Abstract
Objective
In this study, we use a novel automated method for localization and quantitative comparison of magnetoencephalographic (MEG) delta activity in patients with and without recurrent seizures after epilepsy surgery as well as healthy controls.
Methods
We identified the generators of delta activity by source location in frequency domain between 1 and 4 Hz in spontaneous MEG data. Comparison with healthy control subjects by z-transform emphasized relative changes of activation in patients. The individual results were compared to spike localizations and statistical group analysis was performed. Additionally, MEG results were compared to 1–4 Hz activity in invasive EEG (iEEG) in two patients, in whom this data was available.
Results
Patients with recurrent seizures exhibited significantly increased focal MEG delta activity both in comparison to healthy controls and seizure free patients. This slow activity showed a correlation to interictal epileptic activity and was not explained by consequences of the resection alone. In two patients with iEEG, iEEG analysis was concordant with the MEG findings.
Significance
The quantity of delta activity could be used as a diagnostic marker for recurrent seizures. The close relation to epileptic spike localizations and the resection volume of patients with successful second surgery imply involvement in seizure recurrence. This initial evidence suggests a potential application in the planning of second epilepsy surgery.
Keywords: Epilepsy, Delta activity, Surgery, Persisting seizures
Highlights
-
•
We introduce a novel automated method for localization and quantitative comparison of delta activity in epilepsy patients.
-
•
Comparison with healthy controls shows significantly increased slow waves in patients with and without recurring seizures.
-
•
Significant correlation of delta and interictal epileptic activity could not be explained by the resection alone.
-
•
Relation to spike localizations and resection volume imply involvement in seizure recurrence.
-
•
The quantity of delta activity could be used as a diagnostic marker for recurrent seizures.
1. Introduction
In pharmacoresistant cases of focal epilepsy, surgical therapy can lead to substantial improvement of seizure frequency. Up to 85% of patients are completely seizure free after resection (Rosenow and Lüders, 2001). However, this percentage declines to about 60% or less during the three to five years following the procedure (Englot et al., 2012, Noe et al., 2013, Rosenow and Lüders, 2001). Potential reasons for recurrent seizures can be roughly classified into two main putative mechanisms: Incomplete resection and progressive epileptogenesis. The former addresses the notion that incomplete resection of the epileptogenic zone will lead to recurrent seizures (Harroud et al., 2012, Jeha et al., 2007), potentially after a phase of seizure freedom right after epilepsy surgery. The resected tissue may also have inhibited other compartments of an epileptic network, which only become apparent after such “surgical disinhibition”.
Progressive epileptogenesis after epilepsy surgery covers the concept that either the epileptic processes themselves or an underlying pathology causing the epilepsy is not static but develops over time. In this model, the pathology may be more distributed also in focal epilepsies with longer epilepsy durations. After surgery, remaining portions of such pathology would then be capable to regenerate over time and cause recurrent seizures. This is reflected by evidence favoring early epilepsy surgery, e.g. in children (Simasathien et al., 2013).
Almost irrespective of the mechanism, second surgery may be a successful therapy option in cases with recurrent seizures (Mohamed et al., 2007, Siegel et al., 2004). However, focus localizations is more difficult. The resection volume causes breach rhythms and topographical distortions in the surface EEG and renders placement at least of grids and strips for invasive EEG challenging (Mohamed et al., 2007).
Methods for localization of remaining or new epileptic foci in this context could potentially enable second surgery and support planning of the resection. Due to the relative insensitivity of magnetoencephalogragphy (MEG) to conductivity differences (Vorwerk et al., 2014) and thus also alterations due to the resection, MEG based source analysis has been suggested to provide such an approach (Kirchberger et al., 1998, Mohamed et al., 2007). Beyond focus localization, predictors of a potential recurrence of seizures after surgery would have considerable clinical value, e.g. to counsel patients in regard to AED withdrawal, eligibility for driving and general safety considerations.
Interictal spiking in postoperative EEG recordings have been suggested as such a potential predictor of relapses (Di Gennaro et al., 2004, Hildebrandt et al., 2005, Mintzer et al., 2005, Patrick et al., 1995). Occurrence of epileptic spikes six months after epilepsy surgery was shown to correlate well with persisting seizures (Patrick et al., 1995). Vice versa, lack of detection at six months predicted long-term seizure freedom after five years and longer (Hildebrandt et al., 2005). In contrast, preoperative spike rates (Di Gennaro et al., 2004) did not show such correlations. Evidence in regard to earlier EEG recordings is conflicting (Di Gennaro et al., 2004, Mintzer et al., 2005).
Focal delta has been described to occur in concordance with an epileptic focus and have been used for focus localization (Baayen et al., 2003, Geyer et al., 1999, Huppertz et al., 2001, Kaltenhäuser et al., 2007, Patrick et al., 1995). In fact, focal or regional slowing is found in the majority of patients with temporal lobe epilepsies (Gambardella et al., 1995, Geyer et al., 1999) and in about half of patients with extra-temporal lobe epilepsy (Geyer et al., 1999). Occipital and temporal intermittent rhythmic delta activity (OIRDA/TIRDA) has long been recognized as a specific indicator for epilepsy and in case of TIRDA for focus lateralization (Brigo, 2011).
Results about postoperative delta activity are less clear. The structural alterations caused by surgical procedures are thought to generate considerable slowing in postoperative EEG (Amzica and Steriade, 1998). It is therefore difficult to distinguish between this “normal” and any pathophysiologic delta, which may be related to epileptic processes. The few studies on the relation of postoperative delta and the recurrence of seizures relied on visual inspection and evaluated whether and where delta occurred (Patrick et al., 1995). A detailed quantitative analysis has not been performed, at least partially because of the lack of an adequate technique.
Such techniques have been developed to investigate slow waves for preoperative epileptic focus localization before first surgery. Huppertz et al. (2001) and Vanrumste et al. (2005) utilized EEG source analysis and found concordant localizations of visually identified delta, spikes and epileptogenic lesions on MRI. Ishibashi identified slow wave visually in MEG data (Ishibashi et al., 2002). Source analysis yielded localizations on the side of later successful surgery. Notably, this was found in patients with and without mass lesions. The authors suggested that slow wave activity may thus be associated with epileptic processes and is not entirely generated by structural defects. In Kaltenhäuser et al. (2007), we confirmed these findings and additionally reported an overall higher amount of slow wave activity in epilepsy patients compared to healthy controls.
In the presented study, we present a novel automated method to localize slow wave activity in the delta band and enable a quantitative comparison. We apply this method to patients with recurrent seizures after previous epilepsy surgery. We show that these patients present with a significantly higher amount of delta activity in comparison to patients who are seizure free after surgery as well as healthy controls. A close relation to epileptic spike localizations and the resection volume of patients with successful second surgery imply involvement in seizure recurrence.
