Abstract
Background
Intravenous patient-controlled analgesia (IVPCA) is a common method of relieving pain which is a risk factor of postoperative delirium (POD). However, research concerning POD in IVPCA patients is limited.
Objective
We aimed to determine the incidence, risk factors, and phenomenological characteristics of POD in patients receiving IVPCA.
Methods
A prospective, cohort study was conducted in post-general anesthesia IVPCA patients aged ≥60 years. POD was measured by the Nursing Delirium Screening Scale (NuDESC; 0–10). Delirium, pain severity at rest and/or on movement, and side effects of IVPCA during 3 postoperative days were examined twice-daily by the acute pain service team. Pain severity is measured by an 11-point verbal numerical rating scale (11-point VNRS) (0–10). An 11-point VNRS >3 was considered inadequate pain relief. If POD (detected by NuDESC ≥1) is suspected, consulting a neurologist or a psychiatrist to confirm suspected POD is required.
Results
In total, 1,608 patients were included. The incidence rate of POD was 2.2%. Age ≥70 years and American Society of Anesthesiologists physical status >III were the risk factors of POD in IVPCA patients. Approximately three-quarters of all POD cases occurred within the first 2 postoperative days. For pain at rest, patients with inadequate pain relief had significantly greater rates of POD than patients with adequate pain relief (day 1, 8.4% vs 1.5%, P<0.001; day 2, 9.6% vs 2.0%, P=0.028; day 3, 4.1% vs 2.1%, P=0.412). However, the incidence of POD was not associated with movement-evoked pain relief. Most (79.9%) POD cases in IVPCA patients showed either one or two symptoms. The symptoms of POD were ranked from high to low as disorientation (65.7%), illusions/hallucinations (37.1%), inappropriate communication (31.4%), inappropriate behavior (25.7%), and psychomotor retardation (14.2%).
Conclusion
The incidence rate of POD in IVPCA patients was low. Further research is warranted concerning POD and IVPCA pain management.
Keywords: postoperative delirium, postoperative analgesia, patient-controlled analgesia
Introduction
Delirium is an acute and fluctuating brain dysfunction characterized by conscious disturbance and cognitive impairment. Delirium can be recognized as hyperactive, hypoactive, or a mixed type. Postoperative delirium (POD) occurs after surgery and is associated with prolonged hospitalization, greater health care costs, and higher mortalities.1 Risk factors of POD include old age, the American Society of Anesthesiologists (ASA) physical status of III or more, severe illness, types of surgery and anesthesia, postoperative hypoxemia, and severe acute pain.2–5 Interestingly, effective pain management reduces the incidence and severity of POD.6–8 Intravenous patient-controlled analgesia (IVPCA) is a common method of relieving postoperative pain. However, research concerning POD in patients receiving IVPCA is limited.7–9 In particular, phenomenological characteristics of POD in IVPCA patients have not been reported.
Delirium is a clinical diagnosis. Based on the criteria from the Diagnostic and Statistical Manual of Mental Disorders, the confusion assessment method (CAM) that enables nonpsychiatric clinicians to detect delirium was developed and validated in 1990.10 Although it is considered the gold standard assessment tool for delirium,10 accurate clinical information and adequate trained researchers are needed for accurate CAM ratings. Delirium in hospitalized older adults is significantly under-recognized by nurses compared to physician/researchers while using the CAM.11,12 The prevalence of POD grows with an aging population. In a busy hospital environment, a more convenient tool is needed. In 2005, the Nursing Delirium Screening Scale (NuDESC) was developed by Gaudreau et al, which is a five-item observational screening tool13 that nurses can complete easily within 1 minute, thus making it suitable for use in busy wards. More importantly, the NuDESC has high sensitivity and high specificity for examining patients in the surgical wards and post-anesthetic recovery room.14,15
In this study, the NuDESC is used to identify the risk factors, incidence, and phenomenological characteristics of POD in elective noncardiac surgical patients receiving IVPCA. Moreover, the associations between rest/movement-evoked pain and the risk of POD in IVPCA patients were examined.
