Abstract
Background
Although relationships between smoking/high cotinine and type 2 diabetes have consistently been observed, few studies have investigated the relationship between cotinine and underlying pathophysiological defects that characterize diabetes aetiology. This study aimed to test the associations between cotinine and measures of insulin resistance or insulin secretion.
Methods
This analysis included 5,751 non-diabetic adult American from the National Health and Nutrition Examination Survey (NHANES) from 2007–2012. Insulin function was represented with two indexes: insulin resistance index (HOMA-IR) and insulin secretion index (HOMA-B) estimated by homeostasis model assessment. We categorized cotinine levels into quartiles and estimated the odds of HOMA-IR in the 4th quartile and HOMA-B in the 1st quartile among cotinine categories using multiple logistic regression models.
Results
Cotinine concentration was not associated with the risk of high HOMA-IR. Association of cotinine with low HOMA-B existed and differed by race/ethnicity (P for interaction<0.05). High cotinine concentration (in the 4th quartile) was associated with an increased risk of low HOMA-B compared with low cotinine concentrations(1st -2nd quartiles) among white (odds ratio[OR], 1.51 [95% confidence interval[CI], 1.16–1.97]) or black participants (OR, 2.98 [95%CI, 1.90–4.69]) but not among Mexican (OR, 1.79 [95%CI, 0.90–3.53]) or other Hispanic(OR, 1.02 [95%CI, 0.56–1.86]) participants. Such associations remained significant even after further adjustment for HOMA-IR.
Conclusions
High cotinine is associated with decreased insulin secretion function only in white and black non-diabetic U.S. adult population. Results evaluating cotinine in ethnically homogeneous populations may not be broadly generalizable to other racial/ethnic groups.
Introduction
Type 2 diabetes is epidemic. In the U.S., there are about 1.5 million new cases per year, and the crude prevalence of diabetes (diagnosed plus undiagnosed) is reported at 9.6% in adults [1]. Cigarette smoking is another global public health concern, causing the death of about 4 million people every year[2]. A number of previous studies[3] have assessed the association between smoking and the incidence of type of diabetes, suggesting that active smoking could be involved in the development in the glucose abnormalities. Since impaired insulin secretion and insulin resistance are the main pathophysiological components of type 2 diabetes, these two defects are likely be the potential mechanism underlying the smoking-diabetes linkage. Although a few of population-studies [4–7] have investigated the association of smoking and insulin, the conclusions remain controversial.
Cotinine is a major metabolite of nicotine that is used as a marker for both active smoking and tobacco smoke exposure ("passive smoking")[8]. Cotinine is generally preferred over nicotine for such assessments because of its substantially longer half-life[9]. Using cotinine would minimize the bias if smokers do not accurately report their smoking status. A recent U.S. study[10] in a non-diabetic sample reported that both cotinine and self-reported smoking were associated with increased glycated hemoglobin A1c (HbA1c). However, little has been known regarding the role of insulin resistance and β cell function in the linkage of smoking/cotinine and hyperglycemia in large non-diabetic population.
We conducted this study to assess the association of cotinine concentration with insulin resistance and insulin secretion function, using data from the National Health and Nutrition Examination Survey (NHANES) 2007–2012. Since the metabolism of cotinine vary substantially by race/ethnicity[11], we were particularly interested in whether these associations, if exist, differ according to race/ethnicity.
Material and Methods
Study sample
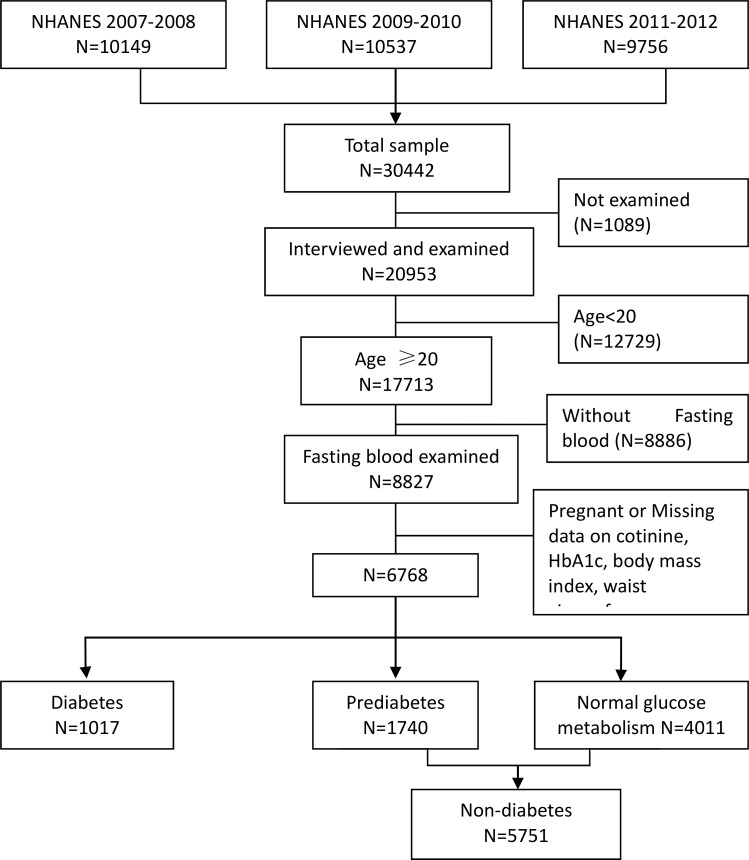
The National Health and Nutrition Examination Survey (NHANES), conducted by the National Center for Health Statistics[12], was designed to be representative of the U.S. civilian non-institutionalized population using complex, multistage probability samples. The NHANES protocol was approved by the National Center for Health Statistics Ethics Review Board[13], and written informed consent was obtained from all participants. Participants were interviewed in homes and subsequently received a physical and laboratory examination in a mobile examination center. We combined three successive waves (2007–2012) of the continuous NHANES for our analysis, generating a total sample of 20,953 individuals. We limited the analysis to adult participants(n = 8,827) with age ≥20 years old, who completed fasting glucose(FPG) and fasting insulin(FPI), HbA1c and cotinine assessment. We further excluded subjects who were pregnant (n = 1,059) or with diabetes (n = 1,017), resulting a final sample size of 5,751 participants (Fig 1).
