Abstract
Stimulation of adipogenesis in mouse preadipocytes requires C/EBPβ as well as activation of the MEK/extracellular signal-regulated kinase (ERK) signaling pathway. In this study, we demonstrate that phosphorylation of C/EBPβ at a consensus ERK/glycogen synthase kinase 3 (GSK3) site regulates adiponectin gene expression during the C/EBPβ-facilitated differentiation of mouse fibroblasts into adipocytes. First, we show that exposure of 3T3-L1 preadipocytes to insulin, dexamethasone (DEX), and isobutylmethylxanthine (MIX) leads to the phosphorylation of C/EBPβ at threonine 188. Pretreating the cells with a MEK1-specific inhibitor (U0126) significantly attenuates this activity. Similarly, these effectors activate the phosphorylation of T188 within an ectopic C/EBPβ overexpressed in Swiss mouse fibroblasts, and this event involves both MEK1 and GSK3 activity. We further show that expression of C/EBPβ (p34kD LAP isoform) in Swiss mouse fibroblasts exposed to DEX, MIX, and insulin induces expression of peroxisome proliferator-activated receptor γ (PPARγ) and some adiponectin but that it does not activate expression of FABP4/aP2. In fact, complete conversion of these fibroblasts into lipid-laden adipocytes, which includes activation of FABP4 and adiponectin expression, requires their exposure to a potent PPARγ ligand such as troglitazone. Expression of a mutant C/EBPβ in which threonine 188 has been modified to alanine (C/EBPβ T188A) can induce PPARγ production in the mouse fibroblasts, but it is incapable of stimulating adiponectin expression in the absence or presence of troglitazone. Interestingly, replacement of T188 with aspartic acid creates a C/EBPβ molecule (C/EBPβ T188D) that possesses adipogenic activity similar to that of the wild-type molecule. The absence of adiponectin expression correlates with a reduced amount of C/EBPα in the adipocytes expressing the T188A mutant suggesting that C/EBPα is required for expression of adiponectin. In fact, ectopic expression of PPARγ in C/EBPα-deficient fibroblasts (NIH 3T3 cells) produces a modest amount of adiponectin, whereas expression of both PPARγ and C/EBPα in NIH 3T3 cells facilitates production of abundant quantities of adiponectin. These data demonstrate that phosphorylation of C/EBPβ at a consensus ERK/GSK3 site is required for both C/EBPα and adiponectin gene expression during the differentiation of mouse fibroblasts into adipocytes.
Initiation of adipogenesis in mouse preadipocytes involves the activation of a series of signaling pathways that lead to expression of a cascade of transcription factors regulating production of many hundreds of proteins responsible for the formation of mature adipocytes (22, 31). It is generally accepted that two principal players in this cascade are peroxisome proliferator-activated receptor γ (PPARγ) and C/EBPα since adipogenesis does not occur in either PPARγ- or C/EBPα-deficient mesenchymal stem cells in vivo or in vitro (2, 20, 29, 30). Induction of transcription of these factors during the early phase of adipogenesis is regulated by C/EBPβ through association with C/EBP regulatory elements within the promoters of the corresponding genes (8, 28, 37, 41). C/EBPβ is a member of the bZIP family of transcription factors that binds to DNA as homodimers or as heterodimers with other C/EBPs. Three isoforms of C/EBPβ (38kD, 34kD, and 20kD in mice) are produced to various extents in different cell types and at specific stages of differentiation through alternative use of three translation initiation sites within a single mRNA (1, 6, 9, 39). The most ubiquitous isoform (34kD), usually referred to as LAP (liver-enriched transcriptional activator protein), is composed of a C-terminal region containing the basic DNA-binding and leucine zipper dimerization domains and a N-terminal region, which contains the transactivation domains (10). A larger isoform (38kD), expressed in a differentiation-associated manner in some cell types including preadipocytes, is identical to LAP except that it contains an additional transactivation domain at the N terminus (11, 18, 24). Both LAP and the 38kD protein are potent transactivators of gene expression, whereas the 20kD isoform, LIP (liver-enriched transcriptional inhibitory protein), represses transcription in a dominant negative manner (6, 10, 16, 18). In fact, LIP contains the C-terminal region but lacks the N-terminal transactivation domains and consequently represses transcription through the formation of inactive dimers with LAP (10).
The transcriptional activity of C/EBPβ is regulated by several mechanisms, including association with other proteins as well as posttranslational modification. In fact, several reports have suggested a role for phosphorylation in regulating C/EBPβ activity in response to a variety of effectors in different cell types (4, 5, 7, 17, 23, 25, 26, 33-35). It has also been suggested that C/EBPβ normally exists in a repressed state and is activated by phosphorylation of a repressor domain located between the N-terminal transactivation domains and the C-terminal bZIP region (19). This domain contains several serines and threonines, all of which are phosphorylated to some extent by a constitutive process (3). The site encompassing threonine 188 (SPPGT188PSP) in murine LAP is a consensus site for both glycogen synthase kinase 3 (GSK3) and extracellular signal-regulated kinase (ERK), which is dephosphorylated in response to growth hormone stimulation of preadipocytes (26). Modification of this site by mutation of the threonine to alanine inhibits the C/EBPβ-associated transcription of a c-fos promoter reporter in response to growth hormone signaling (25).
We have recently shown that exposure of 3T3-L1 preadipocytes to the adipogenic effectors dexamethasone (DEX), isobutylmethylxanthine (MIX), insulin, and fetal bovine serum (FBS) activates a transient burst of MEK/ERK signaling that coincides with the induction of C/EBPβ expression (27). These and other studies have suggested a role for both MEK/ERK signaling and C/EBPβ in regulating PPARγ expression and activity during the early phase of adipogenesis (16). In the present study, we questioned whether the transcriptional activity of C/EBPβ is regulated by this differentiation-associated activation of MEK/ERK through the phosphorylation of the consensus ERK/GSK3 site (T188) within the repressor domain of C/EBPβ. We demonstrate by using an anti-phospho-Thr188 C/EBPβ-specific antibody that effectors which stimulate MEK/ERK signaling in 3T3-L1 preadipocytes also enhance the phosphorylation of T188 in the LAP isoform of C/EBPβ. Expression of LAP in Swiss mouse fibroblasts leads to the induction of PPARγ, which in the presence of a PPARγ ligand (troglitazone) stimulates the conversion of these cells into fat-laden adipocytes, with a corresponding increase in expression of adipogenic genes, including the adiponectin gene. Expression of a mutant LAP in which T188 has been modified to alanine (LAP T188A) is also capable of inducing expression of PPARγ and some markers of adipogenesis in response to troglitazone but is significantly less capable of stimulating C/EBPα and adiponectin gene expression. In addition, ectopic expression of PPARγ in C/EBPα-deficient fibroblasts (NIH 3T3 cells) produces a modest amount of adiponectin, whereas expression of both PPARγ and C/EBPα in NIH 3T3 cells facilitates production of abundant amounts of the adipocytokine. These data demonstrate that phosphorylation of C/EBPβ at a consensus ERK/GSK3 site is required for activation of C/EBPα expression, which leads to adiponectin gene expression during terminal adipogenesis.
MATERIALS AND METHODS
Reagents.
DEX, 3-isobutyl-1-methylxanthine, insulin, aprotinin, LY294002, and Oil Red O were purchased from Sigma (St. Louis, Mo.). Leupeptin and hygromycin were from American Bioanalytical Inc. (Natick, Mass.), while Geneticin, calf serum, and Dulbecco's modified Eagle's medium (DMEM) were supplied by Life Technologies Inc. (Gaithersburg, Md.). FBS was obtained from HyClone (Logan, Utah), [γ-32P]ATP was obtained from Perkin-Elmer Life Sciences (Boston, Mass.), and fibroblast growth factor 2 (FGF-2) was obtained from Peprotech (Rocky Hill, N.J.). Troglitazone was a gift from John Johnson (Parke-Davis/Warner Lambert, Ann Arbor, Mich.).
Antibodies.
