Abstract
Both β-catenin and plakoglobin can stimulate the expression of Lef/Tcf target genes in vitro. β-Catenin is known to associate with Lef/Tcf factors and to participate directly in transactivation in vivo, whereas the role of plakoglobin in transcriptional regulation has been less studied. To analyze the functions of plakoglobin in vivo, we generated transgenic mice expressing in the epidermis N-terminally truncated plakoglobin (ΔN122-PG) lacking the glycogen synthase kinase 3β phosphorylation sites and therefore protected against degradation (transgenic line K5-ΔN122-PG). The expression of ΔN122-PG led to the formation of additional hair germs, hyperplastic hair follicles, and noninvasive hair follicle tumors, a phenotype reminiscent of that induced by expression of N-terminally truncated β-catenin. However, if expressed in β-catenin-null epidermis, ΔN122-PG did not induce new hair follicle germs and follicular tumors. Thus, ΔN122-PG cannot substitute for β-catenin in its signaling functions in vivo and the phenotype observed in K5-ΔN122-PG mouse skin must be due to the aberrant activation of β-catenin signaling. On the other hand, the expression of ΔN122-PG in β-catenin-null skin significantly increased the survival rate of mutant mice, rescued differentiation, and limited excessive proliferation in the interfollicular epidermis, suggesting that plakoglobin may be involved in the intracellular signaling events essential for epidermal differentiation.
β-Catenin and plakoglobin (or γ-catenin) are closely related proteins with similar, but not identical, functions. Both catenins are cytoplasmic components of cadherin-containing cell-cell adherens junctions. Plakoglobin is also an essential constituent of the desmosomal cytoplasmic plaque (reviewed in reference 5).
β-Catenin plays a key role in the Wnt/Tcf signal transduction pathway. In epithelial cells, β-catenin is mainly engaged in the formation of cell-cell junctions, and nonjunctional β-catenin is rapidly degraded by the ubiquitin-proteasome system (1, 20, 36). The activation of Wnt signaling results in the stabilization of nonjunctional β-catenin, its translocation to the nucleus, binding to Lef/Tcf transcription factors, and transactivation of Lef/Tcf target genes. In mammals, these genes include those encoding such important regulators of cellular functions as cyclin D1, c-Myc, c-jun, CD44, and Nr-CAM (http://www.stanford.edu/∼rnusse/pathways/targets.html). Wnt/β-catenin signaling is involved in the regulation of cell fate during development (8), and its aberrant activation due to β-catenin stabilization contributes to tumor progression (reviewed in reference 39). The involvement of plakoglobin in Wnt signaling is still uncertain, although in some cell types Wnt can induce the accumulation of plakoglobin (7, 22, 38). The functional consequences of elevated cellular plakoglobin levels are, however, unclear. Plakoglobin overexpression can induce cell transformation (27), but the restoration of plakoglobin expression in highly tumorigenic cells abolishes tumorigenicity (44). Overexpression of plakoglobin in the epidermis has been reported to decrease proliferation in the interfollicular regions and to shorten the hair follicle cycle, resulting in shorter hairs (9). In Xenopus embryos, ectopic expression of plakoglobin leads to its nuclear accumulation and axis duplication (24), mimicking β-catenin signaling.
The β-catenin and plakoglobin central domains, consisting of armadillo repeats, are structurally similar and bind to numerous protein partners including Tcf factors, cadherins, adenomatous polyposis coli (APC), and axin (51). The N- and C-terminal domains of β-catenin and plakoglobin display less sequence similarity (reviewed in reference 13). However, both catenins can be phosphorylated by glycogen synthase kinase 3β (GSK-3β) on N-terminal serine residues (1, 26), and GSK-3β-mediated phosphorylation leads to proteasomal degradation of β-catenin and plakoglobin (1, 20, 36, 42). Point mutations or deletion of the N-terminal phosphorylation sites result in the stabilization of β-catenin but have no effect on the half-life of plakoglobin (49). Since plakoglobin is localized almost entirely to desmosomes and adherens junctions, with a very limited soluble pool, it is inaccessible to the degradation machinery (42). Nevertheless, the N-terminal GSK-3β phosphorylation sites are important for plakoglobin stability, since overexpression of axin, which promotes its GSK-3β-dependent phosphorylation, leads to plakoglobin degradation (26).
β-Catenin and plakoglobin have transactivation domains at their C termini (21, 45), and β-catenin was suggested to activate target gene transcription by recruiting components of the basal transcription machinery to the corresponding promoter regions (21). Despite the high homology in the armadillo domain, β-catenin has a higher affinity for Tcf transcription factors than plakoglobin (49, 52), and a recent study showed that Tcf-4 has distinct binding sites for the two catenins (34).
Current knowledge concerning the ability of plakoglobin to activate transcription is incomplete. Plakoglobin may stimulate β-catenin signaling by sequestering components of the GSK-3β-APC-axin degradation system, which results in the stabilization of β-catenin (25, 26, 32, 45). Moreover, a recent study demonstrated that plakoglobin transactivates the Lef/Tcf-reporter TOPFLASH and the Nr-CAM and cyclin D1 promoters in β-catenin-null embryonic stem cells (12). Thus, at least in vitro, plakoglobin, like β-catenin, can participate in the regulation of transcription.
The activation of β-catenin signaling is a critical step in hair follicle induction in the embryo, in recruitment of stem cells to the lower, cycling part of hair follicles in anagen, and in lower hair follicle cell differentiation (14, 18, 28, 30, 47). The inhibition of Tcf/β-catenin signaling blocks hair follicle induction and leads to epidermal differentiation and the formation of epidermoid cysts from hair follicle rudiments (23, 35, 46). In contrast, the depletion of plakoglobin does not affect hair follicle outgrowth but severely disturbs epidermal architecture and adhesion due to impaired desmosome assembly (6).
To analyze the functions of plakoglobin in the epidermis, we generated transgenic mice expressing N-terminally truncated plakoglobin (ΔN122-PG, lacking amino acids 1 to 122) and full-length plakoglobin under control of the keratin 5 (K5) promoter which targets transgene expression to the basal layer of the epidermis and the outer root sheath of hair follicles (transgenic lines K5-ΔN122-PG and K5-PG, respectively). ΔN122-PG lacks the GSK-3β phosphorylation sites and is, therefore, protected against degradation. Due to its intact C-terminal and central segments ΔN122-PG retains the transactivation domain, together with binding sites for numerous plakoglobin protein partners, including Lef/Tcf, APC, axin, and classical and desmosomal cadherins.
We found that while K5-PG mice display no visible change in their epidermis, the expression of ΔN122-PG resulted in hyperplasia of the interfollicular epidermis and the formation of additional hair germs, aberrant hyperplastic hair follicles, and noninvasive hair follicle tumors. To determine whether this phenotype required β-catenin-mediated events or was induced by the transgene product per se, we studied the effects of ΔN122-PG expression in β-catenin-null epidermis.
MATERIALS AND METHODS
Generation of transgenic mice.
N-terminally deleted human plakoglobin cDNA (ΔN122-PG) and the full-length human plakoglobin cDNA were hemagglutinin (HA)-tagged at the N terminus and inserted into the blunt-ended BamHI site of the K5 expression cassette (kindly provided by J. L. Jorcano [41]). The resulting constructs were designated K5-ΔN122-PG and K5-PG, respectively (Fig. 1A).
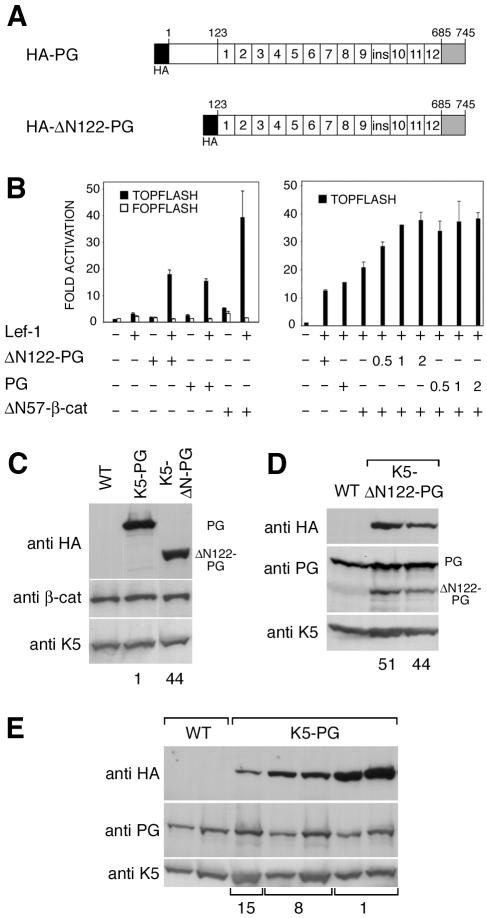
FIG. 1.
