Abstract
Recent publications in The EMBO Journal (Xu et al, 2016) and in Nature Structural & Molecular Biology (Brown et al, 2016) report crystal structures of the Zika virus (ZIKV) NS1 protein. The structures reveal unique surface properties that help explain the specificity of anti‐ZIKV NS1 antibodies. Possible functions of this multifaceted pathogenicity factor are discussed here on the basis of the structures and cautious extrapolation from other flaviviruses.
Subject Categories: Microbiology, Virology & Host Pathogen Interaction; Structural Biology
The contribution of X‐ray crystallography to our understanding of the re‐emerging Zika virus (ZIKV) is impressive. Nine months after the WHO declared the association of ZIKV infection with fetal microcephaly and other neurological disorders a “Public Health Emergency of International Concern”, crystal structures are available for the E protein as well as for all soluble non‐structural protein domains of the virus. Among the reasons for this swift response to the current ZIKV outbreak are methodological advances in X‐ray crystallography in the past few years and the large body of structural results available for the related dengue and West Nile viruses (DENV and WNV). As a consequence, we have more structural than functional data for most of the ZIKV non‐structural (NS) proteins at this stage. It is therefore all the more important to carefully examine the functional implications of these new structures.
One of the latest additions to the structural gallery of ZIKV is non‐structural protein 1 (NS1) (Brown et al, 2016; Xu et al, 2016). This multifunctional pathogenicity factor is the most enigmatic protein of flaviviruses. The ~50‐kD glycoprotein has beneficial effects for the flaviviruses and, paradoxically, others that are protective for the infected host. NS1 is essential for RNA replication of flaviviruses as well as for immune evasion. While NS1 elicits the generation of protective antibodies, it is also involved in direct and antibody‐mediated attack onto target cells. Given the almost complete absence of functional studies on ZIKV NS1, we have to review the huge body of data available for NS1 of other flaviviruses (Muller & Young, 2013) and attempt to cautiously extrapolate from there where possible.
Flavivirus NS1 occurs in three different populations: (i) after translocation to the ER lumen, a membrane‐associated NS1 homodimer plays an essential role in viral RNA replication (Youn et al, 2012); (ii) the NS1 dimer also becomes associated with the plasma membrane of flavivirus‐infected cells, and (iii) NS1 is secreted from infected cells in the form of a hexamer (trimer of dimers) that binds lipid molecules in a central cavity (Gutsche et al, 2011).
Given the > 50% sequence identity, it is not surprising that the new structures of dimeric full‐length ZIKV NS1 reveal the same overall fold as seen for DENV‐2 and WNV NS1 (Akey et al, 2014). Each monomer consists of three domains (Fig 1): an N‐terminal β‐hairpin domain (residues 1–30) that associates with its counterpart from the other protomer through a mini‐domain swap, yielding a β‐roll; a wing domain (residues 31–180) with a discontinuous connector subdomain (residues 31–37 and 152–180); and a C‐terminal β‐ladder domain (residues 181–352). The ZIKV NS1 structures allow the first visualization of a flexible, partly exposed loop within the wing domain, residues 108–128, which was not defined by electron density in the crystal structures of both WNV and DENV‐2 NS1 (Akey et al, 2014). Because of its conserved hydrophobic nature, with residues Trp118, Tyr122, Phe123, and Val124 oriented outwards, the authors propose that this loop may be part of the membrane‐interacting surface of ZIKV NS1, along with the C‐terminus of the β‐roll (Trp28) and the connector subdomain (Phe163) (Fig 1). Recent evidence supports that the N‐terminal part of the flexible loop, in particular Trp115, might be involved in interactions with the E and prM proteins and thereby essential for the production of infectious virions (Scaturro et al, 2015). These authors also showed that DENV‐2 NS1 residues involved in RNA replication are mostly found in the wing domain. In ZIKV NS1, the great majority of these residues are conserved (Fig 1), indicating that the protein might make similar interactions with the replication complex and with the glycoproteins of the capsid.
Figure 1. Three‐dimensional structure of the ZIKV NS1 dimer.