Materials and methods
Patient population
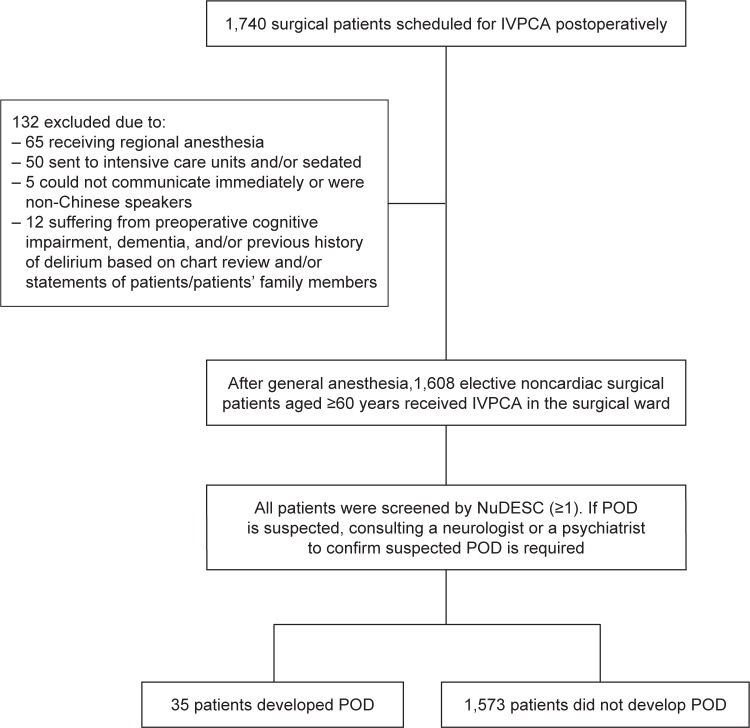
Data for the present study were extracted from the databank of Chi Mei Medical Center by a computerized system. A total of 1,740 surgical patients receiving intravenous morphine via a PCA device for at least 3 continuous postoperative days were assessed from January 2012 to December 2013. Finally, only 1,608 patients aged ≥60 years who received elective noncardiac surgery and general anesthesia were analyzed. Exclusion criteria included intensive care unit patients, those who were sedated, those who could not communicate immediately after surgery, and non-Chinese speakers. Based on chart review and/or statements of patients’ family members, patients suffering from preoperative cognitive impairment and/or dementia were excluded. Preoperative dementia/cognitive impairment was diagnosed if the patient had a previous mini mental state examination with a score <24 or clinical diagnosis of dementia by a geriatrician/physician during the preoperative evaluation.16 Also, based on chart review and/or statements of patients/patients’ family members, patients suffering from previous history of delirium or preoperative delirium were excluded because these are relevant confounders.17 Preoperative delirium was diagnosed if the patient had a previous examination with NuDESC ≥215 or clinical diagnosis of delirium by a physician during the preoperative evaluation. A data collection flowchart is shown in Figure 1.
Figure 1.
Data collection flowchart of POD in surgical patients scheduled for postoperative IVPCA.
Abbreviations: IVPCA, intravenous patient-controlled analgesia; NuDESC, Nursing Delirium Screening Scale; POD, postoperative delirium.
Acute pain service team
The acute pain service team including attending anesthesiologists, residents, pain nurses, and pharmacists provide pain management in Chi Mei Medical Center. PCA machines are routinely set up using the bolus dose without continuous doses. The acute pain service team members regularly visit surgical patients receiving IVPCA at least twice daily. The initial visit is provided by an attending anesthesiologist and a pain nurse, whereas the follow-up visits are usually performed at least twice daily by pain nurses. The first day visit is performed around 4 pm (from 3 to 5 pm) and 9 pm (from 8 to 10 pm). The interval between the first assessment and the surgery is ~4–6 hours. The following day visit is performed around 9 am (from 8 to 10 am) and 4 pm (from 3 to 5 pm). The regular interval in the next 2 days is ~7 hours. Effectiveness of analgesia, delirium assessment, and side effects are assessed in every visit for each patient. If delirium, inadequate pain control, or any problem related to IVPCA is suspected, an anesthesiologist responds immediately and performs an extra visit 2–4 hours later.
Delirium assessment
The NuDESC test assesses disorientation, inappropriate behavior, inappropriate communication, illusions/hallucinations, and psychomotor retardation.13 Each item of the NuDESC is rated as 0, 1, or 2 based on the severity of symptoms (0: absence, 1: minor, and 2: severe). After being trained by a psychiatric expert, the acute pain service team in Chi Mei Medical Center has performed the NuDESC test for screening POD routinely since April 2011. In every visit, the trained anesthesiologists and pain nurses regularly examine each surgical patient receiving IVPCA by using the NuDESC (threshold ≥1), which has high sensitivity, but less specificity.18 If POD is suspected, consulting a neurologist or a psychiatrist to confirm suspected POD is required.