Transactivation by plakoglobin and β-catenin ectopically expressed in keratinocytes and transgene expression in the skin. (A) The plakoglobin constructs used for expression under control of the K5 promoter in the epidermis of transgenic mice. (B) Activation (left) of the TOPFLASH luciferase reporter by ΔN122-PG, plakoglobin, and ΔN57-β-catenin. Pam212 mouse keratinocytes were transiently cotransfected with TOPFLASH or FOPFLASH and, where indicated, with LEF-1 (62.5 ng), ΔN122-PG, full-length plakoglobin, or ΔN57-β-catenin (each, 125 ng) plasmids. Coactivation (right) of TOPFLASH by ΔN57-β-catenin and ΔN122-PG or plakoglobin. To study the effects of plakoglobin on TOPFLASH transactivation by ΔN57-β-catenin, various amounts (62.5, 125, and 250 ng) of ΔN122-PG or full-length plakoglobin plasmids were cotransfected with ΔN57-β-catenin plasmid (125 ng). Numbers (0.5, 1.0, or 2.0) indicated the ratio of plakoglobin to ΔN57-β-catenin plasmid DNA. The values (bars) were corrected for transfection efficiency and represent the activation levels with respect to those obtained with TOPFLASH or FOPFLASH only. The data from a representative experiment performed in triplicate are shown with error bars. (C to E) Expression of ΔN122-PG and plakoglobin in the skin of transgenic mice analyzed by Western blotting. Panel C shows Western blots obtained with skin protein extracts from 3-month-old male K5-ΔN122-PG and K5-PG mice (lines 44 and 1, displaying high levels of transgene expression) probed with anti-HA-tag and anti-β-catenin antibodies. Note similar transgene expression levels in K5-PG and K5-ΔN122-PG skin. Panel D shows protein extracts from 3-month-old male K5-ΔN122-PG mouse skin (lines 44 and 51, displaying high levels of transgene expression). Anti-HA-tag antibody detects transgene product, whereas antibody against the C-terminal part of plakoglobin detects endogenous plakoglobin and transgene product, ΔN122-PG. Panel E shows plakoglobin expression levels in skin samples from 3-month-old male wild-type and K5-PG mice (lines 1, 8, and 15). Skin protein extracts from two wild-type and five K5-PG mice (one from line 15, two from lines 8 and 1) were analyzed with the anti-HA-tag antibody to detect transgene expression and with the antibody against the C-terminal part of plakoglobin recognizing both endogenous plakoglobin and transgene product (total plakoglobin). Note that total plakoglobin levels were not altered in the skin samples of transgenic mice. K5 served as a loading control. WT, wild type.
Transgenic mice were generated by pronuclear injection of the purified K5-ΔN122-PG or K5-PG construct into fertilized oocytes from C57BL/6 mice. Animals were screened for transgene integration by PCR performed on genomic DNA isolated from tail snips. To determine the transgene copy number, the intensity of the radioactive signal for the transgenic probe was compared with genomic DNA from transgenic mice and the signals obtained with a graded copy number of the plasmid containing the transgenic constructs. Signals were quantified by using a Storm 860 PhosphorImager (Molecular Dynamics) and an ImageQuant program.
To generate mice with the K14+/Cre(neo)-βcat lox/null-K5-ΔN122-PG genotype (referred to as K14-βcat−/−-K5-ΔN122-PG), K5-ΔN122-PG females (line 12) were mated with K14+/Cre(neo)-βcat+/null males, and the progeny was genotyped by PCR as previously described (23). K14+/Cre(neo)-βcat +/null-K5-ΔN122-PG mice were mated with β-catenin lox/lox mice, and the resulting progeny was genotyped and analyzed.
Cultured keratinocytes and transfection.
Pam212 keratinocytes were grown in Dulbecco's modified Eagle medium (Gibco BRL, Life Technologies S.A., Cergy Pontoise, France) supplemented with 10% fetal calf serum (Seromed, Biochrom KG, Berlin, Germany), 2 mM l-glutamine (Gibco BRL), and penicillin-streptomycin (Gibco BRL).
Transient transfection experiments were performed with the Geneporter reagent (Gene Therapy Systems Inc., San Diego, Calif.) according to the manufacturer's instructions. Cells were split into 24-well dishes at a density of 5 × 104 cells/well. After 48 h, the cells were transfected with 125 ng of pTOPtkLuciferase (TOPFLASH) or pFOPtkLuciferase (FOPFLASH), 62.5 ng of Renilla reporter constructs (Promega, Madison, Wis.), 62.5 ng of Lef-1, 125 ng of ΔN57-β-catenin (43) plasmid, and, when indicated, with 62.5, 125, or 250 ng of ΔN122-PG or the full-length plakoglobin plasmid.
After a 48-h incubation, cells were scraped into 100 μl of passive lysis buffer (Promega) per well, and after two freeze-thaw cycles, lysates were cleared by centrifugation at 10,000 × g and 4°C for 5 min. Luciferase activity in 20-μl lysate aliquots was measured in a Lumat 9501 luminometer (Berthold, Pforzheim, Germany), by using firefly and Renilla substrates (Promega). The values obtained for firefly luciferase were corrected for transfection efficiency by using Renilla luciferase activity. At least three independent experiments were performed in triplicate in each case.
Whole-mount skin staining.
Paw skin pieces from 6-month-old male wild-type and transgenic mice were spread on microscope slides and fixed overnight in Methacarn (60% methanol, 30% chloroform, 10% acetic acid), rehydrated, and stained with carmine alum for several days. Samples were dehydrated and cleared in xylene for observation.
BrdU incorporation assay, histology, and immunostaining.
To assess cell proliferation, mice were injected intraperitoneally with 0.25 mg of 5-bromo-2′-deoxyuridine (BrdU)/g of body weight 2 h prior to sacrifice. Nuclear BrdU detection was performed on skin sections by using a cell proliferation kit (Amersham) according to the manufacturer's instructions.
Skin samples were fixed with 4% paraformaldehyde in phosphate-buffered saline or Methacarn and processed for embedding in paraffin. For histological analysis and immunohistochemistry, 7-μm sections were cut. For immunohistochemistry, the sections were incubated in 1% H2O2 to block endogenous peroxidase activity. To retrieve nuclear antigens on paraffin-embedded skin sections, slides were incubated for 10 min in 10 mM sodium citrate buffer, pH 6.0, at 90°C.
Cryosections (7 μm) were cut from skin pieces embedded in Tissue-Tek (Miles Diagnostic Division, Elkhart, Ind.) and frozen in isopentane cooled by liquid nitrogen. Before staining, cryosections were fixed in acetone for 10 min at −20°C.
Skin sections were incubated for 60 min in 5% fetal calf serum, then overnight with primary antibodies, and for 2 h at room temperature with appropriate secondary antibodies. Nuclei were stained with 1 μg of DAPI (4′,6′-diamidino-2-phenylindole) (Sigma) per ml for immunofluorescence studies or counterstained with hematoxylin for immunohistochemistry.
Texas Red-, fluorescein isothiocyanate (1:100; Jackson Immunoresearch Laboratories)- or AlexaFluor (1:1,000; Molecular Probes)-conjugated secondary antibodies were used for immunofluorescence labeling, and the Envision+ System HRP kit (Dako) was used for immunohistochemistry.
Immunohistochemical detection of nuclear β-catenin, plakoglobin, HA-tag, c-Myc, and Engrailed-1 was performed with paraformaldehyde-fixed paraffin-embedded tissue samples; detection of C/EBPβ, BrdU, and cyclin D1 was performed with Methacarn-fixed paraffin-embedded tissue and required antigen retrieval. All antikeratin antibodies were used on frozen or paraffin-embedded sections; anti-E-cadherin and antidesmoplakin were used on frozen sections.
RNA isolation, cDNA arrays, and real time reverse transcription (RT)-PCR.
Total RNA was isolated from frozen shaved mouse back skin pieces with RNA-Plus reagent (Bioprobe Systems, Montreuil-Sous-Bois, France). Five-microgram RNA samples were analyzed by cDNA array technology by using a signal transduction pathway finder gene array (SuperArray Inc., Bethesda, Md.) according to manufacturer's instructions. Quantitative analysis of the results was performed by using a PhosphorImager (Molecular Dynamics) and the ImageQuant program.
For real-time RT-PCR, the levels of c-Myc mRNA were normalized to the levels obtained for GAPDH (glyceraldehyde-3-phosphate dehydrogenase). The following primers were used: for mouse c-Myc, CAC CAG CAG CGA CTC TGA A and CCC GAC TCC GAC CTC TTG; for GAPDH, CCA ATG TGT CCG TCG TGG ATC and GTT GAA GTC GCA GGA GAC AAC.
Transmission electron microscopy.
Skin samples were fixed in 2% glutaraldehyde in phosphate-buffered saline, followed by 2% glutaraldehyde in 0.1 M sodium cacodylate-HCl, pH 7.4, washed with 0.1 M sodium cacodylate-HCl, pH 7.4, postfixed with 1% OsO4 in 0.15 M sodium cacodylate-HCl, pH 7.4, dehydrated in a graded series of ethanol and propylene oxide solutions, and embedded in Araldite resin. Ultrathin (60- to 80-nm) sections were prepared with an Ultracut apparatus (Reichert-Jung), contrast stained with uranyl acetate and lead citrate, and observed with a transmission electron microscope (LEO 912).
Protein analysis.