Polyclonal anti-C/EBPα and anti-C/EBPβ, monoclonal anti-PPARγ, and polyclonal antiactin were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.). Polyclonal antiadiponectin (ACRP30) was purchased from Affinity Bioreagents (Golden, Colo.), monoclonal anti-pan-ERK was purchased from Transduction Laboratories (Lexington, Ky.), and monoclonal anti-phospho-ERK was purchased from New England Biolabs (Beverly, Mass.). Polyclonal anti-aP2 was kindly provided by David Bernlohr (University of Minnesota). Polyclonal anti-phospho-188Thr-C/EBPβ was obtained from Cell Signaling Technology Inc. Polyclonal antiperilipin was a gift from Andy Greenberg, (New England Medical Center, Tufts University, Boston, Mass.).
Expression vectors and generation of stable cell lines.
A glutathione S-transferase (GST)-p34C/EBPβ (LAP) bacterial expression plasmid was constructed by subcloning PCR products of C/EBPβ cDNA (16) into BamHI and EcoRI sites of the pGEX-5X-3 vector (Pharmacia Biotech, Uppsala, Sweden). The LAP PCR fragments were generated with the following primers: 5′GCGGATCCCCACCATGGAAGTGGCCAACTT and 5′CCGGAATTCGCATCAAGTCCCGAAACCCGGT. The mutant forms of C/EBPβ (T188A and T188D) were produced by site-directed mutagenesis (QuikChange; Stratagene, La Jolla, Calif.) of the wild-type GST-p34C/EBPβ plasmid with the following primers to introduce the appropriate change in codon 188: T188A, 5′TCCAGCCCGCCCGGAGCTCCGAGCCCCGCCGAC3′ (new SacI site); T188D, 5′TCCAGCCCGCCCGGGGATCCGAGCCCCGCCGAC3′ (new BamHI site). To generate the wild-type and T188A C/EBPβ-pRevTRE expression vectors, BamHI-SalI cDNA fragments from the corresponding C/EBPβ-pGEX-5X-3 plasmids were subcloned into the pRevTRE plasmid (Clontech, Palo Alto, Calif.) digested with BamHI and SalI. To construct the T188D C/EBPβ-pRevTRE expression vector, the corresponding T188D C/EBPβ-pGEX-5X-3 plasmid was subjected to controlled partial digestion with BamHI and SalI and the appropriate fragment corresponding to the C/EBPβ cDNA was purified by preparative agarose gel electrophoresis. This fragment was subcloned into pGEM-T Easy (Promega, Madison, Wis.) to give rise to the T188D C/EBPβ-pGEM-T plasmid. A SalI-SphI fragment of T188D C/EBPβ-pGEM-T was subcloned into pRevTRE digested with SalI and SphI. To generate the pRevTRE-C/EBPα expression vector, a PCR product corresponding to the coding region of C/EBPα (28) was subcloned into pcDNA 3-1 (−)Myc-His (Invitrogen) following digestion with BamHI and HindIII to give rise to the pcDNA 3-1 (−)Myc-His-C/EBPα plasmid. The PCR primers used to produce the coding region of C/EBPα were as follows: 5′CCGGGATCCATGGAGTCGGCCGACTTCTACGA3′ and 5′GATCCCAAGCTTCGCGCAGTTGCCCATGGCCTT3′. A BamHI-PmeI fragment of the pcDNA 3-1 (−)Myc-His-C/EBPα plasmid corresponding to the coding region of C/EBPα linked to coding sequences for the Myc-His tag was then subcloned into the pRev-TRE plasmid digested with BamHI and HpaI, giving rise to the pRev-TRE-C/EBPα-Myc-His retroviral expression vector.
To establish retrovirus-producing cell lines, human embryonic kidney 293T cells were seeded at 80% confluence in a 60-mm-diameter dish on the day of transfection. Individual cultures of cells were transfected with Fugene 6 (Roche Biochemicals Inc., Indianapolis, Ind.) and 2 μg of either the wild-type or mutant C/EBPβ-pREV-TRE vector, the C/EBPα-pREV-TRE vector, or pBabe-PPARγ2, along with 2 μg each of vesicular stomatitis virus G and GP expression plasmids (pVpack; Stratagene). Two days after transfection, culture medium containing high-titer virus was harvested and filtered through a 0.45-μm-pore-size filter. The viral filtrate supernatant was used to infect Swiss mouse 3T3 fibroblasts constitutively expressing the TET activator protein (Swiss TET-off cells; Clontech) or NIH 3T3 cells that were seeded in a 60-mm dish at 25% confluence on the day of infection. Cells were incubated with retrovirus overnight in the presence of 4 μg of Polybrene (Sigma)/ml, and the infection was allowed to proceed for an additional 2 days in fresh growth medium. At this stage, 100 μg of hygromycin and/or puromycin/ml was added to facilitate selection of stable cell lines, which required culture for 2 to 3 weeks in the presence of the antibiotic.
Cell culture.
3T3-L1 preadipocytes were grown in growth medium (DMEM containing 10% calf serum) until confluent and were then maintained in the same medium for an additional 2 to 3 days. Stimulation of the various signaling pathways was induced at 2 to 3 days postconfluence by addition of 1 μM DEX, 0.5 mM MIX, 1.67 μM insulin, 10% FBS, and 1 nM FGF-2. Swiss TET-off cells were maintained in growth medium (DMEM containing 10% FBS) with 100 μg of Geneticin/ml and 1 μg of tetracycline/ml. Swiss TET-off cells expressing either wild-type or mutant C/EBPβ (Swiss-LAP cells) were maintained in the same growth medium as the Swiss TET-off cells except that 100 μg of hygromycin/ml was also included along with the other antibiotics listed above. Differentiation of the Swiss-LAP and NIH-PPARγ ± C/EBPα cell lines was essentially as described previously (13, 16, 21). Cells were plated and cultured without tetracycline for 2 days until they reached confluence. Differentiation was induced at 2 days postconfluence (day 0) by adding fresh DMEM containing 10% FBS, 0.5 mM MIX, 1 μM DEX, 1.67 μM insulin, and 10 μM troglitazone. After 48 h, cells were maintained in DMEM with 10% FBS, 0.83 μM insulin, and 10 μM troglitazone. For experiments with U0126 (10 μM), LY294002 (50 μM), or LiCl (10 mM), postconfluent cells were preincubated with each of the drugs for 30 min prior to addition of other effectors. Oil Red O staining was performed by following the procedure described previously (38). The cells were then photographed by phase-contrast microscopy.
Preparation of whole-cell and nuclear extracts.
Cultured cells were rinsed with phosphate-buffered saline (140 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 8.1 mM Na2HPO4 [pH 7.4]). For whole-cell extracts, cells were harvested in ice-cold extraction buffer (20 mM Tris [pH 7.4], 150 mM NaCl, 0.5% sodium deoxycholate, 2% Nonidet P-40, 0.2% sodium dodecyl sulfate, 1 mM EDTA, 20 mM NaF, 30 mM tetrasodium pyrophosphate, 1 mM dithiothreitol, 1 mM sodium vanadate, 10% glycerol, 5 μM aprotinin, 50 μM leupeptin, 1 mM phenylmethylsulfonyl fluoride [PMSF]) and lysates were incubated on ice for 15 min and then centrifuged at 18,000 × g. For nuclear protein extracts, cells were lysed in lysis buffer (10 mM Tris [pH 7.6], 10 mM NaCl, 3 mM MgCl2, 0.5% Nonidet P-40). After a low-speed centrifugation, nuclei were resuspended in nuclear extraction buffer (20 mM HEPES [pH 7.9], 350 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA [pH 8.0], 25% glycerol, 5 μM aprotinin, 50 μM leupeptin, 1 mM PMSF) and then incubated on ice for 20 min and centrifuged at full speed (13,000 rpm) at 4°C. The resulting supernatants were stored at −80°C, and protein concentrations were determined with the bicinchoninic acid kit (Pierce, Rockford, Ill.).
Gel electrophoresis and immunoblotting.
