Abstract
γ-Aminobutyric acid (GABA) is the major inhibitory transmitter in the mature brain but is excitatory in the developing cortex. We found that mouse zona incerta (ZI) projection neurons form a GABAergic axon plexus in neonatal cortical layer 1, making synapses with neurons in both deep and superficial layers. A similar depolarizing GABAergic plexus exists in the developing human cortex. Selectively silencing mouse ZI GABAergic neurons at birth decreased synaptic activity and apical dendritic complexity of cortical neurons. The ZI GABAergic projection becomes inhibitory with maturation and can block epileptiform activity in the adult brain. These data reveal an early-developing GABAergic projection from the ZI to cortical layer 1 that is essential for proper development of cortical neurons and balances excitation with inhibition in the adult cortex.
During embryonic development, neural activity (1, 2) influences proliferation, migration, and differentiation, as well as circuit refinement (3–5). In immature brains, the neurotransmitter GABA has excitatory effects due to high intracellular chloride (6, 7), contrary to its inhibitory effects in adult brains. GABA in the immature neocortex comes from local interneurons and axonal projections from other brain regions (8–12). The neonatal rodent brain has an excitatory GABAergic plexus projecting widely within cortical layer 1 (13, 14). Here, we show that the zona incerta (ZI) generates the neurons of this GABAergic plexus.
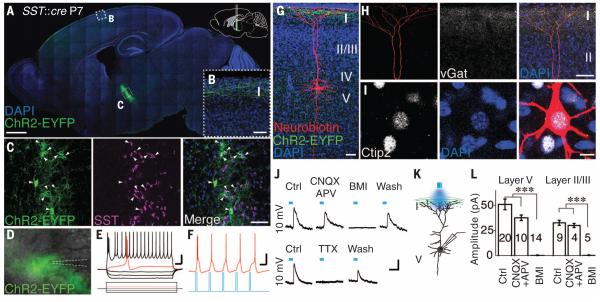
We mapped the ZI pathway in transgenic mice by manipulating channelrhodopsin-2 (ChR2) expression in somatostatin (SST)–expressing neurons in the ZI of neonatal mice (postnatal day P0–P1) (15, 16). Labeling was restricted to ZI GABAergic (Fig. 1A), SST+ (Fig. 1C) neurons 1 week after virus injection, and we observed EYFP+ ZI axonal projections widely distributed in layer 1 of somatosensory and motor cortex (Fig. 1, A and B, and fig. S1). At P7, ChR2 was reliably expressed in ZI neurons, and blue light stimulation induced firing of EYFP+ ZI neurons (Fig. 1, D to F). We filled layer 5 cortical neurons with neurobiotin in acute slices of somatosensory and motor cortex and coimmunostained with the cortical layer 5 marker Ctip2 (Fig. 1I, 16/16 neurons). The apical dendrites of these pyramidal neurons contacted layer 1 EYFP+ axons (Fig. 1G) and colocalized with the GABAergic presynaptic marker vGat (vesicular GABA transporter) (Fig. 1H).
Fig. 1. ZI GABAergic neurons project to cortical layer 1 and form functional synapses with cortical neurons in neonatal mouse brain.
(A) Sagittal view of SST+ ZI neurons specifically labeled with ChR2-EYFP. Locations of images shown in (B) and (C) are indicated. DAPI, 4′,6-diamidino-2-phenylindole. (B) Specific labeling of axons in cortical layer 1. (C) ZI EYFP+ neurons coexpress SST. Arrowheads indicate colocalization. (D) Image of the ZI with superimposed EYFP at P7. (E) Burst-firing of ZI EYFP+ neurons. (F) Action potentials induced by blue light stimulation. (G) Neurobiotin-labeled layer 5 pyramidal cell in P8 SST::cre mouse with ChR2-EYFP injected into the ZI at P0. Note overlap between apical dendritic tufts and EYFP+ axons in layer 1. (H) A high density of vGat expression in layer 1 overlaps with the dendritic tuft. (I) Recorded layer 5 neurons coexpress Ctip2. (J) Schematic showing blue light stimulation of layer 1 EYFP+ axons while recording layer 5 neurons. (K and L) Representative traces (K) and statistical analysis [(L), recorded during P6–P8] show light-evoked synaptic responses at +10 mV with or without receptor antagonists (10 μM CNQX, 50 μM D-APV, 20 μM BMI, or 1 μM TTX). Scale bars, 1 mm (A); 50 μm [(B), (C), (G), and (H)]; 25 mV, 100 ms [(E) and (F)]; 10 μm (I); 20 pA, 50 ms (K). ***P < 0.001. Here and in other figures, numerals within each bar of a graph represent sample size; error bars denote SEM.