Fig 1. Flowchart depicting three successive waves (2007–2012) of continuous NHANES used for analysis.
Cotinine status
Cotinine is used as a biological surrogate for smoking and tabocco exposure. Serum cotinine was measured using isotope dilution-high performance liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometry [14]. We classified cotinine concentrations in all eligible participants into four groups according to quartile, 1st, 2nd, 3rd and 4th quartile or race/ethnic-specific quartile groups.
Glycated hemoglobin, glucose and insulin
HbA1c was measured using high performance liquid chromatography. FPG and FPI were measured in participants examined after an 8-24-h fast, using the hexokinase enzymatic method. Due to the method of insulin assay 2007–2010 switched from Mercodia sandwich ELISA assay to Roche chimilumnescent immunoassay 2011–2012, we converted fasting insulin values from 2011–2012 to make them comparable to values from 2007–2010 in our analysis, using the formula suggested by NHANES[15]:
Other covariates
Demographic information included age, race/ethnicity, and education. Race/ethnicity was categorized as non-Hispanic White, non-Hispanic Black, Mexican American and other Hispanic. Education level was classified as below high school, high school, and above high school. Weight and height were measured using standardized techniques and equipment during clinical examinations. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Waist circumference (WC) was measured at the upper-most lateral border of the ilium. Alcohol consumption was dichotomized as 0, ≤1, 2–3, and ≥4 drinks per week. Physical activity was categorized into three levels (low, moderate and high) according to the total metabolic equivalent score from questionnaires.
Outcomes of Interest
Homeostatic model assessment (HOMA) [16] is a method for assessing insulin resistance and insulin secretion estimated from fasting glucose and insulin or C-peptide concentrations. HOMA has been validated against a variety of physiologic methods and widely used in epidemiology and clinical studies[17]. Insulin resistance and insulin secretion index are calculated as:
Since the studies on HOMA-IR index to detect impaired glucose tolerance or metabolic syndrome are limited and resulted in different cut offs. [18–21], and data on cut off of homeostasis model assessment for insulin secretion is scarce, we used HOMA-IR in top quartile to define the risk of insulin resistance, and HOMA-B in bottom quartile to define the risk of impaired insulin secretion.
Statistical analysis
We accounted for the survey sampling design and used sample weights to generalize estimates to the U.S. population as a whole[12]:
for 2007–2012.
Participant characteristics were tested for differences across cotinine quartile categories. Differences in frequencies were examined by chi-squared tests for categorical variables. Differences in means were tested by ANOVA for continuous variables. To examine the functional forms of the association of cotinine with HOMA-IR and HOMA-B, we applied adjusted penalized smoothing splines. Logistic regression models were used to examine the relationship between cotinine and odds of HOMA-IR in 4th quartile and HOMA-B in 1st quartile. We first compared participants with cotinine in high (4th quartile) and middle(3rd quartile) groups with counterparts with cotinine in low(1st -2nd quartile) group. Second, we performed test for trend across categories of cotinine. Third, we investigated race/ethnicity–cotinine interaction by adding a product term for cotinine levels and race/ethnicity categories to the regression model. The base model included age, gender, race/ethnicity, education attainment and alcohol consumption. The second model added variables possibly cofounding or mediating the associations of interest, such as physical activity levels and waist circumference. Analyses stratified by race/ethnicity were conducted using the same models.
There were 767(11%) participants reporting antihypertensive (beta blocker, diuretic and/or vasodilator) use, which may exert influence on insulin secretion[22]. Sensitive analyses were conducted with further adjusted for antihypertensive use or with exclusion of these participants from the analyses.
Statistical tests were two-sided and P<0.05 was considered statistical significant. We applied GAM in R program (version 2.15.3, R Core Team, Vienna, Australia)[23] to fit spline model. We used survey procedures in SAS software (version 9.2, SAS Institute, Inc., Cary, North Carolina) to account for NHANES sampling design and sample weights.