Proteins were analyzed by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (NEN Life Science, Boston, Mass.) in 25 mM Tris-192 mM glycine. Following transfer, membranes were blocked with 4% nonfat dry milk in phosphate-buffered saline-0.1% Tween 20 and probed with the antibodies specified above. Horseradish peroxidase-conjugated secondary antibodies (Sigma) and an enhanced chemiluminescence (ECL) substrate kit (Perkin-Elmer Life Science) were used for detection of specific proteins.
Electrophoretic mobility shift assay (EMSA).
DNA binding assays were performed on nuclear extracts prepared from wild-type, T188A, and T188D Swiss-LAP cells by following previously described procedures (15, 28). A double-stranded oligonucleotide corresponding to the C/EBP response element within the C/EBPα promoter (5′GATCCGCGTTGCGCCACGATG3′) was end labeled with [γ-32P]ATP by using T4 polynucleotide kinase according to the manufacturer's instructions (New England Biolabs). For supershift analysis, 3 μg of nuclear protein extract was incubated with antibodies at room temperature for 30 min prior to the binding reaction. Each nuclear extract was incubated with 2 μg of poly(dI-dC), 10× binding buffer (100 mM Tris-Cl [pH 7.5], 500 mM NaCl, 10 mM EDTA, 10 mM dithiothreitol, 50% glycerol), 1.5 μl of 50% glycerol, and 10,000 cpm of radiolabeled oligonucleotide in a final volume of 20 μl at room temperature for 30 min. The reaction mixtures were resolved on a nondenaturing 6% polyacrylamide (39:0.5, acrylamide/bisacrylamide) gel at 150 V for 4 h at 4°C in running buffer (25 mM Tris [pH 8.3], 190 mM glycine, 1 mM EDTA, 10% glycerol). Gels were vacuum dried for 1 h before exposure to Biomax MR-1 autoradiography film (Eastman Kodak Co.). Antibodies used for the supershifts were anti-C/EBPβ and anti-C/EBPα, as described above.
RESULTS
Activation of MEK/ERK signaling during the early phase of adipogenesis leads to the phosphorylation of C/EBPβ.
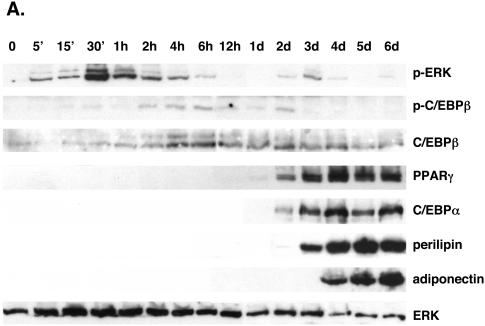
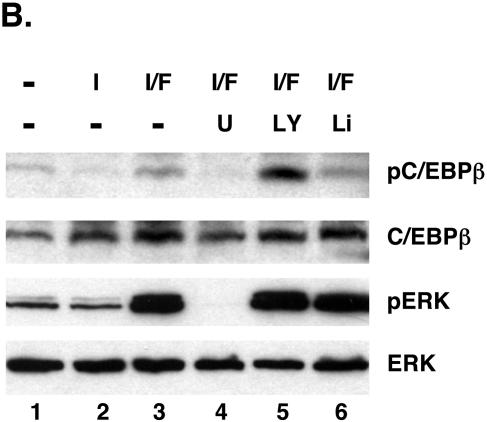
To measure the phosphorylation of C/EBPβ during the differentiation of preadipocytes, we employed a phospho-C/EBPβ-specific antibody, which was produced against a phosphorylated peptide corresponding to the site encompassing threonine 188 of murine C/EBPβ. Figure 1A demonstrates that exposure of confluent 3T3-L1 preadipocytes to DEX, MIX, insulin, and FBS induces MEK1 activity during the initial 60 min of differentiation, as indicated by the phosphorylation of ERK1 and ERK2, which precedes the phosphorylation of C/EBPβ occurring between 1 and 2 h after the start of differentiation. The activation of MEK1 is short-lived since it subsides to quiescent levels by 6 h, whereas C/EBPβ phosphorylation can be detected throughout the first 2 days of adipogenesis. Following these early events, the preadipocytes continue to differentiate into mature adipocytes, as indicated by the sequential expression of the adipogenic genes including the C/EBPβ, PPARγ, C/EBPα, perilipin, and adiponectin genes. This pattern of signaling, in which activation of MEK1 immediately precedes phosphorylation of C/EBPβ, suggests that MEK1 might regulate C/EBPβ activity during adipogenesis. To determine whether MEK1 phosphorylates C/EBPβ in 3T3-L1 preadipocytes, we exposed confluent cells to DEX and MIX in the presence or absence of FGF-2 and/or insulin for 4 h and a Western blot analysis was performed on total cellular proteins. Our previous studies have shown that FGF-2 is a potent inducer of MEK1 activity in 3T3-L1 cells and is a proadipogenic factor; consequently, we employed this effector to enhance the phosphorylation of C/EBPβ. Figure 1B demonstrates that the phospho-C/EBPβ antibody detects a protein that comigrates with the LAP isoform of C/EBPβ in cells exposed to DEX and MIX (lane 1). These conditions also lead to the activation of MEK1, as revealed by the phosphorylation of both ERK1 and ERK2. Exposure of the cells to insulin and FGF-2 enhances the phosphorylation of both C/EBPβ and the ERKs (Fig. 1B, lane 3), whereas exposure to insulin without FGF-2 attenuates the expression of the phospho-C/EBPβ species (Fig. 1B, lane 2). Inhibition of MEK1 activity by exposure of the cells to U0126 blocked the phosphorylation of C/EBPβ by FGF-2 and insulin (Fig. 1, lane 4). Additionally, insulin, which activates both phosphatidylinositol (PI)-3 kinase and Akt/protein kinase B activity and leads to suppression of GSK3 activity, attenuates phosphorylation of C/EBPβ. Treatment of the cells with LY294002, an inhibitor of PI-3 kinase activity, potentiates the ability of FGF-2 to phosphorylate C/EBPβ (Fig. 1B, lane 5), while treatment with LiCl, an inhibitor of GSK3 activity, attenuated this effect of FGF-2. Taken together, the data presented in Fig. 1 demonstrate that activation of MEK1/ERK and/or GSK3 signaling potentiates the phosphorylation of threonine 188 within the LAP isoform of C/EBPβ during the initial stages of adipogenesis in 3T3-L1 preadipocytes.
FIG. 1.
Phosphorylation of C/EBPβ at a consensus ERK/GSK3 site during the differentiation of 3T3-L1 preadipocytes. (A) Proliferating 3T3-L1 preadipocytes were cultured in growth medium until they reached confluence. At 2 days postconfluence (day 0), the quiescent cells were exposed to DEX, MIX, FBS, and insulin, and total cellular protein was harvested at the indicated times. Equal amounts of protein from each sample were subjected to Western blot analysis using antibodies specific for phospho-ERK1/2 (p-ERK), phospho-C/EBPβ (p-C/EBPβ), ERK, C/EBPβ, PPARγ, C/EBPα, perilipin, and adiponectin. (B) Confluent 3T3-L1 preadipocytes were exposed to various combinations of 1.67 μM insulin (I), 1 nM FGF-2 (F), 20 μM U0126 (U), 50 μM LY294002 (LY), and 10 mM LiCl (Li) in the presence of MIX and DEX for 4 h, and total cellular protein was subjected to Western blot analysis using antibodies specific for phospho-C/EBPβ (pC/EBPβ), C/EBPβ, phospho-ERK1/2 (pERK), and ERK.
Activation of ERK or GSK3 in mouse embryo fibroblasts induces the phosphorylation of C/EBPβ on Thr188.
