Abstract
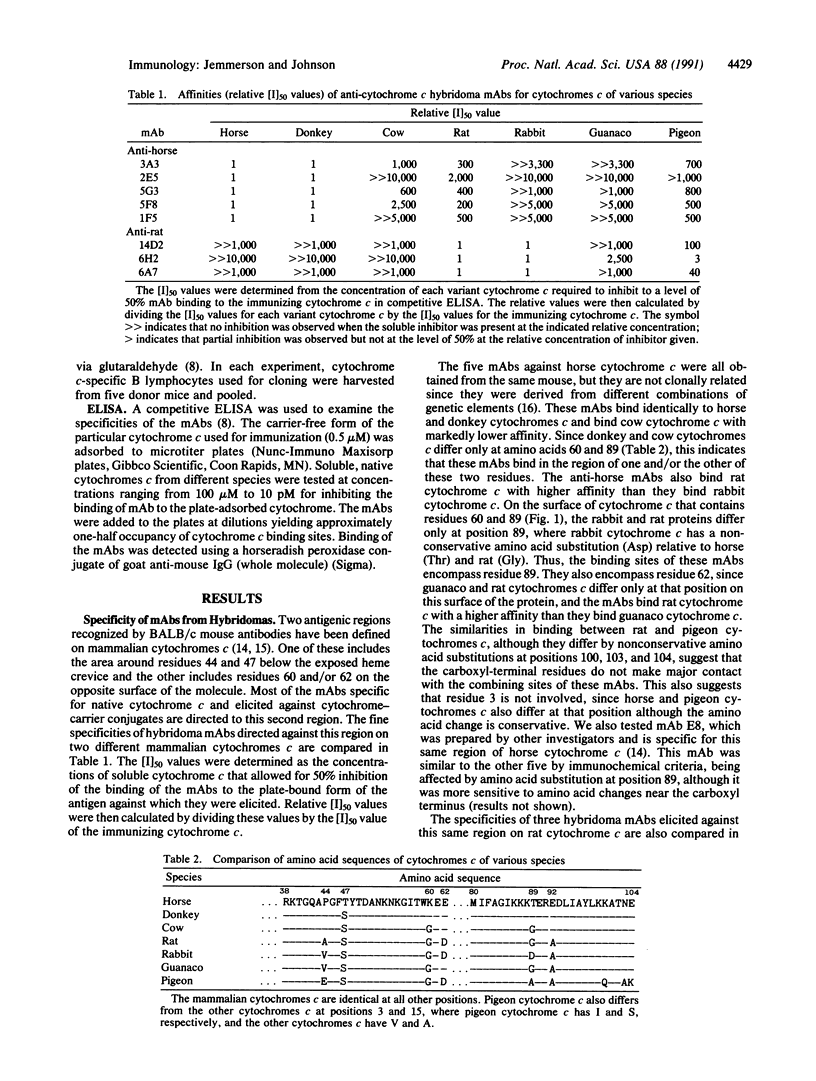
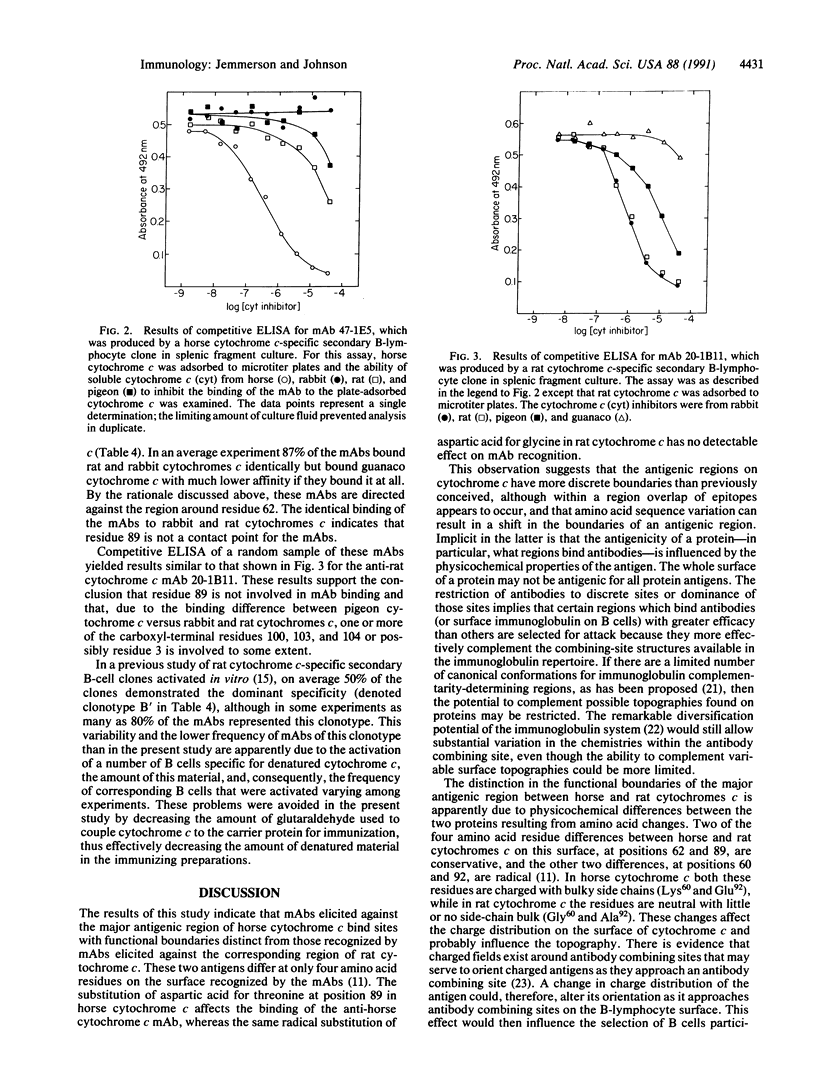
The antigenic sites of horse and rat cytochromes c in the major antigenic region were compared using a panel of variant cytochromes c and a large number of BALB/c mouse monoclonal antibodies (mAbs) in competitive ELISAs. The major antigenic region of cytochrome c is located on the surface opposite of that containing the exposed heme crevice. mAbs specific for this region on rat cytochrome c were affected in binding by amino acid substitutions at positions 62 and at one or more of positions 3, 100, 103, and 104 but not by a substitution at position 89. In contrast, mAbs specific for the same region on horse cytochrome c were affected by amino acid substitutions at positions 62, (probably) 60, and 89. Some, but not all, of the anti-horse cytochrome c mAbs were affected by amino acid substitutions at one or more of positions 3, 100, 103, and 104. Thus, the functional boundaries of this antigenic region are different for these two cytochromes c, as shown primarily by the differential effects of residue 89. This distinction is most likely due to one or more of the four amino acid differences between horse and rat cytochromes c on the antigenic surface that result in different physicochemical properties for the horse and rat proteins. The results suggest that as yet unidentified physicochemical parameters, possibly surface topography and/or charge distribution, influence the focusing of antibodies onto the surface of a protein antigen.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benjamin D. C., Berzofsky J. A., East I. J., Gurd F. R., Hannum C., Leach S. J., Margoliash E., Michael J. G., Miller A., Prager E. M. The antigenic structure of proteins: a reappraisal. Annu Rev Immunol. 1984;2:67–101. doi: 10.1146/annurev.iy.02.040184.000435. [DOI] [PubMed] [Google Scholar]
- Brautigan D. L., Ferguson-Miller S., Margoliash E. Mitochondrial cytochrome c: preparation and activity of native and chemically modified cytochromes c. Methods Enzymol. 1978;53:128–164. doi: 10.1016/s0076-6879(78)53021-8. [DOI] [PubMed] [Google Scholar]
- Bushnell G. W., Louie G. V., Brayer G. D. High-resolution three-dimensional structure of horse heart cytochrome c. J Mol Biol. 1990 Jul 20;214(2):585–595. doi: 10.1016/0022-2836(90)90200-6. [DOI] [PubMed] [Google Scholar]
