Abstract
Objectives
This study aims to analyze the mortality and the length of ICU stay (LOS) of A. baumannii VAP compared to respiratory colonization in patients with mechanical ventilation (MV).
Methods
A prospective cohort study was performed in an ICU of adult patients (February 2010–June 2011). One hundred patients on MV with A. baumannii in lower respiratory airways were recruited, and classified as VAP or airways colonization according to CPIS criteria, with a punctuation ≥6. LOS, 30-days mortality, A. baumannii bacteremia, and clinical features including antibiotic therapy were recorded. Multivariate analysis (linear and Cox regression) and survival analysis (Kaplan-Meier curves) were performed.
Results
Fifty-seven VAP and 43 colonized A. baumannii patients were analyzed. Among the A. baumannii strains, 99% were non-susceptible to carbapenems and the MIC90 of colistin was 0.12 mg/l. Therapy was appropriate in 94.6% of VAP patients, most of them with colistin 6 MIU/day, although in 13 (23.6%) cases colistin was started 48 hours after the onset of VAP. Mortality was similar in both groups (VAP 24.6% vs. colonized 27.9%, p = 0.7). Bacteremia and acute kidney insufficiency were associated with decreased survival (p = 0.02 and p = 0.04, respectively) in VAP patients. LOS was 21.5 (11.5–42.75) vs. 9 (6–22) days for VAP and colonized patients (p = 0.004). VAP (p = 0.003) and age (p = 0.01) were independently related to a longer LOS.
Conclusions
Multidrug-resistant A. baumannii VAP treated with colistin does not have a different mortality compared to lower airways colonization, among patients on mechanical-ventilation, in a setting of high susceptibility to colistin of A. baumannii.
Introduction
Acinetobacter baumannii is among the leading etiologies of hospital-acquired infections worldwide, ventilator-associated pneumonia (VAP) being the most common among them [1]. Attributable mortality of A. baumannii infections can be heterogeneous, depending on underlying conditions and appropriateness of antibiotic therapy, but it may have been as high as 28.5–44.5% [2].The studies that have assessed the attributable mortality of A. baumannii VAP usually included either patients with VAP caused by other pathogens or patients without any pulmonary infection [2–3] in the control group. Since the risk factors for acquisition of A. baumannii can be also associated to higher mortality (wide-spectrum antibiotic therapy, invasive devices and procedures, clinical severity, duration of the ICU stay) [4–5], attributable mortality of A. baumannii could be overestimated despite the efforts made to avoid confusing factors. Thus, mechanically ventilated patients with lower airways colonization by A. baumannii may be the most appropriate control group to weigh up the clinical impact of the A. baumannii VAP, considering that around half of all the patients acquiring A. baumannii in the respiratory tract develop VAP, while the others remain just colonized [6],and that risk factors for acquisition of A. baumannii are similar for both groups [5].
The spread of multidrug-resistant (MDR) strains, most of them resistant to carbapenems, has greatly complicated the management of A. baumannii infections. Colistin, which conserves activity against most clinical isolates of MDR A. baumannii, has emerged as its first-line therapy. However, the traditional dosing regimen of colistin (6million of international units [MIU] daily, tid) was considered insufficient to treat susceptible pathogens with a minimal inhibitory concentration in the upper limit of susceptibility, according to the results of multiple pharmacokinetics studies [7–8]. Consequently, higher dosages of colistin have been generally adopted, although a definitive consensus is lacking [9] and a loading dose is recommended to achieve early effective concentrations in critically ill patients [10]. In our center, where a predominance of carbapenem-resistant A. baumannii has been observed since 2008, the traditional dosages were used until 2011, when a new protocol was slowly adopted (loading dose of 4.5–6 MIU and 9 MIU daily, bid or tid).
The aims of this study were: i) To analyze the impact on mortality and ICU length of stay (LOS) of A. baumannii VAP compared to lower airways colonization in mechanically ventilated patients; ii) To evaluate the efficacy of the different dosages of colistin in A. baumannii VAP. We also analyzed the resistance mechanisms for carbapenems in the resistant A. baumannii isolates.
Patients and Methods
Design
This was a prospective, observational cohort study of patients on invasive mechanical ventilation. The study was approved by the Ethics Committee of the University Hospital Virgen del Rocío. Written informed consent was obtained from a relative of all patients before inclusion in the study.
Setting and period
The study was conducted at the University Hospital Virgen del Rocío, a tertiary-care hospital with 1,251 beds, including 62 adult ICU beds. The enrollment period was from February 2010 to June 2011.
