Abstract
Introduction
To compare the differences in setup errors measured with electronic portal image (EPI) and cone‐beam computed tomography (CBCT) in patients undergoing tangential breast radiotherapy (RT). Relationship between setup errors, body mass index (BMI) and breast size was assessed.
Methods
Twenty‐five patients undergoing postoperative RT to the breast were consented for this study. Weekly CBCT scans were acquired and retrospectively registered to the planning CT in three dimensions, first using bony anatomy for bony registration (CBCT‐B) and again using breast tissue outline for soft tissue registration (CBCT‐S). Digitally reconstructed radiographs (DRR) generated from CBCT to simulate EPI were compared to the planning DRR using bony anatomy in the V (parallel to the cranio‐caudal axis) and U (perpendicular to V) planes. The systematic (Σ) and random (σ) errors were calculated and correlated with BMI and breast size.
Results
The systematic and random errors for EPI (Σ V = 3.7 mm, Σ U = 2.8 mm and σ V = 2.9 mm, σ U = 2.5) and CBCT‐B (Σ V = 3.5 mm, Σ U = 3.4 mm and σ V = 2.8 mm, σ U = 2.8) were of similar magnitude in the V and U planes. Similarly, the differences in setup errors for CBCT‐B and CBCT‐S in three dimensions were less than 1 mm. Only CBCT‐S setup error correlated with BMI and breast size.
Conclusions
CBCT and EPI show insignificant variation in their ability to detect setup error. These findings suggest no significant differences that would make one modality considered superior over the other and EPI should remain the standard of care for most patients. However, there is a correlation with breast size, BMI and setup error as detected by CBCT‐S, justifying the use of CBCT‐S for larger patients.
Keywords: Breast cancer, cone‐beam computed tomography (CBCT), electronic portal image (EPI), radiotherapy, setup errors
Introduction
Radiotherapy (RT) is an essential component of therapy for a substantial majority of breast cancer patients.1, 2 Traditionally RT to the breast was delivered with simple planning techniques involving two opposing tangential beams. This technique has been successful in improving local control.3, 4 In recent years, more highly conformal techniques have been used such as the use of mini‐beams, intensity modulated RT (IMRT) and simultaneously integrated boost, leading to improvements in three‐dimensional (3D) dose distributions.5, 6, 7 However, highly conformal techniques will only be of maximum benefit if patient setup errors can be minimised.
Until recently, electronic portal imaging (EPI) was the standard used for imaging in breast RT for setup verification. However, use of this modality for setup purposes has inherent limitations, as bony anatomy such as chest wall is used as a surrogate for treatment position verification, without assessment of the soft tissue. Cone‐beam computed tomography (CBCT) has been integrated into routine clinical practice in many tumour sites, as it has been shown to offer advantages over EPI.8, 9, 10 The main advantages of using CBCT instead of EPI are the use of 3D instead of two‐dimensional (2D) images of the patient's internal anatomy, and its ability to offer better soft tissue and anatomical contrast.11, 12 In breast RT, only one study has examined this and shown that EPI registration underestimated the bony anatomy setup error compared to CBCT, however, EPI was deemed adequate for tangential breast RT.13 As the breast consists of soft tissue, it is possible that clinically important differences in day‐to‐day soft tissue positioning go unnoticed when assessments based on bony anatomy alone are performed. However, this does not necessarily guarantee that the imaging modality has sufficient positives for each tumour site to be uniformly adopted without evaluation as there are financial costs and potential second malignancy issues to consider with using CBCT.14, 15
The breast is a superficial organ and may have significant potential motion relative to internal organs. Therefore it may be more difficult to be immobilised and this could be more significant in a larger sized breast and/or patient. Several studies have reported the correlation between body mass index (BMI) and setup errors for various tumour sites including endometrial,16 prostate17 and abdominal18 malignancies, however, correlation in breast RT has not been reported. Assessment of the impact of breast size and BMI on setup errors for breast RT is necessary.
Previous studies have used bony anatomy, external patient outline or surgical clips to register the CBCT with a planning reference.13, 19, 20, 21, 22, 23 This study aimed to compare three different methods (EPI, CBCT bony matching and CBCT soft tissue matching) in measuring setup errors in breast RT. The effects of BMI and breast size on the resulting setup errors were also investigated.
