Abstract
A 59‐year‐old woman receiving methotrexate and tacrolimus for rheumatoid arthritis (RA) was referred to our hospital following bilateral ground‐glass opacity observed in her chest X‐ray and elevated serum KL‐6. After methotrexate and tacrolimus cessation, shortness of breath developed and ground‐glass opacity observed in the chest computed tomography rapidly worsened. Bronchoalveolar lavage showed increased lymphocytes, and trans‐bronchial lung biopsy confirmed lymphocytic alveolitis. In addition, the patient had serum antibodies against Trichosporon asahii, a fungal pathogen. The mildew in her bathroom and washing machine which were the source of the fungus were removed, which resulted in no further relapse of the condition. In this patient's case, methotrexate and tacrolimus may have masked and suppressed summer‐type hypersensitivity pneumonitis.
Keywords: Methotrexate, rheumatoid arthritis, summer‐type hypersensitivity pneumonitis, tacrolimus, KL‐6
Introduction
Summer‐type hypersensitivity pneumonitis is the most prevalent form of hypersensitivity pneumonia in Japan 1. Summer‐type hypersensitivity pneumonitis is classically recognized as types III and IV hypersensitivity and is caused by repeated airborne exposure to Trichosporon asahii or Trichosporon mucoides during hot and humid summers 1, 2. Repeated exposure to airborne antigens induces formation of immune complexes with immunoglobulin G as well as complement activation. Particularly, alveolitis occurs via influx of CD4 and CD8 T lymphocytes, pro‐inflammatory cytokines, and mediators. Here we report a case of summer‐type hypersensitivity pneumonitis in a patient with rheumatoid arthritis (RA) during treatment with methotrexate and tacrolimus.
Case Report
A 59‐year‐old woman who had been receiving methotrexate (10 mg/week) and tacrolimus (2 mg/day) for treatment of RA for 2 years, was referred to our Division of Respiratory Medicine following observation of bilateral ground‐glass opacity in her chest X‐ray and an elevated serum KL‐6 of 1097 U/mL (normal, <500 U/mL) in mid‐June 2015. She had no history of smoking; however, mildew was found in her bathroom and washing machine. In addition, other rooms were cluttered and dirty. Chest computed tomography (CT) also showed diffuse ground‐glass opacities (Fig. 1A). Her resting percutaneous oxygen saturation was 96%. Differential diagnoses included Pneumocystis jirovecii pneumonia, cytomegalovirus infection, drug‐induced pneumonia, and acute interstitial pneumonia associated with RA. Although she did not wish to be admitted emergently, methotrexate and tacrolimus were discontinued because of the stable disease status of RA and the possibility of infection or drug‐induced pneumonia. However, she was admitted to our hospital 7 days later due to acute respiratory failure. Her resting and ambulatory percutaneous oxygen saturations were 91 and 78%, respectively. Fine crackles were audible in both lung bases. A subsequent chest CT showed worsening of ground‐glass opacity and appearance of consolidations (Fig. 1B). Bronchoscopy was performed, and the cell counts of bronchoalveolar lavage fluid from the right middle lobe were as follows: lymphocytes (76%), neutrophils (1%), eosinophils (2%), and macrophages (21%). Polymerase chain reaction results were negative for Mycobacterium tuberculosis, P. jirovecii, cytomegalovirus, Mycoplasma pneumoniae, and Legionella pneumoniae. Trans‐bronchial lung biopsy confirmed lymphocytic alveolitis. Serum antibody for T. asahii assessed using antigen‐captured enzyme‐linked immunosorbent assay was positive. High‐dose corticosteroid treatment was started with methylprednisolone (0.5 g on day 1 and 1.0 g on days 2 and 3). Her respiratory failure resolved immediately, and thus, the corticosteroid dose was rapidly tapered and stopped on postadmission day 15. Then, methotrexate and tacrolimus treatments were resumed. Since summer‐type hypersensitivity pneumonitis was suspected, the medical team encouraged her husband to remove all mildew from the bathroom; however, this was not completely accomplished. She came back to hospital after spending 4 days at home. Her serum KL‐6 level was marginally elevated to 703 U/mL as compared to the 636 U/mL value obtained before she returned home (Fig. 2). The medical team again encouraged further removal of mildew from the bathroom and cleaning of all rooms in her house. She was discharged on postadmission day 35. Upon returning home, her KL‐6 level slightly increased again, but then it decreased. Together, with these results and other clinical findings, including being positive for T. asahii antibody, the diagnosis of summer‐type hypersensitivity pneumonitis was made.
Figure 1.
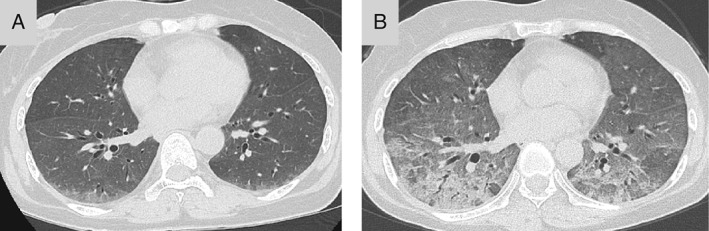
Patient's chest computed tomography (CT) from her first visit to the Division of Respiratory Medicine, which revealed diffuse ground‐glass opacities in the lungs (A). Chest CT at 7 days after cessation of methotrexate and tacrolimus showed worsening of ground‐glass opacities and appearance of consolidations (B).
Figure 2.
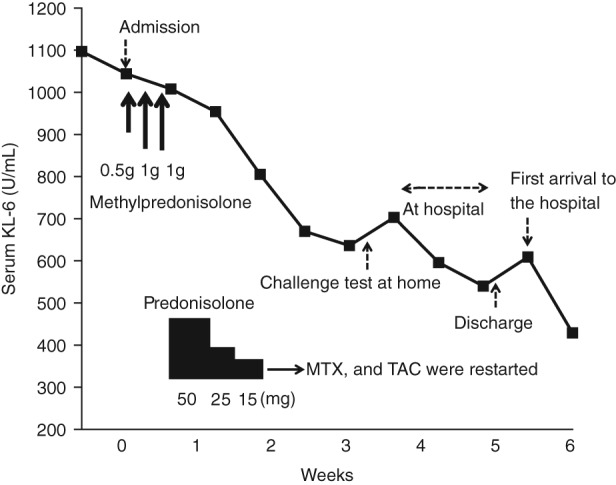
Patient's clinical course. The KL‐6 serum levels decreased after admission but increased after the patient returned home. The KL‐6 serum level decreased again upon a second hospital admission and increased slightly after discharge. MTX, methotrexate; TAC, tacrolimus.
Discussion
Herein, we presented a case of summer‐type hypersensitivity pneumonitis in an RA patient on a methotrexate and tacrolimus treatment regimen. Methotrexate inhibits enzymes involved in purine metabolism, leading to inhibition of T‐cell activation, intercellular adhesion molecule expression by T‐cells, and downregulation of B‐cells 3. Tacrolimus prevents dephosphorylation of nuclear factor of activated T‐cells (NFAT) via calcineurin inhibition 4. For our patient, pneumonitis worsened rapidly after cessation of methotrexate and tacrolimus. No symptoms of fever or granuloma formation in the lung tissue were observed in this patient. It is possible that we simply did not detect granulomata in this patient. However, in patients treated with immunosuppressive agents, granuloma development may not occur. Additionally, it was possible that methotrexate and tacrolimus masked and suppressed the progression of summer‐type hypersensitivity pneumonitis.
For the diagnosis of summer‐type hypersensitivity pneumonitis, antigen‐captured enzyme‐linked immunosorbent assay of serum T. asahii antibody is useful, of which the sensitivity and specificity are 87, and 96%, respectively 5. Our case fulfilled the diagnostic criteria of summer‐type hypersensitivity pneumonitis 1. We used a biomarker of interstitial pneumonia, KL‐6, to orchestrate a challenge test as well as to monitor disease progression. In our patient, KL‐6 decreased after corticosteroid treatment, and it increased after the challenge test.
In conclusion, we have reported a case of summer‐type hypersensitivity pneumonitis developed in a patient with RA treated with immunosuppressive agents. To the best of our knowledge, this is the first case of summer‐type hypersensitivity pneumonitis that worsened after withdrawal of immunosuppressive drugs. Thus, physicians must pay attention to the possibility of accidental suppression of summer‐type hypersensitivity pneumonitis by immunosuppressants.
Disclosure Statements
No conflict of interest declared.
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
Ohkubo, H. , Okayama, M. , Fukumitsu, K. and Niimi, A. (2016) Summer‐type hypersensitivity pneumonitis in a patient with rheumatoid arthritis on methotrexate and tacrolimus, or something similar. Respirology Case Reports, 4 (6), e00194. doi: 10.1002/rcr2.194.
Associate Editor: Conroy Wong
References
- 1. Ando M, Arima K, Yoneda R, et al. 1991. Japanese summer‐type hypersensitivity pneumonitis. Geographic distribution, home environment, and clinical characteristics of 621 cases. Am. Rev. Respir. Dis. 144:765–769. [DOI] [PubMed] [Google Scholar]
- 2. Bourke SJ, Dalphin JC, Boyd G, et al. 2001. Hypersensitivity pneumonitis: current concepts. Eur. Respir. J. 32:81s–92s. [PubMed] [Google Scholar]
- 3. Wessels JA, Huizinga TW, and Guchelaar HJ 2008. Recent insights in the pharmacological actions of methotrexate in the treatment of rheumatoid arthritis. Rheumatology (Oxford) 47:249–255. [DOI] [PubMed] [Google Scholar]
- 4. Scott LJ, McKeage K, Keam SJ, et al. 2003. Tacrolimus: a further update of its use in the management of organ transplantation. Drugs 63:1247–1297. [DOI] [PubMed] [Google Scholar]
- 5. Yoshizawa Y, Furuie T, Otani Y, et al. 2001. Symposium on molecular pathogenesis of respiratory diseases and its clinical implication. 3. Immunological lung disease – recent advances in the pathogenesis of hypersensitivity pneumonitis. Intern. Med. 40:164–167. [DOI] [PubMed] [Google Scholar]