Pain and daily assessment
During the first postoperative days, the intensity of rest/movement-evoked pain, and side effects of IVPCA including nausea, vomiting, and itching are also examined and recorded by acute pain service team members during each visit. Pain severity is measured by an 11-point verbal numerical rating scale (11-point VNRS) (0: no pain; 10: the worst imaginable pain). Patients are categorized as having adequate or inadequate pain relief. An 11-point VNRS score of ≤3 is considered adequate while a score >3 is considered inadequate.
Basic recorded patient characteristics include age, gender, body height/weight, ASA physical status scale, type of anesthesia, preoperative cognitive impairments, dementia, delirium, and comorbidities. Data are prospectively collected and stored electronically.
Ethical approval
This prospective cohort study was approved by the Institutional Review Board (IRB) of Chi Mei Medical Center, Tainan, Taiwan (IRB number: 10009-012) and registered in a publicly accessible database (University Hospital Medical Information Network (UMIN), Japan; http://www.umin.ac.jp/ctr/index.htm; Trial ID: UMIN:lin-yt). The research involved the routine procedures for daily services and presented no risk to the subjects, therefore, the IRB of Chi Mei Medical Center approved the use of an oral consent procedure rather than a written consent form for the use of the medical information of all patients.
Statistical analysis
Potential predictors in IVPCA patients without POD versus with POD were analyzed by univariate and multivariate logistic analysis. Risk of POD was presented as odds ratio (OR) and 95% confidence intervals (CI). All selected predictors and the association of POD development were examined by univariate logistic regression analysis. An entry criterion for multivariate selection was set as P<0.2. Multivariate logistic regression with backward eliminating procedure was applied to determine the final model, and an exit criterion was set as P>0.05. All possible remaining two-way interactions were examined in addition to interactions of any given variable with POD. A stratified regression model was conducted when a significant interaction was found. Adequate pain relief compared to inadequate pain relief in delirious patients and non-delirious patients were measured by chi-square test or Fisher’s exact test. Data were analyzed using STATA 12.0 (Stata Corp, College station, TX, USA) statistical software. A P-value of <0.05 was considered significant.
Results
In this study, 1,608 IVPCA patients who were aged ≥60 years and received general anesthesia were included. Of 1,608 patients, 362 patients were graded as ASA physical status I or II (22.5%), 1,088 patients as ASA III (67.7%), and 158 patients as ASA IV (9.8%). A total of 35 patients were diagnosed with POD using NuDESC with a score of ≥1. The incidence of POD was 2.2% in IVPCA patients.
Risk factors of POD in IVPCA patients
The characteristics of patients are shown in Table 1. Univariate logistic analysis of potential predictors for POD in IVPCA patients identified two predictors – patients aged ≥70 years (crude OR =2.38, 95% CI =1.14–4.99, P=0.018) and ASA physical status >III (crude OR =2.81, 95% CI =1.25–6.30, P<0.001). Gender, comorbidities (hypertension/diabetes mellitus), types of surgery, duration of surgery/anesthesia, total morphine dose/day, and postoperative nausea/vomiting were not associated with the risk of POD (all P>0.05).
Table 1.
