Abstract
miR-3940-5p level was lower in non–small cell lung cancer (NSCLC) tumor tissues than that in the matched tumor-adjacent tissues and correlated with clinicopathological features. Cyclin D1 (CCND1), a key driver of malignant transformation in NSCLC, was overexpressed in many cancers, including NSCLC. The ubiquitin specific peptidase-28 (USP28) was also overexpressed in NSCLC and associated with poor prognosis of NSCLC patients. We searched for miR-3940-5p targets by using TargetScan and miRanda online tools and found that CCND1 and USP28 were potential targets of miR-3940-5p. Based on these findings, we speculated that miR-3940-5p might target CCND1 and USP28 to inhibit NSCLC growth. We determined the expression of miR-3940-5p, CCND1, and USP28 by quantitative real-time polymerase chain reaction and Western blot assays, respectively, and found downregulation of miR-3940-5p and upregulation of CCND1 and USP28 in NSCLC tissues and cell lines. Cell proliferation and apoptosis assays showed that miR-3940-5p suppressed proliferation and promoted apoptosis in NSCLC cells, and silencing CCND1 and USP28 both recapitulated the effects of miR-3940-5p on NSCLC cells. Furthermore, we verified that CCND1 and USP28 were direct targets of miR-3940-5p and also found that the effects of NSCLC cell proliferation and apoptosis by miR-3940-5p were attenuated by overexpression of CCND1 or USP28. The animal experiments also showed that overexpression of miR-3940-5p inhibited the growth of NSCLC tumors in vivo. These results confirmed our speculation that miR-3940-5p inhibits proliferation and induces apoptosis in NSCLC cells by targeting CCND1 and USP28. These findings facilitate a better understanding of the molecular mechanisms underlying NSCLC initiation and progression and provide promising diagnostic markers and therapeutic targets for NSCLC.
Introduction
Lung cancer is the leading cause of cancer-related death in the world, and its pathogenesis is closely associated with tobacco smoking. Lung cancer is classified into two main histological groups including non–small cell lung cancer (NSCLC) and small cell lung cancer, and NSCLC accounts for over 85% of all lung cancer cases [1]. Although great improvements have been achieved in chemotherapy and radiotherapy over the past few decades, the prognosis of the patients with NSCLC is not satisfactory. The overall 5-year survival rate for stage I NSCLC patients after surgery remains up to 83%. And only 5% to 15% stage III NSCLC patients and less than 2% of stage IV NSCLC patients are alive after 5 years [2]. These statistics suggest that effective tools to diagnose at an early age and therapeutic treatments to the late age of the disease are very critical to improve the prognosis of NSCLC patients. Undoubtedly, a better understanding of the molecular mechanisms underlying NSCLC initiation and progression will contribute to develop effective diagnostic tools and therapeutic strategies for NSCLC patients.
MicroRNAs (miRNAs) are small non–protein-coding RNAs of 19 to 25 nucleotides that can regulate their target gene expression through binding to the 3′ untranslated regions (UTRs) of target mRNAs, resulting in degradation, translational repression, or both [3], [4]. It has been reported that miRNAs can posttranscriptionally modulate 30% of human genes, thereby suggesting that miRNAs may play pivotal roles in physiological and pathological processes, including human cancers [5]. Over the past decade, evidence has emerged that many miRNAs are frequently dysregulated in human malignant tumors and are crucial for human cancer initiation, promotion, and progression [6], [7], [8], [9], [10]. And some miRNAs act as oncogenes or tumor suppressor genes, which depend on their target genes. Based on this pivotal role, miRNAs have been considered as putative targets for early diagnosis and treatment of human malignant tumors.
miR-3940-5p, as a newfound miRNA, was recently reported to be reduced in NSCLC and correlated with clinicopathological features [11]. However, the related mRNA targets and molecular mechanisms of miR-3940-5p in NSCLC remain unclear. We used TargetScan and miRanda online tools to identify the potential targets of miR-3940-5p and found that cyclin D1 (CCND1) and the ubiquitin specific peptidase-28 (USP28) are potential targets of miR-3940-5p. CCND1, as an oncogenic driver in human cancer, is also a key driver of malignant transformation in NSCLC [12], [13]. Recently, upregulation of USP28 has been reported to be associated with poor prognosis in NSCLC patients and promote NSCLC cell proliferation [14]. Based on these findings, we speculated that miR-3940-5p might regulate NSCLC cell proliferation and apoptosis by targeting CCND1 and USP28.
In this study, we determined the expression levels of miR-3940-5p, CCND1, and USP28 in NSCLC tissues and cell lines by quantitative real-time polymerase chain reaction (qRT-PCR) and Western blot, respectively, and investigated the associations between miR-3940-5p, CCND1, and USP28 expression and NSCLC cell proliferation and apoptosis. Whereafter, we performed the luciferase reporter assay to identify whether the 3′UTRs of CCND1 and USP28 mRNA are binding targets of miR-3940-5p. We also performed animal experiments to investigate the role of miR-3940-5p in vivo. These findings will provide new clues for the initiation and progression of NSCLC and promising diagnostic markers and therapeutic targets for NSCLC.
Materials and Methods
Tissue Samples
NSCLC tissue samples and matched nontumor normal tissue samples were surgically resected from 20 patients with primary NSCLC at the First Affiliated Hospital of Zhengzhou University. Then tissue samples were immediately flash-frozen in liquid nitrogen, followed by storing at −80°C. No patients were treated before undergoing surgery. The clinicopathologic features of 20 patients were listed in Table 1. The use of the tissue samples was approved by all the patients and by the Ethical Committee of the First Affiliated Hospital of Zhengzhou University.
Table 1.
