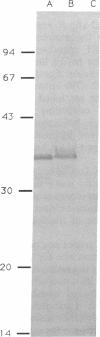
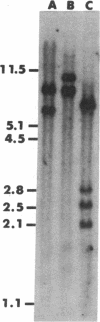
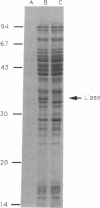
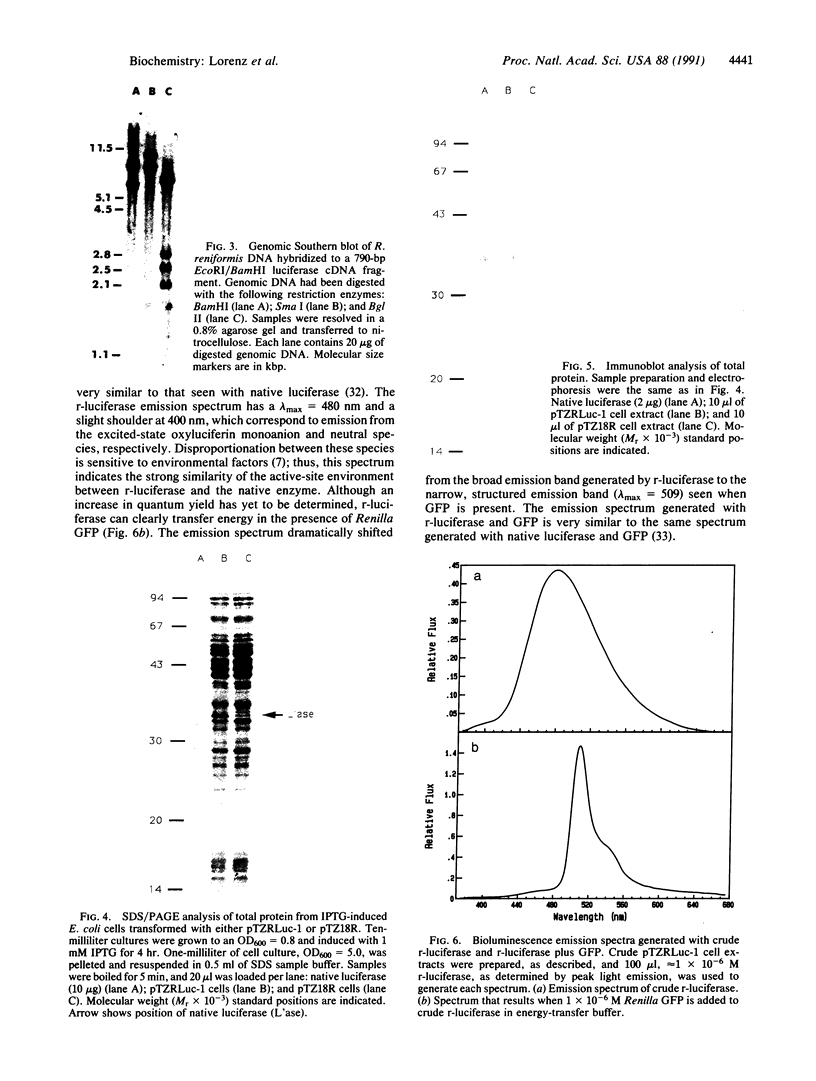
Abstract
Renilla reniformis is an anthozoan coelenterate capable of exhibiting bioluminescence. Bioluminescence in Renilla results from the oxidation of coelenterate luciferin (coelenterazine) by luciferase [Renilla-luciferin:oxygen 2-oxidoreductase (decarboxylating), EC 1.13.12.5]. In vivo, the excited state luciferin-luciferase complex undergoes the process of nonradiative energy transfer to an accessory protein, green fluorescent protein, which results in green bioluminescence. In vitro, Renilla luciferase emits blue light in the absence of any green fluorescent protein. A Renilla cDNA library has been constructed in lambda gt11 and screened by plaque hybridization with two oligonucleotide probes. We report here the isolation and characterization of a luciferase cDNA and its gene product. The recombinant luciferase expressed in Escherichia coli is identical to native luciferase as determined by SDS/PAGE, immunoblot analysis, and bioluminescence emission characteristics.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chaconas G., van de Sande J. H. 5'-32P labeling of RNA and DNA restriction fragments. Methods Enzymol. 1980;65(1):75–85. doi: 10.1016/s0076-6879(80)65012-5. [DOI] [PubMed] [Google Scholar]
- Charbonneau H., Walsh K. A., McCann R. O., Prendergast F. G., Cormier M. J., Vanaman T. C. Amino acid sequence of the calcium-dependent photoprotein aequorin. Biochemistry. 1985 Nov 19;24(24):6762–6771. doi: 10.1021/bi00345a006. [DOI] [PubMed] [Google Scholar]
- Chirala S. S. The nucleotide sequence of the lac operon and phage junction in lambda gt11. Nucleic Acids Res. 1986 Jul 25;14(14):5935–5935. doi: 10.1093/nar/14.14.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gay N. J., Walker J. E. Molecular cloning of a bovine cathepsin. Biochem J. 1985 Feb 1;225(3):707–712. doi: 10.1042/bj2250707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossberger D. Minipreps of DNA from bacteriophage lambda. Nucleic Acids Res. 1987 Aug 25;15(16):6737–6737. doi: 10.1093/nar/15.16.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hart R. C., Matthews J. C., Hori K., Cormier M. J. Renilla reniformis bioluminescence: luciferase-catalyzed production of nonradiating excited states from luciferin analogues and elucidation of the excited state species involved in energy transfer to Renilla green fluorescent protein. Biochemistry. 1979 May 29;18(11):2204–2210. doi: 10.1021/bi00578a011. [DOI] [PubMed] [Google Scholar]
- Hart R. C., Stempel K. E., Boyer P. D., Cormier M. J. Mechanism of the enzyme-catalyzed bioluminescent oxidation of coelenterate-type luciferin. Biochem Biophys Res Commun. 1978 Apr 14;81(3):980–986. doi: 10.1016/0006-291x(78)91447-x. [DOI] [PubMed] [Google Scholar]
- Houmard J., Drapeau G. R. Staphylococcal protease: a proteolytic enzyme specific for glutamoyl bonds. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3506–3509. doi: 10.1073/pnas.69.12.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matthews J. C., Hori K., Cormier M. J. Purification and properties of Renilla reniformis luciferase. Biochemistry. 1977 Jan 11;16(1):85–91. doi: 10.1021/bi00620a014. [DOI] [PubMed] [Google Scholar]
- Matthews J. C., Hori K., Cormier M. J. Substrate and substrate analogue binding properties of Renilla luciferase. Biochemistry. 1977 Nov 29;16(24):5217–5220. doi: 10.1021/bi00643a009. [DOI] [PubMed] [Google Scholar]
- O'Kane D. J., Lee J. Encapsulated radiophosphorescent standards for day-to-day photometer calibration. Photochem Photobiol. 1990 Oct;52(4):723–734. doi: 10.1111/j.1751-1097.1990.tb08673.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Ward W. W., Cormier M. J. An energy transfer protein in coelenterate bioluminescence. Characterization of the Renilla green-fluorescent protein. J Biol Chem. 1979 Feb 10;254(3):781–788. [PubMed] [Google Scholar]
- Wieslander L. A simple method to recover intact high molecular weight RNA and DNA after electrophoretic separation in low gelling temperature agarose gels. Anal Biochem. 1979 Oct 1;98(2):305–309. doi: 10.1016/0003-2697(79)90145-3. [DOI] [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]