Abstract
A pulmonary lymphoepithelioma‐like carcinoma (PLELC) is similar to a lymphoepithelioma, a subtype of nasopharyngeal carcinoma and commonly associated with Epstein–Barr virus infection which is a rare tumour and classified in the group of “other and unclassified carcinoma” in the latest 2015 World Health Organization (WHO) classification. Some reports of lymphoepithelioma‐like carcinoma (LELC) have noted an epidermal growth factor receptor (EGFR) mutation, whereas none have noted a mutation of the echinoderm microtubule‐associated protein‐like 4‐anaplastic lymphoma kinase (EML4‐ALK) fusion gene. This is the first reported case of PLELC with ALK rearrangement. A 76‐year‐old woman underwent a right lower lobectomy and complicated partial resection of the upper lobe with lymph node dissection under complete thoracoscopic approach. A histopathological diagnosis of PLELC was made and the stage was classified as T1aN1(#12l) M0, pl0, G2, Ly1, V1. The results of both ALK immunohistochemistry and EML4‐ALK fusion gene on fluorescence in situ hybridization (FISH) examinations were positive; however, EGFR mutational analysis results showed wild‐type mutation.
Keywords: ALK, EML4‐ALK, lung, lymphoepithelioma‐like carcinoma, pulmonary
Introduction
A pulmonary lymphoepithelioma‐like carcinoma (PLELC) is a rare tumour and is classified in the group of “other and unclassified carcinomas” in the latest 2015 World Health Organization (WHO) classification. It was first reported as an Epstein–Barr virus (EBV)‐associated malignancy in 1987, with about 300 cases since documented. Some reports of lymphoepithelioma‐like carcinoma (LELC) have noted an epidermal growth factor receptor (EGFR) mutation, whereas none have noted a mutation of the echinoderm microtubule‐associated protein‐like 4‐anaplastic lymphoma kinase (EML4‐ALK) fusion gene. This is the first reported PLELC case with ALK rearrangement.
Case Report
A 76‐year‐old woman came to us with a persistent cough and her chest computed tomography (CT) showed a nodule in the right lower lobe. She had never smoked and did not have a travel history. The nodule was located in right segment 6 and measured 2.0 × 1.9 × 1.0 cm, with spiculation and partial enhancement (Fig. 1A) in enhanced CT imaging. Findings of [18F] fluoro‐2‐deoxyglucose positron emission tomography (FDG‐PET) showed significant FDG uptake (SUVmax 7.9) with tumour (Fig. 1B) but none with lymph nodes. The FDG‐PET/CT and the examination by otolaryngologist found no tumour in nasopharyngeal region (Fig.1C). The serum tumour markers, carcinoembryonic antigen (CEA) and soluble cytokeratin 19 fragment (CYFRA), were not elevated. Although bronchoscopic biopsy results did not provide a histological diagnosis, we recommended surgical resection because lung cancer was suspected.
Figure 1.
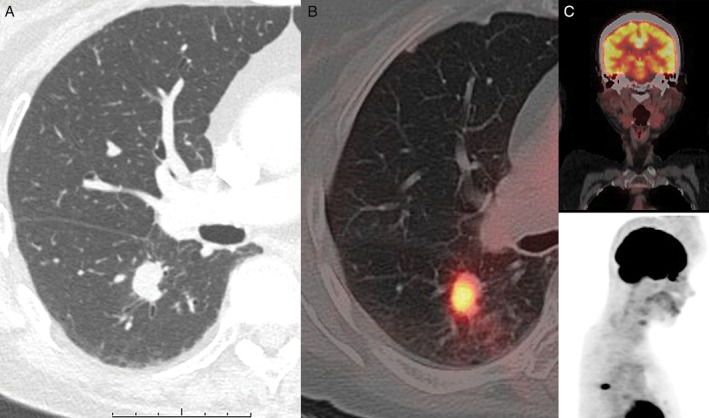
(A) Enhanced chest computed tomography (CT) scan showing nodule in right lower lobe (2.0 × 1.9 × 1.0 cm in size). (B) [18F] Fluoro‐2‐deoxyglucose positron emission tomography (FDG‐PET) image showing significant uptake of FDG (SUVmax 7.9) with main tumour but (C) no uptake with nasopharyngeal area and lymph node.
