Abstract
A transposon mutagenesis screen designed to identify mutants that were defective in peptide-pheromone signaling of the Rgg2/Rgg3 pathway in Streptococcus pyogenes generated insertions in sixteen loci displaying diminished reporter activity. Fourteen unique transposon insertions were mapped to pptAB, an ABC-type transporter recently described to export sex pheromones of Enterococcus faecalis. Consistent with an idea that PptAB exports signaling peptides, the pheromones known as SHPs (short hydrophobic peptides) were no longer detected in cell-free culture supernatants in a generated deletion mutant of pptAB. PptAB exporters are conserved among the Firmicutes, but their function and substrates remain unclear. Therefore, we tested a pptAB mutant generated in Streptococcus mutans and found that while secretion of heterologously expressed SHP peptides required PptAB, secretion of the S. mutans endogenous pheromone XIP (sigX inducing peptide) was only partially disrupted, indicating that a secondary secretion pathway for XIP exists.
Introduction
Streptococcus pyogenes (Group A streptococcus, GAS), is a human-restricted pathogen capable of causing both mild (pharyngitis, impetigo) and life-threatening (necrotizing fasciitis, sepsis) disease, but is also capable of an asymptomatic lifestyle in the host. All GAS genomes sequenced to date encode four Rgg-like transcription-factor proteins: Rgg1 (RopB), which controls the expression of the cysteine protease, SpeB [1,2,3]; Rgg2 and Rgg3, which together regulate genes that contribute to biofilm formation and lysozyme resistance [4,5]; and ComR, which occurs as one of two alleles (M1 or M3) and whose regulon includes genes associated with genetic competence [6]. Rgg proteins are cytoplasmic receptors for peptide pheromones, and with the exception of Rgg1, the pheromones that regulate the activity of each Rgg in GAS have been described [5,6]. Located next to and divergently transcribed from rgg2 and rgg3, are two small ORFs, shp2 and shp3, which encode 22 and 23 amino acid pro-peptide progenitors of the mature pheromones SHP2 and SHP3 (Table 1). In contrast, the small ORF comS, which encodes a 31 or 32 (depending on the allele) amino acid pro-peptide, lies directly downstream of its cognate regulator, comR (Table 1). The active pheromone derived from ComS is termed XIP, and in GAS comprises the C-terminal eight amino acids of the polypeptide.
Table 1. Peptide pheromones known or tested as substrates of PptAB.
| Peptide | Source | Sequence of precursor (mature peptide) | Reference |
|---|---|---|---|
| cOB1 | E. faecalis | MKKRTLWSVITVAVAVLVLGACGNKKS… (272 amino acids in total) | [7] |
| SHPgbs1555 | S. agalactiae | MKKINKALLFTLIMDILIIVGG | [8] |
| SHP2 | S. pyogenes | MKKVNKALLFTLIMDILIIVGG | This study |
| SHP3 | S. pyogenes | MKKISKFLPILILAMDIIIIVGG | This study |
| ComS | S. pyogenes, M1 allele | MLKKYKYYFIFAALLSFKVVQELSAVDWWRL | This study |
| ComS | S. mutans | MFSILTSILMGLDWWSL | This study |
Streptococcus mutans, an oral pathogen and the primary species associated with dental caries, also possesses four Rgg-like regulators. In S. mutans, ComR (SMU.61) is the best-characterized example and has provided an ideal model to study transcriptional control of the alternative sigma factor SigX, the master regulator of competence [9]. As in GAS and other species containing comRS, the S. mutans comS is encoded downstream of comR; the active pheromone comprises the C-terminus of the polypeptide (Table 1). Importantly, ComS alleles across streptococcal species display distinct sequences and pro-peptide lengths [10,11]. Moreover, XIP and SHP pheromone groups are considerably dissimilar from one another.
Although Rgg regulators respond to different pheromones, control different genes, and do not appear to cross-talk, the circuitry required for induction of Rgg2/3 and ComR signaling utilize analogous, and sometimes shared, cellular components. Pheromones are imported into the cell via the oligopeptide permease, Opp, where they interact with their cognate Rgg and initiate gene expression, including at promoters of the pheromone genes, leading to auto-feedback [5,6,9]. Pro-peptides must be exported from the cell and processed before they can function as active signals. The metalloprotease Eep is required for SHP pheromone production in S. pyogenes [5], but is not required to produce XIP in S. mutans [12], and additional processing steps may occur in the final maturation of either peptide [13]. However, the mechanism by which XIP and SHP peptides are exported from the cell remains poorly understood for the model systems of S. pyogenes and S. mutans. Unlike other systems regulating bacteriocin production (e.g., comCDE in S. mutans, sil in GAS), no dedicated transporter has been identified [14,15].
Recently, an ABC transporter called PptAB was identified as contributing to sex pheromone production in Enterococcus faecalis [7], and a separate study confirmed a role for PptAB in an Rgg-SHP signaling pathway in Streptococcus agalactiae [8]. Here, we report the identification of pptAB in a genetic screen and expand the PptAB substrate list to include GAS SHPs.