Results
After the exclusion of participants with diabetes either self-reported or diagnosed with HbA1c criteria, the final sample consisted of 5,751 individuals. Geometric mean (95%CI) of serum cotinine was 0.31(0.25–0.38) ng/mL and varied substantially by race/ethnicity: 0.33(0.25–0.44) ng/mL for white, 0.90(0.63–1.30) ng/mL for black, 0.10(0.07–0.14) ng/mL for Mexican, and 0.17(0.11–0.26) ng/mL for Hispanic. The characteristics of participants in this analysis are presented in Table 1. Gender and ethnicity differed across cotinine categories with Participants with higher proportions of female in lower cotinine categories and the lowest proportion of Mexican or other Hispanics in the highest cotinine category. BMI and WC also differed across cotinine categories with the highest mean BMI and WC in 3rd quartile of cotinine. However, the highest mean HbA1c was observed in 4th quartile of cotinine.
Table 1. Characteristics of adult participants without diabetes in NHANES 2007–2012 by cotinine categories.
| Cotinine categories | |||||
|---|---|---|---|---|---|
| Characteristic | Q1(n = 1496) | Q2(n = 1559) | Q3(n = 1540) | Q4 (= 1531) | P value |
| Age (yrs) | 49.5 (0.5) | 47.7(0.7) | 43.3(0.7) | 41.5(0.5) | <0.0001 |
| BMI (kg/m2) | 27.7 (0.2) | 28.3(0.2) | 29.2(0.3) | 27.3(0.2) | <0.0001 |
| WC (cm) | 95.6 (0.5) | 96.9(0.6) | 99.1(0.7) | 96.0(0.5) | <0.0001 |
| FPG(mmol/L) | 5.43(0.02) | 5.53(0.02) | 5.56(0.02) | 5.50(0.02) | <0.0001 |
| HbA1C(%) | 5.38(0.01) | 5.42(0.01) | 5.39(0.02) | 5.42(0.01) | 0.0129 |
| FPI(uU/mL)a | 10.0(6.8–16.1) | 10.5(6.9–16.9) | 11.2(7.2–17.3) | 9.9(5.9–16.0) | <0.0001 |
| HOMA-IRa | 2.4(1.6–4.0) | 2.6(1.6–4.2) | 2.7(1.7–4.3) | 2.5(1.4–4.1) | <0.0001 |
| HOMA-B(%)a | 108(73–163) | 108(73–163) | 110(76–165) | 103(66–155) | 0.0051 |
| Cotinine(ng/mL)a | 0.01(0.01–0.01) | 0.03(0.02–0.04) | 0.11(0.07–0.25) | 174.00(44.80–290.00) | — |
| Female,n(%) | 892 (63) | 820(56) | 699(46) | 562(39) | <0.0001 |
| Education,n(%) < High school | 268 (10) | 320(13) | 391(19) | 482(28) | |
| High school | 232(14) | 287(18) | 360(26) | 419(30) | |
| > High school | 925 (76) | 879(69) | 686(55) | 502(42) | 0.1575 |
| Race/ethnicity, n(%) Non-Hispanic White | 692 (75) | 620(65) | 612(65) | 760(73) | |
| Non-Hispanic Black | 145(5) | 222(9) | 323(14) | 320(14) | |
| Mexican American | 291(9) | 278(10) | 226(9) | 118(4) | |
| Other Hispanic | 196(6) | 182(6) | 149(6) | 114(4) | |
| Alcohol consumption, n(%) None | 502 (29) | 568(31) | 452(27) | 265(17) | <0.0001 |
| ≤1 Drink/week | 671(47) | 645(45) | 684(47) | 736(52) | |
| 2–3 Drinks/week | 132(13) | 140(12) | 183(17) | 214(17) | |
| ≥4 Drinks/week | 120(11) | 133(11) | 118(9) | 188(14) | <0.0001 |
| Physical Activity, n(%) None/light | 579(35) | 553(33) | 500(30) | 531(34) | |
| Moderate | 542(42) | 535(39) | 473(37) | 363(28) | |
| Vigorous | 304(23) | 398(28) | 464(33) | 509(38) | <0.0001 |
Data are presented as Mean (se), median (IQR) or N (%). BMI, body mass index. WC, waist circumference. HbA1C, Glycated hemoglobin A1c. FPG, fasting glucose. FPI, fasting insulin. HOMA-IR, insulin resistance estimated by homeostasis model assessment. HOMA-B, insulin secretion estimated by homeostasis model assessment.
a, log transformed in ANOVA test. Chi-squared test and ANOVA test were weighted and accounted for NHANES survey design.
HOMA-IR and HOMA-B varied significantly across cotinine categories with the highest HOMA-IR observed in the 3rd quartile of cotinine but the lowest HOMA-B in the 4th quartile of cotinine.