Methods
Patients and planning
Twenty‐five patients undergoing postoperative RT to the breast were prospectively recruited. This sample size was calculated to obtain 80% power to detect a 5‐mm difference within a single plane between the three registration methods. Approval was granted by the local Human Research Ethics Committee for this study. All patients provided written informed consent. The inclusion criteria were patients aged 18 years or older and undergoing breast‐conservation surgery and adjuvant breast tangent RT and no nodal RT. Patients with larger body habitus (BMI larger than 45), bra cup bigger than ‘D’ cup and/or breast volume larger than 1800 cc24 were assessed individually for potential exclusion due to possible collision between the gantry head and patient. This assessment resulted in five patients being excluded from the study. Patients were selected consecutively until 25 cases that met the inclusion criteria were accrued. Patients were positioned supine with a Vacbag on an inclined breast board (MT‐350 Breastboard, CIVCO Medical Solutions, Orange City, IA) with both arms raised above their head. A free breathing non‐contrast fan‐beam computed tomography (CT) scan was performed with 0.2 cm slice thickness using Siemens Somatom Sensation 4 scanner (Siemens Medical Solutions, Germany). CT images with 0.2 cm resolution were used for treatment planning and as a reference for registration with CBCT. A planning digitally reconstructed radiograph (DRR) was also generated for registration with EPI. The following factors were also recorded for correlation measurements with setup errors: BMI and breast size. Patients were categorised according to their breast size into small, medium and large as described by Ramsey et al.25 where breast volumes of <700 cc correspond to small, 700–1100 cc correspond to medium and volumes of >1000 cc correspond to large breasts.
CBCT imaging
The Elekta Synergy XVI system (version 4.5.1, Elekta, Stockholm, Sweden) was used to acquire kilovoltage (kV) CBCT scans of the patients. A total of five images were acquired for each patient in the treatment position prior to radiation delivery at fractions 2, 7, 12, 17 and 22. Scans were acquired at the treatment isocentre. The following scanning protocol was used: approximately 350 2D kV image projections were acquired during a 60 sec, 270° clockwise rotation (180°–90° for right breast and 270°–180° for left breast). The acquisition parameters were 120 kV, 322 mGy and 140 mAs per projection. A S20 collimator cassette was used on all patients giving a nominal irradiated scan length at the isocentre of approximately 26 cm and a similar reconstruction diameter. The three‐dimensional (3D) CBCT scan was reconstructed at 0.2 cm resolution in all three dimensions. The CBCT scans were imported into the planning system (Xio version 4.64, Elekta, Stockholm, Sweden) to generate DRRs to simulate EPIs. A medial tangent EPI with a resolution of 0.2 cm was generated as this field is the standard of practice for image assessment in tangential breast RT in our centre.
Image registration
To complete the CBCT bony registration (CBCT‐B), the scans were rigidly registered in 3D to the planning CT on the bony anatomy using a predefined box‐shaped (‘clipbox’) region of interest containing sternum and ribs on the irradiated side. Automatic registrations were performed by the imaging software and in some instance manual adjustments were performed by a radiation therapist. CBCT soft tissue registration (CBCT‐S) was completed manually where the breast tissue outline was matched to the planning CT. CBCT registrations did not include any degree of rotation. All registrations and measurements were completed offline by a radiation therapist and checked independently by another therapist. No additional training was provided to the radiation therapists to perform this task apart from the general vendor training to use the imaging software.
Data analysis
For each of the simulated EPIs, the following measurements were manually taken in the reference frame of the EPI planar image, described as the V (parallel to the cranio‐caudal axis and the medial tangent beam) and U (perpendicular to V and central beam axis) coordinate system following the approach initially taken by Topolnjak et al.13 The measurements were completed in the V and U coordinates as this is clinically relevant for a tangential technique, where the usual 3D (left–right/cranio‐caudal/anterior–posterior) coordinate system would require additional imaging angles, different to the treatment technique. Cranio‐caudal distance (CCD) is the distance from the inferior skin edge to the inferior field edge at the central plane of the beam and central lung distance (CLD) is the distance between the posterior field edge and the chest wall interface at the central axis of the field (Fig. 1). These measurements were compared with the planning DRR to determine setup errors (Fig. 2).
Figure 1.
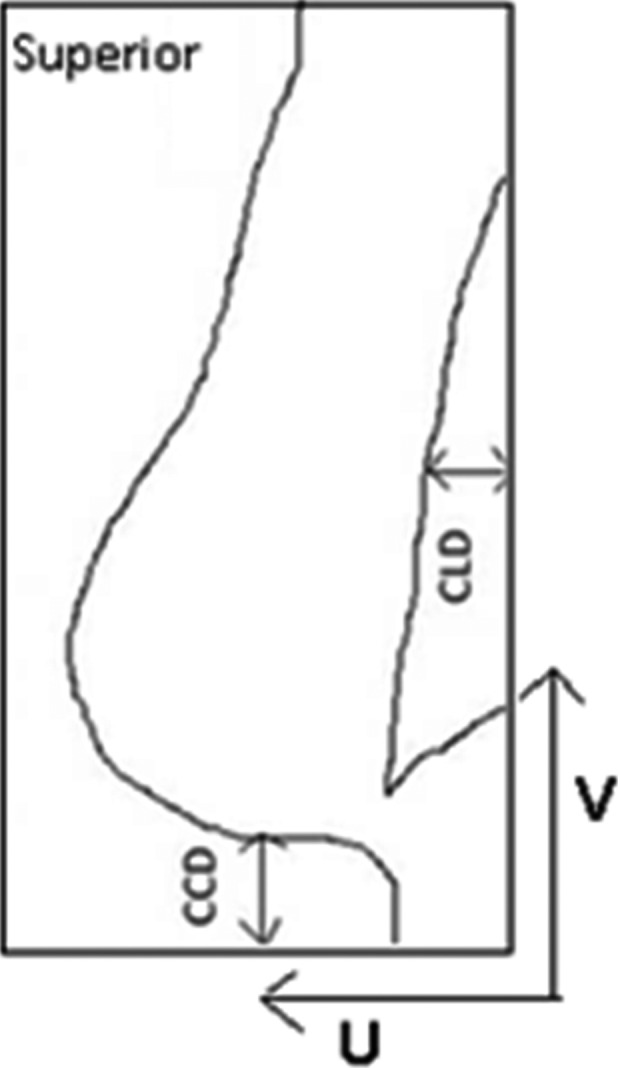
Measurements taken for planning digitally reconstructed radiograph and simulated electronic portal image taken in V: cranio‐caudal distance and U: central lung distance planes.