Patients' Characteristics of Clinical-Pathologic Features
| Characteristics | No. of Patients (n = 20) | Percent (%) |
|---|---|---|
| Age at diagnosis (year) | ||
| ≤60 | 9 | 45 |
| >60 | 11 | 55 |
| Sex | ||
| Male | 13 | 65 |
| Female | 7 | 35 |
| Tumor size (cm) | ||
| ≤3.0 | 15 | 75 |
| >3.0 | 5 | 25 |
| Histological type | ||
| Adenocarcinoma | 14 | 70 |
| Squamous cell carcinoma | 6 | 30 |
Cell Culture and Transfection
The NSCLC cell lines (A549, H460, H520, SPC-A1) and a normal fetal lung fibroblast cell line (MRC5) were purchased from American Type Culture Collection (ATCC, Manassas, VA). MRC5 cells were cultured in DMEM (Invitrogen, Gaithersburg, MD), and NSCLC cells were incubated in RPMI1640 medium (Invitrogen) containing 10% heat-inactivated fetal bovine serum, 100 U/ml of penicillin, and 100 μg/ml of streptomycin. All of the cells were grown in humidified atmosphere containing 5% CO2 at 37°C. A549 cells were transfected with miR-3940-5p mimics, miR-3940-5p inhibitors, small interfering RNA targeting CCND1 (si-CCND1), si-USP28, pcDNA3.1 plasmid containing CCND1 (pcDNA-CCND1), pcDNA-USP28, or their respective controls (GenePharma, Shanghai, China) using Lipofectamine 2000 according to the manufacturer's protocol.
Cell Proliferation Assay
Cell proliferation was determined using Cell Counting Kit 8 (CCK-8; Dojindo, Tokyo, Japan) according to the manufacturer's instructions. Briefly, cells were seeded in 96-well plates at a density of 3 × 103 cells/well. At different time points of 0, 24, 48, and 72 hours, solution reagent was added to each well and incubated at 37°C for 4 hours. The absorbance was measured at 450 nm to assess cell proliferation.
Apoptosis Assay
Cell apoptosis analysis was performed with the Annexin V/FITC and PI apoptosis detection kit (Becton Dickinson, Franklin Lakes, NJ). Briefly, after cells were harvested and suspended with the Annexin-binding buffer, samples were incubated with Annexin V–FITC and PI for 15 minutes at room temperature in the dark. Cell apoptosis was analyzed using flow cytometry (BD Biosciences, Franklin Lakes, NJ) within 1 hour. To detect the effects of miR-3940-5p inhibitors, pcDNA-CCND1, and pcDNA-USP28 on apoptosis rate, cells were cultured in fresh serum-free medium 48 hours after transfection.
RNA Extraction and qRT-PCR
Total RNA was isolated from cultured cells by using TRIzol (Invitrogen) following the manufacturer's protocol. miRNA was reverse transcribed into cDNAs using One Step Prime Script miRNA cDNA Synthesis Kit (Takara, Dalian, China). Total RNA was reverse transcribed into cDNAs with Prime Script RT reagent Kit (Takara). The qRT-PCR assays were performed using the IQ SYBR Green Supermix (Bio-Rad, Hercules, CA). The relative expression of miR-3940-5p was normalized to U6 snRNA. Expression levels of CCND1 and USP28 mRNA were normalized to GAPDH. The fold changes were calculated through quantification using the 2–∆∆Ct method.
Western Blot Analysis
The cultured cells were harvested and lysed with cell lysis buffer containing protease inhibitors. Total protein extract was separated through 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to prewetted polyvinylidene difluoride membranes (Millipore, Billerica, MA). The membranes were then blocked in 5% fat-free milk for 1 hour and probed with antibodies against CCND1 (Cell Signaling, Boston, MA), USP28 (Sigma-Aldrich, St. Louis, MO), and β-actin (Sigma-Aldrich) overnight at 4°C. After washing, the blots were incubated with the horseradish peroxidase–conjugated secondary antibody (Abcam, Cambridge, UK). Signal was visualized with enhanced chemiluminescence detection kit (Thermo scientific, Rockford, IL).
Dual-Luciferase Reporter Assay
The putative target sites of the human CCND1 and USP28 mRNA sequences for miR-3940-5p were designed and synthesized by Sangon Biotech (Shanghai, China). Then the sequences were inserted into the downstream of the firefly luciferase reporter in pmirGLO Dual-Luciferase miRNA Target Expression Vector (Promega, Madison, WI). The mutated putative miR-3940-5p binding sites were also cloned into the same region of the vector. A549 cells were cotransfected with 0.6 μg of the dual-luciferase vector containing either wild-type (WT) or mutant (MUT) 3′UTR and miR-3940-5p mimics or negative control at a final concentration of 100 nM using Lipofectamine 2000 (Invitrogen). Forty-eight hours later, the luciferase activity was detected using the Dual-Luciferase Reporter Assay System (Promega). The luciferase reading of each sample was expressed as a ratio of firefly/Renilla value.
In Vivo Tumor Xenograft Model
In vivo tumor xenograft model was performed as previously described [15]. Briefly, A549 cells (5 × 107) transfected with miR-3940-5p mimics or mimic control were subcutaneously inoculated into the left and right flanks of six nude BALB/c mice (SLAC Laboratory, Shanghai, China), respectively. The tumor size was monitored weekly, and tumor volume was calculated as follows: tumor volume = length × width2/2. After 5 weeks, all nude mice were euthanized, and tumors were removed and weighed. In addition, the expression levels of CCND1 and USP28 protein in xenografts were determined by Western blot.
Statistical Analysis
All the data were presented as the mean ± standard error. All the statistical analyses were performed using SPSS version 16.0 software (SPSS, Chicago, IL). Differences between two groups were determined by Student's t test. P values < .05 were considered statistically significant.