Materials and Methods
Bacterial strains
S. pyogenes and S. mutans were grown in Todd-Hewitt broth (BD) supplemented with 0.2% yeast extract (Amresco) or a chemically-defined medium (CDM; [5,16]) as indicated; broth cultures were grown at 37x° C without shaking, and agar plates were cultured at 37° C with 5% CO2. All cloning was done in E. coli strain BH10c [17] which was routinely cultured in Luria-Bertani broth (BD) at 30° C with agitation. All strains were stored at -80° C in 20% glycerol. Antibiotics were added at the following concentrations when appropriate–S. pyogenes: chloramphenicol (Cm), 3 μg mL-1; erythromycin (Erm), 0.5 μg mL-1; kanamycin (Km), 200 μg mL-1; spectinomycin (Spec), 200 μg mL-1; S. mutans: Cm 7.5 μg mL-1; Erm, 1.5 μg mL-1; Km, 750 μg mL-1; Spec, 500 μg mL-1; and E. coli: Erm, 500μg mL-1; Spec, 150μg mL-1.
Construction of mutant strains and plasmids
To delete pptAB in GAS, a 4094 bp region encompassing the genes and surrounding up- and downstream regions was amplified by PCR using primers JC316/JC317 and cloned into pFED760. pptAB were subsequently deleted by inverse PCR using primers JC318/JC319, and the kanamycin resistance gene, aphA3, was amplified with primers JC320/JC321 and inserted into PacI sites to make pJC251-kan. This knockout plasmid was electroporated into wild-type NZ131 and the Δrgg3 mutant (JCC131), and a two-step temperature dependent selection process was used to identify the mutants of interest [18]. Deletion of pptAB in S. mutans was accomplished similarly using primers JC403/JC404 to amplify the genomic region from wild-type UA159 (pJC297), JC405/JC406 for inverse PCR, and JC320/JC407 for aphA3 cassette amplification. S. mutans strains were transformed with linear PCR product amplified from the resulting plasmid, pJC298, in CDM with the addition of synthetic XIP [9]. To complement the deletion of pptAB in GAS, a 1989 bp PCR fragment was amplified from NZ131 DNA using primers JC322/JC323 and cloned into the multi-copy shuttle vector, pLZ12-Sp, to create ppptAB (pJC252). pPrecA-shp2 (pJC350) and pPrecA-shp3 (pJC352) were constructed by cloning 120 bp or 194 bp fragments containing NZ131 shp2 (primers JC495/SHP2-C9-rev-BglII) or shp3 (primers JC175/JC427), respectively, into pJC303, a pLZ12-Sp-based vector containing the recA promoter directly upstream of a multiple cloning site.
Luciferase assays
Starter cultures of strains of interest were prepared by growing isolated colonies to mid-log phase in CDM (OD600 = 0.5 to 0.6; Spectronic 20+; Thermo), adding glycerol to 20% and freezing at -80° C in aliquots. On the experiment day, starters were thawed and diluted into fresh CDM to a starting OD600 of 0.01 (S. pyogenes) or 0.025 (S. mutans) and incubated at 37° C. For luciferase assays measuring endogenous reporter activity, the OD600 was measured and counts per second (CPS) of 50 μL were assessed by luminometer (Turner BioSystems) after exposure to the decyl aldehyde substrate (Acros); relative light units (RLU) were calculated by dividing CPS by OD600 at each time point. Alternatively, strains of interest were dispensed into a 96-well clear-bottom plate (Greiner), a 1% decyl aldehyde solution was added to interstitial spaces, and the plate was incubated at 37° C with continuous shaking in a microplate reader (Synergy 2, Biotek) with collection of OD600 and luminescence measurements every 20 minutes. For luciferase assays measuring reporter-inducing activity in supernatants, donor cultures were diluted into fresh CDM as described above and grown to an OD600 of 0.5 to 0.6. Donor cells were pelleted by centrifugation, and the supernatants were sterilized by the addition of antibiotics or by filtration. Appropriate luciferase reporter strains were also grown to mid- to late-log phase then diluted into the clarified supernatants to a final OD600 of 0.05, and OD600 and CPS were measured every 30 minutes until maximum RLU were achieved.