Figure 2.
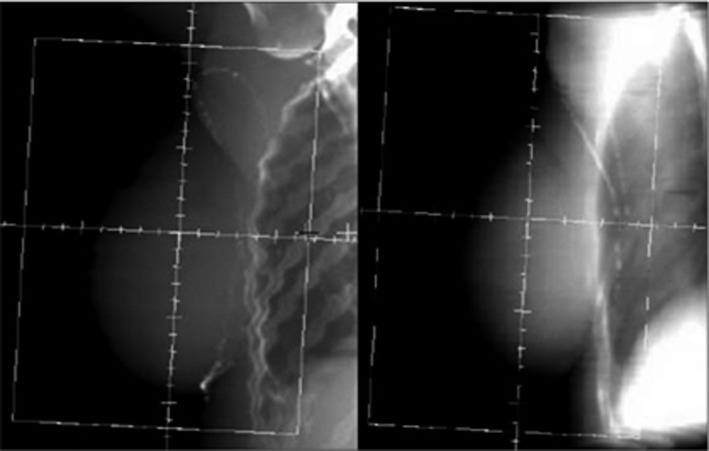
Comparison of planning digitally reconstructed radiograph (left) with the simulated electronic portal image (right).
To compare EPI and CBCT‐B, the CBCT‐B setup errors in the superior–inferior (SI) and anterior–posterior (AP) directions were first transformed into the V and U coordinate system of the EPI plane13 so that a direct comparison could be performed in 2D. The 3D isocentre position shift as determined from the CBCT‐B was transformed to a 2D shift in the EPI plane (V and U coordinate system) as described by Arumugam et al.26 Setup errors were acquired in three dimensions for CBCT‐B and CBCT‐S for direct comparison. Two experienced radiation therapists were involved in all image analysis and measurements to reduce inter‐observer variability. Normality test was performed using SPSS software (IBM SPSS Statistics for Windows, Version 20.0.; IBM Corp., Armonk, NY) to test if the data are normally distributed. Differences in the daily positioning errors were examined using paired Student's t tests to compare results between the three methods. The mean (M), absolute mean and standard deviation (SD) of the setup errors were calculated for each patient for all three methods of image assessment. Setup error has been separated into two main components, systematic error and random error. Systematic errors are the deviations between the planned patient position and the average of the treated positions, while random error is the variability in patient positioning observed between daily treatment verification images.27 The SD of population M expressed the population systematic error (Σ) and the root mean square of the SD of each patient describes the random error (σ).28 Correlation between the setup errors and the following factors was also analysed using Pearson correlation: BMI and breast size. A P < 0.05 was used to denote statistical significance. All statistical tests were performed using SPSS software (IBM SPSS Statistics for Windows).
Results
Patient data
Between May 2011 and February 2014, 25 patients were recruited and consented for the study. Each patient underwent the imaging as per study protocol and a total of 125 CBCT scans were acquired. Only 120 EPIs were available for analysis, five EPIs from five different patients could not be analysed due to poor image quality. Patient characteristics are summarised in Table 1.
Table 1.
Patient characteristics
| No. of patients | 25 |
|---|---|
| Laterality | 10 × Left |
| 15 × Right | |
| Age mean years (range) | 61 (41–79) |
| Mean body mass index (range) | 28.7 (18–44.6) |
| Mean breast volume (range) | 970.2 cc (230–1862 cc) |
| Breast size (range) | 9 × Small (230–694 cc) |
| 5 × Medium (700–1033 cc) | |
| 11 × Large (1128–1862 cc) | |
| Pathology | 2 × Ductal carcinoma in situ |
| 3 × Lobular carcinoma | |
| 20 × Infiltrating ductal carcinoma | |
| Stage | 2 × 0 |
| 15 × IA | |
| 6 × IIA | |
| 2 × IB |
Positioning errors
Normality test showed that all data collected was normally distributed. Differences in the daily positioning errors between the three image registration methods were not statistically significant as indicated by the paired Student's t tests. Table 2 outlines the population mean, absolute mean, systematic (Σ) and random (σ) errors for the three image registration methods. The measured errors were comparable between EPI and CBCT‐B. Similar results were found for setup errors between CBCT‐B and CBCT‐S. The percentage of errors exceeding 5 mm is displayed in Figure 3.