- Carbone F. R., Paterson Y. Monoclonal antibodies to horse cytochrome c expressing four distinct idiotypes distribute among two sites on the native protein. J Immunol. 1985 Oct;135(4):2609–2616. [PubMed] [Google Scholar]
- Carlson S. S., Mross G. A., Wilson A. C., Mead R. T., Wolin L. D., Bowers S. F., Foley N. T., Muijsers A. O., Margoliash E. Primary structure of mouse, rat, and guinea pig cytochrome c. Biochemistry. 1977 Apr 5;16(7):1437–1442. doi: 10.1021/bi00626a031. [DOI] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M., Tramontano A., Levitt M., Smith-Gill S. J., Air G., Sheriff S., Padlan E. A., Davies D., Tulip W. R. Conformations of immunoglobulin hypervariable regions. Nature. 1989 Dec 21;342(6252):877–883. doi: 10.1038/342877a0. [DOI] [PubMed] [Google Scholar]
- Davies D. R., Sheriff S., Padlan E. A. Antibody-antigen complexes. J Biol Chem. 1988 Aug 5;263(22):10541–10544. [PubMed] [Google Scholar]
- Goshorn S. C., Retzel E., Jemmerson R. Common structural features among monoclonal antibodies binding the same antigenic region of cytochrome c. J Biol Chem. 1991 Feb 5;266(4):2134–2142. [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janin J., Chothia C. The structure of protein-protein recognition sites. J Biol Chem. 1990 Sep 25;265(27):16027–16030. [PubMed] [Google Scholar]
- Jemmerson R. Antigenicity and native structure of globular proteins: low frequency of peptide reactive antibodies. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9180–9184. doi: 10.1073/pnas.84.24.9180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemmerson R., Blankenfeld R. Clonal analysis of the BALB/c secondary B cell repertoire specific for a self-antigen, cytochrome c. J Immunol. 1988 Mar 15;140(6):1762–1769. [PubMed] [Google Scholar]
- Jemmerson R., Johnson J. G., Burrell E., Taylor P. S., Jenkins M. K. A monoclonal antibody specific for a cytochrome c T cell stimulatory peptide inhibits T cell responses and affects the way the peptide associates with antigen-presenting cells. Eur J Immunol. 1991 Jan;21(1):143–151. doi: 10.1002/eji.1830210122. [DOI] [PubMed] [Google Scholar]