Univariate logistic analysis of potential predictors for POD in IVPCA patients
| Predictors | No delirium, n (%) N=1,573 (97.9) |
Delirium, n (%) N=35 (2.1) |
Crude odds ratio (95% CI) |
P-value |
|---|---|---|---|---|
| Age groups | ||||
| 60–69 years | 767 (98.7) | 10 (1.3) | 1.0 | |
| ≥70 years | 806 (97.0) | 25 (3.0) | 2.38 (1.14–4.99) | 0.018 |
| Gender | ||||
| Female | 866 (98.1) | 17 (1.9) | 1.0 | |
| Male | 707 (97.6) | 18 (2.4) | 1.30 (0.66–2.54) | 0.554 |
| ASA physical status | ||||
| I, II, III | 1,423 (99.2) | 27 (1.8) | 1.0 | |
| IV | 150 (95.0) | 8 (5.0) | 2.81 (1.25–6.30) | <0.001 |
| Hypertension | ||||
| No | 693 (99.0) | 15 (2.1) | 1.0 | |
| Yes | 880 (97.8) | 20 (2.2) | 1.05 (0.53–2.07) | 0.887 |
| Diabetes mellitus | ||||
| No | 792 (98.3) | 14 (1.7) | 1.0 | |
| Yes | 781 (97.4) | 21 (2.6) | 1.52 (0.77–3.01) | 0.225 |
| Types of surgery | ||||
| Others (thoracotomy, lower abdomen, breast, head, and neck surgery) | 170 (99.4) | 1 (0.6) | 1.0 | |
| Nephrectomy | 70 (97.2) | 2 (2.8) | 4.86 (0.43–54.43) | 0.210 |
| Upper abdomen | 568 (97.3) | 16 (2.7) | 4.79 (0.63–36.37) | 0.139 |
| Orthopedic surgery | 765 (98.0) | 16 (2.0) | 3.56 (0.47–27.00) | 0.335 |
| Duration of surgery | ||||
| <195 min | 1,006 (98.3) | 18 (1.7) | 1.0 | |
| ≥195 min | 567 (97.1) | 17 (2.9) | 1.67 (0.86–3.28) | 0.127 |
| Duration of anesthesia | ||||
| <229 min | 906 (97.7) | 21 (2.3) | 1.0 | |
| ≥229 min | 667 (98.0) | 14 (2.0) | 0.91 (0.46–1.79) | 0.775 |
| Total morphine dose/day | ||||
| ≤20 mg/day | 1,196 (97.71) | 28 (2.29) | 1.0 | |
| >20 mg/day | 377 (98.18) | 7 (1.82) | 0.79 (0.34–1.83) | 0.731 |
| Postoperative nausea/vomiting | ||||
| No | 1,322 (97.6) | 32 (2.4) | 1.0 | |
| Yes | 251 (98.8) | 3 (1.2) | 0.49 (0.15–1.62) | 0.236 |
Notes: Duration of surgery/anesthesia is divided by the mean (195 in surgery, 229 in anesthesia). P<0.05, statistical significance.
Abbreviations: IVPCA, intravenous patient-controlled analgesia; POD, postoperative delirium; CI, confidence interval; ASA, American Society of Anesthesiologists.
As shown in Table 2, multivariate logistic analysis revealed that age ≥70 years (adjusted OR =2.18, 95% CI =1.13–4.69, P=0.023) and the ASA physical status >III (adjusted OR =2.46, 95% CI =1.09–5.56, P=0.029) were the risk factors of POD in IVPCA patients aged ≥60 years. No significant two-way interaction was found among the final prognostic variables (data not shown). Other variables that were examined did not affect the development of POD. Taken together, multivariate regression analyses after adjusting for potential confounders revealed that being aged ≥70 years as well as the ASA physical status >III are independent predictors for POD.
Table 2.
Multivariate logistic analysis of potential predictors for POD in IVPCA patients
| Predictors | Adjusted odds ratio (95% CI) | P-value |
|---|---|---|
| Age groups | 0.023 | |
| 60–69 years | 1.0 | |
| ≥70 years | 2.18 (1.13–4.69) | |
| ASA physical status | 0.029 | |
| I, II, III | 1.0 | |
| IV | 2.46 (1.09–5.56) |
Abbreviations: IVPCA, intravenous patient-controlled analgesia; POD, postoperative delirium; CI, confidence interval; ASA, American Society of Anesthesiologists.
Phenomenological characteristics of POD assessed by NuDESC
A total of 28 patients (80%) experienced POD within the first 2 postoperative days (Table 3). Table 4 shows that 71.4% of these POD patients had a NuDESC score ≤2. Only 11.4% of POD cases had a score ≥5. Based on the rates, the symptoms of POD were ranked from high to low as disorientation (23 patients, 65.7%), illusions/hallucinations (13 patients, 37.1%), inappropriate communication (11 patients, 31.4%), inappropriate behavior (9 patients, 25.7%), and psychomotor retardation (5 patients, 14.2%). In total, 19 patients (54.2%) showed one symptom and nine patients (25.7%) had two symptoms. Only one patient (2.8%) displayed all five symptoms.
Table 3.