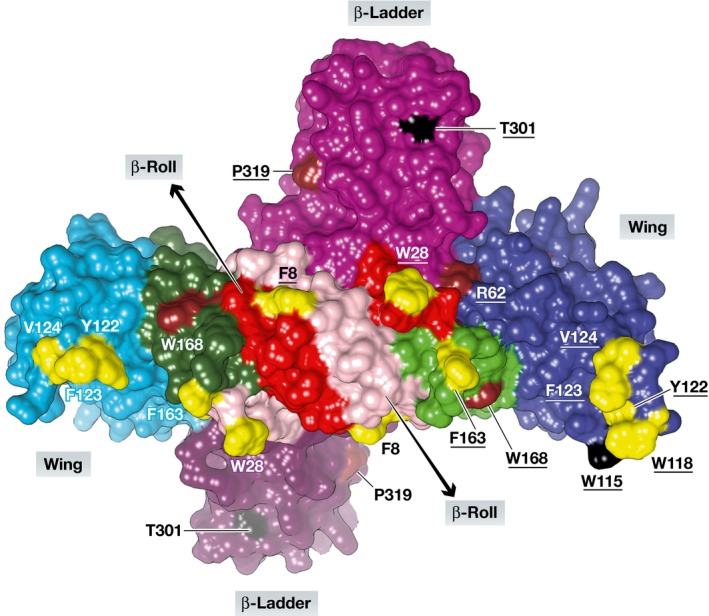
View onto the presumable membrane‐interacting (inner) surface involving the wing and the β‐roll domains of each protomer. The β‐roll domains are pink (protomer A) and red (protomer B), the wing domains are light blue (A) and dark blue (B), the connector subdomains are dark green (A) and light green (B), and the β‐ladder domains (outer surface) are faded dark purple (A) and purple (B). Residues believed to be in contact with the ER membrane are yellow; those proposed to be involved in RNA replication and virion assembly (Scaturro et al, 2015) are brown and black, respectively. Residue numbers from protomer B are underlined. Figure prepared by Dr. Jian Lei using UCSF Chimera and PDB entry 5K6K (Brown et al, 2016).
A hallmark of flavivirus NS1 is its secretion from infected cells in the form of hexamers (sNS1). In the early stages of acute disease, DENV‐2 sNS1 circulates in serum in high concentrations (which are correlated with severity of disease) and is highly immunogenic. Accordingly, sNS1 is used as a diagnostic marker for flavivirus (including ZIKV) infection and as a component of several experimental vaccines. DENV‐2 sNS1 can elicit protective antibodies and also auto‐antibodies that cross‐react with platelets, endothelial cells, components of the extracellular matrix, blood‐clotting proteins, and integrins (see Muller & Young, 2013, for review). It is thought that the action of these auto‐antibodies contributes to the severe forms of dengue known as dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS). For the development of vaccines containing sNS1, it is important to understand which surface epitopes of the protein elicit protective antibodies and which ones lead to the formation of auto‐antibodies. To distinguish between these, the information on the surface properties of sNS1, in particular its electrostatics, as provided by the crystal structures, is of paramount importance. A model of the ZIKV sNS1 hexamer, built on the basis of the X‐ray structure of the dimer, reveals that the electrostatic potential of the outer surface of ZIKV sNS1 is markedly different from those of its DENV‐2 and WNV counterparts (Brown et al, 2016; Xu et al, 2016).
In addition to cross‐reacting anti‐NS1 antibodies, endothelial cells can also be directly destructed by DENV‐2 sNS1 via modification of the glycocalyx, a mechanism partly induced by interaction between sNS1 and Toll‐like receptor 4 (TLR4) (Beatty et al, 2015; Modhiran et al, 2015; Puerta‐Guardo et al, 2016). DENV‐2 sNS1 also activates innate immunity cells by binding to TLR4, leading to the “cytokine storm” typical of dengue shock syndrome (DSS) (Modhiran et al, 2015). Further, sNS1 is involved in immune evasion by binding to various components of the complement system (see Muller & Young, 2013, for review).
Thus, NS1 is clearly involved in the pathogenesis of dengue. However, Zika virus disease is different from dengue and hemorrhagic symptoms have not been observed. To identify the interaction partners of ZIKV NS1 should be a research priority. It is conceivable that ZIKV NS1 might interact with the endothelial cells of the blood–brain barrier to allow for the virus to enter the brain, or with trophoblasts of the maternal–fetal interface. Whether or not such hypothetic interactions involve TLR4 remains to be seen. In any case, the crystal structures of ZIKV NS1 now available will help understand the protein's interactome. The conserved nature of the inner surface of the flaviviral NS1 dimer interacting with the ER membrane, with the replication complex, as well as the glycoproteins of the capsid, suggests that the same mechanisms might be at work for ZIKV as for DENV‐2. However, the different electrostatic properties of the outer face of the sNS1 hexamer of ZIKV suggest that not all NS1‐generated antibodies should cross‐react with DENV sNS1 (Brown et al, 2016; Xu et al, 2016). In fact, the latter prediction has already been confirmed by a recent analysis of anti‐ZIKV antibodies (Stettler et al, 2016) and is also the basis of a ZIKV diagnostic test that exhibits high specificity even in patients with prior dengue infection (Huzly et al, 2016).