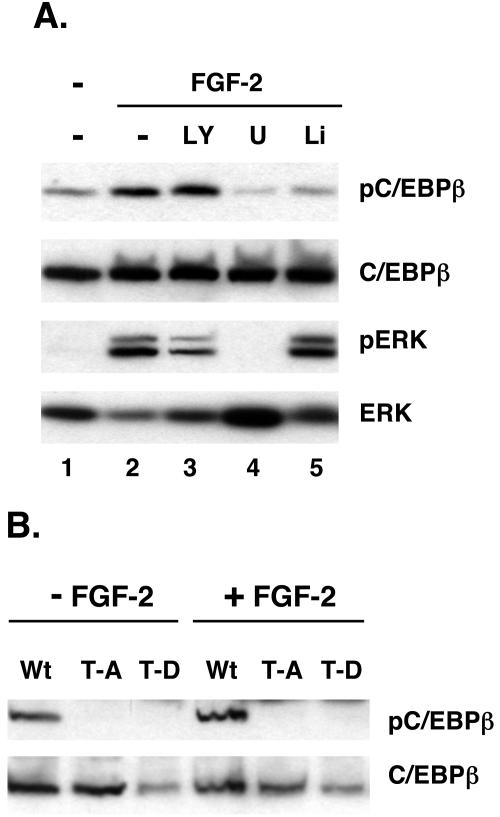
To investigate the role of threonine 188 in regulating C/EBPβ activity, we generated expression vectors corresponding to the LAP isoform of C/EBPβ (wild type) as well as two mutant proteins in which threonine 188 was replaced by either alanine (T188A) or aspartic acid (T188D). To identify the signaling pathways regulating the phosphorylation of C/EBPβ in vivo, we ectopically expressed wild-type LAP and the corresponding T188A and T188D mutant proteins in Swiss mouse fibroblasts using the pRevTET-off expression system. Figure 2A shows an extensive phosphorylation of the wild-type protein in cells exposed to insulin in the presence of MIX and DEX, as revealed by detection of a phosphorylated protein on the Western blot with the phospho-C/EBPβ-specific antibody (Fig. 2A, lane 1). The extent of phosphorylation is enhanced when cells are exposed to FGF-2 along with the other effectors (Fig. 2A, lane 2), which also stimulates MEK activity (phosphorylation of ERK). In fact, inhibition of MEK1 by exposure of the cells to U0126 dramatically attenuates the phosphorylation of C/EBPβ (Fig. 2A, lane 4). As observed in Fig. 1 for the endogenous C/EBPβ in 3T3-L1 preadipocytes, inhibition of PI-3 kinase with LY294002appears to enhance the ability of FGF-2 to induce C/EBPβ phosphorylation (Fig. 2A, lane 3), and this activity is also blocked by treatment of the cells with LiCl (Fig. 2A, lane 5). We also expressed the T188A and T188D mutant C/EBPβs and compared their levels of phosphorylation with that of the wild-type control using the anti-phospho-C/EBPβ antibody (Fig. 2B). As expected, these data show that the antibody does not interact with the mutant proteins on Western blots since they do not contain a threonine to act as an acceptor for phosphate in the Swiss cells. The significance of this analysis is that it demonstrates the specificity of the antibody for phospho-T188 within C/EBPβ and, along with the data in Fig. 2A, confirms that T188 is a target of ERK/GSK3 signaling in Swiss mouse fibroblasts.
FIG. 2.
Phosphorylation of T188 within a consensus ERK/GSK3 site of an ectopic C/EBPβ in response to exposure of Swiss mouse fibroblasts to FGF-2. Swiss mouse fibroblasts expressing either wild-type (Wt), T188A (T-A), or T188D (T-D) forms of C/EBPβ were cultured in growth medium without tetracycline until confluent. Two days later, the cells were exposed to the various effectors in the presence of MIX, DEX, and insulin. (A) The Wt cells were exposed to various combinations of 1 nM FGF-2, 20 μM U0126 (U), 50 μM LY294002 (LY), and 10 mM LiCl (Li) for 30 min and harvested, and the resulting total cell proteins were subjected to Western blot analysis of phospho-C/EBPβ (pC/EBPβ), C/EBPβ, phospho-ERK (pERK), and ERK. (B) Confluent Wt, T-A, and T-D cells were exposed to 1 nM FGF-2 along with DEX, MIX, and insulin for 30 min, and total cell proteins were subjected to Western blot analysis of phospho-C/EBPβ and C/EBPβ.
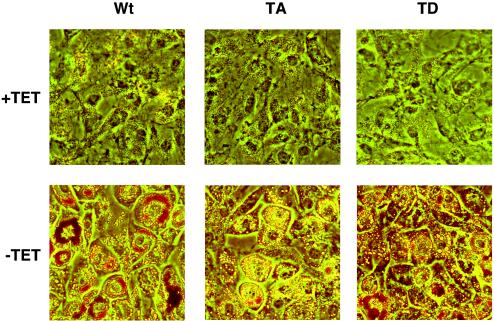
Ectopic expression of wild-type, T188A, and T188D forms of C/EBPβ in Swiss mouse fibroblasts induces accumulation of lipids and morphological differentiation into adipocytic cells.
Our earlier studies have shown that ectopic expression of C/EBPβ in NIH 3T3 fibroblasts induces expression of PPARγ, which, in response to an exogenous PPARγ ligand, leads to lipogenesis and expression of some adipogenic genes, most notably the gene encoding adipose tissue-specific fatty acid binding protein aP2 (36-38). Other adipogenic genes such as the C/EBPα and GLUT4 genes are not expressed; consequently, we chose to investigate the function of C/EBPβ in Swiss mouse fibroblasts, which are more likely to differentiate into mature adipocytes expressing the full complement of adipocyte genes (13). To determine the role of phosphorylation in regulating the adipogenic activity of C/EBPβ, we ectopically expressed LAP and its corresponding ERK/GSK3 site mutant proteins (T188A and T188D) in fibroblasts by culturing the corresponding Swiss-LAP cells (wild type and mutants) for several days in the presence or absence of tetracycline. Confluent cells were then exposed to DEX, MIX, insulin, and troglitazone in the presence or absence of tetracycline. Figure 3 demonstrates that all three cell lines, when cultured in the absence of tetracycline, are capable of undergoing an extensive morphological differentiation, as revealed by the formation of large round cells filled with lipid droplets. It appears that the wild-type cells accumulate more lipid than the T188A mutants even though the latter adopt a round morphology. Culture in the presence of tetracycline to prevent the ectopic expression of the LAP proteins results in maintenance of the original fibroblast morphology throughout the differentiation period.
FIG. 3.
Morphological differentiation of Swiss mouse fibroblasts expressing wild type (Wt), T188A (TA), and T188D (TD) forms of C/EBPβ. The Swiss cell lines corresponding to Wt, TA, and TD forms of C/EBPβ were induced to differentiate for 5 days in the absence or presence of tetracycline (TET), as described in Materials and Methods. The cells were then fixed, stained with Oil Red O, and photographed.
Phosphorylation of C/EBPβ at the ERK/GSK3 site is required for its ability to induce adiponectin gene expression.