Occurrence of postoperative delirium in IVPCA patients
| Postoperative days | Incidence of postoperative delirium | Prevalence of postoperative delirium |
|---|---|---|
| Day 1 | 17 (1.1%) | 17 (1.1%) |
| Day 2 | 10 (0.6%) | 11 (0.6%) |
| Day 3 | 8 (0.5%) | 9 (0.5%) |
Abbreviation: IVPCA, intravenous patient-controlled analgesia.
Table 4.
Phenomenological characteristics of delirium detected by NuDESC in IVPCA patients
| Symptoms | Patients, n (%) |
|---|---|
| NuDESC scores (1–10) | |
| 1 | 16 (45.7) |
| 2 | 9 (25.7) |
| 3–4 | 6 (17.2) |
| ≥5 | 4 (11.4) |
| Features | |
| Disorientation | 23 (65.7) |
| Inappropriate behavior | 9 (25.7) |
| Inappropriate communication | 11 (31.4) |
| Illusions/hallucinations | 13 (37.1) |
| Psychomotor retardation | 5 (14.2) |
| Number of symptoms | |
| 1 | 19 (54.2) |
| 2 | 9 (25.7) |
| 3 | 5 (14.2) |
| 4 | 1 (2.8) |
| 5 | 1 (2.8) |
| Mean (SD) | 1.7 (1.0) |
Abbreviations: NuDESC, Nursing Delirium Screening Scale; IVPCA, intravenous patient-controlled analgesia; SD, standard deviation.
Associations between rest/movement-evoked pain and POD
As shown in Table 5, patients with inadequate rest pain relief (VNRS >3) within the first 3 postoperative days had greater incidence rates of POD than patients with adequate rest pain relief. However, statistical differences were found only on day 1 and 2 (day 1, 8.4% vs 1.5%, P<0.001; day 2, 9.6% vs 2.0%, P=0.028; day 3, 4.1% vs 2.1%, P=0.412). Interestingly, there were no significant differences in the incidence of POD between patients with inadequate and adequate movement-evoked pain relief (day 1, P=0.147; day 2, P=0.726; day 3, P=0.490).
Table 5.
Adequate pain relief compared to inadequate pain relief in delirious patients versus nondelirious patients
| Postoperative days | VNRS score | Pain at rest
|
P-value | Movement-evoked pain
|
P-value | ||
|---|---|---|---|---|---|---|---|
| Delirium, n (%) | No delirium, n (%) | Delirium, n (%) | No delirium, n (%) | ||||
| Day 1 | ≤3 | 23 (65.7) | 1,443 (91.7) | <0.001 | 13 (37.1) | 779 (49.5) | 0.147 |
| >3 | 12 (34.3) | 130 (8.3) | 22 (63.9) | 794 (50.5) | |||
| Day 2 | ≤3 | 32 (91.4) | 1,545 (98.2) | 0.028 | 25 (71.4) | 1,080 (68.7) | 0.726 |
| >3 | 3 (8.6) | 28 (1.8) | 10 (28.6) | 493 (31.3) | |||
| Day 3 | ≤3 | 34 (97.1) | 1,550 (98.5) | 0.412 | 31 (88.6) | 1,326 (84.3) | 0.490 |
| >3 | 1 (2.9) | 23 (1.5) | 4 (11.4) | 247 (15.7) | |||
Notes: VNRS is an 11-point verbal numeric rating pain scale with a score range of 1–10. An 11-point VNRS score of ≤3 is considered adequate pain relief. Categorical variables were measured by chi-square test or Fisher’s exact test. P<0.05, statistical significance.
Abbreviation: VNRS, verbal numerical rating scale.
Discussion
This study is the first prospective study using NuDESC to identify the risk factors and incidence of POD in elective noncardiac surgical patients receiving IVPCA. Risk factors are generally divided into predisposing and precipitating factors. Predisposing factors for POD include advanced age, preexisting dementia, ASA physical status, and functional disabilities. They can be assessed before surgery and identified easily.8,19 In this study, two identified risk factors including age and the ASA physical status are consistent with the previous findings.2,14 Both are predisposing factors that are not modifiable in general.4 Precipitating factors for POD, such as types and duration of surgery, hypoxemia, and postoperative pain, occur intraoperatively and postoperatively.4 Among them, pain is modifiable. In this study, POD incidence in elderly Chinese IVPCA patients was 2.2% which was lower than 5.1%~13% – the overall incidence of POD in elderly Chinese patients.5,20 These results support the previous findings that better pain control lowers the incidence of POD.7,8 However, previous reviews reported that the incidence of POD in general surgical patients ranges from 10% to 46%.21,22 A retrospective study regarding POD showed that the incidence of POD in IVPCA patients undergoing orthopedic surgery in Korea was 8%, which was a result based on chart reviews.9 Possible explanations for the result of the retrospective study are provided. First, delirium is a clinical syndrome. The method of assessing delirium is a major factor which results in the variation of the reported incidence of delirium.4 Second, different types of surgery which introduce considerable heterogeneity may have different inherent risks of delirium.4 Third, race has been found to be statistically related to the development of delirium.23 Accordingly, different methods and more research on populations may yield different results.