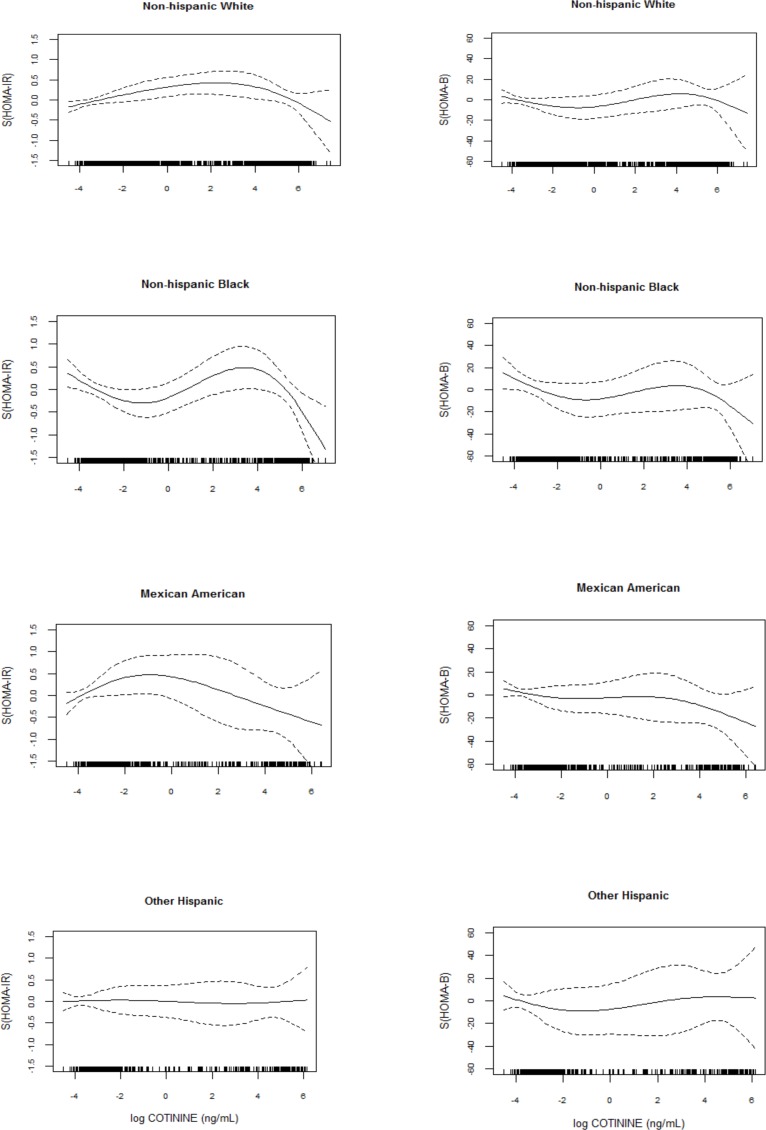
Spline plots (Fig 2) showed that HOMA-IR and HOMA-B fluctuated with cotinine concentration in a similar shape but varied noticeably by race/ethnicity.
Fig 2. Smoothing plots of HOMA-IR and HOMA-B against cotinine by race/ethnicity.
Using logistic regression model, we found no association between cotinine and HOMA-IR among each race/ethnicity participants after fully adjustment (Table 2).
Table 2. Associations of quartiles of cotinine with HOMA-IR by race/ethnicity.
| Model 1 | Model 2 | ||||
|---|---|---|---|---|---|
| Cotinine Quartiles | N of HIR a (%) | Odds Ratio (95%CI) | P Value | Odds Ratio (95%CI) | P Value |
| Whole | |||||
| 1st -2nd | 696(21.8) | 1.00 | 1.00 | ||
| 3rd | 393(26.1) | 1.21(1.01–1.45) | 0.04 | 0.96(0.75–1.22) | 0.72 |
| 4th | 327(21.6) | 0.95(0.77–1.16) | 0.60 | 0.94(0.75–1.18) | 0.61 |
| P trend | 0.87 | 0.60 | |||
| PI | 0.53 | 0.85 | |||
| White | |||||
| 1st -2nd | 267(20.6) | 1.00 | 1.00 | ||
| 3rd | 163(26.6) | 1.29(1.00–1.65) | 0.04 | 0.97(0.69–1.36) | 0.90 |
| 4th | 184(23.8) | 0.94(0.74–1.20) | 0.62 | 0.99(0.74–1.33) | 0.82 |
| P trend | 0.87 | 0.34 | |||
| Black | |||||
| 1st -2nd | 97(26.9) | 1.00 | 1.00 | ||
| 3rd | 96(29.6) | 1.21(0.81–1.82) | 0.38 | 0.84(0.52–1.35) | 0.45 |
| 4th | 73(22.4) | 0.84(0.58–1.23) | 0.35 | 0.82(0.54–1.25) | 0.23 |
| P trend | 0.42 | 0.92 | |||
| Mexican | |||||
| 1st -2nd | 97(26.0) | 1.00 | 1.00 | ||
| 3rd | 44(29.7) | 0.82(0.57–1.19) | 0.29 | 0.90(0.59–1.37) | 0.58 |
| 4th | 29(24.2) | 0.77(0.43–1.38) | 0.38 | 0.64(0.34–1.21) | 0.12 |
| P trend | 0.29 | 0.20 | |||
| Hispanic | |||||
| 1st -2nd | 182(32.3) | 1.00 | 1.00 | ||
| 3rd | 69(30.4) | 1.17(0.74–1.84) | 0.50 | 1.07(0.56–2.07) | 0.73 |
| 4th | 35(28.5) | 1.11(0.65–1.89) | 0.70 | 0.99(0.47–2.07) | 0.85 |
| P trend | 0.61 | 0.98 | |||
a, high HOMA-IR was defined as HOMA in 4 th quartile.
PI, p value for interaction by race/ethnicity.
Model 1, adjusted for age, gender, ethnicity/race, alcohol consumption, education level.
Model 2, Model 1 further adjusted for physical activity and waist circumference.