Results
Downregulation of miR-3940-5p and Upregulation of CCND1 and USP28 in NSCLC Tissues
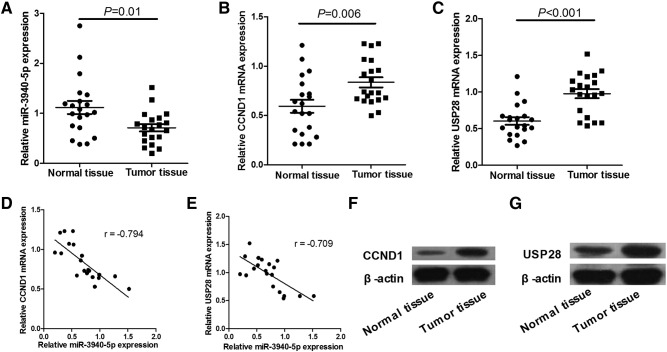
We detected the expression of miR-3940-5p, CCND1, and USP28 mRNA and protein expression in NSCLC tissues by qRT-PCR and Western blot. qRT-PCR assays show that NSCLC tissues have a marked decrease in miR-3940-5p expression and significant increases in CCND1 and USP28 mRNA expression compared with adjacent normal tissues (Figure 1, A–C). In addition, a significant negative correlation was found between expressions of miR-3940-5p versus CCND1 mRNA (r = −0.794; Figure 1D) and USP28 mRNA (r = −0.709; Figure 1E), indicating that miR-3940-5p either directly or indirectly suppressed CCND1 and USP28 transcript levels. Western blot assays show that the expression levels of CCND1 and USP28 protein in tumor tissues are significantly higher than those in their corresponding normal tissues (Figure 1, F and G). In addition, the data show that dysregulation of miR-3940-5p, CCND1, and USP28 is not related to histological type. These results indicate that downregulation of miR-3940-5p and upregulation of CCND1 and USP28 exist in NSCLC tissues, and miR-3940-5p expression is inversely associated with the expression of CCND1/USP28 mRNA.
Figure 1.
miR-3940-5p is downregulated and CCND1 and USP28 are upregulated in NSCLC tissues. (A–C) qRT-PCR shows that NSCLC tissues have a marked decrease in miR-3940-5p expression and significant increases in CCND1 and USP28 mRNA expression compared with adjacent normal tissues. (D and E) miR-3940-5p expression is inversely associated with the expression of CCND1 (r = −0.794)/USP28 (r = −0.709) mRNA in NSCLC tissues. (F and G) The protein levels of CCND1 and USP28 in NSCLC tissues are detected by Western blot. β-Actin serves as an internal control.
miR-3940-5p Suppresses Cell Proliferation and Induces Apoptosis in A549 Cells
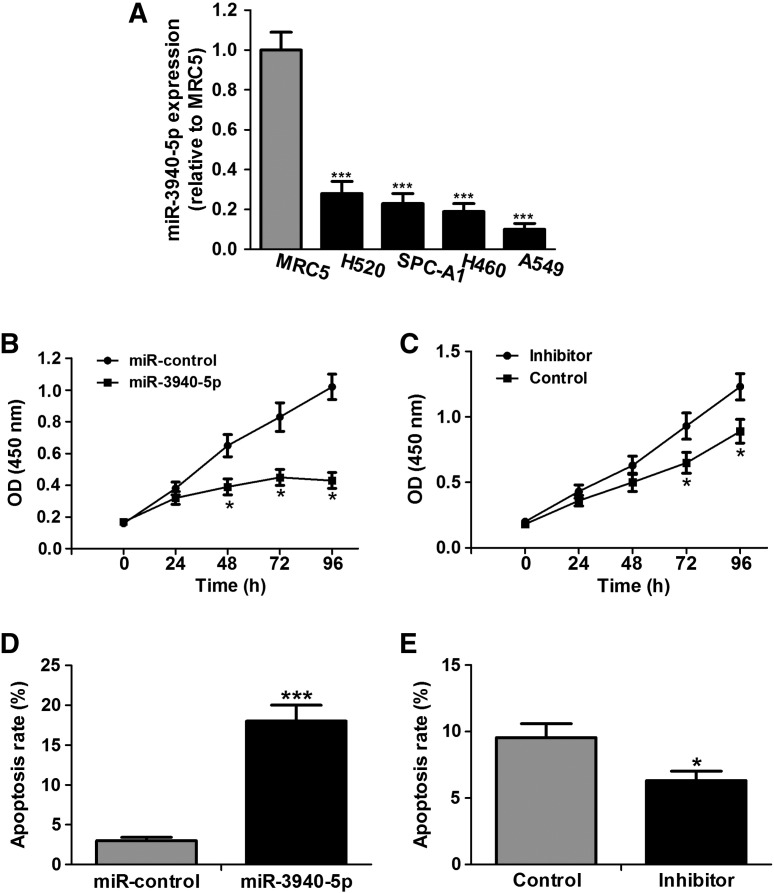
We detected the expression level of miR-3940-5p in normal fetal lung fibroblast cell line MRC5 and NSCLC cell lines A549, H460, H520, and SPC-A1. Data from qRT-PCR show that all NSCLC cell lines lost the endogenous miR-3940-5p compared with MRC5 cell line (Figure 2A). To investigate the effect of miR-3940-5p on NSCLC cell proliferation and apoptosis, we transfected A549 cells with miR-3940-5p mimics or inhibitors, respectively. CCK-8 assay shows that A549 cell proliferation is significantly impaired after the transfection with miR-3940-5p mimics, whereas the proliferation is increased after miR-3940-5p inhibitor treatment (Figure 2, B and C). Flow cytometry presents increased apoptosis in A549 cells transfected with miR-3940-5p mimics and reduced apoptosis after miR-3940-5p inhibitor transfection (Figure 2, D and E). Thus, our data indicate that miR-3940-5p acts as a tumor suppressor in NSCLC cells.
Figure 2.
miR-3940-5p impairs proliferation and initiates apoptosis of A549 cells in vitro. (A) qRT-PCR confirms that miR-3940-5p expression is downregulated in NSCLC cell lines compared with normal lung fibroblast cell line MRC5. U6 snRNA is used as the internal control. (B and C) Proliferation reduction of A549 cells in response to miR-3940-5p overexpression. The growth curves are employed to measure cell proliferation at 24-, 48-, 72-, and 96-hour time points. The data demonstrated are the mean value of three repetitions. (D) Flow cytometry is used to detect apoptosis of A549 cells at 48 hours after transfection with miR-3940-5p mimics or negative control (miR-control). (E) After transfection with miR-3940-5p inhibitor or the negative control (control), cell cultural medium was replaced with fresh serum-free medium, and cell apoptosis was detected at 48 hours post serum deprivation. *P < .05, ***P < .001.