The NuDESC test has been validated for screening delirium in the surgical wards.13–15 In this study, POD patients had relatively low scores measured by the NuDESC. Approximately 80% of POD cases showed either one or two symptoms. Only one patient showed all the five symptoms. This may be due to the effective pain treatment that decreases the risk and severity of POD.6–8 The most common symptom was disorientation, followed by illusions/hallucinations. The least common symptom was psychomotor retardation. In the delirious cancer patients assessed by the memorial delirium assessment scale, the most frequent symptoms are impaired digit span and psychomotor abnormality, followed by disorientation and inattention. Perceptual disturbance (eg, hallucinations) and delusion have relatively lower percentages.24 However, disorientation was the least frequent cognitive deficit in delirious adults assessed by the delirium rating scale-revised-98.25 No consistent phenomenology was found between the results of this study and previous reports. Despite major advancements in delirium research, medical articles concerning the phenomenology of POD are limited. More phenomenological research of POD is needed to find association between symptoms of POD and the etiology, pathophysiology, and treatment.26,27
In the present study, the intensity of patients’ pain was measured with the 11-point VNRS. The 11-point VNRS has been shown to be effective and validated for the assessment of acute pain in older patients and those with a mild/moderate cognitive impairment.28 A concept concerning acute pain at rest and on movement is that adequate relief of rest pain after surgery is important for making the patient comfortable in bed. However, adequate relief of movement-evoked pain after surgery is critical for reducing risks of postoperative cardiopulmonary and thromboembolic complications.28 During the first 2 postoperative days, IVPCA patients with inadequate rest pain relief had a significant increased risk of POD. However, movement-evoked pain was not significantly associated with POD incidence in IVPCA patients. The results are similar to previous findings showing the risk of POD associated with higher rest pain levels but not with movement-evoked pain.7 Taken together, better control of postoperative rest pain by IVPCA is effective in reducing the incidence of POD. As for postoperative opioid consumption, this study revealed no significant association between the POD incidence and the total morphine dose per day. The finding is consistent with that of Sieber et al.29 However, a previous study discovered that patients receiving less parenteral morphine per day were more likely to develop POD than those receiving more analgesics. Inadequately treated pain was considered as a risk factor for POD in older adults.30 By contrast, exposure to high-dose opiates has been found to be a minor precipitating risk factor of POD.31,32 More studies are needed to clarify the associations among uncontrolled pain, opiates doses, and POD. In this study, there was no significant association between the POD incidence and surgical duration as reported previously.33 However, another research study reports that longer surgical duration increases the risk of POD.34 There is no consensus on the effect of longer surgical duration with delirium risk. Similarly, surgical duration is another controversial factor that needs more studies to elucidate its associations with the risk of POD.
This study had several limitations. First, delirium assessment was measured only twice-daily by the acute pain service team. POD incidence may be lower than the true incidence because of the fluctuating nature of POD. Second, the patients were monitored for only 3 postoperative days because most POD occurs during this period. However, delirium may occur after day 3 that may not be detected.2 Third, only Chinese patients were included in this study. Race could possibly play a role in the results as the rate (2.2%) of POD in this study was much lower than that in other studies.9,21,22 Fourth, this research was conducted in a single hospital. More information from multiple centers is required to validate the findings.
Conclusion
The incidence of POD in IVPCA patients was low. The risk of POD was significantly associated with inadequate rest pain relief but not with inadequate movement-evoked pain relief. Most POD cases in IVPCA patients showed either one or two symptoms. Disorientation was the most common, whereas psychomotor retardation was the least common. Further research is warranted concerning POD and IVPCA pain management.