As shown in Table 3, the risks of low HOMA-B were increased with higher cotinine concentration after adjustment for potential covariates. Yet we observed significant heterogeneity of the association of cotinine with HOMA-B by race/ethnicity, with P values for interactions <0.05 across race/ethnicity groups for each model. Cotinine in 4th quartile was associated with significantly higher risks of low HOMA-B among non-Hispanic White participants (OR, 1.47[95%CI, 1.13–1.91]) and non-Hispanic Black participants (OR, 3.04[95%CI, 1.96–4.72]) compared with the reference group. However, there was no evidence of similar associations among either Mexican American (OR, 1.80[95%CI, 0.92–3.53]) or other Hispanic participants (OR, 1.05[95%CI, 0.95–1.88]). Given the relation of HOMA-IR and HOMA-B, we additionally adjusted for HOMA-IR as well as other covariates included in model 2.The linkage of cotinine with HOMA-B remained significant among non-Hispanic White participants and non-Hispanic Black participants (Table 4).
Table 3. Associations of quartiles of cotinine with HOMA-B by race/ethnicity.
| Model 1 | Model 2 | ||||
|---|---|---|---|---|---|
| Cotinine Quartiles | N of LHBb (%) | Odds Ratio(95%CI) | P Value | Odds Ratio(95%CI) | P Value |
| Whole | |||||
| 1st -2nd | 688(23.9) | 1.00 | 1.00 | ||
| 3rd | 309(21.5) | 0.89(0.71–1.10) | 0.28 | 1.13(0.89–1.43) | 0.35 |
| 4th | 440(30.6) | 1.54(1.27–1.88) | <0.0001 | 1.57(1.28–1.94) | <0.0001 |
| P trend | 0.0002 | <0.0001 | |||
| PI | 0.003 | 0.018 | |||
| White | |||||
| 1st -2nd | 391(30.2) | 1.00 | 1.00 | ||
| 3rd | 156(25.5) | 0.88(0.65–1.20) | 0.43 | 1.18(0.85–1.66) | 0.33 |
| 4th | 248(32.0) | 1.48(1.15–1.91) | 0.002 | 1.51(1.16–1.97) | 0.002 |
| P trend | 0.010 | 0.004 | |||
| Black | |||||
| 1st -2nd | 55(15.3) | 1.00 | 1.00 | ||
| 3rd | 61(18.8) | 1.41(0.87–2.29) | 0.17 | 1.77(1.12–2.80) | 0.015 |
| 4th | 100(30.7) | 3.01(1.86–4.88) | <0.0001 | 2.79(1.79–4.69) | <0.0001 |
| P trend | <0.0001 | <0.0001 | |||
| Mexican | |||||
| 1st -2nd | 87(15.5) | 1.00 | 1.00 | ||
| 3rd | 41(18.1) | 1.09(0.72–1.65) | 0.69 | 1.07(0.64–1.78) | 0.80 |
| 4th | 31(25.2) | 1.99(1.22–3.24) | 0.006 | 1.79(0.88–3.48) | 0.11 |
| P trend | 0.016 | 0.17 | |||
| Hispanic | |||||
| 1st -2nd | 78(20.9) | 1.00 | 1.00 | ||
| 3rd | 23(15.5) | 0.81(0.53–1.21) | 0.27 | 0.66(0.41–1.08) | 0.10 |
| 4th | 27(22.5) | 1.05(0.63–1.78) | 0.91 | 1.06(0.57–1.96) | 0.86 |
| P trend | 0.99 | 0.89 |
b, low HOMA-B was defined as HOMA-B in 4 th quartile.
PI, p value for interaction by race/ethnicity.
Model 1, adjusted for age, gender, ethnicity/race, alcohol consumption, education level.
Model 2, Model 1 further adjusted for physical activity and waist circumference.
Table 4. Associations of quartiles of cotinine with HOMA-B further adjusted for HOMA-IR by race/ethnicity.
| Cotinine Quartiles | N of LHBb (%) | Odds Ratio(95%CI) | P Value |
|---|---|---|---|
| White | |||
| 1st -2nd | 391(30.2) | 1.00 | |
| 3rd | 156(25.5) | 1.52(1.08–2.14) | 0.02 |
| 4th | 248(32.0) | 1.73(1.30–2.32) | 0.0002 |
| P trend | 0.0001 | ||
| Black | |||
| 1st -2nd | 55(15.3) | 1.00 | |
| 3rd | 61(18.8) | 1.57(0.80–3.08) | 0.19 |
| 4th | 100(30.7) | 2.66(1.54–4.59) | 0.0004 |
| P trend | 0.0004 | ||
| Mexican | |||
| 1st -2nd | 87(15.5) | 1.00 | |
| 3rd | 41(18.1) | 1.09(0.72–1.65) | 0.42 |
| 4th | 31(25.2) | 2.56(1.03–6.37) | 0.04 |
| P trend | 0.05 | ||
| Hispanic | |||
| 1st -2nd | 78(20.9) | 1.00 | |
| 3rd | 23(15.5) | 0.64(0.35–1.16) | 0.14 |
| 4th | 27(22.5) | 1.22(0.64–2.31) | 0.55 |
| P trend | 0.76 |
b, low HOMA-B was defined as HOMA-B in 1st quartile.
Adjusted for age, gender, ethnicity/race, alcohol consumption, education level, physical activity, waist circumference and HOMA-IR quartiles.