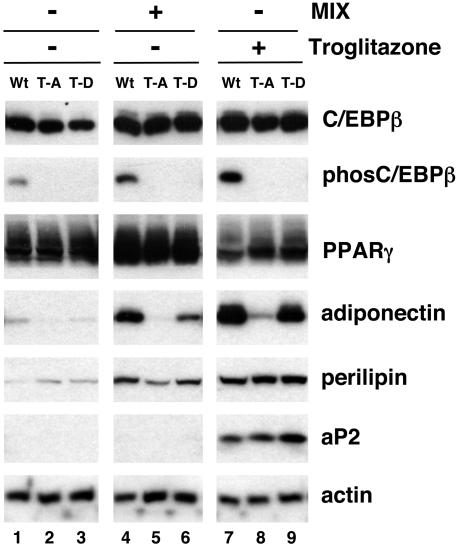
To determine whether these mutant C/EBPs have different effects on adipogenic gene expression, the corresponding Swiss-LAP cells were exposed to DEX and insulin with or without MIX or troglitazone for 5 days and expression of the adipogenic program was assessed by analysis of total cell proteins on Western blots. Exposure of the wild-type cells to DEX and insulin alone in the absence of tetracycline facilitates phosphorylation of the ectopic C/EBPβ and induces expression of PPARγ2 but is incapable of stimulating adipogenesis (Fig. 4, lane 1). Addition of MIX along with DEX and insulin not only causes phosphorylation of C/EBPβ and expression of PPARγ2 but also induces production of adiponectin and perilipin (Fig. 4, lane 4). Interestingly, these conditions are incapable of activating aP2 expression to any significant extent (Fig. 4, lane 4). Activation of PPARγ activity by troglitazone, however, induces aP2 expression and enhances production of the other adipogenic genes (Fig. 4, lane 7). Culture of the T188A mutant cell line in the absence of tetracycline induces abundant expression of C/EBPβ equivalent to the levels produced in the wild-type cells and under all the conditions analyzed is capable of inducing PPARγ2 expression (Fig. 4, lanes 2, 5, and 8). Interestingly, the mutant LAP protein (T188A) in these fibroblasts is not capable of stimulating adiponectin expression to any significant extent compared to wild-type LAP, even in the presence of troglitazone (Fig. 4, compare lanes 5 and 8 with 4 and 7, respectively). Cells that express the T188A protein, however, are capable of synthesizing perilipin and aP2 following exposure to troglitazone. It is noteworthy that the T188D C/EBPβ expresses a significantly higher level of adipogenic activity than the T188A mutant protein but not as high a level as the WT protein. This is due, presumably, to the presence of the negatively charged aspartic acid, which mimics the effect of the phosphorylated threonine 188.
FIG. 4.
Phosphorylation of mouse C/EBPβ at a consensus ERK/GSK3 site (T188) is required for the induction of adiponectin gene expression during the differentiation of mouse fibroblasts. Swiss mouse fibroblast cell lines (wild type [Wt], T188A [T-A], and T188D [T-D]) were grown to confluence in the absence of tetracycline to induce expression of the corresponding C/EBPβ protein. Two days later, the cells were treated with either MIX or troglitazone in the presence of DEX and insulin. The cells were harvested 5 days later and subjected to Western blot analysis of phospho-C/EBPβ (phosC/EBPβ), C/EBPβ, PPARγ, perilipin, adiponectin, aP2, and actin.
Phosphorylation of C/EBPβ at T188 is required to induce expression of C/EBPα as well as adiponectin.
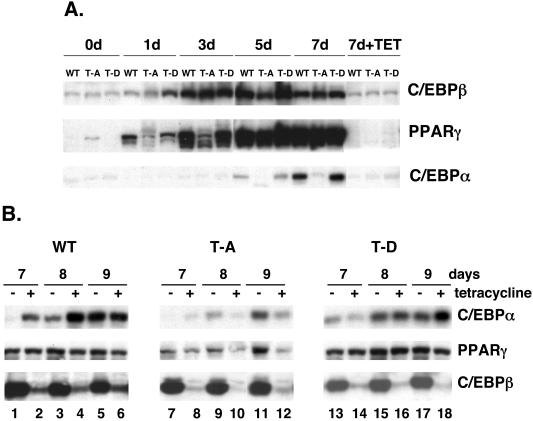
To define the role of phospho-C/EBPβ in regulating adipogenesis, we analyzed the sequential expression of selected adipogenic proteins during the differentiation of wild-type, T188A, and T188D cell lines. Figure 5A demonstrates that exposure of the cells to DEX, MIX, insulin, and troglitazone in the absence of tetracycline leads to an increase in the ectopic C/EBPβ proteins within the initial 24 h, which reaches a maximum of expression by day 3 and remains at these high levels throughout the 7-day culture period. All three cell lines express approximately the same amount of C/EBPβ. PPARγ expression is induced within the initial 1 to 3 days of differentiation and is significantly more apparent at this time in the wild-type and T188D cells than in the T188A cell line. By day 5, it appears that all three cell lines express equivalent quantities of PPARγ. This is in contrast to the expression of C/EBPα, which is abundantly expressed in wild-type and T188D cells but which is virtually absent in cells expressing the T188A mutant protein.
FIG. 5.
Phosphorylation of mouse C/EBPβ at a consensus ERK/GSK3 site (T188) is required for C/EBPα expression and for sustaining terminal adipogenesis. (A) Swiss mouse fibroblast cell lines (wild type [WT], T188A [T-A], and T188D [T-D]) were cultured in growth medium with or without tetracycline until confluent. Two days later, the cells were induced to differentiate by exposure to DEX, MIX, FBS, insulin, and troglitazone for 2 days in the absence or presence of tetracycline. At this stage, cells were treated with insulin and troglitazone in the presence (7d+TET) or absence of tetracycline and harvested at the indicated times. (B) The Swiss-LAP cells (WT, T-A, or T-D) were induced to differentiate in the absence of tetracycline for 6 days as outlined for panel A. At this stage, 1 μg of tetracycline (+) or vehicle (−) was added to the culture medium of each cell line to suppress expression of the ectopic C/EBPβ, and cells were harvested for nuclear proteins at the indicated times. Equal amounts of nuclear proteins from each cell sample (A and B) were subjected to Western blot analysis of C/EBPβ, PPARγ, and C/EBPα.
The data in Fig. 5A suggest that phosphorylation of C/EBPβ is also required to facilitate expression of C/EBPα in response to activation of PPARγ. It is also possible that the T188A mutant C/EBPβ acts as a dominant negative factor which blocks C/EBPα expression. To investigate this possibility, differentiation was induced in three cell lines for 6 days as outlined in Fig. 5A in order to facilitate induction of PPARγ and then ectopic C/EBPβ expression was inhibited by treating the cultures with tetracycline. Figure 5B shows a Western blot analysis of proteins isolated from the cells treated with or without tetracycline for increasing times following the 6-day time period. Addition of tetracycline results in an extensive decrease in expression of the ectopic C/EBPβ in all three cell lines compared to the levels of expression in cells maintained in the absence of tetracycline (Fig. 5B, compare lanes 2, 4, 6, 8, 10, 12, 14, 16, and 18 with 1, 3, 5, 7, 9, 11, 13, 15, and 17). In the wild-type and T188D cells, the decrease in C/EBPβ expression had no effect on the abundant expression of PPARγ or C/EBPα during the 3 days of exposure to tetracycline (Fig. 5B, lanes 2, 4, 6, 14, 16, and 18). These data suggest that, once the ectopic C/EBPβ (wild type or T188D) has established an adipogenic state by inducing PPARγ and C/EBPα, the adipogenic program is self-sustaining, probably as a result of the cross-regulation of PPARγ and C/EBPα. In contrast, suppression of the T188A mutant C/EBPβ at day 6 results in a corresponding down-regulation of PPARγ expression and a decrease in the already low levels of C/EBPα (Fig. 5B, lanes 8, 10, and 12). It appears, therefore, that the T188A C/EBPβ is not acting as a dominant negative molecule but instead is incapable of producing the quantity of C/EBPα required to maintain PPARγ expression during terminal adipogenesis.
Mutant C/EBPβ T188A can bind to a C/EBP response element from the proximal promoter of the C/EBPα gene.