Results and Discussion
A transposon screen identifies an ABC transporter, PptAB
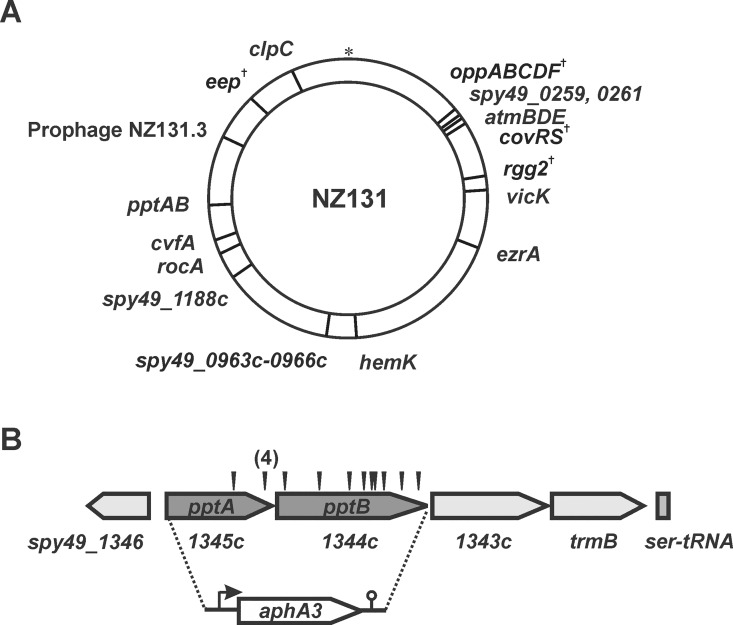
We have previously shown that the primary targets of Rgg2/3-SHP regulation are the shp genes themselves along with their neighboring downstream genes [5]. To identify novel components required for Rgg-SHP signaling, we developed a genetic screen to identify genes involved in secretion, maturation, detection and degradation of SHP pheromones [19]. Briefly, a mariner transposon system [20] was used to mutagenize Δrgg3 strains containing luciferase (lux; JCC198) or β-glucuronidase (gus; JCC233) reporter genes downstream of shp3; in this background, Pshp3 is highly expressed due to the absence of Rgg3, which normally acts as a repressor when pheromone levels are low. Approximately 10,000 individual mutants were screened for loss of reporter activity, and 16 loci were identified in which there were at least three independent transposon insertions from two or more rounds of screening, including genes previously found to be important for Rgg-SHP signaling (e.g., rgg2, eep, opp) [5] (Fig 1A). We recently described how covRS, one of the loci with the highest number of insertions (12 unique insertions), affects signaling through the regulation of the PepO protease [19]. Here, we report that a locus with 14 unique transposon insertions encodes the ABC transporter, PptAB (Fig 1B), an ABC transporter that was recently identified as the exporter for Enterococcus faecalis sex pheromones [7] and confirmed to function in the same capacity for Streptococcus agalactiae Rgg/SHP pheromones [8].
Fig 1. Transposon mutagenesis identifies novel components of the Rgg-SHP quorum sensing circuit.
(A) Sixteen loci were identified according to our criteria (see text). Gene names and chromosomal location are indicated, with the replication origin (*) at twelve o’clock. Components of the QS circuit previously identified are indicated (†). (B) Fourteen unique insertions (arrowheads) mapped to pptAB, a predicted ABC transporter. pptAB were replaced with a cassette containing aphA3, which confers resistance to kanamycin.
PptAB is the primary transporter for SHP pheromones in GAS
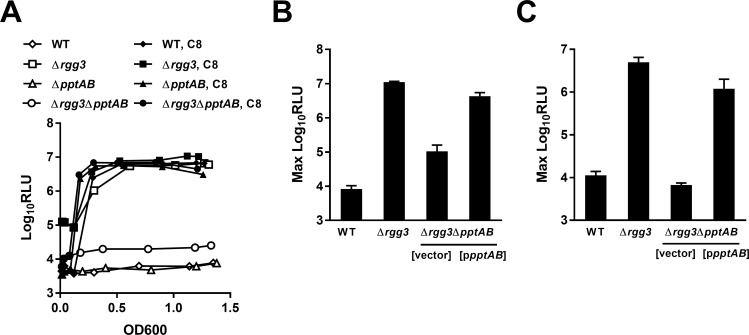
To confirm the role of pptAB in Rgg-SHP signaling in GAS, deletion mutants in which both genes were replaced with a kanamycin-resistance cassette were constructed in both the wild-type NZ131 and Δrgg3 backgrounds, and a Pshp3-lux reporter (pJC219) was integrated into the chromosomes of the resulting strains. As expected, deletion of pptAB in the Δrgg3 background led to a >300-fold decrease in luciferase activity, although the Δrgg3ΔpptAB mutant (JCC209) still had residual reporter activity ~3-fold higher than unstimulated wild-type and the single ΔpptAB mutant (JCC208) (Fig 2A). This residual activity is attributable to loss of direct repression by Rgg3 of the shp promoter, as has been documented in strains incapable of pheromone production [21]. Furthermore, pptAB mutants were still capable of robust Pshp3-lux induction following the addition of synthetic SHP-C8 pheromone, confirming a role for pptAB in signal production but not signal detection. Interestingly, although pptAB are predicted to be co-transcribed as part of a multi-cistronic operon [22], no insertions were identified in downstream genes (spy49_1343c and trmB, a predicted thiamine kinase and tRNA (guanine-N(7)-)-methyltransferase, respectively), suggesting these genes are transcribed independently from a different promoter and/or they are dispensable for pheromone production. Additionally, the pptAB deletion in the Δrgg3 background could be complemented with pptAB alone (ppptAB; pJC252), further supporting the lack of a role for spy49_1343 and trmB in Rgg-SHP signaling (Fig 2B). As SHP pheromones are secreted into the extracellular environment and can be detected in cell-free spent media from producer strains [5], the importance of PptAB for this process was confirmed by quantifying Pshp3-lux-inducing activity in supernatants conditioned by wild-type, Δrgg3, Δrgg3ΔpptAB or complemented strains (Fig 2C).