Surgical resection was performed in order to provide definitive diagnosis and treatment. A right lower lobectomy and complicated partial resection of the upper lobe with lymph node dissection were performed under complete thoracoscopic approach utilizing one window and three ports. The tumour was found to have infiltrated segment 2 and intra‐operative pathological findings revealed pulmonary carcinoma. The operative time was 270 min and blood loss was 130 mL. The patient was discharged on post‐operative day 10 without complications.
The size of the excised mass was 1.8 × 1.5 × 1.4 cm, and adjacent vessel and lymphatic invasion by the tumour were noted (Fig. 2A). Haematoxylin and eosin (HE) staining showed that the nuclei were round with irregular borders, and both the tumour and stromal tissue contained large reactive lymphoplasmacytic cells (Fig. 2B). A histopathological diagnosis of pulmonary LELC was made and the stage was classified as T1aN1(#12l) M0, pl0, G2, Ly1, V1. The results of both ALK immunohistochemistry (Fig. 2C) and EML4‐ALK fusion gene on fluorescence in situ hybridization (FISH) examinations were positive (Fig. 2D). EGFR mutational analysis results showed wild‐type mutation.
Figure 2.
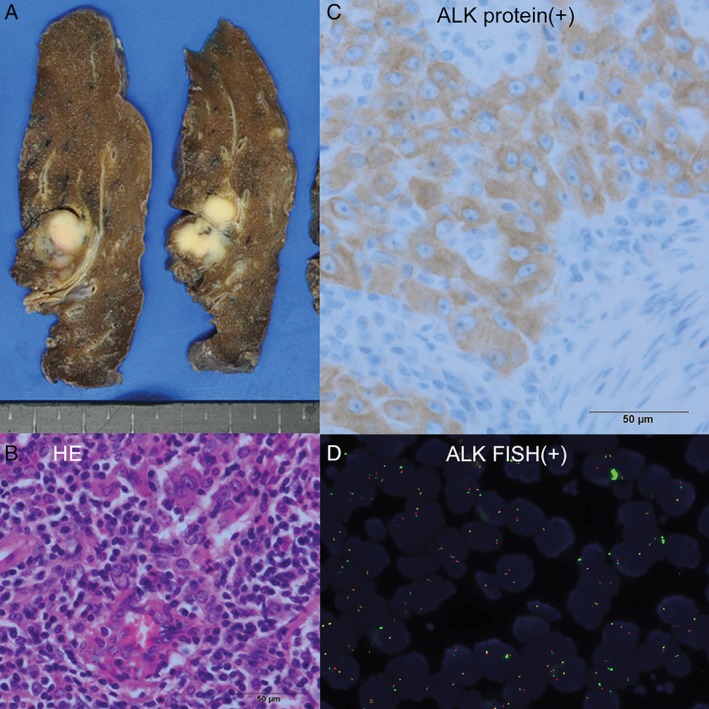
Histopathological findings of the tumour resulting in diagnosis of pulmonary lymphoepithelioma‐like carcinoma. (A) The excised mass was 1.8 × 1.5 × 1.4 cm in size and had infiltrated the upper lobe. (B) Haematoxylin and eosin (HE) staining showed that the tumour consisted of cells with irregular nucleus borders and reactive lymphoplasmacytic cells in both the tumour and stromal tissue. (C) Immunohistological staining for the ALK fusion protein was positive. (D) Echinoderm microtubule‐associated protein‐like 4‐anaplastic lymphoma kinase (EML4‐ALK) fusion gene on fluorescence in situ hybridization (FISH) examinations was positive.
Serum titers of EBV early antigen immunoglobulin (Ig)A were negative and EBV‐specific nuclear antigen was elevated, indicating a prior EBV infection. However, no infection with the virus in tumour cells was shown, as results of EB‐encoded small RNA1 (EBER‐1) on in situ hybridization (ISH) were negative.
The patient did not receive adjuvant chemotherapy and was free from relapse at 1 year after surgery.