2. Material and methods
2.1. Subjects
The study included three groups of subjects, all of them gave informed written consent to participation, in patient P3 (15 years at the time of the MEG recording), the parents additionally gave their written informed consent.
Group P involved 15 patients with pharmacoresistant focal epilepsy after brain surgery (8 male, age 15–54 years, mean 33 years, standard deviation 11 years) and were selected retrospectively. Inclusion criteria were: previous surgery with persisting or recurrent seizures, successful MEG recording. In two of the patients of group P (patient 13 and 15), invasive EEG was recorded as presurgical evaluation for potential second surgery.
Group SF consisted of 10 post-operative seizure-free epilepsy patients (5 male, age 22–57 years, mean 39 years, standard deviation 12 years), which were prospectively recorded for the presented study. Inclusion criteria were previous surgery with seizure freedom for at least 6 months, pharmacoresistant focal epilepsy before surgery, adult age and MEG compatibility. This recruitment strategy resulted in shorter follow-up periods, i.e. time between surgery and MEG recording in group SF (Table 1), since patients are more likely to return for postoperative investigations when they suffer from persisting or recurrent seizures. It has to be expected that some of the seizure free patients will experience recurrent seizures in the future, based on studies of long-term seizure outcomes after epilepsy surgery (Englot et al., 2012, Noe et al., 2013, Rosenow and Lüders, 2001). Such cases may thus already have shown increased delta during our study. This would have diminished the difference between the groups, representing a bias against our findings. Such a bias would lead to less clear differences between the groups. The likeliness that it artificially introduces a difference where there is none or only a weaker one seems less probable. We therefore decided to proceed with this patient selection as our results would then likely under- not overestimate the real difference.
Table 1.
Overview of patients, epilepsy history, and surgery for patients with recurrent seizures (group P) and seizure free patients after epilepsy surgery (group SF). “Age”, “Epilepsy duration” and “years post-OP” are given at the time of the MEG recording for the presented study. In some cases, there was more than one surgical procedure. For each procedure, the time since surgery is listed. MRI findings after first surgery are only mentioned if more than the resection volume of previous surgery was reported. Some of the patients were not operated at our institution; not all information is available in these.
| Patient | Age | Sex | Epilepsy duration (years) | Evidence before first surgery | Location of resection | Histology | Years post-OP | EEG/MRI (after first surgery) |
|---|---|---|---|---|---|---|---|---|
| P1 | 21 | m | 15 | FCD r parietal/occipital | r parietal/occipital | Unclear | 6 | EEG: r frontal, parietal and occipital spikes, l frontal spikes, r parieto-occipital seizures |
| P2 | 15 | f | 5 | FCD | l frontal | FCD 1a | 4.5 | EEG: l frontal spikes, l central seizures, l frontal, central, temporal slowing |
| P3 | 41 | m | 27 | Subependymoma | l temporal | Subependymoma | 5 | EEG: bifrontal spikes and seizures |
| P4 | 31 | m | 25 | FCD | r frontal | Normal MCD |
3.3 2 |
EEG: r fronto-temp. delta-theta; bifrontal spikes |
| P5 | 25 | m | 9 | FCD | l frontal | Normal | 1 | EEG: l fronto-central spikes |
| P6 | 23 | m | 7 | Meningioma | r fronto-central | Meningioma | 8 2 |
EEG: r parietal slowing |
| P7 | 54 | f | 37 | MRI negative | l temporal | Astrogliosis | 10 | EEG: l temp slowing and seizures, bitemporal/parietal spikes MRI: defect l temp, pathological r hippocampus |
| P8 | 32 | m | 13 | Astrocytoma | l temporal | Astrocytoma | 19 5 |
EEG: l temp/par/occ slowing MRI: residual tumor |
| P9 | 39 | f | 27 | FCD | r parietal/occipital | FCD | 6 | EEG: r parieto-occipital seizures MRI: residual FCD |
| P10 | 36 | m | 21 | TLE | r temporal | Hippocampal sclerosis | 9 | EEG: bitemporal seizures and slowing MRI: defect r temp, residual hippocampus |
| P11 | 50 | f | 34 | MRI negative | r temporal | mMCD | 2.5 | EEG: r temp seizures and slowing MRI: defect r temp, progressive gliosis |
| P12 | 23 | f | 18 | MRI negative | r temporal | FCD | 13 | EEG: bitemporal slowing and sharp waves MRI: defect r temp, r hippocampal atrophy |
| P13 | 31 | m | 20 | Cystic lesion | r occipital | Unclear | 19 | EEG: r temp spikes and seizures MRI: cystic lesion r occipital |
| P14 | 44 | f | 30 | TLE | l temporal | Hippocampal sclerosis | 6 | EEG: l temp/par spikes and seizures |
| P15 | 32 | f | 19 | MRI negative | r temporal | Unclear | 13 4 |
EEG: r temp slowing, spikes, seizures |
| SF1 | 31 | f | 2 | Cavernoma | l temporo-mesial | Cavernoma | 1 | EEG: breach rhythm, no spike |
| SF2 | 36 | m | 33 | Hippocampal sclerosis | r temporal | Hippocampal sclerosis | 2 | EEG: breach rhythm, slowing, no spike |
| SF3 | 50 | f | 33 | Hippocampal sclerosis | r temporal | Hippocampal sclerosis | 1 | EEG: breach rhythm, no spike |
| SF4 | 26 | m | 7 | Ganglioglioma/DNET | l temporal | Ganglioglioma | 2.3 | EEG: breach rhythm, slowing, no spike |
| SF5 | 22 | f | 1 | Ganglioglioma | l temporal | Ganglioglioma | 0.5 | EEG: without pathological findings |
| SF6 | 57 | f | 48 | Hippocampal sclerosis | r temporal | Hippocampal sclerosis | 1 | EEG: breach rhythm, slowing, no spike |
| SF7 | 29 | m | 13 | MRI negative | l temporal | Normal | 0.5 | EEG: breach rhythm, slowing, no spike |
| SF8 | 43 | m | 4 | Cavernoma | r temporal | Cavernoma | 0.5 | EEG: without pathological findings |
| SF9 | 54 | m | 6 | Hippocampal sclerosis | l temporal | Hippocampal sclerosis | 0.5 | EEG: breach rhythm, slowing, no spike |
| SF10 | 40 | f | 36 | Hippocampal sclerosis | r temporal | Hippocampal sclerosis | 2 | EEG: breach rhythm, slowing, spike |
m – male, f – female, l – left, r – right, FCD - focal cortical dysplasia, mMCD - mild malformation of cortical development, TLE - temporal lobe epilepsy.