For Western blotting, pieces of shaved dorsal skin (2 to 3 cm2) from wild-type and transgenic mice were flash-frozen in liquid nitrogen, homogenized, and boiled in Laemmli's sample buffer. The supernatants were subjected to sodium dodecyl sulfide-polyacrylamide gel electrophoresis in 10% acrylamide gels. The separated proteins were electrophoretically transferred to polyvinylidene difluoride membranes (Immobilon-P; Millipore) which were probed with antibodies. Immunoblots were developed by using an ECL detection system (Amersham). To examine the subcellular distribution of E-cadherin and catenins, skin specimens from 2-month-old mice were homogenized and extracted with 1 ml of 50 mM Tris-HCl, pH 7.4, buffer containing 100 mM NaCl, 2 mM Ca2Cl, 1% NP-40, 1% Triton X-100, 2 mM phenylmethylsulfonyl fluoride, and 20 μg of aprotinin per ml and centrifuged for 15 min at 15,000 × g at 4°C. To extract the detergent-insoluble fraction, the pellet was boiled in 0.5 ml of Laemmli's sample buffer. Detergent-soluble protein fractions (0.1 mg) and equivalent amounts of the detergent-insoluble fractions were analyzed by Western blotting. For immunoprecipitation, 0.5 mg of the detergent-soluble fractions was used.
Antibodies.
Rabbit anti-β-catenin (Sigma-Aldrich); anti-C/EBPβ (Santa Cruz Biotechnology); anti-Engrailed-1 (Abcam); anti-K1, -K5, -K6, and -K14 (all from Covance); anti c-Myc (N-262; Santa Cruz Biotechnology); rat anti-E-cadherin (Sigma); mouse anti-K10 (Dako); anti-desmoplakin I+II (Boerhinger-Mannheim); anti-BrdU (Pharmingen); and anti-hair keratin AE13 (a gift from T. T. Sun) antibodies were used for immunohistochemistry. Rabbit anti-α-catenin antibody (Sigma) was used for Western blotting; rabbit anti-HA-tag (Santa Cruz Biotechnology) and anti-cyclin D1 (Pharmingen) antibodies were used for immunohistochemistry and Western blotting; rat anti-E-cadherin (Zymed) and mouse anti-β-catenin (Transduction Laboratories) antibodies were used for Western blotting and immunoprecipitation; rat anti-HA tag (Roche) and mouse anti-plakoglobin C terminus (Transduction Laboratories) antibodies were used for immunohistochemistry, Western blotting, and immunoprecipitation.
RESULTS
Generation of transgenic mice and transgene expression in the epidermis.
We first estimated the transactivation capacities of plakoglobin and ΔN122-PG by transiently cotransfecting cultured mouse keratinocytes (cell line Pam212) with the TOPFLASH luciferase reporter and plasmids encoding N-terminally truncated β-catenin (ΔN57-β-catenin), plakoglobin, ΔN122-PG, and Lef-1. In the absence of transfected Lef-1, ΔN57-β-catenin, plakoglobin, and ΔN122-PG only weakly activated transcription from the TOPFLASH reporter, whereas the cotransfection of cells with Lef-1 and catenin constructs resulted in strong activation (Fig. 1B, left panel). Plakoglobin and ΔN122-PG enhanced transcription from the TOPFLASH reporter to similar extents, corresponding to about half of the activation achieved with ΔN57-β-catenin. The catenin constructs only weakly activated transcription from the control FOPFLASH reporter containing mutant Lef/Tcf binding sites. Similar results were obtained with the mouse keratinocyte cell line MCA3D (data not shown).
To investigate whether excess plakoglobin and ΔN122-PG affected the β-catenin-induced activation of TOPFLASH, these two molecules were coexpressed in Pam212 cells, together with ΔN57-β-catenin, Lef-1, and TOPFLASH. The coexpression of plakoglobin or ΔN122-PG (with ratios of plakoglobin to β-catenin plasmid DNA of 0.5, 1.0, and 2.0) resulted in TOPFLASH activation levels higher than those induced by ΔN57-β-catenin and Lef-1 alone (Fig. 1B, right panel), suggesting that plakoglobin and ΔN122-PG were not inhibitory.
The K5 promoter was used to generate transgenic mice expressing plakoglobin and ΔN122-PG in the basal layer of the epidermis. Six founders positive for the plakoglobin transgene and eight founders positive for ΔN122-PG were obtained. Four of the K5-PG and six of the K5-ΔN122-PG founders displayed Mendelian transmission of the transgene to the F1 generation and expressed the transgenes, as shown by Western blot analysis and the immunostaining of skin samples. Southern blot analysis demonstrated that the transgenic mice of all the established lines carried 10 to 20 copies of the transgene (data not shown). Heterozygous transgenic animals were used for subsequent analysis. Low-, medium-, and high-level expressers were detected among the transgenic lines obtained. Skin protein extracts from K5-PG and K5-ΔN122-PG lines with high transgene expression levels contained roughly comparable amounts of transgene products, as shown by immunoblotting with anti-HA-antibody (Fig. 1C). Western blotting performed with anti-plakoglobin antibody recognizing the C terminus revealed endogenous plakoglobin and transgene product, ΔN122-PG, in K5-ΔN122-PG mouse skin (Fig. 1D). Comparison of the corresponding band intensities in Fig. 1D suggested that the transgene product levels in total skin extracts corresponded to approximately 20 to 25% of the endogenous plakoglobin amount. Surprisingly, we did not detect any significant change in the level of total plakoglobin (i.e., endogenous protein plus transgene product) in K5-PG transgenic lines (Fig. 1E). β-Catenin levels were not visibly altered in K5-PG and K5-ΔN122-PG mouse skin extracts (Fig. 1C).
K5-PG mice appeared normal and presented no skin lesions on histological analysis within the 16-month observation period. In contrast, K5-ΔN122-PG mice (both females and males) had enlarged paws and irregularly thickened skin of the muzzle and the tail (Fig. 2A), a phenotype macroscopically similar to that of transgenic mice expressing N-terminally truncated β-catenin in basal keratinocytes (18, 28). Histological analysis revealed that the back skin was also affected although to a lesser extent (data not shown). Manifestation of this phenotype depended on the transgene expression level. Macroscopically obvious skin lesions were only occasionally observed in 10- to 12-month-old animals expressing low levels of ΔN122-PG, whereas in animals with medium (line 12) and high levels of transgene expression (lines 44 and 51), such lesions appeared at the ages of 5 to 6 and 2 to 3 months, respectively. These observations demonstrate that this phenotype results from transgene expression rather than the DNA integration site. The phenotype penetrance was 90% in the lines with high-level transgene expression and 70 to 80% in animals with medium-level expression.
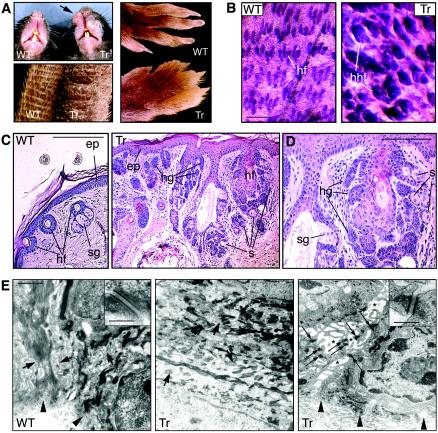
FIG. 2.
Skin lesions in the epidermis of K5-ΔN122-PG mice. (A) Skin lesions on the muzzle, tail, and paws of K5-ΔN122-PG mice. (B) Whole-mount staining of wild-type and K5-ΔN122-PG (line 44) mouse dorsal paw skin. Note the abnormal shape of all visible hair follicles. hf, hair follicle; hhf, hyperplastic hair follicle. (C and D) Hematoxylin and eosin staining of the tail skin sections from 6-month-old wild-type and K5-ΔN122-PG (line 12) mice. Panel D shows one of the abnormal hair follicles shown in panel C but at higher magnification. Note the thickened epidermis (ep), aberrant hair germs (hg), ectopic sebocytes (s). hf, hair follicle, sg, sebaceous gland. Panel E shows an ultrastructural analysis of the epidermis in wild-type and K5-ΔN122-PG mice by transmission electron microscopy. Large arrows indicate cell-cell junctions in wild-type and in K5-ΔN122-PG (central panel, line 12; right panel, line 44) epidermis, small arrows show desmosomes, and arrowheads indicate the basement membrane. Asterisks indicate intercellular gaps observed in basal and spinous layers of the K5-ΔN122-PG mouse epidermis exhibiting high levels of transgene expression and severe phenotype. Insets show individual desmosomes. WT, wild-type, Tr, transgenic. Bars = 0.4 mm (B), 80 μm (C), 40 μm (D), 1.2 μm (E), and 90 nm (insets).
In the most severely affected areas of the skin, all hair follicles were enlarged and abnormal in shape (Fig. 2B). The histological abnormalities observed in K5-ΔN122-PG mice included thickened epidermis, hair and vibrissa follicle hyperplasia, and dermal cysts (Fig. 2C and D and data not shown). Some of the hyperplastic hair follicles found in K5-ΔN122-PG mouse skin were reminiscent of human tumors known as trichofolliculomas (Fig. 2C and D).
In general, cell-cell contacts were not altered in the K5-ΔN122-PG epidermis, as estimated by transmission electron microscopy (Fig. 2E). In animals with severe lesions only, cells in the epidermal basal and suprabasal layers were separated by wider intracellular spaces than those found in wild-type skin (Fig. 2E). Such intercellular gaps have been observed in normal skin in healing wounds (19).
Expression of ΔN122-PG induces hyperplasia of the interfollicular epidermis.