Downregulation of CCND1 and USP28 Inhibits Proliferation and Induces Apoptosis in A549 Cells
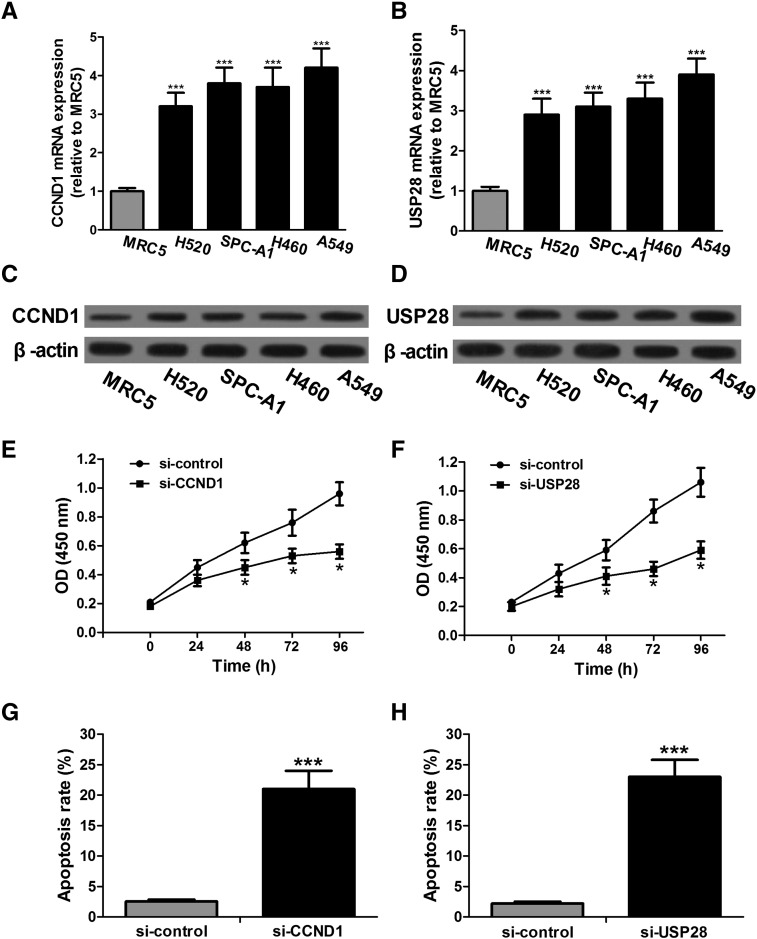
The expression levels of CCND1 and USP28 in different NSCLC cell lines and MRC5 cells were detected by qRT-PCR and Western blot, respectively. Compared with MRC5 cells, all NSCLC cell lines show significant upregulation of CCND1 and USP28 mRNA (Figure 3, A and B). The protein levels of CCND1 and USP28 are also significantly increased in NSCLC cell lines compared with those in MRC5 cells, which are completely consistent with the results of qRT-PCR (Figure 3, C and D). To further investigate the functional roles of CCND1 and USP28 in NSCLC cells, we transfected A549 cells with si-CCND1, si-USP28, or the negative controls and then preformed cell proliferation and apoptosis detection. CCK-8 assay shows that si-CCND1 and si-USP28 significantly inhibit A549 cell proliferation compared with si-controls (Figure 3, E and F). Furthermore, flow cytometry demonstrates that A549 cells transfected with si-CCND1 or si-USP28 have higher apoptosis rate than control cells (Figure 3, G and H). These results indicate that CCND1 and USP28 are upregulated in A549 cells, and knockdown of CCND1 or USP28 obviously restrains cell proliferation and promotes apoptosis.
Figure 3.
Downregulation of CCND1 and USP28 inhibits proliferation and induces apoptosis in A549 cells. (A and B) Upregulated CCND1 and USP28 mRNA is detected by qRT-PCR in NSCLC cell lines compared with that in MRC5 cells. GAPDH is used as endogenous controls. (C and D) The protein levels of CCND1 and USP28 are detected by Western blot. β-Actin serves as an internal control. (E and F) Cell proliferation assay is performed after transfection with si-CCND1, si-USP28, or their respective controls, respectively. si-CCND1 or si-USP28 causes significant cell proliferation reduction, respectively, at 48-, 72-, and 96-hour time points. (G and H) A549 cells with the low endogenous CCND1 or USP28 level after transfection with si-CCND1 or si-USP28 show higher apoptosis rate compared with cells treated with si-controls. *P < .05, ***P < .001.