Finally, group C consisted of 15 healthy controls (7 male, age 25–49 years, mean 33 years, standard deviation 7 years), which were prospectively selected to approximately match the age distribution of groups P and SF.
Table 1 presents a summary of epilepsy history and surgery for each patient of groups P and SF, respectively. The presented research was approved by the ethics committee of the University Hospital Erlangen.
2.2. Findings of presurgical evaluation
Throughout the study, the findings of presurgical evaluation are utilized to investigate the relation between delta activity and the epileptic network. In group P, long-term Video-EEG, MRI, PET and SPECT contributed to presurgical focus localization. The consensus decisions of epilepsy surgery conferences taking all such findings into account, resulted in a hypothetical focus localization, which is addressed as the “clinical focus” in the presented study. Due to the complexity of the investigated cases, second surgery and/or invasive EEG recordings are available only in a small subset of patients. The “clinical focus” thus served as a basis for validation of the delta activity localizations.
2.3. MEG recording
A WHS 3600 MEG device (4D-Neuroimaging, San Diego, USA) with 248 magnetometers was used to record 10 min of continuous MEG data of each subject at rest. Data were recorded with a sampling rate of 2034 Hz and an online high-pass filter of 0.1 Hz. The high sampling rate was used to also allow for high frequency analysis, which is not part of this study.
2.4. MRI processing and source space setup
As part of clinical routine, each patient underwent a magnetic resonance imaging (MRI) examination. The individual subject's high resolution T1-weighted MRI was used for segmentation of the brain compartment to set up a homogeneous single shell volume conduction model with realistic head shape (Nolte, 2003). A three-dimensional source space was built from the triangulation vertices of the inner skull surface. This surface was scaled to form eight concentric shells with decreasing radius, where the number of vertices declined quadratically according to the radius. Consequently, 3800 source positions were distributed over the source space with rather regular resolution. Two tangential dipoles were placed at each position to compute a lead field for source analysis. For control subjects, we had only four individual MRIs available, for the remaining we used the high resolution MRI of the MNI standard brain.
2.5. MEG data processing
Slow wave analysis was done using FieldTrip (Oostenveld et al., 2011) and started with segmentation of the raw data into consecutive non-overlapping trials of 5 s length. We consider this being an adequate value for detecting activity between 1 and 4 Hz, since the frequency resolution of 0.2 Hz provides appropriate sampling in this frequency range. We applied downsampling to 500 Hz for data reduction and more efficient processing. Data segments containing movement artifacts or external interference were rejected and bad channels were corrected by interpolation. We averaged the spectra of all clean trials of the 10 min dataset and performed source localization in frequency domain for each frequency bin using Dynamic Imaging of Coherent Sources (DICS (Gross et al., 2001)). By averaging the DICS results over 1–4 Hz, the generators of delta activity were identified.
In the next step, we morphed the source localization results from the original source space to a standardized target source space utilizing the MNI brain and an algorithm described in MNE software User's Guide (Hämäläinen, 2009). Data were morphed between corresponding shells by linear interpolation of target positions in terms of source triangulation vertex values. Morphing was omitted in control subjects without individual MRI, because the source localization results already existed in the normalized MNI source space.
Once the data of all subjects were expressed in the same source space, the mean and standard deviation of delta activity in healthy controls were computed. Patient data were z-transformed at each vertex by subtraction of control-mean and division by control-standard deviation, which emphasizes relative changes of activation in patients compared to healthy controls. The resulting z-values were then morphed back to their original source space. They were utilized as quantitative measures of delta activity and visualized by overlay on MR images.
This procedure allows relating the amount of delta activity at specific locations to the levels expected in healthy controls at comparable locations. The resection volume introduces some distortion due to brain shift, which is not completely corrected by the method in all cases. However, in healthy controls neighboring source locations generally showed similar amounts of delta activity. Coregistration inaccuracies thus do no result in largely different levels of delta activity for the further analysis.
2.6. MEG spike analysis
In group P, the location of the maximum z-value was related to the location of interictal epileptic spikes. Spike analysis was done by an experienced physician using Curry 7 (Compumedics NeuroScan, Singen, Germany). A digital band-pass filter of 3–70 Hz was applied and epileptic spikes were detected and localized with dipole fit and sLORETA (Pascual-Marqui, 2002). The z-value at the spike location as well as the distance between spike location and the position of the voxel with the maximum delta activity (z-value) were computed for all patients of group P (see Table 2). Since most of the patients in group SF did not have any interictal activity (see Table 1), no spike analysis was performed in this group.
Table 2.
Delta activity in patients with recurrent seizures (group P). Location of the epileptic focus for determination of overlap with delta activity is based on the consensus decision of epilepsy surgery case conferences, which may deviate from spike locations.
| Patient | Maximum z-value | z-Value at spike location | Distance [cm] | Spike frequency (/10 min) | Mono-/multifocal delta distribution | Overlap of delta with focus |
|---|---|---|---|---|---|---|
| P1 | 39.6 | 18.3 | 3.8 | 3 | Mono | Yes |
| P2 | 211.3 | 35.4 | 2.0 | 18 | Mono | Yes |
| P3 | 9.8 | No spikes | – | 0 | Multi | Yes |
| P4 | 33.5 | 6.8 | 1.6 | 8 | Mono | Yes |
| P5 | 44.8 | 29.3 | 1.3 | 12 | Mono | Yes |
| P6 | 37.6 | 14.5 | 9.4 | 2 | Multi | Yes |
| P7 | 7.6 | 0.3 | 8.2⁎ | 4 | Mono | Yes |
| P8 | 157.1 | 64.0 | 4.3 | 7 | Mono | Yes |
| P9 | 5.6 | 4.2 | 0.8 | 20 | Mono | Yes |
| P10 | 8.4 | 3.8 | 1.0 | 2 | Mono | Yes |
| P11 | 29.0 | 9.1 | 2.6 | 1 | Multi | Yes |
| P12 | 146.4 | 50.7 | 1.5 | 20 | Mono | Yes |
| P13 | 34.7 | 26.8 | 0.9 | 76 | Mono | Yes |
| P14 | 56.8 | 1.8 | 3.9 | 2 | Mono | Yes |
| P15 | 177.7 | 41.7 | 2.1 | 2 | Mono | Yes |
| Min | 5.6 | 0.3 | 0.8 | 0 | ||
| Max | 211.3 | 64.0 | 9.4 | 76 | ||
| 1. Quartile | 14.6 | 4.2 | 1.3 | 2 | ||
| Median | 37.6 | 16.4 | 2.1 | 4 | ||
| 3. Quartile | 124.0 | 35.4 | 3.9 | 15 | ||
MEG recording could not replicate VEEG findings, potentially due to short recording duration.