Acknowledgments
The authors would like to thank Dr Yu-Yu Li and Dr Chia-Hung Yu for the collection of data and the nursing staff of the Department of Anesthesiology for the evaluation of patient delirium. The present study was supported by CMHCR10330 provided by Chi Mei Medical Center, Tainan, Taiwan.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Leslie DL, Zhang Y, Bogardus ST, Holford TR, Leo-Summers LS, Inouye SK. Consequences of preventing delirium in hospitalized older adults on nursing home costs. J Am Geriatr Soc. 2005;53(3):405–409. doi: 10.1111/j.1532-5415.2005.53156.x. [DOI] [PubMed] [Google Scholar]
- 2.Marcantonio ER, Goldman L, Mangione CM, et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA. 1994;271(2):134–139. [PubMed] [Google Scholar]
- 3.Zakriya KJ, Christmas C, Wenz JF, Sr, Franckowiak S, Anderson R, Sieber FE. Preoperative factors associated with postoperative change in confusion assessment method score in hip fracture patients. Anesth Analg. 2002;94(6):1628–1632. doi: 10.1097/00000539-200206000-00050. table of contents. [DOI] [PubMed] [Google Scholar]
- 4.Rudolph JL, Marcantonio ER. Review articles: postoperative delirium: acute change with long-term implications. Anesth Analg. 2011;112(5):1202–1211. doi: 10.1213/ANE.0b013e3182147f6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nie H, Zhao B, Zhang YQ, Jiang YH, Yang YX. Pain and cognitive dysfunction are the risk factors of delirium in elderly hip fracture Chinese patients. Arch Gerontol Geriatr. 2012;54(2):e172–e174. doi: 10.1016/j.archger.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Guenther U, Riedel L, Radtke FM. Patients prone for postoperative delirium: preoperative assessment, perioperative prophylaxis, postoperative treatment. Curr Opin Anaesthesiol. 2016;29(3):384–390. doi: 10.1097/ACO.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 7.Lynch EP, Lazor MA, Gellis JE, Orav J, Goldman L, Marcantonio ER. The impact of postoperative pain on the development of postoperative delirium. Anesth Analg. 1998;86(4):781–785. doi: 10.1097/00000539-199804000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Vaurio LE, Sands LP, Wang Y, Mullen EA, Leung JM. Postoperative delirium: the importance of pain and pain management. Anesth Analg. 2006;102(4):1267–1273. doi: 10.1213/01.ane.0000199156.59226.af. [DOI] [PubMed] [Google Scholar]
- 9.Heo DY, Hwang BM. Intravenous patient-controlled analgesia has a positive effect on the prognosis of delirium in patients undergoing orthopedic surgery. Korean J Pain. 2014;27(3):271–277. doi: 10.3344/kjp.2014.27.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Int Med. 1990;113(12):941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 11.Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The confusion assessment method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823–830. doi: 10.1111/j.1532-5415.2008.01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rice KL, Bennett M, Gomez M, Theall KP, Knight M, Foreman MD. Nurses’ recognition of delirium in the hospitalized older adult. Clin Nurse Spec. 2011;25(6):299–311. doi: 10.1097/NUR.0b013e318234897b. [DOI] [PubMed] [Google Scholar]
- 13.Gaudreau JD, Gagnon P, Harel F, Tremblay A, Roy MA. Fast, systematic, and continuous delirium assessment in hospitalized patients: the nursing delirium screening scale. J Pain Symptom Manag. 2005;29(4):368–375. doi: 10.1016/j.jpainsymman.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Radtke FM, Franck M, Schneider M, et al. Comparison of three scores to screen for delirium in the recovery room. Br J Anaesth. 2008;101(3):338–343. doi: 10.1093/bja/aen193. [DOI] [PubMed] [Google Scholar]
- 15.Radtke FM, Franck M, Schust S, et al. A comparison of three scores to screen for delirium on the surgical ward. World J Surg. 2010;34(3):487–494. doi: 10.1007/s00268-009-0376-9. [DOI] [PubMed] [Google Scholar]