Expression of ΔN122-PG driven by the K5 promoter resulted in a thickening of the epidermis, observed, in particular, on the tail, the muzzle, and the dorsal paw surface (Fig. 2 and 3). In wild-type skin, K6 was detected only in hair follicles, whereas most of the basal cells in the epidermis of K5-ΔN122-PG mice stained positive for K6 (Fig. 3A). Epidermal differentiation was not otherwise affected by the presence of the transgene product, as K10 was detected in the suprabasal layers (Fig. 3A), and two other terminal differentiation markers—loricrin and fillagrin—were expressed in the upper layers of the epidermis (data not shown). Foci of BrdU-incorporating cells were found in the interfollicular zones of the epidermal basal layer in K5-ΔN122-PG mice, indicating high rates of proliferation (Fig. 3B). Large cell clusters with cyclin D1-positive nuclei were found in basal keratinocytes of the interfollicular zones of K5-ΔN122-PG epidermis. Far fewer cyclin D1-positive nuclei were detected in the wild-type skin (Fig. 3C). Consistently, protein extracts from K5-ΔN122-PG mouse skin contained higher levels of cyclin D1 than those from wild-type animals (Fig. 3D).
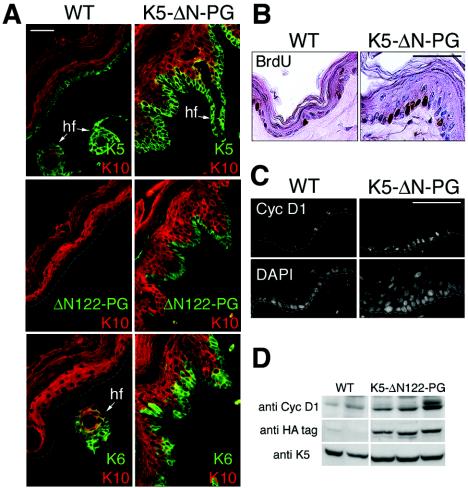
FIG. 3.
Hyperplasia of the epidermis in K5-ΔN122-PG mice. (A) Serial cryosections of the dorsal paw skin from 6-month-old mice. In wild-type skin, K5 was expressed in basal and K10 in suprabasal spinous layers, whereas K6 was limited to hair follicles and was absent from the epidermis. In K5-ΔN122-PG skin (line 44), K6 was expressed in basal keratinocytes, whereas K5 and K10 were expressed normally in basal and suprabasal keratinocytes, respectively. The transgene product was detected in basal cells with an anti-HA-tag antibody. hf, hair follicle. The dotted lines in panels A and C indicate the position of the basement membrane. (B) Immunohistochemistry of BrdU-incorporating cells in wild-type and K5-ΔN122-PG (line 12) 6-month-old male mouse tail epidermis. (C) Distribution of cyclin D1 (Cyc D1) in wild-type and K5-ΔN122-PG (line 12) 6-month-old male mouse tail epidermis, analyzed by immunofluorescence staining. Note the clusters of basal keratinocytes with nuclear cyclin D1 frequently observed in the transgenic mouse skin. (D) Expression of cyclin D1 in wild-type and K5-ΔN122-PG mouse skin. Western blot analysis of skin protein extracts from two wild-type and three K5-ΔN122-PG (line 12) 3-month-old male mice by using anti-cyclin D1 antibody. The blot was stripped and reprobed with anti-HA-antibody to determine transgene product levels and with anti-K5-antibody, which served as a loading control. Bars = 50 μm (A) and 25 μm (B and C). WT, wild type.
Expression of ΔN122-PG induces hair follicle hyperplasia and dermal cysts.
In K5-ΔN122-PG skin, novel hair follicle buds were formed from the outer root sheath of the preexisting hair follicles and from the epidermis (Fig. 2C and D and Fig. 4A and B). The transgene product was detected with anti-HA-antibodies in the outermost cell layer of the aberrant hair follicles (Fig. 4A). Hyperplastic hair follicles were surrounded by a continuous basement membrane, stained with anti-laminin antibodies (Fig. 4B). The basement membrane surrounding abnormal hair follicles was often thickened and contained an unprocessed γ2-laminin chain that was not deposited in the basement membrane in wild-type skin (data not shown).
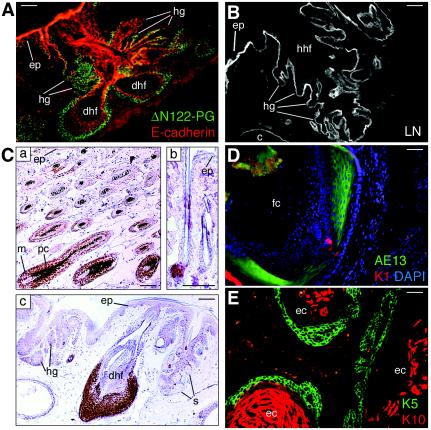
FIG. 4.
Hair follicle hyperplasia in K5-ΔN122-PG transgenic mouse skin. (A) Double immunofluorescence staining of a dorsal paw skin cryosection from a K5-ΔN122-PG 6-month-old male mouse (line 12) with anti-HA-tag revealing ΔN122-PG and anti-E-cadherin antibodies. The transgene product is present in the outer cell layer of the aberrant hair follicles. Note dilated hair follicles, numerous aberrant hair germs, and hair follicles formed from the preexisting follicle. (B) Staining of the basement membrane surrounding the hyperplastic hair follicles found in K5-ΔN122-PG 6-month-old male mouse (line 12) dorsal paw skin with antilaminin antibody. (C) En1 expression in wild-type and K5-ΔN122-PG (line 51) skin. Immunohistochemistry performed on paraformaldehyde-fixed paraffin-embedded skin samples. Sections of back skin from a wild-type mouse showing hair follicles in anagen (a) and telogen (b). En1 was found only in the lower cycling part of hair follicles. In K5-ΔN122-PG mouse tail skin (c), numerous En1-positive cells were detected in aberrant hair follicles. Hair germs and tumors containing ectopic sebocytes showed no En1 staining. (D and E) Dermal cysts in K5-ΔN122-PG transgenic mouse skin (line 44). In a follicular cyst from the dorsal paw skin of a K5-ΔN122-PG mouse (D), numerous cells were stained with AE13 (green), an anti-hair follicle keratin antibody. K1 (red), an early epidermal keratinocyte terminal differentiation marker, was not detected. DAPI staining is shown in blue. In epidermoid cysts in K5-ΔN122-PG mouse muzzle skin, the cells of the outer wall layers expressed K5 (green), whereas K10 (red) was found in the innermost layers. c, cyst; dhf, dilated hair follicle; ec, epidermoid cyst; ep, epidermis; fc, follicular cyst; hhf, hyperplastic hair follicle; hg, hair follicle germ; m, matrix; pc, precortex; s, sebocyte. Bars = 50 μm (A, B, frames a and c of C, D, and E) and 25 μm (C, frame b).
cDNA microarray analysis revealed that the levels of mRNA for En1, the mouse homolog of the Drosophila Engrailed transcription factor, were high in all K5-ΔN122-PG skin samples tested. In wild-type animals, En1 was present mostly in the lower, cycling part of hair follicles, with a particularly strong signal in precortex and matrix cells (Fig. 4C, frames a and b). In K5-ΔN122-PG mice, the enlarged hair follicles and follicular cyst-like structures contained numerous En1-positive cells (Fig. 4C, frame c). En1 expression in hyperplasia indicates its possible origin from the lower hair follicle part. Consistent with this view, little or no staining for En1 was detected in aberrant hair germs formed from the epidermis and in sebocyte-containing hyperplastic hair follicles (Fig. 4C, frame c).
Finally, many multilayered dermal cysts contributed to the increase in skin volume. Some of these cysts stained with the AE13 antibody that recognizes hair-specific cytokeratins (Fig. 4D), whereas others expressed markers of epidermal differentiation (Fig. 4E).
Localization of ΔN122-PG in transgenic mouse skin.
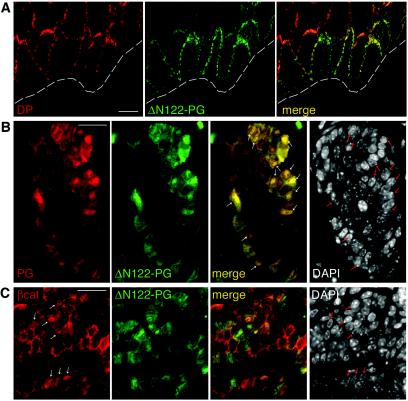
To evaluate the possible activation of β-catenin signaling by ΔN122-PG expression, we analyzed the intracellular distribution of ΔN122-PG and β-catenin in K5-ΔN122-PG mouse skin. In the epidermis, ΔN122-PG was found at cell-cell boundaries, where its distribution correlated with that of desmoplakin (Fig. 5A), suggesting that the transgene product was located in desmosomes. Only a few HA-tag-positive nuclei were detected in the basal cell layer of the epidermis in K5-ΔN122-PG mice (data not shown).
FIG. 5.