CCND1 and USP28 Are Targets of miR-3940-5p
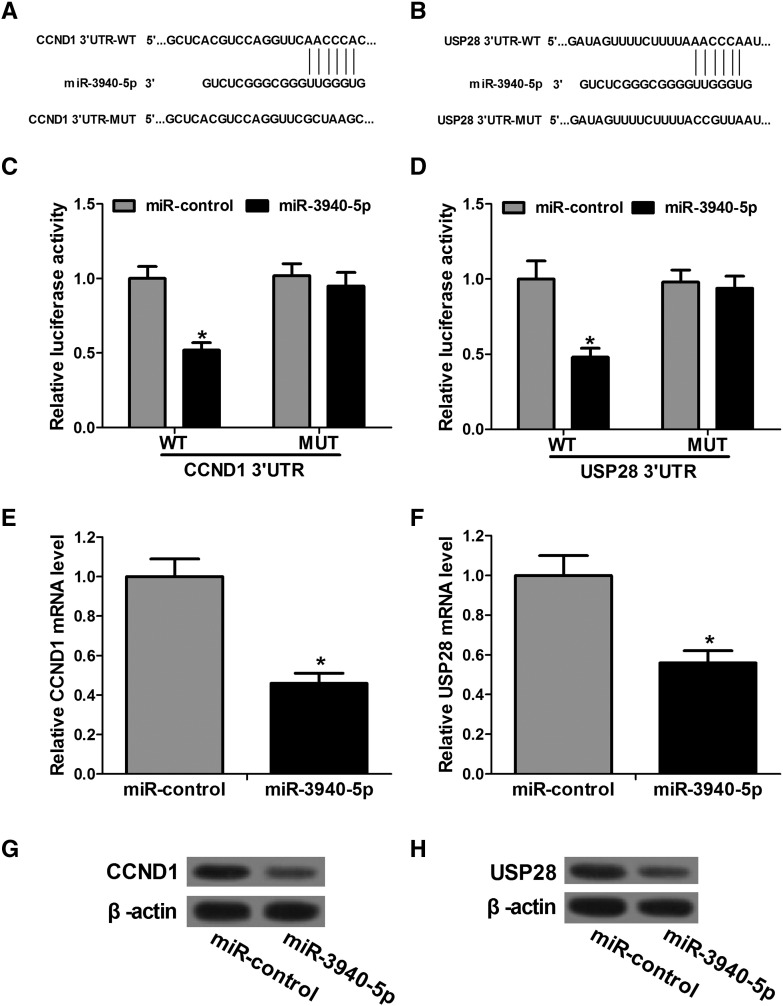
To explore the mechanism underlying miR-3940-5p involvement in proliferation and apoptosis in NSCLC cells, TargetScan and miRanda online tools were used to search for the target genes, and we found that CCND1 and USP28 are putative targets of miR-3940-5p (Figure 4, A and B). The dual-luciferase activity assay was then used to evaluate the possibility of bioinformatical prediction. CCND1 or USP28 3′UTR with or without mutation was cloned into the dual-luciferase vector and then cotransfected with miR-3940-5p mimics or negative control. In line with binding data, the results show that miR-3940-5p markedly suppresses the luciferase activity of the WT CCND1 or USP28 3′UTR without effect on the MUT 3′UTR, which indicates that CCND1 and USP28 are direct targets of miR-3940-5p (Figure 4, C and D). Furthermore, we examined the expression of the endogenous CCND1 and USP28 in A549 cells treated with miR-3940-5p mimics or negative control. As expected, upregulation of miR-3940-5p results in a significant decrease in CCND1 and USP28 expression at both the mRNA and protein levels, which indicates that miR-3940-5p regulates CCND1 and USP28 expression through binding to 3′UTRs of their mRNA, resulting in both degradation and translational repression (Figure 4, E–H). In combination with a significant negative correlation between expressions of miR-3940-5p versus CCND1 mRNA and USP28 mRNA in NSCLC tissues, these results suggest that CCND1 and USP28 are functional targets of miR-3940-5p and miR-3940-5p affects proliferation and apoptosis in A549 cells by targeting CCND1 and USP28.
Figure 4.
CCND1 and USP28 are direct targets of miR-3940-5p. (A and B) Specific locations of the binding sites in miR-3940-5p and 3′UTRs of CCND1 or USP28 are marked. (C and D) The luciferase reporter assay shows that miR-3940-5p significantly suppresses the luciferase activity of reporters containing the WT 3′UTRs of CCND1 or USP28 but has no significant effect on reporters with MUT 3′UTRs. The luciferase activity of each sample is normalized to Renilla luciferase activity. (E and F) Decreased expression of CCND1 or USP28 mRNA is due to miR-3940-5p overexpression in A549 cells. (G and H) The restoration of miR-3940-5p obviously inhibits protein levels of CCND1 and USP28. β-Actin is shown as a loading control. *P < .05.
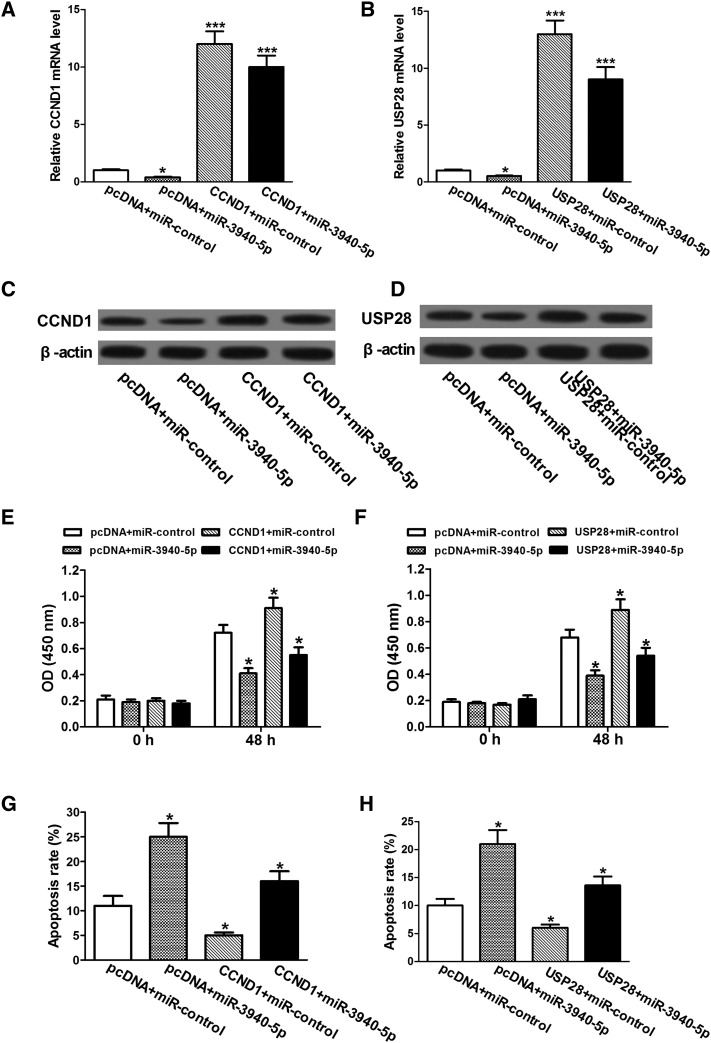
miR-3940-5p Regulates Proliferation and Apoptosis in A549 Cells by Inhibiting CCND1 and USP28 Expression
To explore the functional relevance of miR-3940-5p and its targets CCND1 and USP28, we measured whether CCND1 or USP28 overexpression could reverse the effect of miR-3940-5p on cell proliferation and apoptosis. For this purpose, we transfected pcDNA-CCND1 or pcDNA-USP28 into A549 cells with miR-3940-5p addition. As illustrated in Figure 5, A–D, compared with control cells, CCND1 and USP28 are downregulated at both mRNA and protein levels by ectopic expression of miR-3940-5p; however, pcDNA-CCND1 and pcDNA-USP28 robustly overturn this decreased expression of CCND1 and USP28 induced by miR-3940-5p. Moreover, the upregulation of CCND1 and USP28 partially reverses the impaired proliferation and promoted apoptosis rate induced by miR-3940-5p in A549 cells (Figure 5, E–H). However, overexpression of CCND1 and USP28 fails to fully restore the effect of miR-3940-5p on proliferation and apoptosis probably due to a sufficiently high endogenous level of CCND1 and USP28 already existing in A549 cells. Taken together, our results reveal that miR-3940-5p inhibits proliferation and induces apoptosis in A549 cells by regulation of CCND1 and USP28 expression.