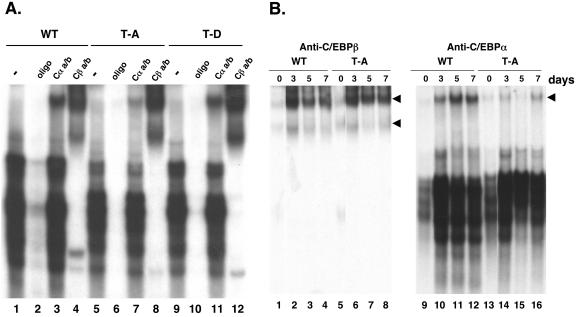
To determine whether the inability of C/EBPβ T188A to stimulate C/EBPα expression stems from its inability to bind to the C/EBP response element within the proximal promoter of the C/EBPα gene, we compared the DNA binding activities of wild-type, T188A, and T188D proteins to a radiolabeled oligonucleotide corresponding to the response element (GCGTTGCGCCACGATG; the response element is underlined). The EMSA presented in Fig. 6A demonstrates that all three proteins expressed in cells that had been differentiated for 5 days avidly bind to the radiolabeled oligonucleotide (lanes 1, 5, and 9), giving rise to several distinct DNA-protein complexes. It is noteworthy that the profile of complexes in the T188A EMSA differs somewhat from those in the wild-type and T188D EMSAs (Fig. 6A, compare lane 5 with 1 and 9). A supershift EMSA using an anti-C/EBPβ antibody confirms that all of these complexes contain C/EBPβ and also demonstrates that the T188A C/EBPβ is as efficient at forming these complexes as the wild-type and T188D proteins. As expected, the abundance of C/EBPα-containing complexes in the T188A cells is significantly lower than that in wild-type or T188D cells, as revealed by the supershift EMSA using an anti-C/EBPα antibody (Fig. 6A, compare lane 7 with 3 and 11). The supershift EMSA presented in Fig. 6B further shows that the T188A C/EBPβ binds to the C/EBP response element as avidly as the wild-type protein throughout the differentiation process (compare lanes 5 to 8 with 1 to 4). In contrast, as observed in Fig. 6A, the abundance of the complexes containing C/EBPα in the T188A cells is significantly lower than that in wild-type cells, consistent with the significantly lower level of expression of the C/EBPα protein in the T188A cells (Fig. 5).
FIG. 6.
Phosphorylation of C/EBPβ at T188 is not required for its ability to bind to a C/EBP regulatory element from the promoter of the C/EBPα gene. Swiss fibroblasts expressing wild-type (WT), T188A (T-A), or T188D (T-D) forms of C/EBPβ were induced to differentiate as described for Fig. 5A, and cells were harvested at either day 7 (A) or the indicated times (B) for preparation of nuclear extracts. Equal amounts of each extract were preincubated for 30 min in the presence or absence of antiserum for either C/EBPβ (Cβa/b) or C/EBPα (Cαa/b) or a 100-fold excess of cold C/EBP-oligonucleotide (C/EBP binding site in the C/EBPα promoter) prior to addition of a 32P-labeled C/EBP-oligonucleotide. The binding of the oligonucleotide to the different C/EBP complexes was analyzed by EMSA, as described in Materials and Methods. Arrowheads, corresponding supershifted complexes.
Induction of adiponectin expression by PPARγ in mouse fibroblasts requires C/EBPα.
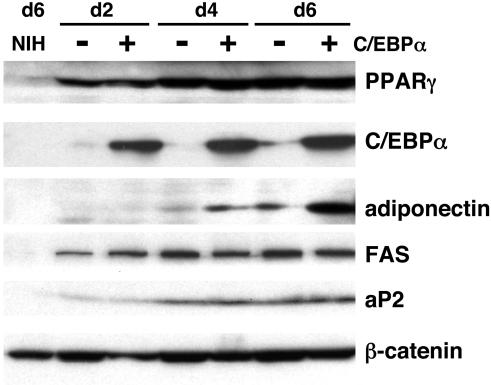
The data presented above suggested that the inability of the T188A mutant protein to activate adiponectin expression in mouse fibroblasts results from its inability to induce expression of C/EBPα. To investigate the role of C/EBPα in regulating adiponectin expression, we generated mouse fibroblasts expressing PPARγ with or without C/EBPα by infecting NIH 3T3 cells (C/EBPα deficient) with retroviral vectors to facilitate expression of C/EBPα and/or PPARγ (i.e., NIH 3T3-Pγ+/Cα− [Cα−] and NIH 3T3-Pγ+/Cα+ [Cα+]). Each cell line was induced to differentiate by exposure to DEX, MIX, insulin, and troglitazone, and total cell proteins prepared at different times were analyzed on Western blots. Figure 7 shows the constitutive expression of PPARγ2 in both cell lines (Cα− and Cα+) and the absence of C/EBPα in the Cα− cell line. Figure 7 also shows an extensive induction of fatty acid synthase and aP2 expression following exposure of both cell lines to the adipogenic inducers. In contrast, adiponectin expression is activated to a significant extent only in the NIH 3T3 fibroblasts expressing both PPARγ2 and C/EBPα (Fig. 7, compare lanes 6 and 7).
FIG. 7.
Induction of adiponectin expression in mouse fibroblasts by PPARγ2 requires expression of C/EBPα. Confluent NIH 3T3 cell lines expressing PPARγ2 with (+C/EBPα) or without (−C/EBPα) were induced to differentiate by exposure to DEX, MIX, FBS, insulin, and troglitazone. At day 2, cells were treated with insulin and troglitazone and harvested at the indicated times for Western blot analysis of PPARγ2, C/EBPα, adiponectin, fatty acid synthase (FAS), aP2, and β-catenin. Noninfected NIH 3T3 fibroblasts were also subjected to the same differentiation protocol for 6 days in order to illustrate the absence of adipogenic proteins.
DISCUSSION
In this report, we demonstrate that phosphorylation of the LAP isoform of C/EBPβ at a consensus ERK/GSK3 site regulates adiponectin gene expression during the C/EBPβ-facilitated differentiation of mouse fibroblasts into adipocytes. The data show that conditional ectopic expression of a mutant form of LAP in which threonine 188 has been replaced with alanine (T188A) is incapable of stimulating adiponectin gene expression, whereas wild-type LAP and a T188D mutant protein express this activity. Phosphorylation of T188 within the consensus ERK/GSK site is likely regulated by proadipogenic effectors by stimulating MEK1 activity during the early phase of adipogenesis in mouse preadipocytes or fibroblasts ectopically expressing C/EBPβ. The inability of the T188A mutant protein to induce adiponectin expression appears to be due to its inability to stimulate C/EBPα expression. Consistent with this notion is the fact that PPARγ2 is capable of stimulating adiponectin expression to a significant extent only in fibroblasts that also express C/EBPα. This finding is in agreement with a recent study suggesting a role for C/EBPα in regulating adiponectin expression in mouse embryo fibroblasts (14).
Identification of T188 in LAP as a target for phosphorylation by ERK and GSK3 in response to exposure of fibroblasts to adipogenic effectors.
The data demonstrate that threonine 188 of the LAP isoform of murine C/EBPβ is a target of ERK and GSK3 in vivo (Fig. 2) as well as in vitro (data not shown). Threonine 188 is located within a domain of LAP that contains several serine and threonine residues that are potential targets for phosphorylation. In fact, a recent study has demonstrated that most of these residues are phosphorylated to some extent by constitutive signaling pathways (3). The studies presented in Fig. 2A that suggest that both ERK and GSK3 can phosphorylate LAP on T188 in response to exposure of mouse fibroblasts to FGF-2 are based on the use of an anti-phospho-C/EBPβ antibody to detect phosphorylated C/EBPβ. The specificity of this antibody for phosphorylated T188 is presented in Fig. 2B, which shows that, in contrast to the wild-type LAP protein, the T188A and T188D mutant proteins do not react with the antibody on Western blots. It appears from the data in Fig. 2A that FGF-2 stimulates the phosphorylation of LAP through at least two separate signaling pathways. The use of U0126 suggests that MEK1/ERK signaling is involved, and the use of both LiCl to block GSK3 and LY294002 to potentially enhance GSK3 activity by attenuating PI-3 kinase/Akt activity supports the involvement of a PI-3 kinase/GSK3 pathway.
Role of T188 in regulating the adipogenic activity of C/EBPβ.