2.7. Statistical analysis
The DICS results of delta activity of the different groups were compared using unpaired t-Tests with prior Lilliefors tests to ensure normality of samples.
Distribution of delta activity in the patient groups was visually classified into two categories: monofocal and multifocal/diffuse. The number of patients in each category was compared using Fisher's exact test.
The ability to diagnose seizure freedom, respectively recurring seizures after epilepsy surgery using the quantity of delta activity, specifically maximum source power in the delta band, was evaluated using receiver-operator-characteristic (ROC) analysis. The ability of delta activity to discern postoperative seizure-freedom from seizure recurrence was investigated by calculating the area under the curve (AUC). AUC values range from 0 to 1. Values of approximately 0.5 show a performance comparable to chance. Higher values demonstrate diagnostic utility regarding a certain outcome. By convention, AUCs < 0.7 are considered poor, ≥ 0.7 to < 0.8 fair, and ≥ 0.8 to < 0.9 good. Higher values demonstrate excellent diagnostic performance.
2.8. Invasive EEG (iEEG) recording
As part of the clinical workup for potential second surgery, two patients of group P underwent long-term invasive Video-EEG recordings with subdural grid and strip electrodes with platinum contacts (AD-Tech Medical Instrument Corporation, Racine, WI, USA). Acquisition was performed on the third day after implantation. Number and location of electrodes were determined based on previous findings of presurgical evaluation. All electrodes were recorded referenced to a surface electrode at the vertex. Patients were monitored using ECG, pulse oximetry and non-invasive blood pressure measurements. Data were recorded using an IT-Med EEG amplifier (Natus Europe GmbH, Planegg, Germany) using an analogue band pass filter from 0.08 to approximately 360 Hz. Data were digitally sampled at 1024 Hz.
2.9. Invasive EEG analysis
A total of one hour of iEEG data per patient was selected from long-term Video-iEEG recordings for several days. Data were taken from waking periods containing minimal artifacts. Spectral analysis was performed on each complete channel (i.e. one hour of data), using Welch's method (Welch, 1967) (Matlab R2010a, The Mathworks, Natick, MA, USA). The resulting power spectral density values in the delta band were plotted and overlaid onto 3D volume renderings of the patient's individual MRI with iEEG in place. Coordinates of iEEG electrodes were taken from post-implantation CT images. Both post-implantation MRI and CT images were registered as part of routine procedures. This method provides an estimation of the overall amount of delta activity in each channel, irrespective of any specific relation to simultaneously occurring spike patterns, e.g. the wave component of spike-waves.
3. Results
3.1. Quantitative comparison
Fig. 1 summarizes the results of delta activity for all groups in a boxplot. Both patient groups show significantly increased delta activity compared to healthy controls (C vs. SF: p = 0.04; C vs. P: p = 0.0005). Furthermore, patients with persisting or recurrent seizures had significantly higher levels of delta activity than patients who became seizure free after surgery (SF vs. P: p = 0.01). The spike frequency ranged from 0 to 76 per 10 min (median 4, Table 2) and did not show a significant correlation to the mean or maximum z-value of the MEG delta activity.
Fig. 1.
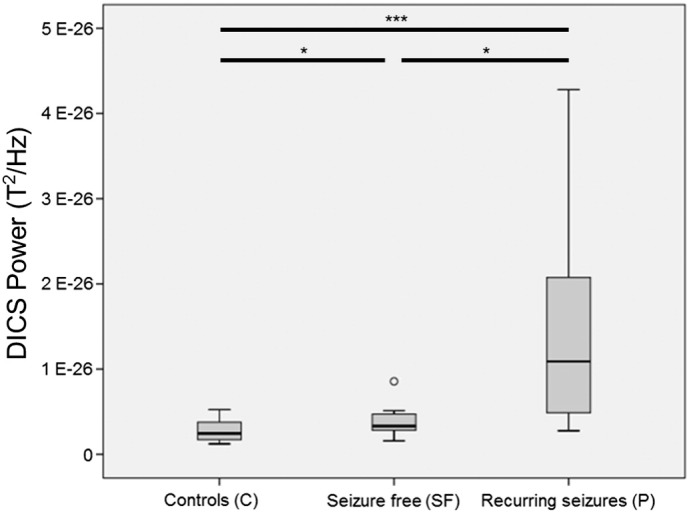
Boxplot with mean overall delta activity in target source space for healthy controls (group C), seizure-free patients (group SF), and patients with seizures (group P). * p < 0.05, *** p < 0.005.
ROC analysis of delta activity as a marker of recurring seizures revealed an area-under-the curve (AUC) value of 0.84. As three patients showed very high delta activity values in group P, a second ROC analysis was calculated excluding these cases. The resulting AUC was 0.80, suggesting an only minor impact of the three cases on the overall performance of delta activity to distinguish seizure-free patients from patients with recurrent seizures after surgery.
3.2. Spatial distribution in MEG
In 12/15 patients of group P, the spatial distribution of increased delta activity was monofocal and located at the borders of the resection volume respectively close to the findings of presurgical evaluation. Three patients had multifocal delta activity showing an overlap with these regions. Maximum z-values ranged from 5.6 to 211.3 (with 25%-quantile Q25 = 14.6, 50%-quantile Q50 = 37.6, 75%-quantile Q75 = 124.0), the distance between maximum z-value and spike location ranged from 0.81 cm to 9.38 cm (Q25 = 1.28 cm, Q50 = 2.09 cm, Q75 = 3.85 cm), where in 13 out of 15 patients the distance was markedly below 4 cm. In Patient P7 with multifocal epilepsy, the MEG-spikes found did not correlate with other diagnostic findings, probably due to insufficient recording time. Patient P6 showed multifocal delta activity, where the global maximum was different from EEG and imaging, but a local maximum was concordant (Table 2).
In group SF the maximum z-values ranged from 1.7 to 14.7 (Q25 = 4.5, Q50 = 7.6, Q75 = 9.3). The spatial distribution was monofocal at the borders of the resection volume in half of the patients the rest had multifocal delta activity showing an overlap with the resection volume (Table 3).
Table 3.