In sensitive analysis, neither adjustment for antihypertensive use nor exclusion of these participants from the analysis altered our results (data not shown).
Discussion
Findings
To our knowledge, no prior study has assessed cotinine-insulin relationships in a large and representative non-diabetic sample. The current study used recent data, representative of the non-diabetic U.S. population, to show that high cotinine concentration is associated with compromised insulin secretion among non-Hispanic White and non-Hispanic Black but not among Mexican American and other Hispanics. There is no association between cotinine concentration and insulin resistance in this population. These findings indicate that compromised insulin secretion but not insulin resistance is operative in the nicotine-diabetes relationship.
How this study fits into the current literature
Insulin, released by pancreatic β cells, is the most important hormone to modulate glucose metabolism. Defects in insulin secretion and/or insulin resistance are generally considered as the two main pathophysiologic mechanism underlying the development of diabetes[24]. Accumulating lines of epidemic evidence [3, 25] have suggested the association of chronic smoking with diabetes. Yet some clinical studies to explore smoking-insulin relations resulted in ambiguous findings. A case-control study in Sweden found that long-term nicotine-containing gum chewing was associated with insulin resistance[26]. Also, a research of 136 healthy Chinese males[27] observed that smoking was correlated with insulin resistance in term of HOMA-IR. In contrast, Mora-Martinez JM et al [6] failed to detect any influence of transdermal administration on insulin sensitivity in healthy individuals. Although Daniel et al[5] demonstrated the smoking-high β cell function linkage in Canadian, findings from a Sweden study[7] argued that the smoking was associated with lower HOMA-B value. Recently, Clair et al[10] found that cotinine, the biomarker of smoking exposure, was associated with increased chronic glycemia in U.S. population without diabetes. But this study failed to investigate cotinine-insulin relationship underlying the cotininine-glycemia linkage. Our analysis furthered Clair et al’s study to examine cotinine-insulin association by using NHANES data 2007–2012 in non-diabetic American. Not only did we discover that cotinine was associated with impaired insulin secretion in terms of HOMA-B, but also we detected race/ethnicity difference in such associations, which has never been reported in previous studies with small sample size.
The results of the present study are plausible. Quite a few research have discovered neuronal nicotinic acetylcholine receptors (nAChRs) expressed on many different non-neuronal cell types including pancreatic islet cells [28–31]. Basal insulin secretion can be modulated by an endogenous pancreatic ganglionic mechanism. There is evidence that nAChRs are present at the ganglionic level in the pancreas and modulate insulin secretion through a complicated intraganglionic mechanism[32]. Direct evidence of the presence of nicotinic receptors on islet β cells has also been shown, based on mRNA expression [31]. Both long term and acute exposure to nicotine led to a reduction in insulin secretion in response to insulin-secreting agonists, including tolbutamide [31]. Acute exposure to nicotine has been shown to inhibit insulin release at both fasting and high glucose levels [31]. Since functional nicotinic receptors are present in pancreatic islets and β cells and nicotine could partially influence pancreatic β cell function, they may represent a potential switch from which to modulate the physiological function of pancreatic cells in tobacco toxicity, i.e., in smokers/those with high cotinine levels. Other studies [33, 34] have also revealed that nicotine exposure can cause β-cell dysfunction, increased β-cell apoptosis, and loss of β-cell mass, mediated via the mitochondrial and /or death receptor pathway; this suggests other possible mechanisms.
The possible reasons behind the racial heterogeneity include inadequate modeling in one or more racial groups, confounding that differs by race, and biological differences. However, results were robust evaluating race-specific quartiles (S1 Table) and we are unable to identify characteristics that could convincingly lead to differential confounding by race. Therefore, our data indicate that biological differences explain much of the observed heterogeneity. Prior studies[11] have described the racial/ethnic differences in the rate of metabolism of nicotine and cotinine as we observed. At the same daily level of cigarette smoking, higher serum cotinine concentrations are detected in blacks than in whites [35, 36]. And race/ethnicity-specific cut point of cotinine [37] for active smoking also supported race/ethnicity disparity in metabolism of nicotine and cotinine. Such difference may provide clue to the reason for the cotinine-insulin secretion associations only observed in whites and blacks.
Strengths of our study
First, it is the first time to use cotinine as the surrogate to smoking exposure assessing smoking- insulin linkage. This method avoided potential bias if smokers do not accurately report their smoking status. Second, the large multi-ethnic population sample allowed us to explore the racial/ethnic heterogeneity in the association of cotinine-insulin if exists, which has never been reported in previous studies. Finally, this analysis was conducted in a nationally representative sample; therefore, our results may be generalized to the entire non-diabetic U.S. adult population.
Some limitations must be considered when interpreting our results. Insulin function includes both stable and dynamic stages. In this study we only focused on the stable stage—fasting state, but did not assess the dynamic stages because loaded plasma insulin levels from OGTT were not available in the NHANES data. Moreover, the sample sizes for Mexican and other Hispanics were relatively small and ranges of cotinine concentration were much narrower than other racial/ethnic groups. Further studies on cotinine-insulin with larger sample size are warranted in Mexican and other Hispanic population. Finally, given that this was a cross-sectional analysis, our study cannot make any inferences regarding causality.