The direct involvement of T188 in regulating the adipogenic activity of C/EBPβ is clearly demonstrated in Fig. 4. Conditional ectopic expression of LAP in nonadipogenic mouse fibroblasts induces PPARγ expression and under specified adipogenic conditions also facilitates conversion of these cells into mature adipocytes based on the accumulation of lipid droplets and expression of adiponectin, perilipin, and FABP4 (aP2). Ectopic expression of the LAP T188A mutant protein in the mouse cells can also stimulate expression of the PPARγ gene and some other adipocyte genes, but it is incapable of inducing adiponectin gene expression. In contrast, the LAP T188D mutant protein expresses a level of adipogenic activity which approaches that of the normal LAP protein. These observations suggest that phosphorylation of C/EBPβ by ERK and/or GSK3 in response to extracellular effectors regulates its adipogenic activity, which includes coordinating the expression of the full complement of genes during terminal adipogenesis, most notably adiponectin. Previous studies by us have shown a positive role for the activation of MEK/ERK signaling during the early phase of adipogenesis in 3T3-L1 preadipocytes (27). Specifically, we demonstrated that inhibition of MEK1 activity with U0126 blocked the expression of PPARγ and C/EBPα in 3T3-L1 preadipocytes and, consequently, prevented the differentiation of these cells into mature adipocytes. The studies presented above, however, demonstrate that preventing the phosphorylation of the consensus ERK/GSK3 domain within C/EBPβ during the differentiation of mouse fibroblasts has a much more selective effect on adipogenic gene expression since induction of PPARγ, perilipin, and aP2 expression occurred in the T188A mutant cells. It is likely that phosphorylation of C/EBPβ is only one of several transcriptional processes that is regulated by MEK/ERK signaling during the early phase of adipogenesis. In fact, recent studies have suggested that clonal expansion, which is required for terminal adipogenesis, is also regulated by MEK/ERK signaling (32).
What is the mechanism by which phosphorylation of C/EBPβ selectively regulates adipogenic gene expression?
It appears from the data in Fig. 4 that the T188A mutant C/EBPβ is as capable of activating PPARγ expression as the wild-type protein in the corresponding Swiss cells following exposure to DEX and insulin with or without MIX or troglitazone. In contrast, the mutant protein is not as effective at inducing C/EBPα expression when cells are exposed to the adipogenic inducers, including troglitazone (Fig. 5). This effect does not appear to be due to any significant decrease in the ability of the T188A C/EBPβ to bind to a C/EBP regulatory element located in the minimal promoter of the C/EBPα gene (Fig. 6). It is possible, however, that the phosphorylation-defective C/EBPβ may recruit corepressors to the C/EBPα gene and not the PPARγ gene. Recent studies have suggested that the transcriptional regulation of the PPARγ gene by C/EBPs differs from that of other C/EBP target genes. In fact, the C/EBP response element within the proximal promoter of the PPARγ2 gene does not conform to the usual consensus sequence (12). The element consists of two overlapping sequences that direct the interaction of C/EBP with nuclear factor of activated T cells (NFAT) to form an enhancer complex responsible for NFAT-mediated activation of PPARγ2 gene transcription (40). It is conceivable, therefore, that phosphorylation of T188 of C/EBPβ is not required for the formation of this unique enhancer complex. In contrast, however, the C/EBP regulatory element within the C/EBPα promoter conforms to a consensus sequence similar to that present in many other C/EBP target genes. Furthermore, studies have shown that C/EBPβ and C/EBPα are potent activators of C/EBPα gene transcription through direct interaction with this response element in the proximal promoter. The data presented above are consistent, therefore, with the notion that the unphosphorylated form of C/EBPβ (T188A) is incapable of activating C/EBPα transcription. It follows that the absence of C/EBPα is responsible for the lack of adiponectin expression since the studies shown in Fig. 7 clearly demonstrate that expression of adiponectin during adipogenesis depends on C/EBPα expression.
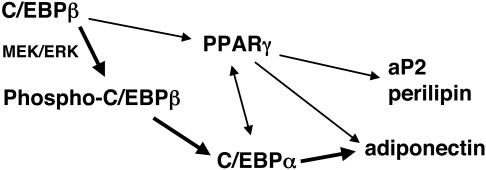
In conclusion, these studies identify an important role for phosphorylation of C/EBPβ at a consensus ERK/GSK3 site in regulating the expression of adiponectin during adipogenesis. The model presented in Fig. 8 illustrates a signaling pathway leading to adiponectin which includes phosphorylation of C/EBPβ and activation of C/EBPα. It is likely that this pathway also requires activation of PPARγ since terminal adipogenesis depends on PPARγ activity. These observations, however, raise the possibility of identifying targets that may facilitate modulation of adiponectin expression without affecting other features of adipocyte function. For instance, promoting phosphorylation of C/EBPβ in insulin-resistant adipocytes may enhance secretion of adiponectin without increasing accumulation of lipid and, in so doing, may be an effective therapy for metabolic syndrome.
FIG. 8.
Transcription factors and signaling pathways regulating adiponectin expression during adipogenesis. This model is based on this study and other studies referred to in the text.
Acknowledgments
This work was supported by USPHS grants DK51586 and DK58825.
REFERENCES
- 1.An, M. R., C. C. Hsieh, P. D. Reisner, J. P. Rabek, S. G. Scott, D. T. Kuninger, and J. Papaconstantinou. 1996. Evidence for posttranscriptional regulation of C/EBPα and C/EBPβ isoform expression during the lipopolysaccharide-mediated acute-phase response. Mol. Cell. Biol. 16:2295-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barak, Y., M. C. Nelson, E. S. Ong, Y. Z. Jones, P. Ruiz-Lozano, K. R. Chien, A. Koder, and R. M. Evans. 1999. PPARγ is required for placental, cardiac, and adipose tissue development. Mol. Cell 4:585-595. [DOI] [PubMed] [Google Scholar]
- 3.Bradley, M. N., L. Zhou, and S. T. Smale. 2003. C/EBPβ regulation in lipopolysaccharide-stimulated macrophages. Mol. Cell. Biol. 23:4841-4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buck, M., V. Poli, T. Hunter, and M. Chojkier. 2001. C/EBPβ phosphorylation by RSK creates a functional XEXD caspase inhibitory box critical for cell survival. Mol. Cell 8:807-816. [DOI] [PubMed] [Google Scholar]
- 5.Buck, M., V. Poli, P. van der Geer, M. Chojkier, and T. Hunter. 1999. Phosphorylation of rat serine 105 or mouse threonine 217 in C/EBP beta is required for hepatocyte proliferation induced by TGFα. Mol. Cell 4:1087-1092. [DOI] [PubMed] [Google Scholar]
- 6.Calkhoven, C. F., C. Muller, and A. Leutz. 2000. Translational control of C/EBPα and C/EBPβ isoform expression. Genes Dev. 14:1920-1932. [PMC free article] [PubMed] [Google Scholar]
- 7.Chinery, R., J. A. Brockman, D. T. Dransfield, and R. J. Coffey. 1997. Antioxidant-induced nuclear translocation of CCAAT/enhancer-binding protein β. A critical role for protein kinase A-mediated phosphorylation of Ser299. J. Biol. Chem. 272:30356-30361. [DOI] [PubMed] [Google Scholar]
- 8.Clarke, S. L., C. E. Robinson, and J. M. Gimble. 1997. CAAT/enhancer binding proteins directly modulate transcription from the peroxisome proliferator-activated receptor γ2 promoter. Biochem. Biophys. Res. Commun. 240:99-103. [DOI] [PubMed] [Google Scholar]
- 9.Darlington, G. J., S. E. Ross, and O. A. MacDougald. 1998. The role of C/EBP genes in adipocyte differentiation. J. Biol. Chem. 273:30057-30060. [DOI] [PubMed] [Google Scholar]