Intracellular distribution of the transgene product in K5-ΔN122-PG skin. (A) Confocal microscopy, double immunofluorescence staining of a cryosection through K5-ΔN122-PG mouse (line 12) epidermis with anti-desmoplakin (DP) and anti-HA-tag antibodies to detect ΔN122-PG. In the epidermis, ΔN122-PG was found at cell-cell boundaries colocalized with desmoplakin. The dotted line indicates the position of the basement membrane. (B and C) Double immunofluorescence staining of paraformaldehyde-fixed paraffin-embedded sections through a hair follicle tumor from K5-ΔN122-PG mouse dorsal paw skin (line 12). Panel B shows labeling with antibodies against the C-terminal part of plakoglobin, revealing the endogenous protein as well as the transgene product, and against the HA-tag, detecting ΔN122-PG. In the cells of the inner layers where the K5 promoter is not active, cell-cell boundaries stained with anti-plakoglobin antibody only, whereas both antibodies stained numerous nuclei in the basal cell layer. Arrows indicate plakoglobin and HA-tag-positive nuclei. Note that only HA-positive nuclei stained with anti-plakoglobin antibody. Panel C shows labeling with anti-β-catenin and anti-HA-tag antibodies. Arrows indicate nuclei strongly stained for β-catenin. DAPI was used to stain nuclei. Bars = 5 μm (A) and 12 μm (B and C).
In the hyperplastic hair follicles formed in K5-ΔN122-PG skin, HA-tag staining was observed at cell-cell boundaries and in the nuclei of the outer (basal) cell layer (Fig. 5B). β-Catenin was also present in the nuclei of many basal cells (Fig. 5C), indicating that the expression of ΔN122-PG led to the stabilization and nuclear translocation of β-catenin.
In β-catenin-null epidermis, ΔN122-PG does not induce the formation of new hair germs but restores the differentiation.
The observed effects of ΔN122-PG expression in the epidermis could be attributed either entirely to β-catenin, which was stabilized and accumulated in the nuclei due to the presence of the transgene product, or, at least in part, to the intrinsic ability of ΔN122-PG to activate transcription via Lef/Tcf or other factors. To examine these possibilities, we introduced ΔN122-PG into basal keratinocytes of β-catenin-null epidermis (mice referred to as K14-βcat−/−-K5-ΔN122-PG).
The phenotypic characteristics of K14-βcat−/−-K5-ΔN122-PG mice are summarized in Table 1. K5 promoter-driven expression of ΔN122-PG in mice displaying conditional inactivation of the β-catenin gene increased survival rate, as in the 2-month observation period, 6 of 11 K14-βcat−/− mice and only 1 of 8 K14-βcat−/−-K5-ΔN122-PG mice died. K14-βcat−/− mice weighed 30 to 60% less than their wild-type littermates and opened their eyes only partially, probably due to defects in the eyelid epidermis. In contrast, six of the eight K14-βcat−/−-K5-ΔN122-PG mice were smaller than wild-type mice by only 10 to 15% and had macroscopically normal eyelids. However, like K14-βcat−/− mice, K14-βcat−/−-K5-ΔN122-PG littermates presented alopecia.
TABLE 1.
Effects of ΔN122-PG expression on the phenotype of K14-βcat−/− mice
| Parameter | No. (%) of mice (no. of mice/total no. of mice analyzed)
|
|
|---|---|---|
| K14-βcat−/− | K14-βcat−/−-K5-ΔN122-PG | |
| Death within 2 months of observation | 55 (6/11) | 12.5 (1/8) |
| Decrease in weight of 30 to 60% | 100 (11/11) | 25 (2/8) |
| Eyelid defects | 100 (11/11) | 25 (2/8) |
| Partial alopecia | 100 (11/11) | 100 (8/8) |
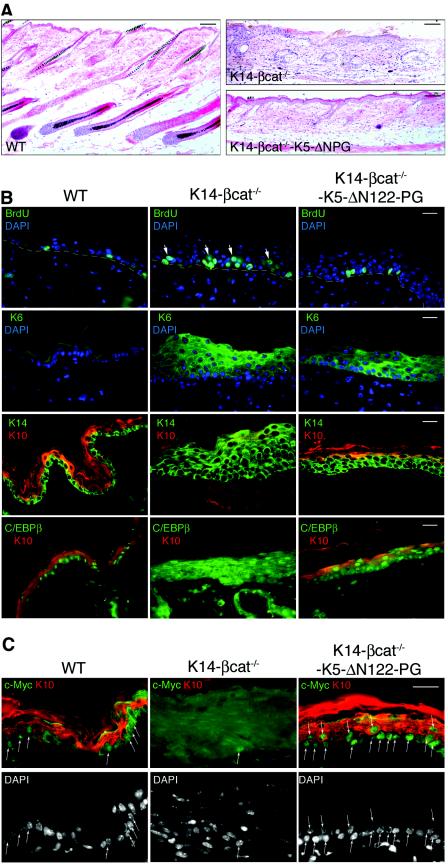
Histological analysis of skin samples from 2- and 6-month-old mice confirmed that the expression of ΔN122-PG in β-catenin-null epidermis neither induced normal hair follicle cycling nor led to the formation of new hair germs or hair follicle tumors detected in the skin of K5-ΔN122-PG mice (Fig. 6A). These results suggest that β-catenin-mediated events rather than intrinsic plakoglobin signaling activity induced the skin lesions in K5-ΔN122-PG mice.
FIG.6.
Partial restoration of differentiation in the β-catenin-null epidermis expressing ΔN122-PG. (A) Sections through wild-type and mutant (K14-βcat−/− and K14-βcat−/−-K5-ΔN122-PG) 1-month-old male mouse back skin stained with hematoxylin and eosin. Note the abnormally thickened epidermis in K14-βcat−/− mouse skin and an improvement in epidermal architecture in K14-βcat−/−-K5-ΔN122-PG mouse. In wild-type skin, hair follicles are in anagen, while in mutant mouse skin, hair follicle cycling is arrested. (B) Proliferation and differentiation marker expression in wild-type and mutant (K14-βcat−/− and K14-βcat−/−-K5-ΔN122-PG) mouse epidermis. Sections through Methacarn-fixed paraffin-embedded back skin from 1-month-old mice. Note that the expression of ΔN122-PG in β-catenin-null epidermis decreases proliferation and the expression of K6 and K14 and restores expression of K10 and nuclear localization of C/EBPβ in the suprabasal layers of the epidermis. Arrows indicate BrdU-positive nuclei in suprabasal layers of β-catenin-null epidermis. (C) c-Myc expression in wild-type and mutant 2-month-old mouse epidermis. Paraformaldehyde-fixed paraffin-embedded back skin sections. Note numerous basal and suprabasal keratinocytes with nuclear c-Myc staining in K14-βcat−/−-K5-ΔN122-PG skin. Arrows indicate c-Myc-positive nuclei. Bars = 60 μm (A) and 15 μm (B and C). WT, wild type.
In young animals, β-catenin-null epidermis was found to be abnormally thick and hyperproliferative (23). In animals older than 4 to 5 months, this phenotype was less obvious. Since an earlier paper describing the phenotype of β-catenin-null skin focused on hair follicle defects, in this study, we examined interfollicular β-catenin-null epidermis in more detail. In β-catenin-null epidermis, proliferating cells were present in basal and suprabasal layers, whereas staining for basal cell K14 and for K6, a hyperproliferation marker, was observed in all cell layers (Fig. 6B). In addition, many areas lacking K10, a marker of terminal keratinocyte differentiation, were found in the epidermis of K14-βcat−/− mice (Fig. 6B). As K10 expression is regulated by the transcription factor C/EBPβ (29, 50), we compared the distribution of C/EBPβ in mutant and wild-type epidermis. While in wild-type skin C/EBPβ was concentrated in the nuclei of basal and some suprabasal keratinocytes, in β-catenin-null epidermis, the anti-C/EBPβ antibody in addition stained the cytoplasm in all cell layers (Fig. 6B). This abnormal intracellular localization suggests that activation of C/EBPβ in β-catenin-null epidermis may be impaired.
In the K14-βcat−/−-K5-ΔN122-PG epidermis, BrdU-incorporating cells were found mostly in the basal layer. The expression of K6 and K5 or K14 was downregulated in the upper layers of the epidermis, whereas normal K10 expression was restored; and the intracellular distribution of C/EBPβ was rather similar to that observed in wild-type skin, with C/EBPβ found essentially in the nuclei of suprabasal cells (Fig. 6B). Thus, the expression of ΔN122-PG significantly restored the differentiation of β-catenin-null interfollicular epidermis.
Expression of ΔN122-PG leads to c-Myc up-regulation in β-catenin-null epidermis.
c-Myc, shown to be activated in some cells overexpressing plakoglobin (27), is also an important regulator of epidermal keratinocyte differentiation (4, 16, 17, 48). We therefore examined by real-time RT-PCR the effects of ΔN122-PG on c-Myc expression in the epidermis. Expression of ΔN122-PG in wild-type epidermis and inactivation of the β-catenin gene did not alter c-Myc mRNA levels. In contrast, in K14-βcat−/−-K5-ΔN122-PG epidermis, c-Myc levels were two times higher (Table 2). Immunofluorescence analysis of 1- to 2-month-old K14-βcat−/−-K5-ΔN122-PG mouse skin sections with anti-c-Myc antibodies detected nuclear c-Myc in many basal and suprabasal keratinocytes, while only weak diffuse staining and rare c-Myc-positive nuclei were observed in β-catenin-null epidermis (Fig. 6C).
TABLE 2.
