j.bone.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 36.Aigner T, et al. Histopathology atlas of animal model systems—Overview of guiding principles. Osteoarthritis Cartilage. 2010;18(Suppl 3):S2–S6. doi: 10.1016/j.joca.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 37.Batista MA, et al. Nanomechanical phenotype of chondroadherin-null murine articular cartilage. Matrix Biol. 2014;38:84–90. doi: 10.1016/j.matbio.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yasuhara R, et al. Roles of β-catenin signaling in phenotypic expression and proliferation of articular cartilage superficial zone cells. Lab Invest. 2011;91(12):1739–1752. doi: 10.1038/labinvest.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou Y, Park M, Cheung E, Wang L, Lu XL. The effect of chemically defined medium on spontaneous calcium signaling of in situ chondrocytes during long-term culture. J Biomech. 2015;48(6):990–996. doi: 10.1016/j.jbiomech.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zimmerman BK, et al. Role of interstitial fluid pressurization in TMJ lubrication. J Dent Res. 2015;94(1):85–92. doi: 10.1177/0022034514553626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xia Y, Moody JB, Burton-Wurster N, Lust G. Quantitative in situ correlation between microscopic MRI and polarized light microscopy studies of articular cartilage. Osteoarthritis Cartilage. 2001;9(5):393–406. doi: 10.1053/joca.2000.0405. [DOI] [PubMed] [Google Scholar]