- 10.Descombes, P., and U. Schibler. 1991. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell 67:569-579. [DOI] [PubMed] [Google Scholar]
- 11.Eaton, E. M., M. Hanlon, L. Bundy, and L. Sealy. 2001. Characterization of C/EBPβ isoforms in normal versus neoplastic mammary epithelial cells. J. Cell Physiol. 189:91-105. [DOI] [PubMed] [Google Scholar]
- 12.Elberg, G., J. M. Gimble, and S. Y. Tsai. 2000. Modulation of the murine peroxisome proliferator-activated receptor γ2 promoter activity by CCAAT/enhancer-binding proteins. J. Biol. Chem. 275:27815-27822. [DOI] [PubMed] [Google Scholar]
- 13.El-Jack, A. K., J. K. Hamm, P. F. Pilch, and S. R. Farmer. 1999. Reconstitution of insulin-sensitive glucose transport in fibroblasts requires expression of both PPARγ and C/EBPα. J. Biol. Chem. 274:7946-7951. [DOI] [PubMed] [Google Scholar]
- 14.Gustafson, B., M. M. Jack, S. W. Cushman, and U. Smith. 2003. Adiponectin gene activation by thiazolidinediones requires PPAR γ2, but not C/EBPα: evidence for differential regulation of the aP2 and adiponectin genes. Biochem. Biophys. Res. Commun. 308:933-939. [DOI] [PubMed] [Google Scholar]
- 15.Hamm, J. K., A. K. el Jack, P. F. Pilch, and S. R. Farmer. 1999. Role of PPARγ in regulating adipocyte differentiation and insulin-responsive glucose uptake. Ann. N. Y. Acad. Sci. 892:134-145. [DOI] [PubMed] [Google Scholar]
- 16.Hamm, J. K., B. H. Park, and S. R. Farmer. 2001. A role for C/EBPβ in regulating peroxisome proliferator-activated receptor gamma activity during adipogenesis in 3T3-L1 preadipocytes. J. Biol. Chem. 276:18464-18471. [DOI] [PubMed] [Google Scholar]
- 17.Hanlon, M., T. W. Sturgill, and L. Sealy. 2001. ERK2- and p90(Rsk2)-dependent pathways regulate the CCAAT/enhancer-binding protein-beta interaction with serum response factor. J. Biol. Chem. 276:38449-38456. [DOI] [PubMed] [Google Scholar]
- 18.Kowenz-Leutz, E., and A. Leutz. 1999. A C/EBPβ isoform recruits the SWI/SNF complex to activate myeloid genes. Mol. Cell 4:735-743. [DOI] [PubMed] [Google Scholar]
- 19.Kowenz-Leutz, E., G. Twamley, S. Ansieau, and A. Leutz. 1994. Novel mechanism of C/EBPβ (NF-M) transcriptional control: activation through derepression. Genes Dev. 8:2781-2791. [DOI] [PubMed] [Google Scholar]
- 20.Linhart, H. G., K. Ishimura-Oka, F. DeMayo, T. Kibe, D. Repka, B. Poindexter, R. J. Bick, and G. J. Darlington. 2001. C/EBPα is required for differentiation of white, but not brown, adipose tissue. Proc. Natl. Acad. Sci. USA 98:12532-12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moldes, M., Y. Zuo, R. F. Morrison, D. Silva, B. H. Park, J. Liu, and S. R. Farmer. 2003. Peroxisome-proliferator-activated receptor gamma suppresses Wnt/β-catenin signalling during adipogenesis. Biochem. J. 376:607-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrison, R. F., and S. R. Farmer. 1999. Insights into the transcriptional control of adipocyte differentiation. J. Cell Biochem. 1999(Suppl. 32-33):59-67. [DOI] [PubMed] [Google Scholar]
- 23.Nakajima, T., S. Kinoshita, T. Sasagawa, K. Sasaki, M. Naruto, T. Kishimoto, and S. Akira. 1993. Phosphorylation at threonine-235 by a ras-dependent mitogen-activated protein kinase cascade is essential for transcription factor NF-IL6. Proc. Natl. Acad. Sci. USA 90:2207-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pedersen, T. A., E. Kowenz-Leutz, A. Leutz, and C. Nerlov. 2001. Cooperation between C/EBPα TBP/TFIIB and SWI/SNF recruiting domains is required for adipocyte differentiation. Genes Dev. 15:3208-3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piwien-Pilipuk, G., O. MacDougald, and J. Schwartz. 2002. Dual regulation of phosphorylation and dephosphorylation of C/EBPβ modulates its transcriptional activation and DNA binding in response to growth hormone. J. Biol. Chem. 277:44557-44565. [DOI] [PubMed] [Google Scholar]
- 26.Piwien-Pilipuk, G., D. Van Mater, S. E. Ross, O. A. MacDougald, and J. Schwartz. 2001. Growth hormone regulates phosphorylation and function of CCAAT/enhancer-binding protein beta by modulating Akt and glycogen synthase kinase-3. J. Biol. Chem. 276:19664-19671. [DOI] [PubMed] [Google Scholar]
- 27.Prusty, D., B. H. Park, K. E. Davis, and S. R. Farmer. 2002. Activation of MEK/ERK signaling promotes adipogenesis by enhancing PPARγ and C/EBPα gene expression during the differentiation of 3T3-L1 preadipocytes. J. Biol. Chem. 277:46226-46232. [DOI] [PubMed] [Google Scholar]
- 28.Rana, B., Y. Xie, D. Mischoulon, N. L. R. Bucher, and S. R. Farmer. 1995. The DNA binding activity of C/EBP transcription factors is regulated in G1 phase of the hepatocyte cell cycle. J. Biol. Chem. 270:18123-18132. [DOI] [PubMed] [Google Scholar]
- 29.Rosen, E. D., C. H. Hsu, X. Wang, S. Sakai, M. W. Freeman, F. J. Gonzalez, and B. M. Spiegelman. 2002. C/EBPα induces adipogenesis through PPARγ unified pathway. Genes Dev. 16:22-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosen, E. D., P. Sarraf, A. E. Troy, G. Bradwin, K. Moore, D. S. Milstone, B. M. Spiegelman, and R. M. Mortensen. 1999. PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell 4:611-617. [DOI] [PubMed] [Google Scholar]
- 31.Rosen, E. D., C. J. Walkey, P. Puigserver, and B. M. Spiegelman. 2000. Transcriptional regulation of adipogenesis. Genes Dev. 14:1293-1307. [PubMed] [Google Scholar]
- 32.Tang, Q. Q., T. C. Otto, and M. D. Lane. 2003. Mitotic clonal expansion: a synchronous process required for adipogenesis. Proc. Natl. Acad. Sci. USA 100:44-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trautwein, C., C. Caelles, P. van der Geer, T. Hunter, M. Karin, and M. Chojkier. 1993. Transactivation by NF-IL6/LAP is enhanced by phosphorylation of its activation domain. Nature 364:544-547. [DOI] [PubMed] [Google Scholar]
- 34.Trautwein, C., P. van der Geer, M. Karin, T. Hunter, and M. Chojkier. 1994. Protein kinase A and C site-specific phosphorylations of LAP (NF-IL6) modulate its binding affinity to DNA recognition elements. J. Clin. Investig. 93:2554-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wegner, M., Z. Cao, and M. G. Rosenfeld. 1992. Calcium-regulated phosphorylation within the leucine zipper of C/EBPβ. Science 256:370-373. [DOI] [PubMed] [Google Scholar]
- 36.Wu, Z., N. L. R. Bucher, and S. R. Farmer. 1996. Induction of peroxisome proliferator-activated receptor gamma during conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPβ, C/EBPδ, and glucocorticoids. Mol. Cell. Biol. 16:4128-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu, Z., Y. Xie, N. L. R. Bucher, and S. R. Farmer. 1995. Conditional ectopic expression of C/EBPβ in NIH 3T3 cells induces PPARγ and stimulates adipogenesis. Genes Dev. 9:2350-2363. [DOI] [PubMed] [Google Scholar]
- 38.Wu, Z., Y. Xie, R. F. Morrison, N. L. Bucher, and S. R. Farmer. 1998. PPARγ induces the insulin-dependent glucose transporter GLUT4 in the absence of C/EBPα during the conversion of 3T3 fibroblasts into adipocytes. J. Clin. Investig. 101:22-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiong, W., C. C. Hsieh, A. J. Kurtz, J. P. Rabek, and J. Papaconstantinou. 2001. Regulation of CCAAT/enhancer-binding protein-beta isoform synthesis by alternative translational initiation at multiple AUG start sites. Nucleic Acids Res. 29:3087-3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang, T. T., and C. W. Chow. 2003. Transcription cooperation by NFAT.C/EBP composite enhancer complex. J. Biol. Chem. 278:15874-15885. [DOI] [PubMed] [Google Scholar]
- 41.Yeh, W. C., Z. Cao, M. Classon, and S. L. McKnight. 1995. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev. 9:168-181. [DOI] [PubMed] [Google Scholar]