Fig 2. Rgg-SHP signaling in a S. pyogenes pptAB mutant.
(A) Luciferase expression from Pshp3 reporters integrated into wild-type (WT; NZ131), Δrgg3 (JCC131), and Δrgg3ΔpptAB (JCC209) GAS strains with (closed symbols) and without (open symbols) the addition of 100nM synthetic SHP3-C8 (C8) peptide. Data shown are representative of experiments performed at least three times. (B) Maximum Pshp3-lux reporter activity in WT, Δrgg3, or Δrgg3ΔpptAB strains carrying a plasmid encoding pptAB (pJC252) or empty vector (pLZ12-Sp). (C) Maximum Pshp3-lux reporter-inducing activity in conditioned supernatants prepared from WT, Δrgg3, or Δrgg3ΔpptAB donor strains expressing pptAB (pJC252) or empty vector (pLZ12-Sp). Donor cultures were grown to OD ~0.5, cells were removed by centrifugation and filtration, and Pshp3-lux activity of a Δrgg3 shpGGG reporter strain (BNL204) was measured. For B and C, data shown are the mean and SD from at least three experiments.
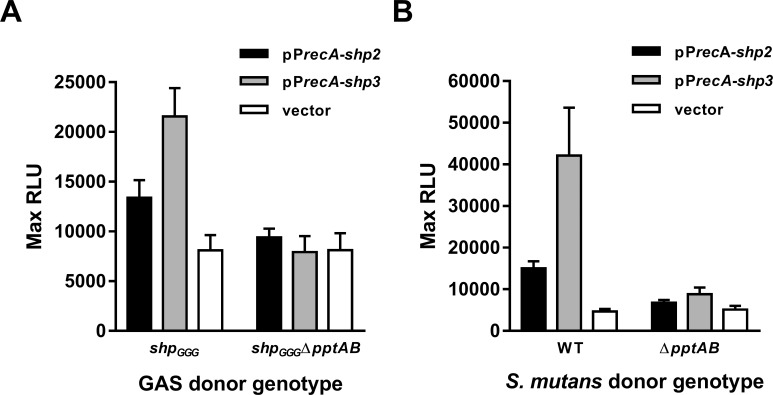
In GAS, transcription of shp genes is subject to auto-feedback, wherein induction of Rgg-SHP signaling leads to increased expression of the pheromones themselves [5]. To uncouple SHP production from the influence of Rgg2 and Rgg3 and to separate production of the pheromones from their downstream function, full-length shp2 and shp3 were cloned under the recA promoter (pPrecA-shp2, pJC350; pPrecA-shp3, pJC352), which is constitutively expressed. These plasmids were used to transform strains in which the start codons of both shp genes had been mutated to GGG (shpGGG; BNL170), rendering the strains unable to produce pheromone from chromosomal loci or undergo auto-induction. Spent culture supernatants were prepared from donor strains BNL170 or a shpGGGΔpptAB strain (JCC218) carrying pJC350, pJC352, or the empty vector, and pheromone production was quantified by measuring the luciferase activity induced in a Pshp3-lux reporter strain (BNL204). As expected, supernatants from shpGGG but otherwise wild-type strains contained Pshp3-inducing activity, but media from shpGGG ΔpptAB or donors with empty vector did not (Fig 3A).
Fig 3. PptAB exports SHP pheromones in GAS and S. mutans.
(A) Maximum Pshp3-lux reporter-inducing activity in culture supernatants prepared from (A) GAS and (B) S. mutans WT and ΔpptAB donor strains expressing PrecA-shp2 (pJC350) or PrecA-shp3 (pJC352). Both GAS donor strains are deleted for chromosomal copies of shp2 and shp3 (shpGGG; BNL170 and JCC218, respectively). Donor cultures were grown to OD ~0.5, cells were removed by centrifugation, supernatants were filtered or chemically sterilized, and Pshp3-lux activity of a Δrgg3 shpGGG reporter strain (BNL204) was measured. Data shown are the mean and SD from at least three experiments.
Interestingly, although both shp2 and shp3 were expressed from the same promoter (PrecA), luciferase-inducing activity was always higher in pPrecA-shp3 supernatants. Our lab has previously shown that shp3 has greater potential to activate Rgg-SHP signaling than shp2; using a series of gene replacements and by constructing chimeric shps, it was determined that the difference in activation potential lies in the non-effector N-terminal portion of the peptides [23]. Furthermore, mature pheromones (C-terminal eight amino acids; Table 1) bind the Rgg proteins and induce Pshp signaling with similar EC50 values [13,23]. However, we have also shown that an aminopeptidase, PepO, degrades SHPs and limits signaling, with SHP2-C8 inactivated more efficiently than SHP3-C8 [19]. Thus, it is possible that the difference between SHP2- and SHP3-containing supernatants observed here arises from differences in processing or degradation, or even in efficiency of export by PptAB. However, there remains a significant reduction in activity in supernatants prepared from the ΔpptAB mutant versus wild-type (p<0.0001, Student’s t-test), regardless of SHP identity, confirming its importance in export of both SHP pheromones. Additionally, induction of signaling in wild-type (shp-intact) cells leads to positive feedback at both shp promoters; therefore, the functional consequences of differences between the two peptides are most likely minimal in wild-type cells under normal conditions.