Table 2.
Summary of errors for the three image registration methods
| Setup errors in V and U planes | Setup errors in three dimensions | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EPI (mm) | CBCT‐B (mm) | CBCT‐B (mm) | CBCT‐S (mm) | |||||||
| V | U | V | U | LR | SI | AP | LR | SI | AP | |
| Mean | −0.71 | −0.48 | −2.20 | −0.67 | −0.69 | −2.20 | +0.80 | −0.80 | −1.30 | +0.10 |
| Absolute | 3.46 | 2.91 | 3.72 | 3.40 | 3.28 | 3.72 | 2.67 | 3.72 | 3.38 | 2.68 |
| Σ | 3.69 | 2.83 | 3.49 | 3.42 | 3.41 | 3.49 | 2.24 | 3.75 | 2.96 | 2.15 |
| σ | 2.91 | 2.52 | 2.82 | 2.77 | 2.98 | 2.82 | 2.74 | 3.00 | 3.07 | 2.90 |
Σ = systematic error, σ = random error, (−) = left/superior/anterior, (+) = right/inferior/posterior. LR, left–right; SI, superior–inferior; AP, anterior–posterior; EPI, electronic portal imaging; CBCT‐B, cone‐beam computed tomography (CT) bony registration; CBCT‐S, cone‐beam CT soft tissue registration.
Figure 3.
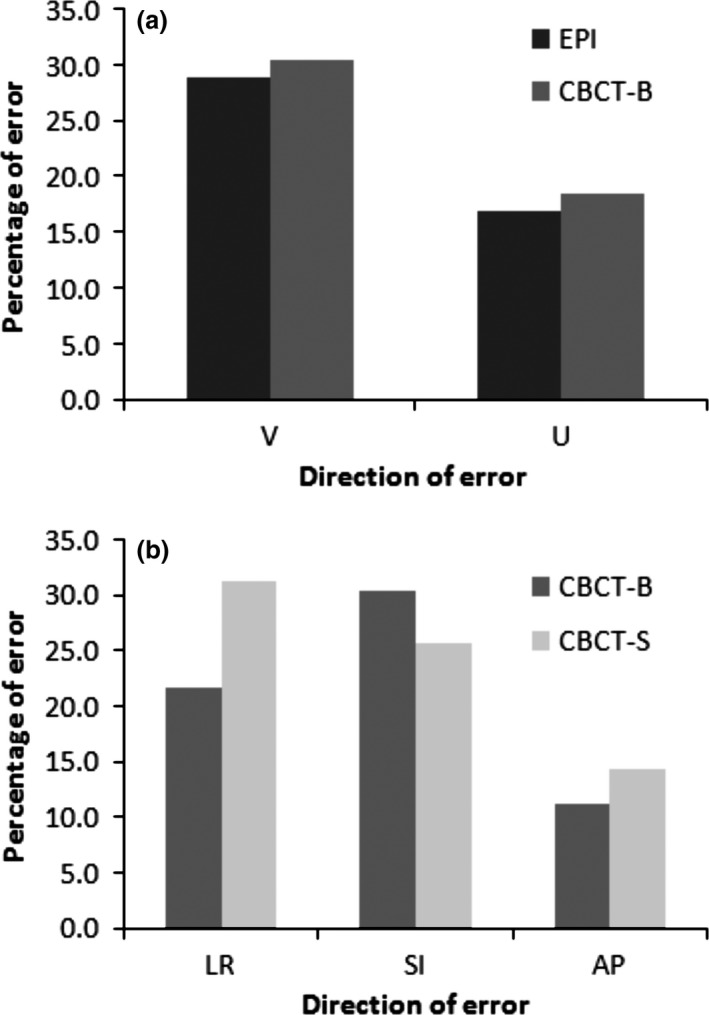
The percentage of errors exceeding 5 mm comparing (A) electronic portal image and cone‐beam computed tomography bone registration (CBCT‐B) in the V: cranio‐caudal distance and U: central lung distance planes, and (B) CBCT‐B and cone‐beam CT soft tissue registration (CBCT‐S) in the left–right (LR), superior–inferior and anterior–posterior directions.
Correlation between setup errors and other factors
A positive Pearson correlation (r) was established for breast size and BMI, with r = 0.709. Setup errors measured with CBCT‐S showed statistically significant correlation with BMI (Fig. 4) with r values of 0.619, 0.771 and 0.703 for left–right, SI and AP directions respectively. Similar significant correlation was found between CBCT‐S setup error and breast size with r values of 0.659, 0.614 and 0.609 for left–right, SI and AP directions respectively. No other correlation was statistically significant with other registration methods and factors.
Figure 4.
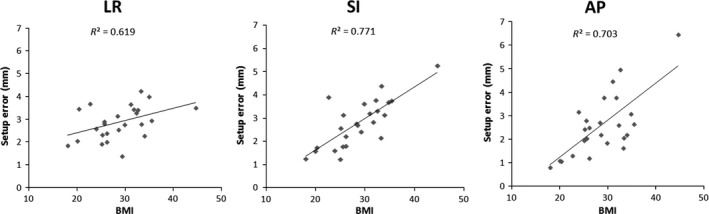
Correlation between setup error and body mass index in left–right (LR), superior–inferior and anterior–posterior directions for cone‐beam computed tomography soft tissue registration.