Blue light stimulation of layer 1 evoked synaptic responses in layer 4 and layer 5 pyramidal neurons. The light-evoked responses were not sensitive to α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) or N-methyl-d-aspartate (NMDA) receptor antagonists [6-cyano-2, 3-dihydroxy-7-nitro-quinoxaline (CNQX) and d-2-amino-5-phosphonovalerate (D-APV), respectively] but were abolished by the GABAA receptor antagonist bicuculline (BMI) (Fig. 1, J to L) and reversibly blocked by tetrodotoxin (TTX, n = 4; Fig. 1K). Stimulation of layer 1 axons also induced GABAergic responses from layer 2/3 neurons, supporting observations that the layer 1 GABAergic plexus connects with pyramidal neurons from multiple layers in neonatal mice (13).
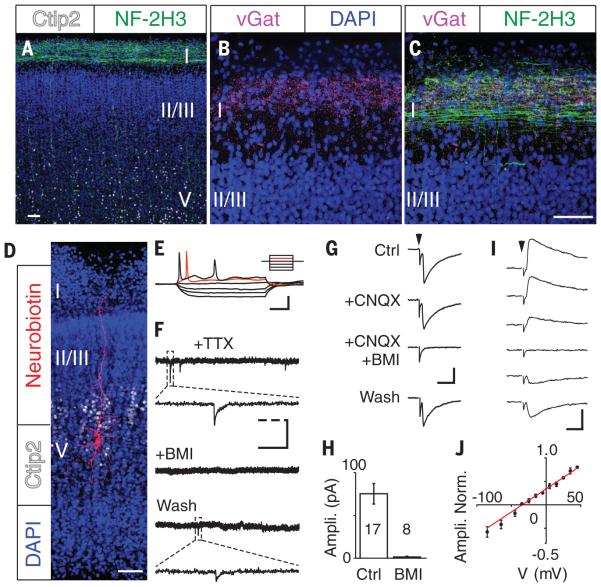
Morphologically, the human brain has abundant GABAergic synapses in cortical layer 1 as early as gestational week (GW) 12 (17, 18). In the second trimester, subplate neurons show spontaneous firing and synaptic activity (19, 20). Examining the expression and distribution of GABAergic axons in human cortex at GW 24, we found that both the axon marker neurofilament-2H3 and the GABAergic presynaptic marker vGat were expressed in cortical layer 1 (Fig. 2, A to C). In acute brain slices from GW 22, pyramidal neurons in deep cortical layers had high membrane resistance (1.1 ± 0.1 gigaohms, n = 15), low membrane capacitance (23.0 ± 2.9 pF, n = 15), and fired only one or two action potentials (Fig. 2, D and E) (19). Cortical neurons expressed GABAA receptors (fig. S2) and displayed typical GABAergic miniature synaptic currents (Fig. 2F). Thus, human cortical neurons express functional GABAA receptors and GABAergic synapses in the late second trimester.
Fig. 2. Synaptic responses in developing human cortical neurons from layer 1 GABAergic axons.
(A to C) Neurofilament-2H3 (NF-2H3) (A) and vGat (B) are coexpressed (C) in human fetal cortical layer 1. (D) Cortical neuron filled intracellularly with neurobiotin. Ctip2, cortical layer 5 marker; DAPI, nuclear marker. (E) Action potential firing of a layer 5 neuron under a stepped-current protocol ranging from −30 pA to 50 pA. (F) Miniature postsynaptic potentials of cortical neurons are sensitive to BMI. (G) Synaptic responses in layer 5 neurons evoked from cortical layer 1 were insensitive to CNQX but were reversibly blocked by BMI. (H) Summarized results showing average amplitude of evoked PSC responses in the presence or absence of BMI. (I and J) Sample traces (I) and summarized result (J) of evoked synaptic responses at holding potentials from −65 mV (bottom) to 35 mV (top), with 20 mV interval. Scale bars, 50 μm [(A) to (D)]; 25 mV, 200 ms (E); 40 pA, 2 s, 0.2 s (dashed line) (F); 100 pA, 10 ms [(G) and (I)].