Criteria of inclusion and exclusion
Adult patients (≥18 years) admitted to the ICU and requiring invasive mechanical ventilation for more than 48 hours, and having at least one culture of trachea-bronchial aspirate (BAS) with A. baumannii isolation. Patients with history of previous endotracheal intubation in the preceding 365 days, tracheotomy or cystic fibrosis, were excluded.
Recruitment and follow up
Daily, two of the researchers identified the new candidates among ICU patients with MV. After obtaining the informed consent, their baseline characteristics were collected in a standardized form and BAS cultures were performed every three days while intubated, up to 30 days. If A. baumannii was isolated, patients were definitively included in the study cohort and followed until hospital discharge, death or 30 days, which ever occurred first. All clinical decisions were made by the physicians in charge of the patients.
Variables
Patient demographics, primary diagnosis, in-hospital admission department, APACHE II [11] and Charlson comorbidity Score [12] were recorded at the moment of inclusion. Antimicrobial therapy reception, and development of any cause of septic shock or acute kidney injury (AKI) were recorded if they happened at any time during the follow-up, as well as ICU LOS and mortality.
Patients were classified as A. baumannii VAP or lower airways colonization according to the CPIS score, being classified as VAP those cases with a punctuation ≥6 [13]. Septic shock [14] and AKI [15] were defined according to standard criteria. Appropriate antimicrobial therapy was considered when VAP patients received at least one drug active against the A. baumannii isolates. Mortality was defined as death from any cause within 30 days after the first A. baumannii isolation in colonized patients or after the onset of pneumonia in infected patients. ICU stay was measured from the first isolation of A. baumannii.
Microbiological procedures
BAS were processed immediately for quantitative cultures. One hundred μL aliquots of serial ten-fold dilutions were plated on Columbia sheep blood agar and incubated at 37°C. Colony forming units (CFUs) were counted after 24 h and expressed as log10 CFU/mL. Blood cultures were processed in a BACTEC™ system. A. baumannii identification was performed by biochemical tests in an automatized multi-test system (MicroScan Walkaway™), and by matrix-assisted laser desorption ionization–time-of-flight (MALDI-TOF) analysis using a MALDI biotyper (Bruker Daltonics). Among isolates from BAS, the first one from colonized patients and the first at the diagnosis in patients with VAP were selected for antimicrobial susceptibility testing. Minimum inhibitory concentrations for imipenem, sulbactam, ceftazidime, amikacin, ciprofloxacin, tigecycline, colistin, and rifampin were performed by the broth microdilution according to the CLSI criteria [16]. Isolates were defined as multi-drug resistant (MDR) according to the standard criteria [17]. In those isolates resistant to imipenem, PCR amplifications were done to detect the presence of the oxacillinasesblaOXA51, blaOXA23, blaOXA24, blaOXA58, and the metallo-β-lactamases (MBL) IMP and VIM genes [18–19]. Expression of blaOXA51 was assessed testing by PCR the presence of the sequence ISAba1 upstream and the gene encoding blaOXA51 [20].
Sample size
For the sample size, assuming 49,25% mortality in patients with A. baumannii VAP (average mortality of A. baumannii VAP in two studies performed in our centre [3,21]) and 20% in respiratory colonization [6], and an infected and colonized ratio of 1:1, a minimum of 40 evaluable patients per group were required, with an alpha error of 5% and a power of 80%.
Statistical analysis
Discrete variables were expressed as counts (percentage) and continuous variables as the median and interquartile range or mean and standard deviation, as appropriate. ICU stay analysis was made only on patients who survived until discharge from the ICU. Logarithmic transformation was performed if length of stay variables did not show linear distribution. Differences in categorical variables were calculated using a two-sided likelihood ratio chi-square test or Fisher exact test, and the t-test or Mann-Whitney test was used for continuous variables, when appropriate. Survival was analyzed by Kaplan-Meier curves. Back stepped Cox regression was used to assess the factors independently associated to survival. Results are presented as the hazard ratio (HR) and 95% confidence interval (CI). Linear regression was used for multivariate analysis in the case of ICU LOS, including the variables with a p<0.05 in the bivariate analysis. Statistical significance was defined as p<0.05. The PASW 18.0 package was used.
Results
During the study period, 285 eligible patients were identified in the ICU. A. baumannii was isolated from respiratory samples in 100 of them: 57 were classified as VAP, with an average CPIS score of 6.36±1.14, and 43 as lower airways colonization, respectively (Fig 1). The elapsed time from intubation to the first isolate was 9 (5–14) days.
Fig 1. Flow chart on the enrollment of patients in the study and final classification as ventilator-associated pneumonia (VAP) or colonization in those with isolation of Acinetobacter baumannii in trachea-bronchial aspirate (BAS).