- 16.Lee HB, Mears SC, Rosenberg PB, Leoutsakos JM, Gottschalk A, Sieber FE. Predisposing factors for postoperative delirium after hip fracture repair in individuals with and without dementia. J Am Geriatr Soc. 2011;59(12):2306–2313. doi: 10.1111/j.1532-5415.2011.03725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fong TG, Davis D, Growdon ME, Albuquerque A, Inouye SK. The interface between delirium and dementia in elderly adults. Lancet Neurol. 2015;14(8):823–832. doi: 10.1016/S1474-4422(15)00101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neufeld KJ, Leoutsakos JS, Sieber FE, et al. Evaluation of two delirium screening tools for detecting post-operative delirium in the elderly. Br J Anaesth. 2013;111(4):612–618. doi: 10.1093/bja/aet167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inouye SK. Delirium in older persons. N Engl J Med. 2006;354:1157–1165. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- 20.Dai YT, Lou MF, Yip PK, Huang GS. Risk factors and incidence of postoperative delirium in elderly Chinese patients. Gerontology. 2000;46(1):28–35. doi: 10.1159/000022130. [DOI] [PubMed] [Google Scholar]
- 21.Deiner S, Silverstein JH. Postoperative delirium and cognitive dysfunction. Br J Anaesth. 2009;103(Suppl 1):i41–i46. doi: 10.1093/bja/aep291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mittal V, Muralee S, Williamson D, et al. Review: delirium in the elderly: a comprehensive review. Am J Alzheimers Dis Other Demen. 2011;26(2):97–109. doi: 10.1177/1533317510397331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livingston RL, Zucker DK, Isenberg K, Wetzel RD. Tricyclic antidepressants and delirium. J Clin Psychiatry. 1983;44(5):173–176. [PubMed] [Google Scholar]
- 24.Boettger S, Boettger S, Breitbart W. The phenomenology of delirium: presence, severity, and relationship between symptoms. J Geriatr. 2014;2014:1–6. [Google Scholar]
- 25.Meagher DJ, Moran M, Raju B, et al. Phenomenology of delirium. Assessment of 100 adult cases using standardised measures. Br J Psychiatry. 2007;190:135–141. doi: 10.1192/bjp.bp.106.023911. [DOI] [PubMed] [Google Scholar]
- 26.Stagno D, Gibson C, Breitbart W. The delirium subtypes: a review of prevalence, phenomenology, pathophysiology, and treatment response. Palliat Support Care. 2004;2(2):171–179. doi: 10.1017/s1478951504040234. [DOI] [PubMed] [Google Scholar]
- 27.Gupta N, de Jonghe J, Schieveld J, Leonard M, Meagher D. Delirium phenomenology: what can we learn from the symptoms of delirium? J Psychosom Res. 2008;65(3):215–222. doi: 10.1016/j.jpsychores.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 28.Breivik H, Borchgrevink PC, Allen SM, et al. Assessment of pain. Br J Anaesth. 2008;101(1):17–24. doi: 10.1093/bja/aen103. [DOI] [PubMed] [Google Scholar]
- 29.Sieber FE, Mears S, Lee H, Gottschalk A. Postoperative opioid consumption and its relationship to cognitive function in older adults with hip fracture. J Am Geriatr Soc. 2011;59(12):2256–2262. doi: 10.1111/j.1532-5415.2011.03729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrison RS, Magaziner J, Gilbert M, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. 2003;58(1):76–81. doi: 10.1093/gerona/58.1.m76. [DOI] [PubMed] [Google Scholar]
- 31.Marcantonio ER. Postoperative delirium a 76-year-old woman with delirium following surgery. JAMA. 2012;308(1):73–81. doi: 10.1001/jama.2012.6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marino J, Russo J, Kenny M, Herenstein R, Livote E, Chelly JE. Continuous lumbar plexus block for postoperative pain control after total hip arthroplasty. A randomized controlled trial. J Bone Joint Surg Am. 2009;91(1):29–37. doi: 10.2106/JBJS.H.00079. [DOI] [PubMed] [Google Scholar]
- 33.Benoit AG, Campbell BI, Tanner JR, et al. Risk factors and prevalence of perioperative cognitive dysfunction in abdominal aneurysm patients. J Vasc Surg. 2005;42(5):884–890. doi: 10.1016/j.jvs.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 34.Yildizeli B, Ozyurtkan MO, Batirel HF, Kuscu K, Bekiroglu N, Yuksel M. Factors associated with postoperative delirium after thoracic surgery. Ann Thorac Surg. 2005;79(3):1004–1009. doi: 10.1016/j.athoracsur.2004.06.022. [DOI] [PubMed] [Google Scholar]