Delta activity in seizure free patients after epilepsy surgery (group SF). Location of the epileptic focus for determination of overlap with delta activity is based on the consensus decision of epilepsy surgery case conferences, which may deviate from spike locations.
| Patient | Maximum z-value | Mono-/multifocal delta distribution | Overlap of delta with clinical focus |
|---|---|---|---|
| SF1 | 4.5 | Multi | Yes |
| SF2 | 7.2 | Multi | Yes |
| SF3 | 3.0 | Mono | Yes |
| SF4 | 8.8 | Multi | Yes |
| SF5 | 12.9 | Multi | Yes |
| SF6 | 14.7 | Mono | Yes |
| SF7 | 9.3 | Mono | Yes |
| SF8 | 1.7 | Mono | Yes |
| SF9 | 4.7 | Mono | Yes |
| SF10 | 8.0 | Multi | Yes |
| Min | 1.7 | ||
| Max | 14.7 | ||
| 1. Quartile | 4.5 | ||
| Median | 7.6 | ||
| 3. Quartile | 9.3 | ||
Fisher's exact test for mono-/multifocal distribution of delta activity in groups P and SF reached the level of a tendency (p = 0.091).
3.3. Spatial distribution in iEEG and second surgery
Both patients with iEEG showed frequent spiking in the one hour used for data analysis. P13 presented with over 1000 spikes in different locations (Fig. 2) and P15 with over 200 spikes in more than half of the 58 electrodes.
Fig. 2.
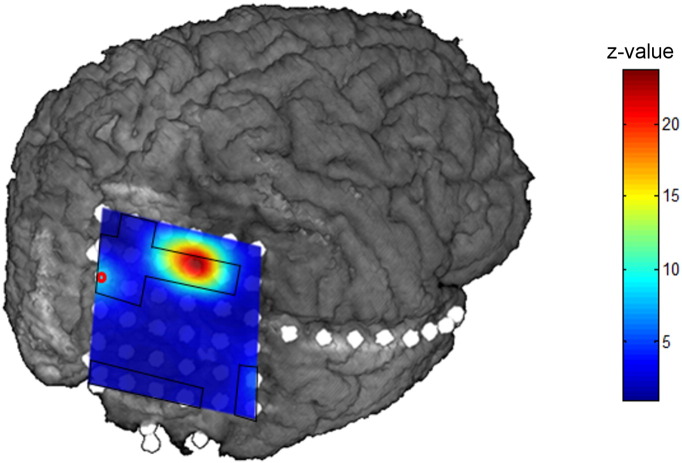
Results of iEEG delta activity of patient P13, visualized on the patient's MRI volume. The seizure onset zone is marked by a red circle, interictal areas are marked by a black line.
In these patients, the delta increase in iEEG was limited to a few electrodes, which also showed interictal activity. Conversely however, the majority of electrodes with interictal activity did not show increased delta. In P13, a delta increase was also visible in the seizure onset zone; albeit to a lower degree than in interictal areas (see Fig. 2). In P15, there was no overlap with the seizure onset electrodes.
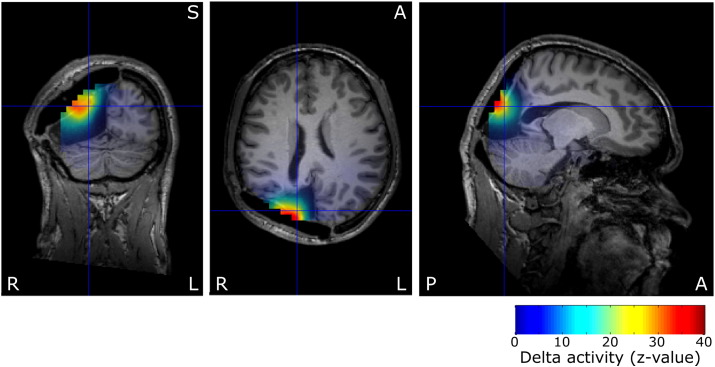
At the time of writing, patients P4, P13 and P15 underwent reoperation. Results of this study were not used for planning of surgery. P4 and P13 were seizure free (Engel 1A(Engel, 1993) at 6 months post-OP), whereas P15 still suffered from seizures (Engel 4B, 3 years post-OP). Fig. 3 shows the z-values of patient P13 overlaid on the post-surgical MRI. The focal increase of delta activation as well as the spikes are located clearly within the resection volume. In contrast, the delta maximum in P15, both in MEG and iEEG, has not been resected.
Fig. 3.
Preoperative delta activity z-values of patient P13 on post-surgical MRI after second surgery, centered on spike location. The reoperation resected the cyst and surrounding cortex, including the delta maximum and spike localizations. The delta activity findings were not used for planning of the surgical procedure.
4. Discussion
We investigated delta activity after epilepsy surgery and compared patients with recurrent seizures to seizure free patients and healthy controls. Using an automated method, we observed significant differences between the groups. The overall level of delta activity was significantly higher with recurrent seizures and did not depend on the resection alone. The contrast was clear enough to be potentially useful as a predictor of successful therapy. To our knowledge, this has never been published before. Furthermore, the localization of postoperative delta activity could potentially contribute to focus localization for second surgery.
4.1. Quantitative and qualitative differences
Our results show that patients with recurrent seizures after surgery have increased focal delta activity, both in comparison to healthy controls and patients who are seizure free after surgery. While the former would be expected corresponding to the frequent observation of focal slowing after intracranial surgery (Di Gennaro et al., 2004), the latter is not readily explained by this conventional interpretation and has only been reported sparsely (Patrick et al., 1995). It rather suggests that certain aspects of slowing are correlated with the recurrence of seizures. In fact, patients suffering from seizures before epilepsy surgery also presented with similar increased levels of slow activity. Previous studies have described such focal preoperative slow activity in patients with seizures, independent also of large lesions (Ishibashi et al., 2002, Kaltenhäuser et al., 2007, Vanrumste et al., 2005). Therefore, if epilepsy surgery is not successful and seizures are not stopped, it seems reasonable to assume, that this focal slow activity also persists.
The topography of delta activity further argues against a generation only due to the resection itself. Both in MEG and invasive EEG, the topography was highly focal, constrained to areas at the border of the resected volume. If the only origin would in fact be the resection, the resulting distribution would be expected to be more diffuse and extended over larger areas along the border. The area of increased slow activity was related to the areas exhibiting interictal spikes in both modalities. However, while there was an overlap, the zones of slowing and spiking were not identical. Other studies also observed such a focal distribution in unoperated patients, which yielded localizations concordant with those of interictal spikes (Kaltenhäuser et al., 2007, Vanrumste et al., 2005).
In contrast, Di Gennaro et al. (2004) did not find an association between postoperative slowing and seizure outcome, while the presence of interictal spikes was correlated with persisting seizures. The study visually analyzed 21 electrode scalp EEG recordings, while we used a high number of MEG sensors and source level analysis. Furthermore, while we only recorded resting state activity, Di Gennaro et al. applied photo stimulation and hyperventilation. Both the different methodology and recording paradigm may have obfuscated an association between slowing and seizure outcome.