We characterized the morphology and location of recorded cortical neurons by filling cells with neurobiotin and postimmunostaining with streptavidin-549 and the deep cortical layer marker Ctip2. Ctip2 marker expression (Fig. 2D and fig. S3) identified layer 5 pyramidal neurons with apical dendrites extending to cortical layer 1 (Fig. 2D). We recorded robust evoked synaptic responses only when stimulating layer 1 (Fig. 2G), but not deep cortical layers (−0.1 ± 0.6 pA, n = 10) or the subplate (−1.6 ± 0.5 pA, n = 10). Evoked responses were insensitive to the AMPA receptor antagonist CNQX but were blocked by BMI (Fig. 2, G and H). The evoked synaptic currents reversed at −34.7 mV, close to the Cl− equilibrium potential [−31.1 mV = −46.0 mV (by Nernst equation) + 14.9 mV (junction potential)], consistent with mediation through GABAA receptors (Fig. 2, I and J).
Neurons in human layer 1 cortex had GABAergic responses by GW 20 that were not evident at GW 16 or 18. By GW 22, the majority of deep-layer neurons demonstrated GABAergic synaptic responses. We did not detect evoked responses in GW 24 layer 2/3 neurons, which are still very immature (fig. S4). To evaluate the potential contribution of layer 1 local neurons to the GABAergic response of pyramidal neurons, we applied glutamate locally in layer 1. Although this induced robust firing of layer 1 neurons, it did not evoke synaptic responses in layer 4 and layer 5 pyramidal neurons (fig. S5). Thus, a GABAergic axon plexus arising from long-projection neurons is present in layer 1 in second-trimester human cortex.
GABA responses are depolarizing in the mouse brain during the first postnatal week (fig. S6) as a result of high intracellular chloride ion concentration [Cl−] (6, 7). To study GABA responses in GW 22 cortical neurons, we performed gramicidin-perforated patch recordings to avoid perturbing the intracellular [Cl−]. GABA responses reversed at −44.4 mV (fig. S7) and were depolarizing because the resting membrane potential of cortical neurons at this stage was −64.8 ± 1.6 mV (n = 14). We then monitored intracellular Ca2+ in response to GABA by loading neurons with a membrane-permeable Ca2+ indicator, Oregon Green BAPTA-1 AM. Local GABA application induced robust intracellular Ca2+ elevation in layer 5 cortical neurons (fig. S7). Thus, as in mouse (21–23), GABA is depolarizing in human fetal cortex when functional GABAergic synapses are first established.
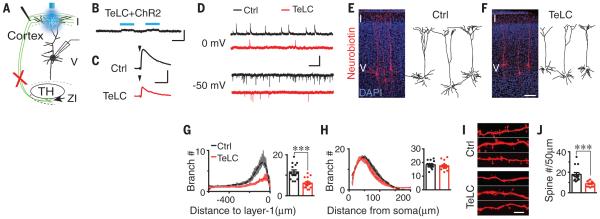
To explore the effect of the layer 1 GABAergic axon plexus on cortical development, we selectively blocked synaptic GABA release from mouse ZI neurons by selective expression of tetanus toxin light chain (TeLC), which blocks synaptic vesicle release (24). At P0–P1, we injected AAV virus containing Cre-dependent TeLC fused with 2A– green fluorescent protein under human elongation factor 1a (EF1α) promoter (AAV1-EF1α-DIO-TeLC-2A-GFP) stereotaxically into the ZI of SST::Cre;Ai14 mice. We detected GFP expression in the ZI within 1 week (fig. S8). To test the efficiency of TeLC blockade, we first co-injected AAV1-DIO-ChR2 with AAV1-DIO-TeLC-2A-GFP unilaterally into the ZI. Blue light stimulation of cortical layer 1 failed to induce synaptic currents in layer 5 pyramidal neurons from acute brain slices obtained one week post viral injection (0.12 ± 0.20 pA, n = 16; Fig. 3, A and B). To evaluate the extent to which layer 1 GABAergic synaptic release is impaired by TeLC expression in the ZI, we recorded GABAergic responses in layer 5 pyramidal neurons evoked by electrical stimulation of layer 1. We found that the amplitude of evoked GABAergic responses was reduced by 79.5% in neurons ipsilateral to the TeLC injection site (34.1 ± 1.8 pA, n = 18) relative to the contralateral hemisphere (166.0 ± 6.4 pA, n = 13, P < 0.001; Fig. 3C). Given that TeLC-2A-GFP labeled 70.7% of ZI SST+ neurons (n = 1465/2072; fig. S8), these data suggest that ZI projections provide a major GABAergic synaptic input to layer 1 in the first postnatal week.