Discussion
To our knowledge, this is the first study investigating three image registration methods for breast RT. In this study, setup errors have been evaluated in 25 breast cancer patients. The novel aspect investigated is the utilisation of 3D soft tissue matching rather than bone matching alone, and a comparison with simulated 2D EPI. The relationship between setup errors and BMI and breast size was also assessed.
CBCT‐B, in comparison with EPI, seemed to detect a smaller error in the V coordinate but greater in the U coordinate. However, the largest difference between the two registration methods was only 0.6 mm in the U coordinates and this difference was not statistically significant, nor considered clinically significant. Similarly, setup errors measured with CBCT‐B and CBCT‐S were consistent, with the largest individual difference (data not shown) observed in the SI direction (0.5 mm). A previous study13 examining the differences between EPI and CBCT for bony matching in breast found that EPI‐based setup errors were smaller than the CBCT‐based setup errors, which is the opposite of what we observed in the SI direction. The previous study acquired both EPI and CBCT images before treatment and potentially introduced intrafraction motion between the two image acquisitions, whereas the current study generated simulated EPI from the CBCT scan to eliminate the time interval factor. The resolution of the simulated EPI (2 mm) in the current study is lower compared to real EPI acquired on the linear accelerator which has a resolution of 0.4 mm, which may result in uncertainty in measurements. However, since the magnitude of change would likely be reduced with higher image resolution, this could strengthen our findings. This study suggests that EPI and bone landmarks are sufficient surrogates for patient setup for tangential breast RT and minor soft tissue differences do not affect the setup error considerably.
In a recent study, Topolnjak et al.13 found Σ u = 2.2/σ u = 2.9 mm and Σ v = 3.3/σ v = 2.9 mm for EPI‐based setup errors. Hurkmans et al.27 conducted an overview of setup errors for breast RT and found Σ CLD = 2.7–3.1 mm and Σ CCD = 1–14.4 mm depending on immobilisation and imaging modality (EPI/film) used. Lirette et al.29 utilised EPI and found Σ CLD = 3.9/σ CLD = 3.4 mm and Σ CCD = 3.1/σ CCD = 1.7 mm. Another study using EPI found Σ CLD = 4.4 and Σ CCD = 6.1 mm.30 The results are similar to ours in the U (CLD) direction but larger in the V (CCD) direction, perhaps due to differences in immobilisation in some of these earlier EPI‐based studies. The values of Σ by CBCT‐B and CBCT‐S are generally higher than other CBCT studies, but the values of σ are consistent with most other studies13, 19, 20, 21, 22, 23 as outlined in Table 3. The patient setup used in this study was generally similar to other CBCT‐based studies, most using immobilisation device to support patients' arms for stability. The results for CBCT‐S were also similar to those reported by Padilla et al.31 where optical surface verification tools were used to measure setup errors with approximately 1 mm difference in systematic and random errors between the two studies.
Table 3.
Comparison of results with other cone‐beam computed tomography studies
| LR (mm) | SI (mm) | AP (mm) | ||||
|---|---|---|---|---|---|---|
| Σ | σ | Σ | σ | Σ | σ | |
| Veldeman et al.19 (clips matching) | 1.5 | 7.3 | 1.4 | 2.3 | 2.8 | 3.2 |
| Kirby et al.20 (bony matching) | 1.8 | 2.7 | 1.9 | 3.1 | 1.8 | 2.6 |
| Kirby et al.20 (clips matching) | 1.3 | 2.8 | 1.5 | 3.2 | 1.8 | 2.4 |
| Topolnjak et al.21 (bony matching) | 2.4 | 2.8 | 2.3 | 4.1 | 1.5 | 3.6 |
| Topolnjak et al.21 (soft tissue matching) | 2.2 | 3.3 | 1.8 | 3.8 | 0.7 | 3.7 |
| Topolnjak et al.13 (bony matching) | 3.1 | 2.2 | 3.8 | 2.8 | 2.5 | 2.6 |
| Kim et al.22 (clips matching) | 4.3 | 2.2 | 2.5 | 1.6 | 3.1 | 1.7 |
| White et al.23 (bony matching) | 2.7 | 2.4 | 2.4 | 2.9 | 1.7 | 2.2 |
| This study (bony matching) | 3.4 | 3.0 | 3.5 | 2.8 | 2.2 | 2.7 |
| This study (soft tissue matching) | 3.8 | 3.0 | 3.0 | 3.1 | 2.2 | 2.9 |
LR, left–right; SI, superior–inferior; AP, anterior–posterior; Σ, systematic error; σ, random error.