Our findings however do not suggest that all slow activity after surgery originates from the epileptic network. This is demonstrated by the increased levels of slow activity even in seizure free patients after surgery. In these cases, the most likely explanation is indeed that the resection itself induces regional slowing to a certain degree.
Different forms of focal or regional slow activity are well known in clinical EEG. Intermittent regional delta activity (IRDA), i.e. frontal IRDA or occipital IRDA are sometimes observed in patients with epilepsy but do not show any clear relationship to occurrence of seizure or any specific focus localization(Brigo, 2011). In contrast, temporal intermittent regional delta activity (TIRDA) shows a high correlation with temporo-mesial epilepsy. It has been demonstrated that focal slowing in iEEG occurs in the irritative and seizure onset zone especially if the neocortex is involved (Brigo, 2011). While there are similarities, focal slow activity as observed in our study and reported Ishibashi et al. (2002); Kaltenhäuser et al. (2007); Vanrumste et al. (2005) seems to be a different entity, with only limited or at least unclear overlap.
4.2. Hypothetical neurophysiologic pathomechanisms
Regarding the underlying pathomechanisms of the generation of focal delta activity, there are three putative candidates: spike wave components, deafferentiation delta and cortical intrinsic delta.
Hypothetically, the detected focal delta activity could correspond to the wave component of spike wave complexes. This could potentially explain the focal distribution, which shows maxima close to spike localizations, overlapping but not identical with these. However, even with frequent spiking, spikes and spike wave have to be regarded as rare events in comparison to the amount of background resting state data. It seems therefore unlikely, that in general just a few spikes would result in delta activity with z-values up to 100 and more. Even in patient P3, a z-value of 9.8 is reached, even though no spikes were found in the same dataset.
Deafferentiation delta can be observed, when thalamic inputs to cortical neurons are disrupted. In athalamic cats (Ball et al., 1977), cortical delta is enhanced. Similar observations have been reported for isolated hemispheres (Kellaway et al., 1966). In contrast, electric stimulation of structures of the thalamus and brainstem disrupts this activity (Steriade et al., 1993). Amzica and Steriade provide an overview (Amzica and Steriade, 1998). The common interpretation of slowing after epilepsy surgery is thus, that it is generated cortically due to resective deafferentiation. Larger lesions, such as tumors may also lead to partial deafferentiation of thamalo-cortical connections and therefore disinhibit this type of intrinsic, in a sense thalamic-dependent cortical delta.
The second type of cortical delta is hypothesized to be driven by intrinsically bursting neurons (IB), occurring due to depolarization, and primarily independent of a lack of thalamic excitation. Intrinsic properties of IB cells lead to a depolarization response which consists of cycles of delta crowned by a burst of action potentials (100–250 Hz). It has been argued that these burst spikes are highly efficient to recruit larger populations of cortical neurons (Amzica and Steriade, 1998). In addition, fast spiking (FS) interneurons have been shown to play an active role in the generation of cortical delta (Carracedo et al., 2013). For both cell types an involvement in seizures, spike waves and polyspike waves have been illustrated (Fujiwara-Tsukamoto et al., 2010, Timofeev and Steriade, 2004). Furthermore, studies of epilepsy after long-term changes after traumatic deafferentiation (Nita et al., 2006), in genetic models of cortical malformations (Jacobs et al., 1999) and after status epilepticus (Sanabria et al., 2002) show an increase of IB cells and/or enhanced bursting properties.
The focal delta which we observed in patients with recurrent seizures may thus be a correlate of active and potentially increased IB and FS populations. The resective deafferentiation may add more delta activity due to disconnected thalamic excitation. In case of seizure free patients after surgery, the observed delta activity may exclusively consist of the deafferentiation type.
4.3. Clinical relevance
Postoperative levels of delta activity differed significantly between patients with and without persisting or recurrent seizures. ROC-analysis yielded an AUC value of 0.84, which in principle enables an excellent differentiation between the groups. In comparison, the AUC value for predicting seizure freedom after surgery of various clinical, radiological and electrophysiological routine parameters reached values up to 0.76, however limited to temporal lobe epilepsy (Goellner et al., 2013). Before actual clinical application is viable, the time course of the occurrence of focal slow activity has to be investigated. Due to the cross-sectional nature of our study, it was not possible to determine, whether postoperative slow activity also existed before surgery or whether there were significant changes in an individual patient. It also remains unclear, if there are novel postoperative increases and whether they occur within days, weeks or months after surgery and before the first recurring seizure.
It is known, that seizures may still recur up to two to five years after surgery (de Tisi et al., 2011). At least a percentage of seizure free patients may therefore develop seizures again in the long-term. The slight but significant increase in comparison to healthy controls may thus also originate from the same mechanisms as in the patients with already recurring seizures.
Analysis of focal delta activity may be also helpful to plan second surgery or guide implantation of invasive electrodes in patients with recurrent seizures, potentially independent of spikes (Ishibashi et al., 2002, Kaltenhäuser et al., 2007, Vanrumste et al., 2005). The three examples of P4, P13 and P15 illustrate this aspect: Areas of increased delta remained in situ in P15, who developed recurrent seizures, while P4 and P13 were seizure free after a resection which also included the delta areas. While spikes in P4 occurred over both frontal lobes, delta was limited to the side of resection. If reproduced with more patients, taking slow activity into account for resection is reminiscent of “clusterectomy”, i.e. taking localizations of interictal spikes into account. It has been repeatedly shown, that this enables significantly higher rates of seizure freedom (Mu et al., 2014, Vadera et al., 2013).
4.4. Limitations
The investigation of patients with recurrent seizures was retrospective, due to the limited number of cases who are reevaluated in detail after previous epilepsy surgery. Therefore, our study suffers from the problems which are typical for retrospective analyses: potential selection bias, influence on the non-automated portions of our analysis, etc. In contrast, patients for the seizure free group were recruited consecutively from the clinical routine. While this approach may benefit from the advantages of prospective studies, it led to a higher proportion of temporal lobe epilepsy cases in this group. There may therefore be a distortion in regard to the delta between groups. Lobe- and pathology-specific differences, as well as reasons for the broad range of the amount of delta activity in patients with recurrent seizures would have to be addressed in larger studies. Furthermore, differences in anti-epileptic medication have to be taken into account, which was difficult to address with the comparably low number of patients of our study. Furthermore, the time between measurements and previous seizures would also be interesting to investigate the relation of focal slow activity and postictal slowing. So far, it remains unknown, whether these are two different entities or whether they share a common basis.
Finally, investigations of the time course of delta, as well as longitudinal studies are warranted to evaluate the ideal time point for postoperative recordings. Such results could shed light on mechanisms of seizure recurrence.