Figure 5.
miR-3940-5p regulates proliferation and apoptosis in A549 cells by inhibiting CCND1 and USP28 expression. (A and B) The mRNA levels of CCND1 and USP28 are determined by qRT-PCR. GAPDH is used as endogenous controls. (C and D) Western blot analysis for protein levels of CCND1 and USP28. β-Actin is used as loading control. (E and F) Upregulation of CCND1 or USP28 in A549 cells transfected with miR-3940-5p mimics partly relieves the inhibition of cell proliferation induced by miR-3940-5p. (G and H) After transfection, cell cultural medium was replaced with fresh serum-free medium. Cell apoptosis was detected at 48 hours post serum deprivation. Overexpression of CCND1 or USP28 in A549 cells transfected with miR-3940-5p mimics partly reverses the induction of apoptosis by miR-3940-5p. *P < .05, ***P < .001.
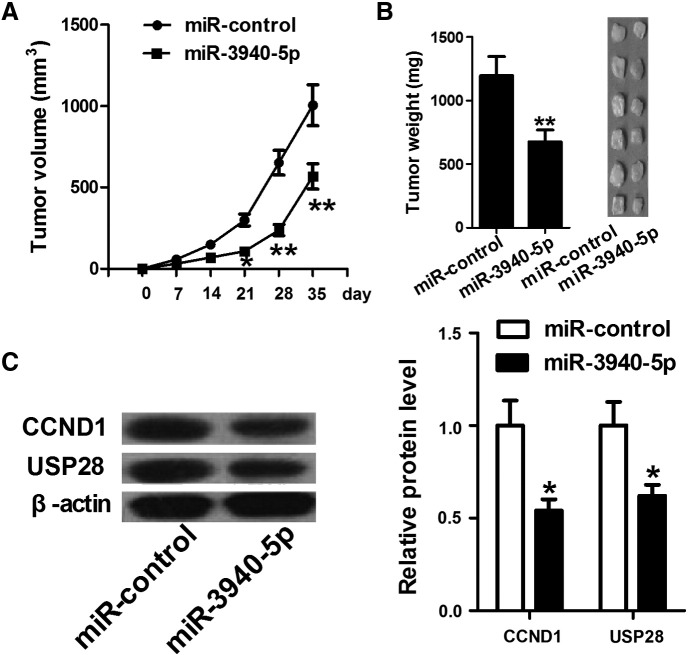
miR-3940-5p Reduces Tumor Growth in Xenograft Models of NSCLC
The in vitro data showed a tumor-suppressor role for miR-3940-5p in NSCLC. We further investigated the effect of miR-3940-5p on NSCLC cell tumorigenicity. Results showed that the tumor volume in the miR-3940-5p group is significantly reduced compared with that in the control group (Figure 6A). Accordingly, the tumor weight in the miR-3940-5p group is significantly lighter than that in the control group on day 35 following surgery resection (Figure 6B). In addition, the expression levels of CCND1 and USP28 protein in xenografts were examined by Western blot. Results show that CCND1 and USP28 protein expression levels are decreased compared with the control group (Figure 6C), suggesting that miR-3940-5p negatively regulates CCND1 and USP28 in vivo.
Figure 6.
miR-3940-5p inhibits tumor growth in xenograft models of NSCLC. (A) Tumor volume in the miR-3940-5p group is significantly reduced compared with that in the control group. (B) The picture and weight of xenografts on day 35 following surgery resection. (C) Compared with the control group, the miR-3940-5p group has dramatic decreases in CCND1 and USP28 protein expression. *P < .05, **P < .01.
Discussion
The recent discovery of miRNAs has received significant attention in cancer research [16], [17]. Evidence suggests that the dysregulated expression of miRNAs occurs frequently in cancers and this abnormity either contributes to carcinogenesis or reflects the initiation and progression of cancers [6], [7], [8], [9]. For example, in NSCLC, miR-21 and miR-205 suppress tumor suppressor PTEN and promote growth and invasion [18], [19]. Loss of miR-200c expression induces an aggressive, invasive, and chemoresistant phenotype in NSCLC [20]. miR-449c and miR-126 target c-Myc and EGFL7, respectively, and inhibit NSCLC progression [21], [22].
Recently, miR-3940-5p was found to be lower in NSCLC tumor tissues than in the matched tumor-adjacent tissues and correlated with clinicopathological features [11]. However, the role and molecular mechanism of miR-3940-5p in NSCLC remain unclear. Here, we first determined the expression level of miR-3940-5p in NSCLC tissues and cells by qRT-PCR and found that miR-3940-5p is significantly downregulated in NSCLC tissues and cells. MTT and flow cytometry analysis shows that miR-3940-5p represses proliferation and promotes apoptosis in NSCLC cells. Then we further investigated the molecular mechanisms by which miR-3940-5p exerts regulatory effects on NSCLC cell proliferation and apoptosis. We searched the TargetScan and miRanda databases and found that CCND1 and USP28 are potential targets of miR-3940-5p.