Effects of ΔN122-PG on c-Myc expression in normal and β-catenin-null epidermis
| Genotype | Expression ± SEM (no. of mice analyzed)a |
|---|---|
| Wild-type | 1.14 ± 0.12 (4) |
| K5-ΔN122-PG | 1.01 ± 0.31 (4) |
| K14-βcat−/− | 1.02 ± 0.21 (5) |
| K14-βcat−/−-K5-ΔN122-PG | 2.00 ± 0.6 (4) |
RNA was isolated from skin extract from 2-month-old mice. Values obtained for c-Myc by real-time RT-PCR were normalized to those of GAPDH.
Subcellular distribution of catenins and E-cadherin in wild-type and mutant epidermis.
Expression of ΔN122-PG in the epidermis may induce changes in cell-cell adhesion. We therefore examined the effects of ΔN122-PG on the composition of E-cadherin-catenin complexes and the distribution of E-cadherin and catenins in detergent-soluble and detergent-insoluble cellular fractions from wild-type and mutant epidermis from 2-month-old mice (Fig. 7A and B).
FIG. 7.
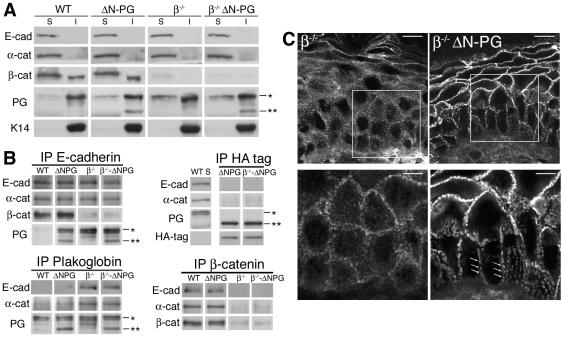
Subcellular distribution of E-cadherin and catenins in wild-type and mutant mouse skin. (A) Distribution of E-cadherin and catenins between detergent-soluble (S) and insoluble (I) fractions of skin extracts by Western blotting (B) Immunoprecipitation with antibodies against E-cadherin, β-catenin, plakoglobin, and anti-HA-tag. The immunoprecipitates were analyzed by Western blotting. The detergent-soluble fraction from wild-type skin extract (WT S) served as a control for HA-tag immunoprecipitate. *, position of full-length plakoglobin; **, position of ΔN122-PG. (C) Confocal microscopy. Cryosections through 2-month-old K14-βcat−/− (β−/−) and K14-βcat−/−-K5-ΔN122-PG (β−/−ΔN-PG) mouse epidermis. Arrows indicate characteristic patchy staining with anti-desmoplakin antibodies at cell-cell boundaries in K14-βcat−/−-K5-ΔN122-PG epidermis. Bars = 10 μm (upper panels) and 5 μm (lower panels).
Western blot analysis of these subcellular fractions showed no significant differences in the levels of E-cadherin and catenins in skin extracts from mutant and wild-type mice (Fig. 7A). In all samples, E-cadherin and α-catenin were found essentially in the detergent-soluble fractions. A small amount of E-cadherin and α-catenin was also detected in the detergent-insoluble fractions, if the exposure time for the immunoblot development was increased (data not shown). β-Catenin was present in both the detergent-soluble and detergent-insoluble fractions of wild-type and K5-ΔN122-PG skin extracts. Although most of the plakoglobin was detergent-insoluble in all extracts, a slightly greater amount of detergent-soluble plakoglobin was detected in K5-ΔN122-PG, K14-βcat−/−, and K14-βcat−/−-K5-ΔN122-PG skin. The amount of ΔN122-PG in the detergent-soluble fractions was below detection levels.
The detergent-soluble fractions of skin extracts were used for immunoprecipitation assays (Fig. 7B). In K5-ΔN122-PG skin, the amount of α-catenin coprecipitating with E-cadherin was similar to that in wild-type skin. Thus, the expression of ΔN122-PG did not significantly prevent the binding of α-catenin to E-cadherin-containing complexes and the formation of adherens junctions. In contrast to what was observed for wild-type skin samples, endogenous plakoglobin and ΔN122-PG were detected in E-cadherin immunoprecipitates from K5-ΔN122-PG extracts, suggesting that, to some extent, these molecules may replace β-catenin in E-cadherin-containing complexes. However, only very small amounts of E-cadherin and α-catenin were found in immunoprecipitates from K5-ΔN122-PG skin extracts obtained with anti-plakoglobin antibody. This indicates that only a small fraction of plakoglobin was associated with E-cadherin and α-catenin. Moreover, the anti-HA antibody did not precipitate detectable amounts of E-cadherin or α-catenin from K5-ΔN122-PG skin extracts. Thus, the expression of ΔN122-PG did not interfere with the formation of adherens junctions in the epidermis.
In β-catenin-null epidermis (K14-βcat−/− and K14-βcat−/−-K5-ΔN122-PG mice), the amount of α-catenin coprecipitating with E-cadherin was similar to that in wild-type extracts (Fig. 7B). Consistent with the recent findings of Posthaus et al., plakoglobin coprecipitated with E-cadherin, whereas E-cadherin and α-catenin were found in immunoprecipitates obtained with anti-plakoglobin antibody (40). This result suggests that, in β-catenin-null epidermis, plakoglobin replaced β-catenin in adherens junctions.
We investigated whether the recruitment of plakoglobin to adherens junctions affected desmosomes by analyzing the distribution of desmoplakin in β-catenin-null epidermis by confocal immunofluorescence microscopy. Weak irregular staining was observed in the basal and suprabasal layers of K14-βcat−/− epidermis, whereas ΔN122-PG expression resulted in stronger staining and a characteristically patchy distribution of desmoplakin at cell-cell boundaries (Fig. 5A and Fig. 7C).
DISCUSSION
The expression of stabilized N-terminally truncated β-catenin under control of the K14 promoter has been shown to activate Tcf/β-catenin signaling aberrantly in basal keratinocytes, inducing de novo hair follicle formation and tumors originating from the upper and lower hair follicles (18, 28). We found that K5-ΔN122-PG mice presented a similar skin phenotype, with hair follicle hyperplasia displaying differentiation features characteristic of both the upper (i.e., hair keratin-negative, trichofolliculoma-like, and sebocyte-containing tumors) and lower (i.e., hair keratin-positive, expressing En1) hair follicles. These results suggest that ΔN122-PG activates the Tcf/β-catenin pathway or induces another signal that substitutes for that elicited by β-catenin. However, in β-catenin-null skin, ΔN122-PG did not induce the formation of new hair germs and follicular tumors, suggesting that the abnormal hair follicles found in K5-ΔN122-PG mouse skin appear due to the aberrant activation of β-catenin signaling. Thus, even though in vitro, ΔN122-PG activates transcription from Lef/Tcf target genes, it cannot substitute for β-catenin in its signaling functions in vivo. A recent study demonstrated that the interaction of plakoglobin with the adherens junction components E-cadherin and α-catenin could activate β-catenin signaling (33). Consistent with this, immunoprecipitation assays showed that in K5-ΔN122-PG, but not in wild-type skin, a small amount of plakoglobin was present in E-cadherin-containing complexes.
Part of the α-catenin-binding site (2) is deleted in ΔN122-PG, and this molecule may therefore interfere with the recruitment of α-catenin to adherens junctions, thereby affecting cell-cell adhesion in the epidermis. Several lines of evidence suggest that this is not the case. First, the amount of α-catenin coprecipitating with E-cadherin was not altered by transgene expression, implying that ΔN122-PG did not prevent the binding of α-catenin to E-cadherin-containing complexes. Second, ΔN122-PG was essentially restricted to the desmosomes, with only very small amounts found in complex with E-cadherin, as E-cadherin could not be immunoprecipitated with anti-HA antibody. This observation is consistent with the findings of Chitaev et al., who demonstrated that the affinity of plakoglobin for desmosomal cadherins is several times greater than that for E-cadherin (11). Finally, ΔN122-PG expression significantly improved the architecture and differentiation of β-catenin-null epidermis, suggesting that a dominant-negative effect of the transgene product on epidermal cell-cell adhesion is very unlikely.
In contrast to K5-ΔN122-PG mice, transgenic mice from the K5-PG lines did not display an abnormal skin phenotype. Although the levels of transgene product differed significantly in animals from different K5-PG lines, the total levels of plakoglobin (including the transgene product and endogenous plakoglobin) remained similar to those found in wild-type skin. These data are consistent with the observation that the expression of N-terminally truncated, rather than full-length, plakoglobin leads to the cytoplasmic accumulation of ectopic and endogenous plakoglobin in A431 squamous carcinoma cells (37).
Charpentier et al. recently reported that moderate ectopic expression of N-terminally truncated or full-length-plakoglobin in basal keratinocytes under control of the K14 promoter (transgenic lines K14-PG and K14-ΔN80-PG) resulted in a mild phenotype different from that described here (9). In these transgenic mice, during the two first growth cycles hair follicles prematurely (2 days earlier than wild-type littermates) terminated the growth phase, anagen, and entered into the regression phase, telogen (9). The shortened hair follicle cycle resulted in somewhat shorter hairs. The phenotype became less obvious in the older mice, as noted by the authors, and all the data presented in the paper were obtained with mice younger than 45 days. In contrast, in K5-ΔN122-PG mice, macroscopically visible skin lesions appeared rather late, at the age of 2 to 3, 5 to 6, and 10 to 12 months in the animals with high, medium, and low transgene expression levels, respectively. The exact reasons for this difference in the phenotypes are not known. Although the K5 and K14 promoters have similar expression patterns and target transgenes to basal epithelia, the spatial and temporal regulation of the expression of the constructs used in the two studies might be slightly different. Further, the Swiss Webster genetic background may favor the subtle, early phenotype observed by Charpentier et al.