In summary, our study indicates that high cotinine concentration/ smoking is associated with compromised stable insulin secretion in a white and black non-diabetic U.S. population. Further longitudinal and experimental research is needed to examine causal relationships from nicotine exposure to β-cell dysfunction and race/ethnicity heterogeneity should be considered when explain the study result.
Supporting Information
(DOCX)
(DOC)
(DOCX)
Acknowledgments
We thank Tami R. Bartell in the Mary Ann and J. Milburn Smith Child Health Research Program, Children's Hospital of Chicago Research Center, for English editing.
Abbreviations
- HbA1c
Glycated hemoglobin A1c
- NHANES
National Health and Nutrition Examination Survey
- BMI
Body mass index
- WC
Waist circumference
- HOMA
Homeostatic model assessment
- FPG
Fasting plasma glucose
- FPI
Fasting plasma insulin
- nAChRs
neuronal nicotinic acetylcholine receptors
Data Availability
Data are available from the NHANES website, http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm.
Funding Statement
This work was supported in part by 100 Talents Program of the Chinese Academy of Sciences (to X Liu). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Cowie CC, Rust KF, Byrd-Holt DD, Gregg EW, Ford ES, Geiss LS, et al. (2010) Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988–2006. Diabetes Care 33: 562–568. 10.2337/dc09-1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The World Health Report. Reducing Risks, Promoting Health Life. Available: http://www.who.int/whr/2002/en/whr02_en.pdf?ua=1. accessed Feb,25th 2014
- 3.Willi C, Bodenmann P, Ghali WA, Faris PD,Cornuz J (2007) Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 298: 2654–2664. 10.1001/jama.298.22.2654 [DOI] [PubMed] [Google Scholar]
- 4.Ronnemaa T (1996) Smoking Is Independently Associated With High Plasma Insulin Levels in Nondiabetic Men. Diabetes Care 19: 1229–1232. [DOI] [PubMed] [Google Scholar]
- 5.Daniel M,Cargo MD (2004) Association between smoking, insulin resistance and beta-cell function in a North-western First Nation. Diabet Med 21: 188–193. [DOI] [PubMed] [Google Scholar]
- 6.Mora-Martinez JM, Gonzalez-Ortiz M, Balcazar-Munoz BR,Martinez-Abundis E (2004) Acute effect of the transdermal administration of nicotine on insulin sensitivity in healthy individuals with and without a family history of type 2 diabetes mellitus in the first branch. Metab Syndr Relat Disord 2: 227–233. 10.1089/met.2004.2.227 [DOI] [PubMed] [Google Scholar]
- 7.Ostgren CJ, Lindblad U, Ranstam J, Melander A,Rastam L (2000) Associations between smoking and beta-cell function in a non-hypertensive and non-diabetic population. Skaraborg Hypertension and Diabetes Project. Diabet Med 17: 445–450. [DOI] [PubMed] [Google Scholar]
- 8.Center for Disease Control and Prevention. National Health and Nutrition Examination Survey,2005–2006 Data Documentation, Codebook, and Frequencies,Serum Cotinine (COT_D). Available: http://www.cdc.gov/nchs/nhanes/nhanes2005-2006/COT_D.htm#Component_Description. accessed Feb. 25th 2013
- 9.Jarvis MJ, Russell MA, Benowitz NL,Feyerabend C (1988) Elimination of cotinine from body fluids: implications for noninvasive measurement of tobacco smoke exposure. Am J Public Health 78: 696–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clair C, Bitton A, Meigs JB,Rigotti NA (2011) Relationships of cotinine and self-reported cigarette smoking with hemoglobin A1c in the U.S.: results from the National Health and Nutrition Examination Survey, 1999–2008. Diabetes Care 34: 2250–2255. 10.2337/dc11-0710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez-Stable EJ, Herrera B, Jacob P 3rd,Benowitz NL (1998) Nicotine metabolism and intake in black and white smokers. JAMA 280: 152–156. [DOI] [PubMed] [Google Scholar]
- 12.NHANES Analysis Course. Available: http://www.cdc.gov/nchs/tutorials/NHANES/NHANESAnalyses/NHANES_Analyses_intro.htm accessed Apr. 25th 2013
- 13.Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS). National Center for Health Statistics (NCHS) National Center for Health Statistics Ethics Review Board. Available: http://www.cdc.gov/nchs/nhanes/irba98.htm. accessed Oct. 10th 2015
- 14.Bernert JT Jr., Turner WE, Pirkle JL, Sosnoff CS, Akins JR, Waldrep MK, et al. (1997) Development and validation of sensitive method for determination of serum cotinine in smokers and nonsmokers by liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Clin Chem 43: 2281–2291. [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data[1999–2012]. Available: http://www.cdc.gov/nchs/data/nhanes/survey_content_99_12.pdf. accessed Sep. 25th 2014
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF,Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 17.Wallace TM, Levy JC,Matthews DR (2004) Use and abuse of HOMA modeling. Diabetes Care 27: 1487–1495. [DOI] [PubMed] [Google Scholar]