S. mutans PptAB exports GAS SHP pheromones
pptAB are conserved among Firmicutes within the orders Bacillales and Lactobacillales, including in several pathogenic species, and the transporter was previously shown to export signaling peptides in E. faecalis [7] and S. agalactiae [8] in addition to S. pyogenes as described above. PptA and PptB of GAS share 88% and 69% similarity with homologues in S. mutans. To test whether the S. mutans transporter could export heterologous SHP pheromones, wild-type (UA159) and ΔpptAB (JCC263) strains were transformed with the PrecA-shp2 and PrecA-shp3 expression constructs (pJC350 and pJC352, respectively), and the resulting strains were used to produce conditioned media. Similar to experiments with GAS donor strains, supernatants from wild-type cells contained higher levels of Pshp3 reporter-inducing activity than those from ΔpptAB (Fig 3B), suggesting that the criteria by which this ABC transporter recognizes substrates are conserved across different species. Although the S. mutans genome encodes four Rgg-like regulators, with the exception of ComRS, the peptides and functions of these proteins have not been well characterized. However, given our findings, PptAB seems a possible candidate for transport of these substrates. As with GAS-conditioned supernatants, we observed greater luciferase-inducing activity in pPrecA-shp3 supernatants than pPrecA-shp2 supernatants. It is worth noting that both Eep, a metalloprotease involved in SHP processing [5], and PepO are conserved in S. mutans; therefore, the same factors that contribute to the differences between SHP2 and SHP3 observed in GAS may be in play in this species. Finally, although there was a significant reduction in activity from supernatants produced by wild-type versus ΔpptAB donor strains, supernatants from ΔpptAB still contained activity that was significantly different from the vector-only control (pPrecA-shp2, p = 0.0002; pPrecA-shp3, p<0.0001; Student’s t-test). Whether this difference is due to export, albeit inefficient, by another unidentified ABC transporter or to non-specific cell lysis during growth has not been determined.
PptAB contributes to but is not required for export of competence pheromones
As mentioned above, both GAS and S. mutans possess homologues of ComR, an important regulator of competence [6,9]. These proteins are 68% similar and are activated upon binding their cognate XIP pheromone (Table 1). ComR activation induces expression of sigX, which is in turn required for expression of competence-related genes. Like the GAS shp promoter, robust S. mutans PsigX induction depends on auto feedback of the ComRS sensory system, since in addition to PsigX, ComR positively regulates expression of comS. The importance of ComRS in the natural transformation of S. mutans has been demonstrated [9,24], and our lab has also shown that ComRS signaling is functional in GAS, as evidenced by the induction of a PsigX-lux reporter upon addition of synthetic XIP, although laboratory conditions favoring spontaneous PsigX induction and transformation have remained elusive [6]. Recently, natural transformation was demonstrated for GAS grown in a biofilm model, suggesting that other host-derived factors/signals are required and our current in vitro conditions do not recapitulate the correct environment [25].
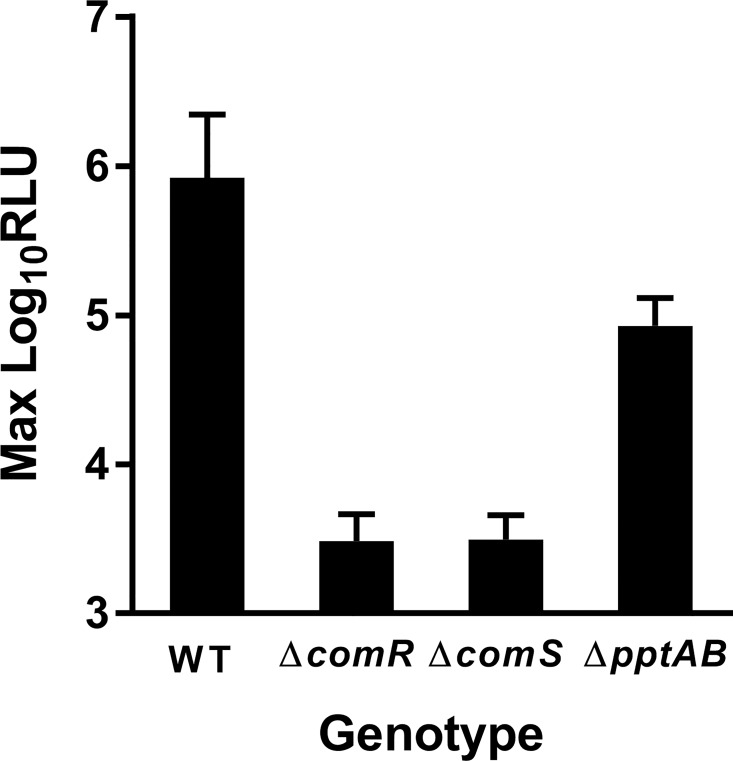
To ask whether PptAB is important for ComRS signaling, we took advantage of the observation that when grown in CDM, S. mutans spontaneously develops a high level of competence during late-logarithmic growth, an event that is slightly preceded by expression from the PsigX promoter and coincides with the accumulation of XIP in conditioned supernatants [9,12,24,26]. The growth and luciferase activity of wild-type S. mutans and the pptAB mutant (UA159 and JCC263, respectively) containing a PsigX-luxAB reporter (pWAR304) were measured over time. The pptAB mutant exhibited an ~10-fold reduction in average maximum RLU compared to wild-type, but still had PsigX activity ~25-fold greater than comR or comS mutants, which are defective for signaling suggesting that PptAB may contribute to, but is not required for, the production of XIP (Fig 4).