The notion that overexpression of plakoglobin can lead to the stimulation of β-catenin signaling in the mouse epidermis is in agreement with the results obtained earlier in Xenopus and in cultured cells of different origins (25, 26, 32, 45). In addition, this study as well as the paper by Charpentier et al. (9) showed that plakoglobin and ΔN-PG constructs activated TOPFLASH when transiently transfected into keratinocytes. Therefore, it can be suggested that relatively mild expression levels of the plakoglobin transgenes may inhibit hair follicle growth, whereas a more important increase of the total intracellular plakoglobin level achieved due to ΔN-PG expression is required to stimulate β-catenin signaling. Thus, the data presented by Charpentier et al. and our findings may be considered complementary rather than contradictory.
A phenotype reminiscent of that described in this study was obtained by expression of N- and C-terminally truncated β-catenin in transgenic mouse epidermis (15). On the one hand, ΔNΔC-β-catenin expression may lead to the stabilization of endogenous β-catenin and aberrant activation of Tcf/β-catenin signaling (52). On the other hand, this molecule interferes with Tcf-mediated transactivation, acting as a dominant-negative inhibitor that lacks the transactivation domain. Consistently, in cultured keratinocytes ΔNΔC-β-catenin inhibits activation of the TOPFLASH reporter (15). In contrast, in our study ΔN122-PG, when coexpressed with β-catenin, further augmented the activation of TOPFLASH, suggesting that different molecular mechanisms underlie the phenotypes resulting from expression of ΔN122-PG and ΔNΔC-β-catenin.
We found that ΔN122-PG expression significantly improved the differentiation of interfollicular epidermis and increased the survival rate in mice with conditional inactivation of the β-catenin gene in the skin. Decreased survival of K14-βcat−/− mice was also observed in the earlier study (23). The death of pups occurred essentially at weeks 2 to 5 after birth and was found to be increased around weaning. This might be due to a defect in the esophagus of K14-βcat−/− mice, since the K14 promoter is also active in this tissue. Mutant mice which survived this early crisis period were found to be viable and fertile (23).
The alterations in cell growth and differentiation observed in β-catenin-null epidermis can be interpreted as hyperplasia of the basal layer or the uncontrolled proliferation of transient amplifying cells (23). Interestingly, the depletion of β-catenin does not alter keratinocyte proliferation in culture (40), suggesting that other factors characteristic of in vivo tissue organization, rather than the lack of β-catenin-signaling per se, are responsible for the phenotype of the β-catenin-null epidermis. Although plakoglobin was found to substitute for β-catenin in adherens junctions, no increase in the level of plakoglobin was detected in β-catenin-null skin. Thus, the recruitment of plakoglobin to adherens junctions may result in the depletion of this molecule from desmosomes, which may in turn lead to an increase in proliferation and changes in differentiation. The expression of ΔN122-PG may restore the level of plakoglobin in the desmosomal compartment, thereby rescuing the differentiation of β-catenin-null keratinocytes. Consistently, the expression of ΔN122-PG in β-catenin-null skin led to the restoration of a desmoplakin distribution pattern similar to that in wild-type skin. Several studies have suggested that desmosomal components are involved in the regulation of epidermal differentiation (3, 10, 31), but their precise role in intracellular signaling events remains to be elucidated.
Alternatively, or in addition, plakoglobin may be directly involved in the transcriptional regulation of genes not involved in hair follicle induction but essential for epidermal differentiation, such as c-Myc. The results of a recent study demonstrating that in β-catenin-null embryonic stem cells, plakoglobin can transactivate the promoters of Nr-CAM, cyclin D1, and Lef/Tcf reporter are in favor of such interpretation (12). In either case, the capacity of ΔN122-PG to rescue the differentiation of β-catenin-null epidermis strongly suggests that plakoglobin, either as an essential desmosomal constituent or as a signaling molecule regulating transcription, may influence epidermal differentiation.
Acknowledgments
We are grateful to Avri Ben-Ze'ev for encouraging us to study the signaling functions of plakoglobin in vivo and for advice on the manuscript. We particularly thank C. Goujet and the personnel of the Service d'Expérimentation Animale et de Transgenèse, Villejuif, especially R. DuchÂteau and A. Loeuillet, for taking care of the transgenic mice. We also thank J. L. Jorcano for providing the K5 promoter construct and T. T. Sun and H. Clevers for donating reagents. We are grateful to F. Gaill and to M. J.-P. Lechaire for performing transmission electron microscopy and to M.-A. Deugnier and D. Medina for valuable discussions.
This work was supported by the Association pour la Recherche sur le Cancer (ARC 4440) and an ACI 2001 grant from the Ministère de la Recherche, France. J.T. is supported by a grant from the Association pour la Recherche sur le Cancer. M.M.F. is Chargé de Recherche, and M.A.G. is Directeur de Recherche at the Institut de la Santé et de la Recherche Médicale.
REFERENCES
- 1.Aberle, H., A. Bauer, J. Stappert, A. Kispert, and R. Kemler. 1997. Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 16:3797-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aberle, H., H. Schwartz, H. Hoschuetzky, and R. Kemler. 1996. Single amino acid substitutions in proteins of the armadillo gene family abolish their binding to alpha-catenin. J. Biol. Chem. 271:1520-1526. [DOI] [PubMed] [Google Scholar]
- 3.Allen, E., Q. C. Yu, and E. Fuchs. 1996. Mice expressing a mutant desmosomal cadherin exhibit abnormalities in desmosomes, proliferation, and epidermal differentiation. J. Cell Biol. 133:1367-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold, I., and F. M. Watt. 2001. c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and differentiation of their progeny. Curr. Biol. 11:558-568. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Ze'ev, A., and B. Geiger. 1998. Differential molecular interactions of β-catenin and plakoglobin in adhesion, signaling and cancer. Curr. Opin. Cell Biol. 10:629-639. [DOI] [PubMed] [Google Scholar]
- 6.Bierkamp, C., H. Schwarz, O. Huber, and R. Kemler. 1999. Desmosomal localization of β-catenin in the skin of plakoglobin null-mutant mice. Development 126:371-381. [DOI] [PubMed] [Google Scholar]
- 7.Bradley, R. S., P. Cowin, and A. M. Brown. 1993. Expression of Wnt-1 in PC12 cells results in modulation of plakoglobin and E-cadherin and increased cellular adhesion. J. Cell Biol. 123:1857-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cadigan, K. M., and R. Nusse. 1997. Wnt signaling: a common theme in animal development. Genes Dev. 11:3286-3305. [DOI] [PubMed] [Google Scholar]
- 9.Charpentier, E., R. M. Lavker, E. Acquista, and P. Cowin. 2000. Plakoglobin suppresses epithelial proliferation and hair growth in vivo. J. Cell Biol. 149:503-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chidgey, M., C. Brakebusch, E. Gustafsson, A. Cruchley, C. Hail, S. Kirk, A. Merritt, A. North, C. Tselepis, J. Hewitt, C. Byrne, R. Fassler, D. Garrod. 2001. Mice lacking desmocollin 1 show epidermal fragility accompanied by barrier defects and abnormal differentiation. J. Cell Biol. 155: 821-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chitaev, N. A., R. E. Leube, R. B. Troyanovsky, L. G. Eshkind, W. W. Franke, S. M. Troyanovsky. 1996. The binding of plakoglobin to desmosomal cadherins: patterns of binding sites and topogenic potential. J. Cell Biol. 133:359-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conacci-Sorrell, M. E., T. Ben-Yedidia, M. Shtutman, E. Feinstein, P. Einat, and A. Ben-Ze'ev. 2002. Nr-CAM is a target gene of the β-catenin/LEF-1 pathway in melanoma and colon cancer and its expression enhances motility and confers tumorigenesis. Genes Dev. 16:2058-2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cowin, P., and B. Burke. 1996. Cytoskeleton-membrane interactions. Curr. Opin. Cell Biol. 8:56-65. [DOI] [PubMed] [Google Scholar]
- 14.DasGupta, R., and E. Fuchs. 1999. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 126:4557-4568. [DOI] [PubMed] [Google Scholar]
- 15.DasGupta, R., H. Rhee, and E. Fuchs. 2002. A developmental conundrum: a stabilized form of β-catenin lacking the transcriptional activation domain triggers features of hair cell fate in epidermal cells and epidermal cell fate in hair follicle cells. J. Cell Biol. 158:331-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frye, M., C. Gardner, E. R. Li, I. Arnold, and F. M. Watt. 2003. Evidence that Myc activation depletes the epidermal stem cell compartment by modulating adhesive interactions with the local microenvironment. Development 130:2793-2808. [DOI] [PubMed] [Google Scholar]