All three registration methods in this study utilised different regions of interest. The chest wall was used for the EPI registration, whereas the registration for CBCT‐B included the sternum and ribs. Due to differences in anatomy visibility between 2D (EPI) and 3D (CBCT‐B) modalities, it was not possible to use same regions of interest for assessment. As for CBCT‐S registration, only breast tissue was used for registration with no reference to bony landmark. BMI and breast size positively correlated with magnitude of setup errors measured with CBCT‐S, but not with setup errors measured with EPI and CBCT‐B. The main reason for this could be due to the fact that registration with CBCT‐S was focused on actual soft tissue and breast outline matching; hence for patients with large breast size or BMI, larger setup errors are detected with CBCT‐S due to more variation in the soft tissue setup differences in this group of patients. As for bony matching, patient setup error could be consistent regardless of their breast size as breast motion (soft tissue displacement and/or soft tissue deformation) is not taken into account. However, Offerman et al.32 found no correlation between breast size, BMI, height, weight and age and degree of daily shift on a tomotherapy unit for treatment of breast cancer. The main reason for the differing results could be due to the variation in patient position, flat in the Offerman study versus inclined in the current study. The inclined position may be more unstable causing larger patients and breasts more susceptible to setup variation.
This study demonstrated correlation between BMI and breast size with setup errors measured with CBCT‐S. The next logical step would be to assess whether CBCT‐S has a role when evaluating large breast patient setups. The sample size of 25 patients was sufficient to assess the difference in setup error between EPI and CBCT for the entire cohort, and to assess the correlation between setup errors and BMI and breast size. However, this sample size was insufficient to assess the role of CBCT‐S in larger patients as there were only 11 patients. Given the small sample size, we cannot exclude the possibility that with a sufficient sample of larger patients, we might see a clinically and statistically significant difference that would warrant the use of CBCT‐S for larger patients. However, registration by CBCT‐S alone with no attention to bony landmarks may not be ideal for some patients. If the soft tissue registration adjusts the chest wall depth encompassed by the beam, this may result in inadequate coverage of the chest wall which could be important for some patients. We suggest that any image registration method used should be thoroughly assessed. The utility of CBCT‐S in patients with large BMI and breast size could be further assessed in a dosimetric study to investigate the impact of setup error. However, it is also to be noted that patients with very large body habitus cannot be assessed as they were excluded from the study due to collision risk between the gantry head and patient.
The use of CBCT to improve the setup accuracy in breast RT can increase dose to organs at risk significantly,15, 33, 34, 35 and potentially increasing the patients' likelihood of developing a secondary cancer.36 The associated imaging dose and risks should be carefully considered before use of CBCT for breast especially now that we have identified no statistically significant benefit with CBCT‐B use. Furthermore, the time taken to acquire a CBCT scan and perform image registration could be greater compared to EPI, further introducing the potential for patient movement. This may be more important for larger patients who are more likely to roll, with longer time on the treatment couch. Time studies comparing CBCT and EPI acquisition times are scarce for breast RT, however, Perrier et al. reported the times for image acquisition comparing CBCT and EPI in prostate cancer, and found that the mean times were 4.6 min (SD: 4.5 min) for CBCT and 3.6 min (SD: 1.6 min) for EPI.37 There are also treatment planning steps that can be taken to address minor daily variations and further reduce the necessity for CBCT use in tangential breast RT. For example, at our institution, we ensure that the minimum field size for forward planned breast IMRT is 9 cm2 and standard multi‐leaf collimator blocks are used without complex shapes. This would further lessen the need for high precision and reduce the need for daily imaging.
This study was powered to detect a difference in error of 5 mm as this is the standard imaging tolerance for tangential breast RT in our institution. However, in some clinical situations (e.g. close proximity to the heart, previous treatment to the contralateral breast and cases with small clinical target volume to planning target volume margin), setup errors of less than 5 mm may be important. In this situation, further investigation may be required with a larger sample size to detect a specified difference.
Setup errors are dependent on a number of factors including patient body habitus and immobilisation devices used. This study is based on patient population and immobilisation technique from a single institution. For this reason, the results of this study may not translate directly to other institutions and should be carefully reviewed before any change in clinical practice. The current study is focused on accuracy of whole breast irradiation but does not address errors in targeting the lumpectomy cavity which is important for some techniques such as accelerated partial breast irradiation and simultaneous integrated boost. However, EPI and CBCT‐B could be ruled out in identifying the lumpectomy cavity, hence highlighting the role of CBCT‐S for these techniques. CBCT may also confer particular advantage in detecting changes in seroma size, shape and position during the course of treatment. Another advantage of CBCT over 2D is the ability to detect left–right difference as well as any rotation that has occurred. This would have potential clinical implications for treatment of left‐sided breast cancers where heart sparing is of importance.
Conclusion
CBCT and EPI show insignificant variation in their ability to detect setup error. These findings suggest no significant differences that would make one modality considered superior over the other and EPI should remain as the standard of care. However, there is a correlation with breast size and BMI and setup error as detected by CBCT‐S. Given the small sample size, we cannot exclude the possibility that with a sufficient sample of larger patients, we might see a clinically and statistically significant difference that would justify the use of CBCT‐S for larger patients. Overall, this study suggests that CBCT is not justified for the routine setup of tangential breast RT and the associated imaging dose and risks should be carefully considered before use of CBCT for breast.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgement
The authors thank Michael Jameson, BSc, and Sankar Arumugam, PhD, for their assistance in this study. The authors also thank Wei Xuan, PhD, for providing statistical advice.