- 18.Wongwananuruk T, Rattanachaiyanont M, Leerasiri P, Indhavivadhana S, Techatraisak K, Angsuwathana S, et al. (2012) The Usefulness of Homeostatic Measurement Assessment-Insulin Resistance (HOMA-IR) for Detection of Glucose Intolerance in Thai Women of Reproductive Age with Polycystic Ovary Syndrome. Int J Endocrinol 2012: 571035 10.1155/2012/571035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madeira IR, Carvalho CN, Gazolla FM, de Matos HJ, Borges MA,Bordallo MA (2008) [Cut-off point for Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) index established from Receiver Operating Characteristic (ROC) curve in the detection of metabolic syndrome in overweight pre-pubertal children]. Arq Bras Endocrinol Metabol 52: 1466–1473. [DOI] [PubMed] [Google Scholar]
- 20.Yamada C, Mitsuhashi T, Hiratsuka N, Inabe F, Araida N,Takahashi E (2011) Optimal reference interval for homeostasis model assessment of insulin resistance in a Japanese population. J Diabetes Investig 2: 373–376. 10.1111/j.2040-1124.2011.00113.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esteghamati A, Ashraf H, Khalilzadeh O, Zandieh A, Nakhjavani M, Rashidi A, et al. (2010) Optimal cut-off of homeostasis model assessment of insulin resistance (HOMA-IR) for the diagnosis of metabolic syndrome: third national surveillance of risk factors of non-communicable diseases in Iran (SuRFNCD-2007). Nutr Metab (Lond) 7: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lithell HO (1991) Effect of antihypertensive drugs on insulin, glucose, and lipid metabolism. Diabetes Care 14: 203–209. [DOI] [PubMed] [Google Scholar]
- 23.(2013). RCT R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
- 24.Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, et al. (2007) Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care 30: 753–759. 10.2337/dc07-9920 [DOI] [PubMed] [Google Scholar]
- 25.Carlsson S, Midthjell K,Grill V (2004) Smoking is associated with an increased risk of type 2 diabetes but a decreased risk of autoimmune diabetes in adults: an 11-year follow-up of incidence of diabetes in the Nord-Trondelag study. Diabetologia 47: 1953–1956. 10.1007/s00125-004-1554-9 [DOI] [PubMed] [Google Scholar]
- 26.Eliasson B, Taskinen MR,Smith U (1996) Long-term use of nicotine gum is associated with hyperinsulinemia and insulin resistance. Circulation 94: 878–881. [DOI] [PubMed] [Google Scholar]
- 27.Gao S, Wang YH,Li M (2012) Cigarette smoking increases levels of retinol-binding protein-4 in healthy men with normal glucose tolerance. Chin Med J (Engl) 125: 1686–1689. [PubMed] [Google Scholar]
- 28.Conti-Fine BM, Navaneetham D, Lei S,Maus AD (2000) Neuronal nicotinic receptors in non-neuronal cells: new mediators of tobacco toxicity? Eur J Pharmacol 393: 279–294. [DOI] [PubMed] [Google Scholar]
- 29.Ejiri K, Taniguchi H,Baba S (1989) Participation of nicotinic receptor in hormone release from isolated rat islets of Langerhans. Diabetes Res Clin Pract 6: 53–59. [DOI] [PubMed] [Google Scholar]
- 30.Nelson L, Gardner ME,Young MJ (1982) Regulation of calcium distribution in bovine sperm cells: cytochemical evidence for motility control mechanisms. Cell Motil 2: 225–242. [DOI] [PubMed] [Google Scholar]
- 31.Yoshikawa H, Hellstrom-Lindahl E,Grill V (2005) Evidence for functional nicotinic receptors on pancreatic beta cells. Metabolism 54: 247–254. 10.1016/j.metabol.2004.08.020 [DOI] [PubMed] [Google Scholar]
- 32.Stagner JI,Samols E (1986) Modulation of insulin secretion by pancreatic ganglionic nicotinic receptors. Diabetes 35: 849–854. [DOI] [PubMed] [Google Scholar]
- 33.Bruin JE, Gerstein HC, Morrison KM,Holloway AC (2008) Increased pancreatic beta-cell apoptosis following fetal and neonatal exposure to nicotine is mediated via the mitochondria. Toxicol Sci 103: 362–370. 10.1093/toxsci/kfn012 [DOI] [PubMed] [Google Scholar]
- 34.Bruin JE, Petre MA, Raha S, Morrison KM, Gerstein HC,Holloway AC (2008) Fetal and neonatal nicotine exposure in Wistar rats causes progressive pancreatic mitochondrial damage and beta cell dysfunction. PLoS One 3: e3371 10.1371/journal.pone.0003371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caraballo RS, Giovino GA, Pechacek TF, Mowery PD, Richter PA, Strauss WJ, et al. (1998) Racial and ethnic differences in serum cotinine levels of cigarette smokers: Third National Health and Nutrition Examination Survey, 1988–1991. JAMA 280: 135–139. [DOI] [PubMed] [Google Scholar]
- 36.Wagenknecht LE, Cutter GR, Haley NJ, Sidney S, Manolio TA, Hughes GH, et al. (1990) Racial differences in serum cotinine levels among smokers in the Coronary Artery Risk Development in (Young) Adults study. Am J Public Health 80: 1053–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benowitz NL, Bernert JT, Caraballo RS, Holiday DB,Wang J (2009) Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol 169: 236–248. 10.1093/aje/kwn301 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOC)
(DOCX)
Data Availability Statement
Data are available from the NHANES website, http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm.