Abstract
A 61‐year‐old never‐smoking woman with stage IV lung adenocarcinoma with initially unknown epidermal growth factor receptor (EGFR) status, lung metastasis, pleural dissemination, and malignant pleural effusion in 2007 received 10 prior anti‐cancer regimens including gefitinib as second‐ and ninth‐line treatments, with minimal efficacy with gefitinib. EGFR mutation analysis performed in pleural effusion specimens during ninth‐line gefitinib was negative. In 2015, she developed progressive disease with peritoneal dissemination. Few/absent bowel sounds and no gross features of mechanical obstruction on the imaging led to the diagnosis of paralytic ileus. Blood test revealed elevated C‐reactive protein (CRP). EGFR mutation analysis revealed S768I point mutation in EGFR exon 20 without the T790M resistance mutation in pleural and peritoneal effusion cytology specimens. She received afatinib (20 mg once daily) as 11th‐line treatment, with resolution of peritoneal carcinomatosis and CRP normalization, confirmed by follow‐up computed tomography. At 12 months after afatinib initiation, there was no symptomatic recurrence or disease exacerbation.
Keywords: Afatinib, non‐small cell lung cancer, paralytic ileus, peritoneal carcinomatosis
Introduction
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs), such as gefitinib and afatinib, are currently considered standard therapy regimens for non‐small cell lung cancer (NSCLC) harbouring EGFR mutations due to high response rate and prolonged progression‐free survival (PFS) observed in several randomized clinical trials (RCTs). However, gefitinib treatment was associated with shorter PFS and lower response rates in patients with uncommon EGFR mutations 1. Moreover, the efficacy of EGFR‐TKI in gastrointestinal motility disorders is unknown. We report good response of refractory paralytic ileus due to peritoneal carcinomatosis in an afatinib‐treated NSCLC patient harbouring an uncommon EGFR mutation.
Case Report
A 61‐year‐old never‐smoker Japanese woman who was diagnosed in 2007 with stage IV lung adenocarcinoma of right middle lobe with initially unknown EGFR status and single lung metastasis of right upper lobe, pleural dissemination, and malignant pleural effusion received the following anticancer drug regimens in the following order: carboplatin (CBDCA)/paclitaxel (PTX)/bevacizumab (BEV), gefitinib, cisplatin/pemetrexed (PEM), gemcitabine, S1, docetaxel, amrubicin, CBDCA/PTX/BEV, gefitinib, and PEM/BEV. EGFR status of the patient was unknown during initial diagnosis due to the lack of commercially available EGFR mutation tests. Gefitinib, approved for inoperable or recurrent NSCLC in July 2002 in Japan, was administered as second‐ and ninth‐line treatment. The sum of target lesions of primary disease and lung metastasis showed 14% decrease in size using Response Evaluation Criteria in Solid Tumours (RECIST) during second‐line gefitinib treatment, as observed by computed tomography (CT). Gefitinib as ninth‐line treatment, however, had little efficacy in reducing tumour size, and EGFR mutation analysis of right pleural effusion using the Scorpion Amplification Refractory Mutation System® (ARMS®) was negative for EGFR mutations at that time.
During 10th‐line treatment, she developed progressive disease due to peritoneal dissemination (Fig. 1A) and was admitted to our hospital with severe abdominal distension and pain. Abdominal auscultation yielded few/absent bowel sounds; serum C‐reactive protein (CRP) was elevated; and abdominal X‐ray and CT showed multiple fluid levels (Fig. 1B), ascites, and dilatation of bowel loops without gross mechanical obstruction (Fig. 1C). She was diagnosed with paralytic ileus due to progressive disease with peritoneal carcinomatosis. Treatment with systemic corticosteroids, octreotide acetate, and metoclopramide hydrochloride, and drainage of stomach contents through nasogastric tube, and peritoneal drainage for 1.5 months after admission was ineffective.
Figure 1.
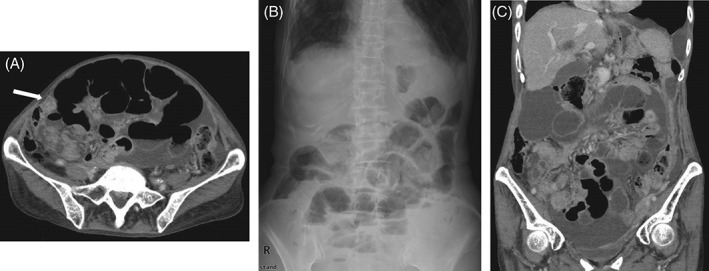
(A) Abdominal computed tomography (CT) scan showing severe abdominal distension and peritoneal dissemination (arrow) before afatinib treatment. (B) Abdominal X‐ray showing multiple fluid levels. (C) Abdominal CT scan showing ascites and dilatation of bowel loops without mechanical obstruction.
During her stay for paralytic ileus, EGFR mutation analysis revealed S768I point mutation in EGFR exon 20 without T790M resistance mutation by cytology analysis of both pleural and peritoneal effusion specimens using the Cobas® kit. Therefore, with consideration of potential adverse effects, she was started on once‐daily oral 20 mg afatinib, as 11th‐line treatment. Although she experienced grade 1 oral mucositis and paronychia during afatinib treatment, abdominal distension and pain quickly resolved, and CRP levels quickly returned to normal, to support response to afatinib therapy. Follow‐up CT scan on day 57 showed marked improvement in peritoneal dissemination with afatinib (Fig. 2). At 12‐month follow‐up evaluation, she continued afatinib treatment without symptom recurrence or disease exacerbation.
Figure 2.
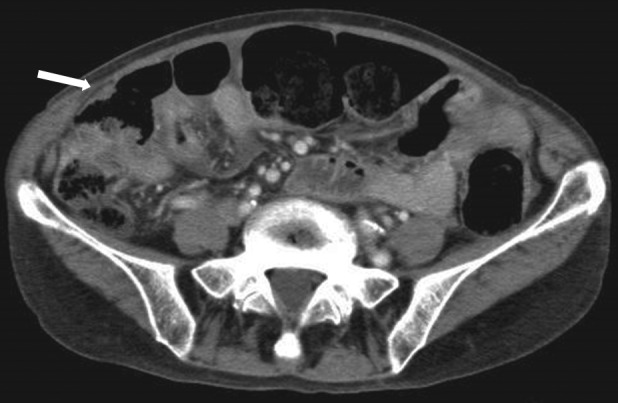
Abdominal computed tomography scan showing resolved abdominal distension and peritoneal dissemination (arrow) after 8 weeks of afatinib treatment (partial response).
Discussion
Peritoneal carcinomatosis is a rare but serious complication in advanced lung cancer. In our patient, paralytic ileus due to peritoneal carcinomatosis of NSCLC caused severe abdominal distension and pain. Furthermore, initial therapeutic approaches were ineffective, whereas afatinib was successful. To the best of our knowledge, this is the first report of an NSCLC patient harbouring an uncommon EGFR mutation and progressive disease presenting with peritoneal carcinomatosis and paralytic ileus who was effectively treated with afatinib.
Possible explanation of afatinib efficacy in our patient is its activity against the uncommon S768I mutation in EGFR. The combined post hoc analysis of LUX‐Lung 2, 3, and 6 indicated that afatinib was effective in patients with certain uncommon EGFR mutations, especially G719X, L861G, and S768I 2. However, afatinib can cause serious side effects such as diarrhoea. Pharmacokinetics of afatinib in patients with paralytic ileus due to peritoneal carcinomatosis of lung cancer is unknown. A pharmacokinetics study on alvimopan, a mu‐opioid antagonist for post‐operative ileus, found that its plasma concentrations and absorption were higher in surgical subjects who developed post‐operative paralytic ileus than in non‐surgical subjects 3, suggesting that absorption and plasma levels of therapeutics might be higher during paralytic ileus. Meanwhile, therapeutic efficacy of reduced‐dose afatinib (20 mg) was similar to that achieved with normal dose in the LUX‐Lung 3 RCT 4. Therefore, based on the demonstrated feasibility and efficacy of lower‐dose afatinib, and to ensure that acceptable plasma concentrations could be achieved, once‐daily oral afatinib at 20 mg, was administered in this patient with paralytic ileus due to peritoneal carcinomatosis.
The Scorpion ARMS® and Cobas® EGFR mutation tests can detect 1–5% of mutations to total DNA, with comparable sensitivity. Precise reasons underlying the change in EGFR mutation status from negative during ninth‐line treatment to positive at the time of paralytic ileus are unknown. The number of tumour cells in first pleural effusion specimen for cytology might have been insufficient to detect EGFR mutations. Alternatively, the number of tumour cells harbouring EGFR mutations in pleural effusion might have increased gradually, thus cells can be detected during second thoracentesis, as EGFR TKI‐sensitive and ‐resistant clones were shown to heterogeneously exist in lung tumours 5.
In conclusion, afatinib was effective for paralytic ileus due to peritoneal carcinomatosis in a patient with NSCLC harbouring an uncommon EGFR mutation. Treatment with afatinib, which sometimes causes severe diarrhoea, might be feasible even in patients with gastrointestinal motility disorders such as paralytic ileus.
Disclosure Statements
Authors received honoraria from AstraZeneca KK, Boehringer Ingelheim, Pfizer Japan Inc., Eli Lilly Japan K.K., Chugai Pharmaceutical Co., Ltd, and Ono Pharmaceutical Co., Ltd.
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
Kobayashi, H. , Wakuda, K. and Takahashi, T. (2016) Effectiveness of afatinib in lung cancer with paralytic ileus due to peritoneal carcinomatosis. Respirology Case Reports, 4 (6), e00197. doi: 10.1002/rcr2.197.
Associate Editor: Wai‐Cho Yu
References
- 1. Wu JY, Yu CJ, Chang YC, et al. 2011. Effectiveness of tyrosine kinase inhibitors on "uncommon" epidermal growth factor receptor mutations of unknown clinical significance in non‐small cell lung cancer. Clin. Cancer Res. 17(11):3812–3821. [DOI] [PubMed] [Google Scholar]
- 2. Yang JC, Sequist LV, Geater SL, et al. 2015. Clinical activity of afatinib in patients with advanced non‐small‐cell lung cancer harbouring uncommon EGFR mutations: a combined post‐hoc analysis of LUX‐Lung 2, LUX‐Lung 3, and LUX‐Lung 6. Lancet Oncol. 16(7):830–838. [DOI] [PubMed] [Google Scholar]
- 3. Foss JF, Fisher DM, and Schmith VD 2008. Pharmacokinetics of alvimopan and its metabolite in healthy volunteers and patients in postoperative ileus trials. Clin. Pharmacol. Ther. 83(5):770–776. [DOI] [PubMed] [Google Scholar]
- 4. Yang JC‐H, Ahn M‐J, Dickgreber NJ, et al. 2015. Influence of dose adjustment on afatinib safety and efficacy in patients (pts) with advanced EGFR mutation‐positive (EGFRm+) non‐small cell lung cancer (NSCLC). J. Clin. Oncol. 33(suppl): abstract 8073. [Google Scholar]
- 5. Oxnard GR, Arcila ME, Chmielecki J, et al. 2011. New strategies in overcoming acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in lung cancer. Clin. Cancer Res. 17(17):5530–5537. [DOI] [PMC free article] [PubMed] [Google Scholar]