Fig 4. ComRS signaling in a S. mutans pptAB mutant.
Maximum luciferase activity of WT (UA159), ΔcomR (MW02), ΔcomS (MW05) and ΔpptAB (JCC263) mutants carrying a multi-copy PsigX-lux reporter (pWAR304). Cells were grown to mid-log phase in CDM then diluted to an OD600 ~0.05 in a 96-well plate, and growth and luciferase activity were measured every 20 minutes in a Synergy 2 plate reader (Biotek). Data shown are the mean and SD from three experiments.
In an attempt to uncouple comS expression from the positive-feedback loop and to further explore the role of PptAB in ComS/XIP secretion, the GAS M1 allele, comSM1_GAS, or the S. mutans gene, comSUA159, was cloned downstream of the PrecA promoter to make pJC354 and pJC371, respectively. These plasmids were then transferred to donor strains. In NZ131, the native comR contains a duplication of three amino acids in the C-terminal domain of the protein rendering it nonfunctional. Therefore, a strain was constructed in which the NZ131 gene was replaced with a functional copy from MGAS8232 (called NZ131 comRMGAS8232 or MW361; see S1 File), and the ability of this strain to induce transcription from PsigX in response to XIP was confirmed (S1 Fig). Unfortunately, conditioned supernatants collected from donor strains expressing PrecA-comS varied widely in PsigX-inducing activity and failed to clarify the role of PptAB in ComS export (S2 and S3 Figs). For GAS ComS/XIP, conditions that lead to expression of the gene in vitro remain unknown, and endogenously-produced pheromone has never been detected. Thus, it is possible that even though transcription was under the control of a constitutive promoter, additional factors needed for productive pheromone synthesis are lacking in both GAS and S. mutans during growth under these conditions. For S. mutans ComS/XIP, supernatants collected at time points early enough to avoid PsigX auto-induction may not have had time to accumulate detectable XIP in the supernatants. Furthermore, growth phase and culture pH can have a significant effect on cells’ response to ComS, thus it is possible the conditions tested here were not favorable for robust pheromone detection or production [27]. Finally, it has been proposed that processed XIPs in some streptococci remain in close association with the cell surface, which would complicate its detection by the method used here [28].
Concluding Remarks
We have shown here that the ABC transporter, PptAB, plays an important role in the export of SHP pheromones in GAS, adding this species to a growing list organisms whose cell-cell signaling circuitry relies on this transporter for efficient export of signaling molecules from the cell. PptAB is conserved among many bacteria, including many Firmicute species. Indeed, we found that the S. mutans homologue could export GAS pheromones. Interestingly however, PptAB was not critical for ComRS signaling in this species, suggesting that the exporter must exhibit some substrate specificity and/or the competence pheromone preferentially uses an as-of-yet unidentified exporter. The S. mutans genome contains three Rgg-like regulators in addition to ComR, and the role of PptAB in the export of this species’ endogenous SHP pheromones remains to be determined. Finally, if PptAB has evolved to export signaling peptides, it is possible that some of the phenotypes exhibited by pptAB mutants in other species, including exoprotein secretion, competence, sporulation in B. subtilis [29,30,31], and cell wall structure and composition in S. aureus [32], may be regulated by small peptides.
Supporting Information
NZ131 in which the native comR allele was replaced with MGAS8232 comR (MW361) and carrying a multi-copy PsigX-lux reporter (pWAR200) was grown in CDM containing synthetic M1 GAS XIP at the indicated concentrations. OD600 and CPS were measured until maximum RLU were achieved.
(TIF)
Maximum PsigX-lux reporter activity induced by supernatants from NZ131 comRMGAS8232 (MW361), UA159, and UA159ΔpptAB (JCC263) donor strains expressing the GAS M1 comS allele from the recA promoter (pJC354). Donor strains were grown to an OD600 of 0.5 to 0.6, and supernatants were clarified by centrifugation and the addition of erythromycin. NZ131 comRMGAS8232 containing the GAS PsigX-lux reporter (pWAR200) was diluted into the supernatants, and OD600 and CPS were measured until maximum RLU were achieved.
(TIF)
Maximum PsigX-lux reporter activity induced by conditioned supernatants from UA159 and UA159ΔpptAB (JCC263) donors expressing the S. mutans comS from the recA promoter (pJC371). Donor strains were grown to an OD600 of 0.5 to 0.6, and supernatants were clarified by centrifugation and the addition of erythromycin. A ΔcomS strain containing the S. mutans PsigX-lux reporter (MW17) was diluted into the supernatants, and OD600 and CPS were measured until maximum RLU were achieved.