J Med Radiat Sci 63 (2016) 224–231
References
- 1. Cancer Management Guidelines: Breast. Vancouver, BC: British Columbia Cancer Agency, 2003. [Google Scholar]
- 2. Delaney G, Barton M, Jacob S. Estimation of an optimal radiotherapy utilization rate for breast carcinoma: A review of the evidence. Cancer 2003; 98: 1977–86. [DOI] [PubMed] [Google Scholar]
- 3. Fisher B, Anderson S, Bryant J, et al. Twenty‐year follow‐up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002; 347: 1233–41. [DOI] [PubMed] [Google Scholar]
- 4. Veronesi U, Cascinelli N, Mariani L, et al. Twenty‐year follow‐up of a randomized study comparing breast‐conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002; 347: 1227–32. [DOI] [PubMed] [Google Scholar]
- 5. Kestin LL, Sharpe MB, Frazier RC, et al. Intensity modulation to improve dose uniformity with tangential breast radiotherapy: Initial clinical experience. Int J Radiat Oncol Biol Phys 2000; 48: 1559–68. [DOI] [PubMed] [Google Scholar]
- 6. Vicini FA, Sharpe M, Kestin L, et al. Optimizing breast cancer treatment efficacy with intensity‐modulated radiotherapy. Int J Radiat Oncol Biol Phys 2002; 54: 1336–44. [DOI] [PubMed] [Google Scholar]
- 7. van der Laan HP, Dolsma WV, Maduro JH, Korevaar EW, Hollander M, Langendijk JA. Three‐dimensional conformal simultaneously integrated boost technique for breast‐conserving radiotherapy. Int J Radiat Oncol Biol Phys 2007; 68: 1018–23. [DOI] [PubMed] [Google Scholar]
- 8. Borst GR, Sonke JJ, Betgen A, Remeijer P, van Herk M, Lebesque JV. Kilo‐voltage cone beam CT setup measurements for lung cancer patients; first clinical results and comparison with electronic portal‐imaging device. Int J Radiat Oncol Biol Phys 2007; 68: 555–61. [DOI] [PubMed] [Google Scholar]
- 9. Chen YJ, Han C, Liu A, et al. Setup variations in radiotherapy of esophageal cancer: Evaluation by daily megavoltage computed tomographic localization. Int J Radiat Oncol Biol Phys 2007; 68: 1537–45. [DOI] [PubMed] [Google Scholar]
- 10. Moseley DJ, White EA, Wiltshire KL, et al. Comparison of localization performance with implanted fiducial markers and cone‐beam computed tomography for on‐line image‐guided radiotherapy of the prostate. Int J Radiat Oncol Biol Phys 2007; 67: 942–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Mourik A, van Kranen S, den Hollander S, Sonke JJ, van Herk M, van Vliet‐Vroegindeweij C. Effects of setup errors and shape changes on breast radiotherapy. Int J Radiat Oncol Biol Phys 2011; 79: 1557–64. [DOI] [PubMed] [Google Scholar]
- 12. Boda‐heggemann J, Lohr F, Wenz F, Flentje M, Guckenberger M. kV cone‐beam CT‐based IGRT. Strahlenther Onkol 2011; 187: 284–91. [DOI] [PubMed] [Google Scholar]
- 13. Topolnjak R, Sonke JJ, Nijkamp J, et al. Breast patient setup error assessment: Comparison of electronic portal image devices and cone‐beam computed tomography matching results. Int J Radiat Oncol Biol Phys 2010; 78: 1235–43. [DOI] [PubMed] [Google Scholar]
- 14. Amols H, Jaffray D. Image‐guided radiotherapy is being overvalued as a clinical tool in radiation oncology. Med Phys 2006; 33: 3583–6. [DOI] [PubMed] [Google Scholar]
- 15. Donovan E, James H, Bonora M, Yarnold J, Evans P. Second cancer incidence risk estimates using BEIR VII models for standard and complex external beam radiotherapy for early breast cancer. Med Phys 2012; 39: 5814–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lin LL, Hertan L, Rengan R, Teo BK. Effect of body mass index on magnitude of setup errors in patients treated with adjuvant radiotherapy for endometrial cancer with daily image guidance. Int J Radiat Oncol Biol Phys 2012; 83: 670–5. [DOI] [PubMed] [Google Scholar]
- 17. Wong JR, Gao Z, Merrick S, et al. Potential for higher treatment failure in obese patients: Correlation of elevated body mass index and increased daily prostate deviations from the radiation beam isocentres in an analysis of 1465 computed tomographic images. Int J Radiat Oncol Biol Phys 2009; 75: 49–55. [DOI] [PubMed] [Google Scholar]
- 18. Choi M, Fuller CD, Wang SJ, et al. Effect of body mass index on shifts in ultrasound‐based image‐guided intensity‐modulated radiation therapy for abdominal malignancies. Radiother Oncol 2009; 91: 114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Veldeman L, De Gersem W, Speleers B, et al. Alternated prone and supine whole‐breast irradiation using IMRT: Setup precision, respiratory movement and treatment time. Int J Radiat Oncol Biol Phys 2012; 82: 2055–64. [DOI] [PubMed] [Google Scholar]