- Louie G. V., Hutcheon W. L., Brayer G. D. Yeast iso-1-cytochrome c. A 2.8 A resolution three-dimensional structure determination. J Mol Biol. 1988 Jan 20;199(2):295–314. doi: 10.1016/0022-2836(88)90315-4. [DOI] [PubMed] [Google Scholar]
- Metcalf E. S., Klinman N. R. In vitro tolerance induction of neonatal murine B cells. J Exp Med. 1976 Jun 1;143(6):1327–1340. doi: 10.1084/jem.143.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotný J., Handschumacher M., Haber E., Bruccoleri R. E., Carlson W. B., Fanning D. W., Smith J. A., Rose G. D. Antigenic determinants in proteins coincide with surface regions accessible to large probes (antibody domains). Proc Natl Acad Sci U S A. 1986 Jan;83(2):226–230. doi: 10.1073/pnas.83.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi H., Hata Y., Tanaka N., Kakudo M., Sakurai T., Aihara S., Morita Y. Structure of rice ferricytochrome c at 2.0 A resolution. J Mol Biol. 1983 May 25;166(3):407–418. doi: 10.1016/s0022-2836(83)80092-8. [DOI] [PubMed] [Google Scholar]
- Reichlin M. Use of glutaraldehyde as a coupling agent for proteins and peptides. Methods Enzymol. 1980;70(A):159–165. doi: 10.1016/s0076-6879(80)70047-2. [DOI] [PubMed] [Google Scholar]
- Smith-Gill S. J., Lavoie T. B., Mainhart C. R. Antigenic regions defined by monoclonal antibodies correspond to structural domains of avian lysozyme. J Immunol. 1984 Jul;133(1):384–393. [PubMed] [Google Scholar]
- Tainer J. A., Getzoff E. D., Paterson Y., Olson A. J., Lerner R. A. The atomic mobility component of protein antigenicity. Annu Rev Immunol. 1985;3:501–535. doi: 10.1146/annurev.iy.03.040185.002441. [DOI] [PubMed] [Google Scholar]
- Takano T., Dickerson R. E. Conformation change of cytochrome c. I. Ferrocytochrome c structure refined at 1.5 A resolution. J Mol Biol. 1981 Nov 25;153(1):79–94. doi: 10.1016/0022-2836(81)90528-3. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Westhof E., Altschuh D., Moras D., Bloomer A. C., Mondragon A., Klug A., Van Regenmortel M. H. Correlation between segmental mobility and the location of antigenic determinants in proteins. Nature. 1984 Sep 13;311(5982):123–126. doi: 10.1038/311123a0. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Wilson I. A., Skehel J. J. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981 Jan 29;289(5796):373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]