(TIF)
Methods describing construction of strains and plasmids used for experiments in Supporting Information, and reference list for Supporting Information.
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We are grateful to Lauren Mashburn-Warren and Breah LaSarre for construction of some of the strains and plasmids used in this study, and to Dr. Donald Morrison and members of the Federle Lab for thoughtful comments and discussion.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by grants from the NIH NIAID #AI091779 and the Burroughs Wellcome Fund Investigators in the Pathogenesis of Infectious Dieseases.
References
- 1.Chaussee MS, Ajdic D, Ferretti JJ (1999) The rgg gene of Streptococcus pyogenes NZ131 positively influences extracellular SPE B production. Infect Immun 67: 1715–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hollands A, Aziz RK, Kansal R, Kotb M, Nizet V, Walker MJ (2008) A naturally occurring mutation in ropB suppresses SpeB expression and reduces M1T1 group A streptococcal systemic virulence. PLoS One 3: e4102 10.1371/journal.pone.0004102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyon WR, Gibson CM, Caparon MG (1998) A role for trigger factor and an rgg-like regulator in the transcription, secretion and processing of the cysteine proteinase of Streptococcus pyogenes. Embo J 17: 6263–6275. 10.1093/emboj/17.21.6263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang JC, Jimenez JC, Federle MJ (2015) Induction of a quorum sensing pathway by environmental signals enhances group A streptococcal resistance to lysozyme. Mol Microbiol 97: 1097–1113. 10.1111/mmi.13088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang JC, LaSarre B, Jimenez JC, Aggarwal C, Federle MJ (2011) Two group A streptococcal peptide pheromones act through opposing Rgg regulators to control biofilm development. PLoS Pathog 7: e1002190 10.1371/journal.ppat.1002190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mashburn-Warren L, Morrison DA, Federle MJ (2012) The cryptic competence pathway in Streptococcus pyogenes is controlled by a peptide pheromone. J Bacteriol 194: 4589–4600. 10.1128/JB.00830-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varahan S, Harms N, Gilmore MS, Tomich JM, Hancock LE (2014) An ABC transporter is required for secretion of peptide sex pheromones in Enterococcus faecalis. MBio 5: e01726–01714. 10.1128/mBio.01726-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez-Pascual D, Gaudu P, Fleuchot B, Besset C, Rosinski-Chupin I, Guillot A, et al. (2015) RovS and its associated signaling peptide form a cell-to-cell communication system required for Streptococcus agalactiae pathogenesis. MBio 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mashburn-Warren L, Morrison DA, Federle MJ (2010) A novel double-tryptophan peptide pheromone controls competence in Streptococcus spp. via an Rgg regulator. Mol Microbiol 78: 589–606. 10.1111/j.1365-2958.2010.07361.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fontaine L, Wahl A, Flechard M, Mignolet J, Hols P (2015) Regulation of competence for natural transformation in streptococci. Infect Genet Evol 33: 343–360. 10.1016/j.meegid.2014.09.010 [DOI] [PubMed] [Google Scholar]
- 11.Shanker E, Morrison DA, Talagas A, Nessler S, Federle MJ, Prehna G (2016) Pheromone recognition and selectivity by ComR proteins among Streptococcus species. PLoS Pathog 12: e1005979 10.1371/journal.ppat.1005979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan R, Rukke HV, Ricomini Filho AP, Fimland G, Arntzen MO, Thiede B, et al. (2012) Extracellular identification of a processed type II ComR/ComS pheromone of Streptococcus mutans. J Bacteriol 194: 3781–3788. 10.1128/JB.00624-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aggarwal C, Jimenez JC, Nanavati D, Federle MJ (2014) Multiple length peptide-pheromone variants produced by Streptococcus pyogenes directly bind Rgg proteins to confer transcriptional regulation. J Biol Chem 289: 22427–22436. 10.1074/jbc.M114.583989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hale JD, Heng NC, Jack RW, Tagg JR (2005) Identification of nlmTE, the locus encoding the ABC transport system required for export of nonlantibiotic mutacins in Streptococcus mutans. J Bacteriol 187: 5036–5039. 10.1128/JB.187.14.5036-5039.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hidalgo-Grass C, Ravins M, Dan-Goor M, Jaffe J, Moses AE, Hanski E (2002) A locus of group A Streptococcus involved in invasive disease and DNA transfer. Mol Microbiol 46: 87–99. [DOI] [PubMed] [Google Scholar]
- 16.van de Rijn I, Kessler RE (1980) Growth characteristics of group A streptococci in a new chemically defined medium. Infect Immun 27: 444–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howell-Adams B, Seifert HS (2000) Molecular models accounting for the gene conversion reactions mediating gonococcal pilin antigenic variation. Mol Microbiol 37: 1146–1158. [DOI] [PubMed] [Google Scholar]