- 17.Gandarillas, A., and F. M. Watt. 1997. c-Myc promotes differentiation of human epidermal stem cells. Genes Dev. 11:2869-2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gat, U., R. DasGupta, L. Degenstein, and E. Fuchs. 1998. De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated β-catenin in skin. Cell 95:605-614. [DOI] [PubMed] [Google Scholar]
- 19.Grose R, C. Hutter, W. Bloch, I. Thorey, F. M. Watt, R. Fassler, C. Brakebusch, S. Werner. 2002. A crucial role of beta 1 integrins for keratinocyte migration in vitro and during cutaneous wound repair. Development 129:2303-2315. [DOI] [PubMed] [Google Scholar]
- 20.Hart, M., J. P. Concordet, I. Lassot, I. Albert, R. del los Santos, H. Durand, C. Perret, B. Rubinfeld, F. Margottin, R. Benarous, and P. Polakis. 1999. The F-box protein β-TrCP associates with phosphorylated β-catenin and regulates its activity in the cell. Curr. Biol. 9:207-210. [DOI] [PubMed] [Google Scholar]
- 21.Hecht, A., C. M. Litterst, O. Huber, and R. Kemler. 1999. Functional characterization of multiple transactivating elements in beta-catenin, some of which interact with the TATA-binding protein in vitro. J. Biol. Chem. 274:18017-18025. [DOI] [PubMed] [Google Scholar]
- 22.Hinck, L., W. J. Nelson, and J. Papkoff. 1994. Wnt-1 modulates cell-cell adhesion in mammalian cells by stabilizing beta-catenin binding to the cell adhesion protein cadherin. J. Cell Biol. 124:729-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huelsken, J., R. Vogel, B. Erdmann, G. Cotsarelis, and W. Birchmeier. 2001. β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 105:533-545. [DOI] [PubMed] [Google Scholar]
- 24.Karnovsky, A., and M. W. Klymkowsky. 1995. Anterior axis duplication in Xenopus induced by the over-expression of the cadherin-binding protein plakoglobin. Proc. Natl. Acad. Sci. USA 92:4522-4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klymkowsky, M. W., B. O. Williams, G. D. Barish, H. E. Varmus, and Y. E. Vourgourakis. 1999. Membrane-anchored plakoglobins have multiple mechanisms of action in Wnt signaling. Mol. Biol. Cell 10:3151-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kodama, S., S. Ikeda, T. Asahara, M. Kishida, and A. Kikuchi. 1999. Axin directly interacts with plakoglobin and regulates its stability. J. Biol. Chem. 274:27682-27688. [DOI] [PubMed] [Google Scholar]
- 27.Kolligs, F. T., B. Kolligs, K. M. Hajra, G. Hu, M. Tani, K. R. Cho, and E. R. Fearon. 2000. γ-Catenin is regulated by the APC tumor suppressor and its oncogenic activity is distinct from that of β-catenin. Genes Dev. 14:1319-1331. [PMC free article] [PubMed] [Google Scholar]
- 28.Lo Celso, C., D. M. Prowse, and F. M. Watt. 2004. Transient activation of β-catenin signaling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development 131:1787-1799. [DOI] [PubMed] [Google Scholar]
- 29.Maytin, E. V., J. C. Lin, R. Krishnamurthy, N. Batchvarova, D. Ron, P. J. Mitchell, and J. F. Habener. 1999. Keratin 10 gene expression during differentiation of mouse epidermis requires transcription factors C/EBP and AP-2. Dev. Biol. 216:164-181. [DOI] [PubMed] [Google Scholar]
- 30.Merrill, B. J., U. Gat, R. DasGupta, and E. Fuchs. 2001. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 15:1688-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merritt, A. J., M. Y. Berika, W. Zhai, S. E. Kirk, B. Ji, M. J. Hardman, D. R. Garrod. 2002. Suprabasal desmoglein 3 expression in the epidermis of transgenic mice results in hyperproliferation and abnormal differentiation. Mol. Cell. Biol. 22:5846-5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller, J. R., and R. T. Moon. 1997. Analysis of the signaling activities of localization mutants of β-catenin during axis specification in Xenopus. J. Cell Biol. 139:229-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miravet, S., J. Piedra, J. Castano, I. Raurell, C. Franci, M. Dunach, and A. Garcia de Herreros. 2003. Tyrosine phosphorylation of plakoglobin causes contrary effects on its association with desmosomes and adherens junction components and modulates β-catenin-mediated transcription. Mol. Cell. Biol. 23:7391-7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miravet, S., J. Piedra, F. Miro, E. Itarte, A. Garcia de Herreros, and M. Dunach. 2002. The transcriptional factor Tcf-4 contains different binding sites for β-catenin and plakoglobin. J. Biol. Chem. 277:1884-1891. [DOI] [PubMed] [Google Scholar]
- 35.Niemann, C., D. M. Owens, J. Huelsken, W. Birchmeier, and F. M. Watt. 2002. Expression of ΔNLef1 in mouse epidermis results in differentiation of hair follicles into squamous epidermal cysts and formation of skin tumours. Development 129:95-109. [DOI] [PubMed] [Google Scholar]
- 36.Orford, K., C. Crockett, J. P. Jensen, A. M. Weissman, and S. W. Byers. 1997. Serine phosphorylation-regulated ubiquitination and degradation of beta-catenin. J. Biol. Chem. 272:24735-24738. [DOI] [PubMed] [Google Scholar]
- 37.Palka, H. L., and K. J. Green. 1997. Roles of plakoglobin end domains in desmosome assembly. J. Cell Sci. 110:2359-2371. [DOI] [PubMed] [Google Scholar]
- 38.Papkoff, J., B. Rubinfeld, B. Schryver, and P. Polakis. 1996. Wnt-1 regulates free pools of catenins and stabilizes APC-catenin complexes. Mol. Cell. Biol. 16:2128-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Polakis, P. 2000. Wnt signaling and cancer. Genes Dev. 14:1837-1851. [PubMed] [Google Scholar]
- 40.Posthaus, H., L. Williamson, D. Baumann, R. Kemler, R. Caldelari, M. M. Suter, H. Schwarz, and E. Muller. 2002. β-Catenin is not required for proliferation and differentiation of epidermal mouse keratinocytes. J. Cell Sci. 115:4587-4595. [DOI] [PubMed] [Google Scholar]
- 41.Ramirez, A., A. Bravo, J. L. Jorcano, and M. Vidal. 1994. Sequences 5′ of the bovine keratin 5 gene direct tissue- and cell-type-specific expression of a lacZ gene in the adult and during development. Differentiation 58:53-64. [DOI] [PubMed] [Google Scholar]
- 42.Sadot, E., I. Simcha, K. Iwai, A. Ciechanover, B. Geiger, and A. Ben-Ze'ev. 2000. Differential interaction of plakoglobin and β-catenin with the ubiquitin-proteasome system. Oncogene 19:1992-2001. [DOI] [PubMed] [Google Scholar]
- 43.Salomon, D., P. A. Sacco, S. G. Roy, I. Simcha, K. R. Johnson, M. J. Wheelock, and A. Ben-Ze'ev. 1997. Regulation of β-catenin levels and localization by overexpression of plakoglobin and inhibition of the ubiquitin-proteasome system. J. Cell Biol. 139:1325-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simcha, I., B. Geiger, S. Yehuda-Levenberg, D. Salomon, and A. Ben-Ze'ev. 1996. Suppression of tumorigenicity by plakoglobin: an augmenting effect of N-cadherin. J. Cell Biol. 133:199-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simcha, I., M. Shtutman, D. Salomon, I. Zhurinsky, E. Sadot, B. Geiger, and A. Ben-Ze'ev. 1998. Differential nuclear translocation and transactivation potential of β-catenin and plakoglobin. J. Cell Biol. 141:1433-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Genderen, C., R. M. Okamura, I. Farinas, R. G. Quo, T. G. Parslow, L. Bruhn, and R. Grosschedl. 1994. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 8:2691-2703. [DOI] [PubMed] [Google Scholar]
- 47.Van Mater, D., F. T. Kolligs, A. A. Dlugosz, and E. R. Fearon. 2003. Transient activation of beta-catenin signaling in cutaneous keratinocytes is sufficient to trigger the active growth phase of the hair cycle in mice. Genes Dev. 15:1219-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waikel, R. L., Y. Kawachi, P. A. Waikel, X. J. Wang, and D. R. Roop. 2001. Deregulated expression of c-Myc depletes epidermal stem cells. Nat. Genet. 28:165-168. [DOI] [PubMed] [Google Scholar]
- 49.Williams, B. O., G. D. Barish, M. W. Klymkowsky, and H. E. Varmus. 2000. A comparative evaluation of β-catenin and plakoglobin signaling activity. Oncogene 19:5720-5728. [DOI] [PubMed] [Google Scholar]
- 50.Zhu, S., H.-S. Oh, M. Shim, E. Sterneck, P. F. Johnson, and R. C. Smart. 1999. C/EBPβ modulates the early events of keratinocyte differentiation involving growth arrest and keratin 1 and keratin 10 expression. Mol. Cell. Biol. 19:7181-7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhurinsky, J., M. Shtutman, and A. Ben-Ze'ev. 2000. Plakoglobin and β-catenin: protein interactions, regulation and biological roles. J. Cell Sci. 113:3127-3139. [DOI] [PubMed] [Google Scholar]
- 52.Zhurinsky, J., M. Shtutman, and A. Ben-Ze'ev. 2000. Differential mechanisms of LEF/TCF family-dependent transcriptional activation by β-catenin and plakoglobin. Mol. Cell. Biol. 20:4238-4252. [DOI] [PMC free article] [PubMed] [Google Scholar]