CCND1, a member of the D-type cyclin family, is a common oncogene of human cancers, which exerts carcinogenic action by regulating cell cycle. CCND1 promotes G1-S progression by forming active holoenzymes with CDK4 and CDK6 and sequentially phosphorylates the retinoblastoma proteins (pRb). Then pRb releases the E2F transcription factors, which trigger their downstream genes to modulate the G1/S phase transition [23]. The overexpression of CCND1 has been demonstrated in many cancers, such as NSCLC, breast cancer, lymphomas, gliomas, and nasopharyngeal carcinoma [12], [24], [25], [26]. Many studies reported that CCND1 can be regulated by miRNAs and affect the initiation and progression of cancers. For example, miR-138 suppresses nasopharyngeal carcinoma growth and tumorigenesis by targeting the CCND1 [27]. miR-15a and miR-16-1 downregulate CCND1 and induce apoptosis and cell cycle arrest in osteosarcoma [27]. miR-449b inhibits the proliferation of SW1116 colon cancer stem cells partly by downregulation of CCND1 expression [28]. miR-490-3p and miR-545 target CCND1 and inhibit NSCLC cell proliferation [29], [30].
USP28, a member of USP family, is a key regulator of the DNA damage checkpoint and is overexpressed in many cancers, such as colon cancer, breast carcinomas, and NSCLC [14], [31]. Popov et al. [32] reported that USP28 can control MYC stability, and the stabilization of MYC is required for proliferation of several tumor cells types. Recently, Zhang et al. [14] reported that overexpression of USP28 promotes NSCLC cell proliferation and apoptosis inhibition and is associated with poor prognosis in NSCLC patients.
Based on these findings, it was tempting to speculate that miR-3940-5p exerts regulatory effects on NSCLC cell proliferation and apoptosis via negatively regulating CCND1 and USP28 expression. We determined the expression levels of CCND1 and USP28 and found that CCND1 and USP28 expressions are both significantly upregulated in NSCLC tissues and cells. The results of MTT and flow cytometry analysis show that silencing both CCND1 and USP28 by siRNA recapitulates the effects of miR-3940-5p on NSCLC cells. Luciferase assay confirmed that miR-3940-5p targets CCND1 and USP28 directly. An inverse correlation is also found between miR-3940-5p and CCND1/USP28 expression in NSCLC tissues and A549 cells. In addition, we determined the expression levels of CCND1 and USP28 in A549 cells following the treatment of miR-3940-5p mimics. Not surprisingly, an obvious decrease of CCND1 and USP28 expression at both mRNA and protein levels is observed in the A549 cells transfected with miR-3940-5p mimics. Moreover, the effects of A549 cell proliferation and apoptosis by miR-3940-5p are partly restored. These results confirmed our speculation that miR-3940-5p inhibits NSCLC cell proliferation and promotes apoptosis by targeting CCND1 and USP28. Furthermore, animal study also confirmed that overexpression of miR-3940-5p suppresses the growth of NSCLC tumors and negatively regulates CCND1 and USP28 in vivo.
Altogether, miR-3940-5p is downregulated in NSCLC tissues and cells and inhibits NSCLC cell proliferation and promotes apoptosis, whereas CCND1 and USP28 are upregulated, and silencing both CCND1 and USP28 recapitulates the effects of miR-3940-5p on NSCLC cells. The role of miR-3940-5p is mediated by the target genes CCND1 and USP28. These findings facilitate a better understanding of the molecular mechanisms underlying NSCLC initiation and progression and provide promising diagnostic markers and therapeutic targets for NSCLC.
Conflict of Interest
The authors declared that no conflict of interest exists.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Higgins MJ, Ettinger DS. Chemotherapy for lung cancer: the state of the art in 2009. Expert Rev Anticancer. 2009;9:1365–1378. doi: 10.1586/era.09.115. [DOI] [PubMed] [Google Scholar]
- 3.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 5.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 6.Asangani I, Rasheed S, Nikolova D, Leupold J, Colburn N, Post S, Allgayer H. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–2136. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 7.Park S-M, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang P, Chen L, Zhang J, Chen H, Fan J, Wang K, Luo J, Chen Z, Meng Z, Liu L. Methylation-mediated silencing of the miR-124 genes facilitates pancreatic cancer progression and metastasis by targeting Rac1. Oncogene. 2014;33:514–524. doi: 10.1038/onc.2012.598. [DOI] [PubMed] [Google Scholar]
- 9.Gao J, Li L, Wu M, Liu M, Xie X, Guo J, Tang H, Xie X. MiR-26a inhibits proliferation and migration of breast cancer through repression of MCL-1. PLoS One. 2013;8:e65138. doi: 10.1371/journal.pone.0065138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jansson MD, Lund AH. MicroRNA and cancer. Mol Oncol. 2012;6:590–610. doi: 10.1016/j.molonc.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun Y, Su B, Zhang P, Xie H, Zheng H, Xu Y, Du Q, Zeng H, Zhou X, Chen C. Expression of miR-150 and miR-3940-5p is reduced in non–small cell lung carcinoma and correlates with clinicopathological features. Oncol Rep. 2013;29:704–712. doi: 10.3892/or.2012.2152. [DOI] [PubMed] [Google Scholar]