- 18.Degnan BA, Fontaine MC, Doebereiner AH, Lee JJ, Mastroeni P, Dougan G, et al. (2000) Characterization of an isogenic mutant of Streptococcus pyogenes Manfredo lacking the ability to make streptococcal acid glycoprotein. Infect Immun 68: 2441–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilkening RV, Chang JC, Federle MJ (2016) PepO, a CovRS-controlled endopeptidase, disrupts Streptococcus pyogenes quorum sensing. Mol Microbiol 99: 71–87. 10.1111/mmi.13216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Breton Y, McIver KS (2013) Genetic manipulation of Streptococcus pyogenes (the Group A Streptococcus, GAS). Curr Protoc Microbiol 30: Unit 9D 3. [DOI] [PMC free article] [PubMed]
- 21.Lasarre B, Aggarwal C, Federle MJ (2013) Antagonistic Rgg regulators mediate quorum sensing via competitive DNA binding in Streptococcus pyogenes. MBio 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dehal PS, Joachimiak MP, Price MN, Bates JT, Baumohl JK, Chivian D, et al. (2010) MicrobesOnline: an integrated portal for comparative and functional genomics. Nucleic Acids Res 38: D396–400. 10.1093/nar/gkp919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaSarre B, Chang JC, Federle MJ (2013) Redundant group a streptococcus signaling peptides exhibit unique activation potentials. J Bacteriol 195: 4310–4318. 10.1128/JB.00684-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desai K, Mashburn-Warren L, Federle MJ, Morrison DA (2012) Development of competence for genetic transformation of Streptococcus mutans in a chemically defined medium. J Bacteriol 194: 3774–3780. 10.1128/JB.00337-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marks LR, Mashburn-Warren L, Federle MJ, Hakansson AP (2014) Streptococcus pyogenes biofilm growth in vitro and in vivo and its role in colonization, virulence, and genetic exchange. J Infect Dis 210: 25–34. 10.1093/infdis/jiu058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wenderska IB, Lukenda N, Cordova M, Magarvey N, Cvitkovitch DG, Senadheera DB (2012) A novel function for the competence inducing peptide, XIP, as a cell death effector of Streptococcus mutans. FEMS Microbiol Lett 336: 104–112. 10.1111/j.1574-6968.2012.02660.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo Q, Ahn SJ, Kaspar J, Zhou X, Burne RA (2014) Growth phase and pH influence peptide signaling for competence development in Streptococcus mutans. J Bacteriol 196: 227–236. 10.1128/JB.00995-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gardan R, Besset C, Gitton C, Guillot A, Fontaine L, Hols P, et al. (2013) Extracellular life cycle of ComS, the competence-stimulating peptide of Streptococcus thermophilus. J Bacteriol 195: 1845–1855. 10.1128/JB.02196-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leskela S, Kontinen VP, Sarvas M (1996) Molecular analysis of an operon in Bacillus subtilis encoding a novel ABC transporter with a role in exoprotein production, sporulation and competence. Microbiology 142 (Pt 1): 71–77. [DOI] [PubMed] [Google Scholar]
- 30.Leskela S, Wahlstrom E, Hyyrylainen HL, Jacobs M, Palva A, Sarvas M, et al. (1999) Ecs, an ABC transporter of Bacillus subtilis: dual signal transduction functions affecting expression of secreted proteins as well as their secretion. Mol Microbiol 31: 533–543. [DOI] [PubMed] [Google Scholar]
- 31.Kontinen VP, Sarvas M (1988) Mutants of Bacillus subtilis defective in protein export. J Gen Microbiol 134: 2333–2344. 10.1099/00221287-134-8-2333 [DOI] [PubMed] [Google Scholar]
- 32.Jonsson IM, Juuti JT, Francois P, AlMajidi R, Pietiainen M, Girard M, et al. (2010) Inactivation of the Ecs ABC transporter of Staphylococcus aureus attenuates virulence by altering composition and function of bacterial wall. PLoS One 5: e14209 10.1371/journal.pone.0014209 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
NZ131 in which the native comR allele was replaced with MGAS8232 comR (MW361) and carrying a multi-copy PsigX-lux reporter (pWAR200) was grown in CDM containing synthetic M1 GAS XIP at the indicated concentrations. OD600 and CPS were measured until maximum RLU were achieved.
(TIF)
Maximum PsigX-lux reporter activity induced by supernatants from NZ131 comRMGAS8232 (MW361), UA159, and UA159ΔpptAB (JCC263) donor strains expressing the GAS M1 comS allele from the recA promoter (pJC354). Donor strains were grown to an OD600 of 0.5 to 0.6, and supernatants were clarified by centrifugation and the addition of erythromycin. NZ131 comRMGAS8232 containing the GAS PsigX-lux reporter (pWAR200) was diluted into the supernatants, and OD600 and CPS were measured until maximum RLU were achieved.
(TIF)
Maximum PsigX-lux reporter activity induced by conditioned supernatants from UA159 and UA159ΔpptAB (JCC263) donors expressing the S. mutans comS from the recA promoter (pJC371). Donor strains were grown to an OD600 of 0.5 to 0.6, and supernatants were clarified by centrifugation and the addition of erythromycin. A ΔcomS strain containing the S. mutans PsigX-lux reporter (MW17) was diluted into the supernatants, and OD600 and CPS were measured until maximum RLU were achieved.
(TIF)
Methods describing construction of strains and plasmids used for experiments in Supporting Information, and reference list for Supporting Information.
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.