- 20. Kirby AM, Evans P, Helyer SJ, Donovan E, Convery H, Yarnold J. A randomised trial of supine versus prone breast radiotherapy (SuPr study): Comparing set‐up errors and respiratory motion. Radiother Oncol 2011; 100: 221–6. [DOI] [PubMed] [Google Scholar]
- 21. Topolnjak R, Vliet‐Vroegindeweij CV, Sonke JJ, et al. Breast‐conserving therapy: Radiotherapy margins for breast tumor bed boost. Int J Radiat Oncol Biol Phys 2008; 72: 941–8. [DOI] [PubMed] [Google Scholar]
- 22. Kim LH, Wong J, Yan D. On‐line localization of the lumpectomy cavity using surgical clips. Int J Radiat Oncol Biol Phys 2007; 69: 1305–9. [DOI] [PubMed] [Google Scholar]
- 23. White EA, Cho J, Vallis KA, et al. Cone beam computed tomography guidance for setup of patients receiving accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys 2007; 68: 547–54. [DOI] [PubMed] [Google Scholar]
- 24. Dundas K, Atyeo J, Cox J. What is a large breast? Measuring and categorizing breast size for tangential breast radiation therapy. Australas Radiol 2007; 51: 589–93. [DOI] [PubMed] [Google Scholar]
- 25. Ramsey C, Chase D, Scaperoth D, Arwood D, Oliver A. Improved dose homogeneity to the intact breast using three‐dimensional treatment planning: Technical considerations. Med Dosim 2000; 25: 1–6. [DOI] [PubMed] [Google Scholar]
- 26. Arumugam S, Sidhom M, Xing A, Holloway L. An online x‐ray based position validation system for prostate hypofractionated radiotherapy. Med Phys 2016; 43: 961–74. [DOI] [PubMed] [Google Scholar]
- 27. Hurkmans CW, Remeijer P, Lebesque JV, Mijnheer BJ. Set‐up verification using portal imaging; review of current clinical practice. Radiother Oncol 2001; 58: 105–20. [DOI] [PubMed] [Google Scholar]
- 28. Yang DS, Yoon WS, Chung SY, et al. Set‐up uncertainty during breast radiotherapy. Strahlenther Onkol 2013; 189: 315–20. [DOI] [PubMed] [Google Scholar]
- 29. Lirette A, Pouliot J, Aubin M, Larochelle M. The role of electronic portal imaging in tangential breast irradiation: A prospective study. Radiother Oncol 1995; 37: 241–5. [DOI] [PubMed] [Google Scholar]
- 30. Fein DA, McGee KP, Schultheiss TE, Fowble BL, Hanks GE. Intra‐ and interfractional reproducibility of tangential breast fields: A prospective on‐line portal imaging study. Int J Radiat Oncol Biol Phys 1996; 34: 733–40. [DOI] [PubMed] [Google Scholar]
- 31. Padilla L, Kang H, Washington M, Hasan Y, Chmura SJ, Al‐Hallaq H. Assessment of interfractional variation of the breast surface following conventional patient positioning for whole‐breast radiotherapy. J Appl Clin Med Phys 2014; 15: 4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Offerman S, Lamba M, Lavigne R. Effect of breast volume on treatment reproducibility on a tomotherapy unit in the treatment of breast cancer. Int J Radiat Oncol Biol Phys 2011; 80: 417–21. [DOI] [PubMed] [Google Scholar]
- 33. Harrison RM, Wilkinson M, Rawlings DJ, Moore M. Doses to critical organs following radiotherapy and concomitant imaging of the larynx and breast. Br J Radiol 2007; 80: 898–995. [DOI] [PubMed] [Google Scholar]
- 34. Quinn A, Holloway L, Koh E‐S, et al. Radiation dose and contralateral breast cancer risk associated with megavoltage cone‐beam computed tomographic image verification in breast radiation therapy. Pract Radiat Oncol 2013; 3: 93–100. [DOI] [PubMed] [Google Scholar]
- 35. Batumalai V, Quinn A, Jameson M, Delaney G, Holloway L. Imaging dose in breast radiotherapy: Does breast size affect the dose to the organs at risk and the risk of secondary cancer to the contralateral breast? J Med Radiat Sci 2015; 62: 32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xu XG, Bednarz B, Paganetti H. A review of dosimetry studies on external‐beam radiation treatment with respect to second cancer induction. Phys Med Biol 2008; 53: R193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Perrier L, Morelle M, Pommier P, et al. Cost of prostate image‐guided radiation therapy: Results of a randomized trial. Radiother Oncol 2013; 106: 50–8. [DOI] [PubMed] [Google Scholar]