Disclosure of conflicts of interest
None of the authors have any conflict of interest to disclose.
Ethical publication statement
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Acknowledgements
Funding: This work was supported by the Deutsche Forschungsgemeinschaft (DFG STE 380/15-1). We acknowledge support by Deutsche Forschungsgemeinschaft and Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU) within the funding programme Open Access Publishing.
References
- Amzica F., Steriade M. Electrophysiological correlates of sleep delta waves. Electroencephalogr. Clin. Neurophysiol. 1998;107:69–83. doi: 10.1016/s0013-4694(98)00051-0. [DOI] [PubMed] [Google Scholar]
- Baayen J.C., de Jongh A., Stam C.J., de Munck J.C., Jonkman J.J., Kasteleijn-Nolst Trenité D.G.A., Berendse H.W., van Cappellen van Walsum A.M., Heimans J.J., Puligheddu M., Castelijns J.A., Vandertop W.P. Localization of slow wave activity in patients with tumor-associated epilepsy. Brain Topogr. 2003;16:85–93. doi: 10.1023/b:brat.0000006332.71345.b7. [DOI] [PubMed] [Google Scholar]
- Ball G.J., Gloor P., Schaul N. The cortical electromicrophysiology of pathological delta waves in the electroencephalogram of cats. Electroencephalogr. Clin. Neurophysiol. 1977;43:346–361. doi: 10.1016/0013-4694(77)90258-9. [DOI] [PubMed] [Google Scholar]
- Brigo F. Intermittent rhythmic delta activity patterns. Epilepsy Behav. 2011;20:254–256. doi: 10.1016/j.yebeh.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Carracedo L.M., Kjeldsen H., Cunnington L., Jenkins A., Schofield I., Cunningham M.O., Davies C.H., Traub R.D., Whittington M.A. A neocortical delta rhythm facilitates reciprocal interlaminar interactions via nested theta rhythms. J. Neurosci. 2013;33:10750–10761. doi: 10.1523/JNEUROSCI.0735-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Tisi J., Bell G.S., Peacock J.L., McEvoy A.W., Harkness W.F.J., Sander J.W., Duncan J.S. The long-term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: a cohort study. Lancet. 2011;378:1388–1395. doi: 10.1016/S0140-6736(11)60890-8. [DOI] [PubMed] [Google Scholar]
- Di Gennaro G., Quarato P.P., Sebastiano F., Esposito V., Onorati P., Mascia A., Romanelli P., Grammaldo L.G., Falco C., Scoppetta C., Eusebi F., Manfredi M., Cantore G. Postoperative EEG and seizure outcome in temporal lobe epilepsy surgery. Clin. Neurophysiol. 2004;115:1212–1219. doi: 10.1016/j.clinph.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Engel J. Lippincott Williams & Wilkins; 1993. Surgical Treatment of the Epilepsies. [Google Scholar]
- Englot D.J., Wang D.D., Rolston J.D., Shih T.T., Chang E.F. Rates and predictors of long-term seizure freedom after frontal lobe epilepsy surgery: a systematic review and meta-analysis. J. Neurosurg. 2012;116:1042–1048. doi: 10.3171/2012.1.JNS111620. [DOI] [PubMed] [Google Scholar]
- Fujiwara-Tsukamoto Y., Isomura Y., Imanishi M., Ninomiya T., Tsukada M., Yanagawa Y., Fukai T., Takada M. Prototypic seizure activity driven by mature hippocampal fast-spiking interneurons. J. Neurosci. 2010;30:13679–13689. doi: 10.1523/JNEUROSCI.1523-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambardella A., Gotman J., Cendes F., Andermann F. Focal intermittent delta activity in patients with mesiotemporal atrophy: a reliable marker of the epileptogenic focus. Epilepsia. 1995;36:122–129. doi: 10.1111/j.1528-1157.1995.tb00970.x. [DOI] [PubMed] [Google Scholar]
- Geyer J.D., Bilir E., Faught R.E., Kuzniecky R., Gilliam F. Significance of interictal temporal lobe delta activity for localization of the primary epileptogenic region. Neurology. 1999;52:202–205. doi: 10.1212/wnl.52.1.202. [DOI] [PubMed] [Google Scholar]
- Goellner E., Bianchin M.M., Burneo J.G., Parrent A.G., Steven D.A. Timing of early and late seizure recurrence after temporal lobe epilepsy surgery. Epilepsia. 2013;54:1933–1941. doi: 10.1111/epi.12389. [DOI] [PubMed] [Google Scholar]
- Gross J., Kujala J., Hamalainen M., Timmermann L., Schnitzler A., Salmelin R. Dynamic imaging of coherent sources: studying neural interactions in the human brain. Proc. Natl. Acad. Sci. U. S. A. 2001;98:694–699. doi: 10.1073/pnas.98.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen M.S. 2009. MNE software User's Guide Version 2.7 [WWW Document] (URL http://www.martinos.org/meg/manuals/MNE-manual-2.7.pdf accessed 12.9.15) [Google Scholar]
- Harroud A., Bouthillier A., Weil A.G., Nguyen D.K. Temporal lobe epilepsy surgery failures: a review. Epilepsy Res. Treat. 2012;2012:201651. doi: 10.1155/2012/201651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt M., Schulz R., Hoppe M., May T., Ebner A. Postoperative routine EEG correlates with long-term seizure outcome after epilepsy surgery. Seizure. 2005;14:446–451. doi: 10.1016/j.seizure.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Huppertz H.J., Hof E., Klisch J., Wagner M., Lücking C.H., Kristeva-Feige R. Localization of interictal delta and epileptiform EEG activity associated with focal epileptogenic brain lesions. NeuroImage. 2001;13:15–28. doi: 10.1006/nimg.2000.0680. [DOI] [PubMed] [Google Scholar]
- Ishibashi H., Simos P.G., Castillo E.M., Maggio W.W., Wheless J.W., Kim H.L., Venkataraman V., Sanders D.K., Breier J.I., Zhang W., Davis R.N., Papanicolaou A.C. Detection and significance of focal, interictal, slow-wave activity visualized by magnetoencephalography for localization of a primary epileptogenic region. J. Neurosurg. 2002;96:724–730. doi: 10.3171/jns.2002.96.4.0724. [DOI] [PubMed] [Google Scholar]
- Jacobs K.M., Kharazia V.N., Prince D.A. Mechanisms underlying epileptogenesis in cortical malformations. Epilepsy Res. 1999;36:165–188. doi: 10.1016/s0920-1211(99)00050-9. [DOI] [PubMed] [Google Scholar]