Its multitude of interactions with other flaviviral proteins and with host components makes NS1 an attractive target for antiviral drug discovery (Watterson et al, 2016). Once the structural information on ZIKV NS1 now available will have been complemented by the urgently awaited functional studies, it will be possible to target specific functions of ZIKV NS1.
See also: X Xu et al (October 2016) and WC Brown et al (September 2016)
References
- Akey DL, Brown WC, Dutta S, Konwerski J, Jose J, Jurkiw TJ, DelProposto J, Ogata CM, Skiniotis G, Kuhn RJ, Smith JL (2014) Flavivirus NS1 structures reveal surfaces for associations with membranes and the immune system. Science 343: 881–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty PR, Puerta‐Guardo H, Killingbeck SS, Glasner DR, Hopkins K, Harris E (2015) Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Sci Transl Med 7: 304ra141 [DOI] [PubMed] [Google Scholar]
- Brown WC, Akey DL, Konwerski JR, Tarrasch JT, Skiniotis G, Kuhn RJ, Smith JL (2016) Extended surface for membrane association in Zika virus NS1 structure. Nat Struct Mol Biol 23: 865–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutsche I, Coulibaly F, Voss JE, Salmon J, d'Alayer J, Ermonval M, Larquet E, Charneau P, Krey T, Mégret F, Guittet E, Rey FA, Flamand M (2011) Secreted dengue virus nonstructural protein NS1 is an atypical barrel‐shaped high‐density lipoprotein. Proc Natl Acad Sci USA 108: 8003–8008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huzly D, Hanselmann I, Schmidt‐Chanasit J, Panning M (2016) High specificity of a novel Zika virus ELISA in European patients after exposure to different flaviviruses. Euro Surveill 21: 30203 [DOI] [PubMed] [Google Scholar]
- Modhiran N, Watterson D, Muller DA, Panetta AK, Sester DP, Liu L, Hume DA, Stacey KJ, Young PR (2015) Dengue virus NS1 protein activates cells via Toll‐like receptor 4 and disrupts endothelial cell monolayer integrity. Sci Transl Med 7: 304ra142 [DOI] [PubMed] [Google Scholar]
- Muller DA, Young PR (2013) The flavivirus NS1 protein: molecular and structural biology, immunology, role in pathogenesis and application as a diagnostic biomarker. Antiviral Res 98: 192–208 [DOI] [PubMed] [Google Scholar]
- Puerta‐Guardo H, Glasner DR, Harris E (2016) Dengue virus NS1 disrupts the endothelial glycocalyx, leading to hyperpermeability. PLoS Pathog 12: e1005738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaturro P, Cortese M, Chatel‐Chaix L, Fischl W, Bartenschlager R (2015) Dengue virus non‐structural protein 1 modulates infectious particle production via interaction with the structural proteins. PLoS Pathog 11: e1005277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, Vanzetta F, Minola A, Jaconi S, Mele F, Foglierini M, Pedotti M, Simonelli L, Dowall S, Atkinson B, Percivalle E, Simmons CP, Varani L, Blum J, Baldanti F et al (2016) Specificity, cross‐reactivity, and function of antibodies elicited by Zika virus infection. Science 353: 823–826 [DOI] [PubMed] [Google Scholar]
- Watterson D, Modhiran N, Young PR (2016) The many faces of the flavivirus NS1 protein offer a multitude of options for inhibitor design. Antiviral Res 130: 7–18 [DOI] [PubMed] [Google Scholar]
- Xu X, Song H, Qi J, Liu Y, Wang H, Su C, Shi Y, Gao GF (2016) Contribution of intertwined loop to membrane association revealed by Zika virus full‐length NS1 structure. EMBO J 35: 2170–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn S, Li T, McCune BT, Edeling MA, Fremont DH, Cristea IM, Diamond MS (2012) Evidence for a genetic and physical interaction between nonstructural proteins NS1 and NS4B that modulates replication of West Nile virus. J Virol 86: 7360–7371 [DOI] [PMC free article] [PubMed] [Google Scholar]