- 12.Gautschi O, Ratschiller D, Gugger M, Betticher DC, Heighway J. Cyclin D1 in non–small cell lung cancer: a key driver of malignant transformation. Lung Cancer. 2007;55:1–14. doi: 10.1016/j.lungcan.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 13.Kim JK, Diehl JA. Nuclear cyclin D1: an oncogenic driver in human cancer. J Cell Physiol. 2009;220:292–296. doi: 10.1002/jcp.21791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Xu B, Qiang Y, Huang H, Wang C, Li D, Qian J. Overexpression of deubiquitinating enzyme USP28 promoted non–small cell lung cancer growth. J Cell Mol Med. 2015;19:799–805. doi: 10.1111/jcmm.12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu X, Li H, Long L, Hui L, Chen H, Wang X, Shen H, Xu W. miR-126 enhances the sensitivity of non–small cell lung cancer cells to anticancer agents by targeting vascular endothelial growth factor A. Acta Biochim Biophys Sin. 2012;44:519–526. doi: 10.1093/abbs/gms026. [DOI] [PubMed] [Google Scholar]
- 16.Hummel R, Hussey DJ, Haier J. MicroRNAs: predictors and modifiers of chemo- and radiotherapy in different tumour types. Eur J Cancer. 2010;46:298–311. doi: 10.1016/j.ejca.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 17.Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov. 2010;9:775–789. doi: 10.1038/nrd3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.J-g Z, Wang J-j, Zhao F, Liu Q, Jiang K, Yang G-h. MicroRNA-21 (miR-21) represses tumor suppressor PTEN and promotes growth and invasion in non–small cell lung cancer (NSCLC) Clin Chim Acta. 2010;411:846–852. doi: 10.1016/j.cca.2010.02.074. [DOI] [PubMed] [Google Scholar]
- 19.Lei L, Huang Y, Gong W. miR-205 promotes the growth, metastasis and chemoresistance of NSCLC cells by targeting PTEN. Oncol Rep. 2013;30:2897–2902. doi: 10.3892/or.2013.2755. [DOI] [PubMed] [Google Scholar]
- 20.Ceppi P, Mudduluru G, Kumarswamy R, Rapa I, Scagliotti GV, Papotti M, Allgayer H. Loss of miR-200c expression induces an aggressive, invasive, and chemoresistant phenotype in non–small cell lung cancer. Mol Cancer Res. 2010;8:1207–1216. doi: 10.1158/1541-7786.MCR-10-0052. [DOI] [PubMed] [Google Scholar]
- 21.Miao L-J, Huang S-F, Sun Z-T, Gao Z-Y, R-x Z, Liu Y, Wang J. MiR-449c targets c-Myc and inhibits NSCLC cell progression. FEBS Lett. 2013;587:1359–1365. doi: 10.1016/j.febslet.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Sun Y, Bai Y, Zhang F, Wang Y, Guo Y, Guo L. miR-126 inhibits non–small cell lung cancer cells proliferation by targeting EGFL7. Biochem Biophys Res Commun. 2010;391:1483–1489. doi: 10.1016/j.bbrc.2009.12.098. [DOI] [PubMed] [Google Scholar]
- 23.Tashiro E, Tsuchiya A, Imoto M. Functions of cyclin D1 as an oncogene and regulation of cyclin D1 expression. Cancer Sci. 2007;98:629–635. doi: 10.1111/j.1349-7006.2007.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wessendorf S, Schwaenen C, Kohlhammer H, Kienle D, Wrobel G, Barth TF, Nessling M, Möller P, Döhner H, Lichter P. Hidden gene amplifications in aggressive B-cell non-Hodgkin lymphomas detected by microarray-based comparative genomic hybridization. Oncogene. 2003;22:1425–1429. doi: 10.1038/sj.onc.1206297. [DOI] [PubMed] [Google Scholar]
- 25.Xie L, Xu L, He Z, Zhou W, Wang L, Zhang L, Lan K, Ren C, Liu W, Yao K. Identification of differentially expressed genes in nasopharyngeal carcinoma by means of the Atlas human cancer cDNA expression array. J Cancer Res Clin. 2000;126:400–406. doi: 10.1007/PL00008488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Büschges R, Weber RG, Actor B, Lichter P, Collins VP, Reifenberger G. Amplification and expression of cyclin D genes (CCND1 CCND2 and CCND3) in human malignant gliomas. Brain Pathol. 1999;9:435–442. doi: 10.1111/j.1750-3639.1999.tb00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, Lv X-B, Wang X-P, Sang Y, Xu S, Hu K, Wu M, Liang Y, Liu P, Tang J. MiR-138 suppressed nasopharyngeal carcinoma growth and tumorigenesis by targeting the CCND1 oncogene. Cell Cycle. 2012;11:2495–2506. doi: 10.4161/cc.20898. [DOI] [PubMed] [Google Scholar]
- 28.Fang Y, Gu X, Li Z, Xiang J, Chen Z. miR-449b inhibits the proliferation of SW1116 colon cancer stem cells through downregulation of CCND1 and E2F3 expression. Oncol Rep. 2013;30:399–406. doi: 10.3892/or.2013.2465. [DOI] [PubMed] [Google Scholar]
- 29.Gu H, Yang T, Fu S, Chen X, Guo L, Ni Y. MicroRNA-490-3p inhibits proliferation of A549 lung cancer cells by targeting CCND1. Biochem Biophys Res Commun. 2014;444:104–108. doi: 10.1016/j.bbrc.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 30.Du B, Wang Z, Zhang X, Feng S, Wang G, He J, Zhang B. MicroRNA-545 suppresses cell proliferation by targeting cyclin D1 and CDK4 in lung cancer cells. PLoS One. 2014;9:e88022. doi: 10.1371/journal.pone.0088022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J, Tan Q, Yan M, Liu L, Lin H, Zhao F, Bao G, Kong H, Ge C, Zhang F. miRNA-200c inhibits invasion and metastasis of human non–small cell lung cancer by directly targeting ubiquitin specific peptidase 25. Mol Cancer. 2014;13:1–14. doi: 10.1186/1476-4598-13-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Popov N, Wanzel M, Madiredjo M, Zhang D, Beijersbergen R, Bernards R, Moll R, Elledge SJ, Eilers M. The ubiquitin-specific protease USP28 is required for MYC stability. Nat Cell Biol. 2007;9:765–774. doi: 10.1038/ncb1601. [DOI] [PubMed] [Google Scholar]