- Jeha L.E., Najm I., Bingaman W., Dinner D., Widdess-Walsh P., Lüders H. Surgical outcome and prognostic factors of frontal lobe epilepsy surgery. Brain. 2007;130:574–584. doi: 10.1093/brain/awl364. [DOI] [PubMed] [Google Scholar]
- Kaltenhäuser M., Scheler G., Rampp S., Paulini A., Stefan H. Spatial intralobar correlation of spike and slow wave activity localisations in focal epilepsies: a MEG analysis. NeuroImage. 2007;34:1466–1472. doi: 10.1016/j.neuroimage.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Kellaway P., Gol A., Proler M. Electrical activity of the isolated cerebral hemisphere and isolated thalamus. Exp. Neurol. 1966;14:281–304. doi: 10.1016/0014-4886(66)90115-4. [DOI] [PubMed] [Google Scholar]
- Kirchberger K., Hummel C., Stefan H. Postoperative multichannel magnetoencephalography in patients with recurrent seizures after epilepsy surgery. Acta Neurol. Scand. 1998;98:1–7. doi: 10.1111/j.1600-0404.1998.tb07370.x. [DOI] [PubMed] [Google Scholar]
- Mintzer S., Nasreddine W., Passaro E., Beydoun A. Predictive value of early EEG after epilepsy surgery. J. Clin. Neurophysiol. 2005;22:410–414. doi: 10.1097/01.WNP.0000173201.86774.D6. [DOI] [PubMed] [Google Scholar]
- Mohamed I.S., Otsubo H., Ochi A., Elliott I., Donner E., Chuang S., Sharma R., Holowka S., Rutka J., Snead O.C. Utility of magnetoencephalography in the evaluation of recurrent seizures after epilepsy surgery. Epilepsia. 2007;48:2150–2159. doi: 10.1111/j.1528-1167.2007.01271.x. [DOI] [PubMed] [Google Scholar]
- Mu J., Rampp S., Carrette E., Roessler K., Sommer B., Schmitt F.C., De Tiège X., Hamer H., Boon P., Pauli E., Bluemcke I., Zhou D., Buchfelder M., Stefan H. Clinical relevance of source location in frontal lobe epilepsy and prediction of postoperative long-term outcome. Seizure. 2014;23:553–559. doi: 10.1016/j.seizure.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Nita D.A., Cissé Y., Timofeev I., Steriade M. Increased propensity to seizures after chronic cortical deafferentation in vivo. J. Neurophysiol. 2006;95:902–913. [Google Scholar]
- Noe K., Sulc V., Wong-Kisiel L., Wirrell E., Van Gompel J.J., Wetjen N., Britton J., So E., Cascino G.D., Marsh W.R., Meyer F., Horinek D., Giannini C., Watson R., Brinkmann B.H., Stead M., Worrell G.A. Long-term outcomes after nonlesional extratemporal lobe epilepsy surgery. JAMA Neurol. 2013;70:1003–1008. doi: 10.1001/jamaneurol.2013.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte G. The magnetic lead field theorem in the quasi-static approximation and its use for magnetoencephalography forward calculation in realistic volume conductors. Phys. Med. Biol. 2003;48:3637–3652. doi: 10.1088/0031-9155/48/22/002. [DOI] [PubMed] [Google Scholar]
- Oostenveld R., Fries P., Maris E., Schoffelen J.-M. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Marqui R.D. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find. Exp. Clin. Pharmacol. 2002;(Suppl. 24 D):5–12. [PubMed] [Google Scholar]
- Patrick S., Berg A., Spencer S.S. EEG and seizure outcome after epilepsy surgery. Epilepsia. 1995;36:236–240. doi: 10.1111/j.1528-1157.1995.tb00990.x. [DOI] [PubMed] [Google Scholar]
- Rosenow F., Lüders H. Presurgical evaluation of epilepsy. Brain. 2001;124:1683–1700. doi: 10.1093/brain/124.9.1683. [DOI] [PubMed] [Google Scholar]
- Sanabria E.R.G., da Silva A.V., Spreafico R., Cavalheiro E.A. Damage, reorganization, and abnormal neocortical hyperexcitability in the pilocarpine model of temporal lobe epilepsy. Epilepsia. 2002;43(Suppl. 5):96–106. doi: 10.1046/j.1528-1157.43.s.5.31.x. [DOI] [PubMed] [Google Scholar]
- Siegel aM., Cascino G.D., Meyer F.B., McClelland R.L., So E.L., Marsh W.R., Scheithauer B.W., Sharbrough F.W. Resective reoperation for failed epilepsy surgery: seizure outcome in 64 patients. Neurology. 2004;63:2298–2302. doi: 10.1212/01.wnl.0000147476.86575.a7. [DOI] [PubMed] [Google Scholar]
- Simasathien T., Vadera S., Najm I., Gupta A., Bingaman W., Jehi L. Improved outcomes with earlier surgery for intractable frontal lobe epilepsy. Ann. Neurol. 2013;73:646–654. doi: 10.1002/ana.23862. [DOI] [PubMed] [Google Scholar]
- Steriade M., Amzica F., Nuñez A. Cholinergic and noradrenergic modulation of the slow (approximately 0.3 Hz) oscillation in neocortical cells. J. Neurophysiol. 1993;70:1385–1400. doi: 10.1152/jn.1993.70.4.1385. [DOI] [PubMed] [Google Scholar]
- Timofeev I., Steriade M. Neocortical seizures: initiation, development and cessation. Neuroscience. 2004;123:299–336. doi: 10.1016/j.neuroscience.2003.08.051. [DOI] [PubMed] [Google Scholar]
- Vadera S., Jehi L., Burgess R.C., Shea K., Alexopoulos A.V., Mosher J., Gonzalez-Martinez J., Bingaman W. Correlation between magnetoencephalography-based “clusterectomy” and postoperative seizure freedom. Neurosurg. Focus. 2013;34 doi: 10.3171/2013.4.FOCUS1357. [DOI] [PubMed] [Google Scholar]
- Vanrumste B., Jones R.D., Bones P.J., Carroll G.J. Slow-wave activity arising from the same area as epileptiform activity in the EEG of paediatric patients with focal epilepsy. Clin. Neurophysiol. 2005;116:9–17. doi: 10.1016/j.clinph.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Vorwerk J., Cho J.H., Rampp S., Hamer H., Knösche T.R., Wolters C.H. A guideline for head volume conductor modeling in EEG and MEG. NeuroImage. 2014;100:590–607. doi: 10.1016/j.neuroimage.2014.06.040. [DOI] [PubMed] [Google Scholar]
- Welch P. The use of fast Fourier transform for the estimation of power spectra: a method based on time averaging over short, modified periodograms. IEEE Trans. Audio Electroacoust. 1967;15:70–73. [Google Scholar]