Fig. 3. Reduced synaptic activity and apical dendritic complexity after blocking ZI GABAergic transmission in neonatal mice.
(A) Schematic showing layer 5 neurons recorded with light or electrical stimulation of layer 1. Red cross indicates blocked ZI activity. TH, thalamus. (B) Light stimulation of layer 1 fails to induce synaptic responses in P7 SST::Cre mice where TeLC and ChR2 were coexpressed in the ZI (TeLC+ChR2) at P0 (compare to Fig. 1K). (C) Reduced amplitude of layer 1 electrical stimulation–induced GABAergic responses (at 0 mV) in SST::Cre mice expressing TeLC in the ZI. (D) Reduced frequency of spontaneous GABAergic (at 0 mV) and glutamatergic (at −50 mV) responses in layer 5 neurons (P7–P9). (E and F) Morphology of P7 layer 5 cortical neurons in control-injected (E) or TeLC-injected (F) hemisphere. (G and H) Linear Sholl plots of apical (G) or basal (H) dendritic branches, showing best-fit polynomials (dashed lines) and SEM (shaded regions). Statistical results show maximum number of intersections. (I and J) Confocal microscopy images (I) and statistical results (J) show decreased spines in apical dendrites (***P < 0.001). Scale bars, 100 pA, 50 ms (B); 20 pA, 50 ms (C); 20 pA, 2s (D); 100 μm (F); 5 μm (I). In (G), (H), and (J), error bars denote SEM.
We next examined cortical neuron development with ZI GABAergic transmission blocked by unilateral injection of AAV1-DIO-TeLC-2A-GFP into P0–P1 SST::Cre mice. The frequency of spontaneous GABAergic and glutamatergic synaptic currents was reduced 1 week after injection (Fig. 3D and fig. S9), but the amplitudes were unchanged (fig. S9). The number of dendritic branches in the apical dendritic tuft was decreased in layer 5 neurons ipsilateral to the TeLC injection. Basal dendritic branches were unaffected (Fig. 3, E to H). We also observed reduced spine numbers in apical dendrites after TeLC treatment (Fig. 3, I and J). Thus, layer 1 GABAergic synaptic activity is crucial for normal development of synapses and dendrites in pyramidal neurons.
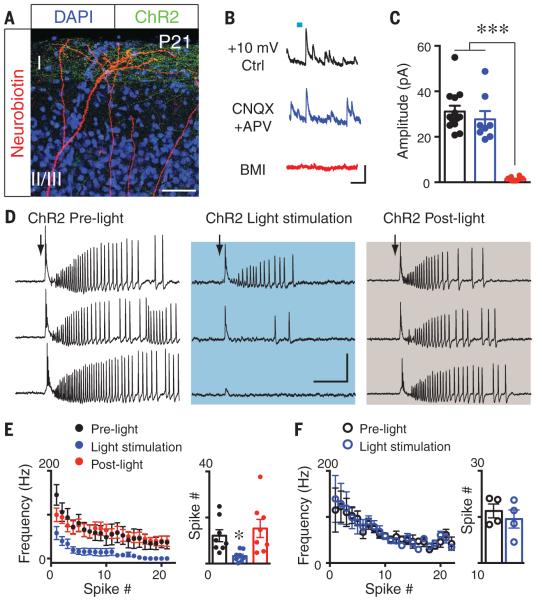
GABA responses become hyperpolarizing after the first postnatal week in mice (fig. S6), due to a lower intracellular [Cl−], and thus become inhibitory (7, 25–27). To explore whether a functional ZI GABAergic pathway persists in the mature mouse brain, we used selective labeling of ChR2 layer 1 ZI axons in P21 mice (Fig. 4A) and found that blue light stimulation induced robust GABAergic synaptic currents in pyramidal neurons (Fig. 4, B and C). To evaluate the role of ZI GABAergic projections in cortical circuit function, we used a slice model in which cortical excitation was enhanced by Mg2+-free artificial cerebrospinal fluid (28). We then used cell-attached recordings of cortical layer 4 neurons to preserve intracellular Cl− integrity. Electrical stimulation of adjacent regions within the same cortical layer induced epileptiform activity in recorded neurons (fig. S10). Coactivation of ZI axons by blue light stimulation of layer 1 ChR2-expressing axons reversibly suppressed the induced epileptiform activity (Fig. 4, D and E) but had no effect in slices from the contralateral hemisphere lacking ChR2 expression (Fig. 4F and fig. S10). Thus, ZI GABAergic projections are inhibitory in the mature mouse brain.