Discussion
A PLELC is similar to a lymphoepithelioma, a subtype of nasopharyngeal carcinoma and commonly associated with EBV infection, and is generally seen in Southeast Asia. It is considered that ISH findings of EBER‐1 are the most sensitive; ISH is considered the standard test used to detect EBV within tumour cells. EBV infection of tumour cells has been proven in many cases reported in Asia, whereas it is rare in Caucasians. It is possible that there is another factor related to carcinogenesis besides EBV infection.
Pre‐operative diagnosis of PLELC may be difficult, because histopathological diagnosis is obtained from surgically resected tissues in far more cases than with bronchoscopic biopsy findings. 18FDG‐PET may be a useful pre‐operative examination, as shown by a previous report that found strong accumulation in a PLELC 1, as also seen in the present case.
Chang et al. reported a high level of programmed cell death‐ligand 1 (PD‐L1) expression and infrequent driver mutations in PLELCs as compared with conventional non‐small cell lung cancer (NSCLC) 2. Thus, it appears that an EGFR mutation is uncommon in PLELC, although some have detected mutation at rates ranging from 1.8% to 17.4% 2, 3, 4. The kinds of mutations vary, including exons 18, 19, 20, and 21. The positive rate of PD‐L1 in PLELC has been reported to be as high as 74.3% 2 to 75.8% 4. The presence of the ALK fusion gene was noted by Mano et al. in 2007 and that is also found in approximately 5–8% of NSCLC cases, especially adenocarcinoma. Four studies of ALK rearrangement were presented in 2015, in which none of 232 cases (11 investigated by Wong 5, 66 by Chang 2, 42 by Wang 3, and 113 by Fang 4), had that aberration. Therefore, this is the first report of a PLELC with the ALK fusion gene.
The outcome of PLELC is better as compared with other NSCLC cases, with a 5‐year survival rate ranging from 59.5% to 62%. Complete resection is necessary to obtain a cure, as there is no standard chemotherapy regimen for this rare tumour. Combination chemotherapy with taxane and platinum agents has shown favourable responses, similar to nasopharyngeal carcinoma. There are also a few reports using EGFR‐TKI, ALK inhibitor, and anti‐PD‐1 antibody treatments, which may be useful in PLELC patients. It is important to investigate whether those new drugs are an effective therapeutic option for patients affected with PLELC.
Conclusion
This is the first report of PLELC with ALK rearrangement. ALK inhibitor may play an important role for prognosis in this case.
Disclosure Statements
No conflict of interest declared.
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
Ose, N. , Kawai, T. , Ishida, D. , Kobori, Y. , Takeuchi, Y. and Senba, H. (2016) Pulmonary lymphoepithelioma‐like carcinoma with echinoderm microtubule‐associated protein‐like 4‐anaplastic lymphoma kinase (EML4‐ALK) fusion gene. Respirology Case Reports, 4 (6), e00200. doi: 10.1002/rcr2.200.
Associate Editor: Fu‐Qiang Wen
References
- 1. Dong A, Zhang J, Wang Y, et al. 2015. FDG PET/CT in primary pulmonary lymphoepithelioma‐like carcinoma. Clin. Nucl. Med. 40:134–137. [DOI] [PubMed] [Google Scholar]
- 2. Chang YL, Yang CY, Lin MW, et al. 2015. PD‐L1 is highly expressed in lung lymphoepithelioma‐like carcinoma: a potential rationale for immunotherapy. Lung Cancer 88:254–259. [DOI] [PubMed] [Google Scholar]
- 3. Wang L, Lin Y, Cai Q, et al. 2015. Detection of rearrangement of anaplastic lymphoma kinase (ALK) and mutation of epidermal growth factor receptor (EGFR) in primary pulmonary lymphoepithelioma‐like carcinoma. J. Thorac. Dis. 7:1556–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fang W, Hong S, Chen N, et al. 2015. PD‐L1 is remarkably over‐expressed in EBV‐associated pulmonary lymphoepithelioma‐like carcinoma and related to poor disease‐free survival. Oncotarget 6:33019–33032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wong DW, Leung EL, So KK, et al. 2009. The EML4‐ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild‐type EGFR and KRAS. Cancer 115:1723–1733. [DOI] [PubMed] [Google Scholar]