Fig. 4. ZI GABAergic connections remain functional in mature mouse brain and can suppress epileptiform activity.
(A) Layer 1 ChR2-EYFP axons overlap with distal dendrites of layer 5 pyramidal neurons in P21 SST::Cre mice. (B and C) Representative (B) and statistical (C) results show that layer 1 light stimulation– induced synaptic currents in layer 5 neurons (at +10 mV) are GABAergic (***P < 0.001). (D) Epileptiform activity is reversibly blocked by light stimulation of layer 1 ChR2-EYFP+ axons from ZI (50 ms, 10 Hz, 20 pulses, 30 s interval). (E and F) Both spike frequency and numbers were reversibly reduced upon light stimulation (*P < 0.05) (E), but not in controls lacking ChR2 expression (F). Scale bars, 50 μm (A); 50 pA, 100 ms (B); 40 mV, 0.2 s (D). In (C), (E), and (F), error bars denote SEM.
The circuit identified here is one of the earliest to appear in cortical development. While the importance of cortical interneurons to circuit function has been well documented (29, 30), our study shows that the ZI circuit originating in the diencephalon supports synaptogenesis, apical but not basal dendritic branching (Fig. 3, E to H), and spine development (Fig. 3, I and J) in cortical neurons. In the absence of ZI activity, we found decreased inhibitory postsynaptic current (IPSC) frequency in upper- and lower-layer neurons; we found decreased excitatory postsynaptic current (EPSC) frequency only in layer 5 neurons (Fig. 3, E to H, and fig. S11), possibly because they receive different presynaptic inputs that could target distinct dendritic domains (31). GABA signaling elevates [Ca2+] (32), which could be restricted to the apical dendritic tuft (33) and may modulate apical dendritic development. Neurotrophic factors such as reelin and semaphorin 3A modulate apical dendritic branching and spine density in an activity-dependent manner (34, 35); thus, it will be interesting to examine whether the developmental effects of ZI activity are mediated by neurotrophic factors (4, 36, 37). Relatively modest structural or functional changes in cortical neurons can have substantial behavioral impacts. The defects in dendritic arborization, spine density, and synaptic activity of cortical neurons observed upon blocking ZI activity resemble those implicated in a variety of neurodevelopmental diseases (9, 38–41); thus, the ZI pathway may play a role in disease etiology (42).
Supplementary Material
ACKNOWLEDGMENTS
We thank J. Huguenard, J. Paz, S. H. Wang, W. Walantus, and Y. Y. Wang for technical assistance; C. R. Yu for TeLC plasmid; G. P. Feng and Y. Zhou for TeLC-2A-GFP viral vectors; K. Deisseroth for ChR2 vectors; A. Alvarez-Buylla and C. Arnold for the stereotaxic injection rig; Kriegstein laboratory members for discussions; and W. P. Ge, S. Mayer, C. Gertz, and T. Nowakowski for critical reading of the manuscript. Supported by National Institute of Neurological Disorders and Stroke grant R37 NS35710 (A.R.K.) and a CARE & CURE Pediatric Epilepsy Fellowship from the Epilepsy Foundation of Greater Los Angeles (J.C.). The supplement contains additional data.
Footnotes
SUPPLEMENTARY MATERIALS
www.sciencemag.org/content/350/6260/554/suppl/DC1
Materials and Methods
Supplementary Text
Figs. S1 to S11
References (43–47)
REFERENCES AND NOTES
- 1.Torres F, Anderson C. J. Clin. Neurophysiol. 1985;2:89–103. doi: 10.1097/00004691-198504000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Khazipov R, Luhmann HJ. Trends Neurosci. 2006;29:414–418. doi: 10.1016/j.tins.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Katz LC, Shatz CJ. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 4.Spitzer NC. Nature. 2006;444:707–712. doi: 10.1038/nature05300. [DOI] [PubMed] [Google Scholar]
- 5.Zhang LI, Poo MM. Nat. Neurosci. 2001;4(suppl.):1207–1214. doi: 10.1038/nn753. [DOI] [PubMed] [Google Scholar]
- 6.Cherubini E, Gaiarsa JL, Ben-Ari Y. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- 7.Owens DF, Boyce LH, Davis MB, Kriegstein AR. J. Neurosci. 1996;16:6414–6423. doi: 10.1523/JNEUROSCI.16-20-06414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demarque M, et al. Neuron. 2002;36:1051–1061. doi: 10.1016/s0896-6273(02)01053-x. [DOI] [PubMed] [Google Scholar]
- 9.Saunders A, et al. Nature. 2015;521:85–89. doi: 10.1038/nature14179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caputi A, Melzer S, Michael M, Monyer H. Curr. Opin. Neurobiol. 2013;23:179–186. doi: 10.1016/j.conb.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 11.Melzer S, et al. Science. 2012;335:1506–1510. doi: 10.1126/science.1217139. [DOI] [PubMed] [Google Scholar]
- 12.Picardo MA, et al. Neuron. 2011;71:695–709. doi: 10.1016/j.neuron.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dammerman RS, Flint AC, Noctor S, Kriegstein AR. J. Neurophysiol. 2000;84:428–434. doi: 10.1152/jn.2000.84.1.428. [DOI] [PubMed] [Google Scholar]
- 14.Lin CS, Nicolelis MA, Schneider JS, Chapin JK. Science. 1990;248:1553–1556. doi: 10.1126/science.2360049. [DOI] [PubMed] [Google Scholar]
- 15.Kolmac C, Mitrofanis J. Anat. Embryol. 1999;199:265–280. doi: 10.1007/s004290050227. [DOI] [PubMed] [Google Scholar]
- 16.Taniguchi H, et al. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molliver ME, Kostović I, van der Loos H. Brain Res. 1973;50:403–407. doi: 10.1016/0006-8993(73)90741-5. [DOI] [PubMed] [Google Scholar]
- 18.Zecevic N. Cereb. Cortex. 1998;8:245–252. doi: 10.1093/cercor/8.3.245. [DOI] [PubMed] [Google Scholar]
- 19.Moore AR, et al. Cereb. Cortex. 2009;19:1795–1805. doi: 10.1093/cercor/bhn206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore AR, Zhou WL, Jakovcevski I, Zecevic N, Antic SD. J. Neurosci. 2011;31:2391–2398. doi: 10.1523/JNEUROSCI.3886-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tyzio R, et al. J. Neurosci. 1999;19:10372–10382. doi: 10.1523/JNEUROSCI.19-23-10372.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ben-Ari Y. Nat. Rev. Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 23.Owens DF, Kriegstein AR. Nat. Rev. Neurosci. 2002;3:715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- 24.Schiavo G, et al. Nature. 1992;359:832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- 25.Ganguly K, Schinder AF, Wong ST, Poo M. Cell. 2001;105:521–532. doi: 10.1016/s0092-8674(01)00341-5. [DOI] [PubMed] [Google Scholar]
- 26.Luhmann HJ, Prince DA. J. Neurophysiol. 1991;65:247–263. doi: 10.1152/jn.1991.65.2.247. [DOI] [PubMed] [Google Scholar]
- 27.Rivera C, et al. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- 28.Glykys J, et al. Neuron. 2009;63:657–672. doi: 10.1016/j.neuron.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Powell EM, et al. J. Neurosci. 2003;23:622–631. doi: 10.1523/JNEUROSCI.23-02-00622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cobos I, et al. Nat. Neurosci. 2005;8:1059–1068. doi: 10.1038/nn1499. [DOI] [PubMed] [Google Scholar]
- 31.Petreanu L, Mao T, Sternson SM, Svoboda K. Nature. 2009;457:1142–1145. doi: 10.1038/nature07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang DD, Kriegstein AR. J. Neurosci. 2008;28:5547–5558. doi: 10.1523/JNEUROSCI.5599-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schiller J, Schiller Y, Stuart G, Sakmann B. J. Physiol. 1997;505:605–616. doi: 10.1111/j.1469-7793.1997.605ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chai X, et al. Cereb. Cortex. 2014 10.1093/cercor/bhu216. [Google Scholar]
- 35.Polleux F, Morrow T, Ghosh A. Nature. 2000;404:567–573. doi: 10.1038/35007001. [DOI] [PubMed] [Google Scholar]
- 36.Poo MM. Nat. Rev. Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 37.Wong RO, Ghosh A. Nat. Rev. Neurosci. 2002;3:803–812. doi: 10.1038/nrn941. [DOI] [PubMed] [Google Scholar]
- 38.Cossart R, et al. Nat. Neurosci. 2001;4:52–62. doi: 10.1038/82900. [DOI] [PubMed] [Google Scholar]
- 39.Garey LJ, et al. J. Neurol. Neurosurg. Psychiatry. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM. Nat. Neurosci. 2011;14:285–293. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rubenstein JL, Merzenich MM. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitrofanis J. Neuroscience. 2005;130:1–15. doi: 10.1016/j.neuroscience.2004.08.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.