Colonized and VAP patients had similar demographic and clinical characteristics, as detailed in Table 1. Fifty-three (92.9%) infected patients received appropriate therapy with colistin and one (1.7%) with tigecycline; in 13 (23.6%) cases, colistin was started 48 hours after the onset of VAP. Among patients who received colistin, loading dose was not used and all of them received dosages of 2 MIU of sodium colistimethate thrice daily, titrated on renal function when appropriate.
Table 1. Distribution of baseline and clinical features among patients with A. baumannii lower airways colonization and those with A. baumannii VAP.
| Colonization | VAP | p | |
|---|---|---|---|
| (n = 43) | (n = 57) | ||
| Age | 66 (43–70) | 51 (43.5–69.5) | 0.186 |
| med (IQR) | |||
| Female sex | 17 (39.5) | 22 (38.6) | 0.920 |
| n (%) | |||
| Charlson score | 1 (0–2) | 0 (0–2) | 0.529 |
| med (IQR) | |||
| Chronic heart failure | 7 (16.3) | 6 (10.5) | 0.397 |
| n (%) | |||
| COPD | 5 (11.6) | 6 (10.5) | 0.862 |
| n (%) | |||
| DM | 9 (20.9) | 11 (19.3) | 0.840 |
| n (%) | |||
| AIDS | 0 | 1 (1.8) | 0.383 |
| n (%) | |||
| Chronic liver disease | 1 (2.3) | 2 (3.5) | 0.215 |
| n (%) | |||
| Medical patient | 24 (52.2) | 22 (38.6) | 0.084 |
| n (%) | |||
| Surgical patient | 8 (18.6) | 17 (29.8) | 0.200 |
| n (%) | |||
| Trauma patient | 11 (25.6) | 18 (31.6) | 0.510 |
| n (%) | |||
| APACHE II | 17 (12–21.25) | 17 (13–20) | 0.947 |
| med (IQR) | |||
| Previous antibiotic therapy | 40 (93) | 53 (92.9%) | 0.994 |
| n (%) | |||
| Carbapenems | 13 (30.2) | 33 (57.9) | 0.006 |
| n (%) | |||
| Piperacillin-tazobactan | 10 (23.2) | 25 (43.8) | 0.032 |
| n (%) | |||
| Quinolones | 14 (32.6) | 14 (24.6) | 0.378 |
| n (%) | |||
| Vancomycin | 6 (13.9) | 22 (38.6) | 0.007 |
| n (%) | |||
| Acute kidney injury | 13 (30.2) | 27 (47.4) | 0.085 |
| n (%) | |||
| Septic shock | 17 (39.5) | 30 (52.6) | 0.194 |
| n (%) | |||
| Surgery | 19 (44.2) | 38 (66.7) | 0.025 |
| n (%) | |||
| Mortality | 12 (27.9) | 14 (24.6) | 0.700 |
| n (%) |
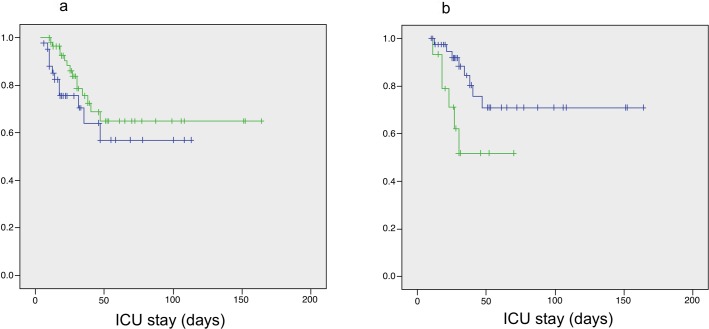
There were no differences in the mortality of patients with A. baumannii VAP regarding to those with airways colonization (Table 1), and so did their survival rate according to the Kaplan-Meier analysis (p = 0.3) (Fig 2A). Patients with bacteremic VAP had a trend to higher mortality than VAP patients without bacteremia (42.9% vs. 20.9%, p = 0.1); and the survival Kaplan-Meier analysis showed lower survival in bacteremic patients (p = 0.02) (Fig 2B). In the multivariate survival analysis, colistin was associated to a better survival in patients with VAP, while bacteremia and AKI were related to poor prognosis (Table 2).
Fig 2.
a) Survival Kaplan-Meyer analysis between Acinetobacter baumannii colonized and ventilator-associated pneumonia (VAP) patients (Blue: Colonized patients; Green: VAP patients); b) Survival Kaplan-Meyer analysis among patients with Acinetobacter baumannii ventilator-associated pneumonia, with or without bacteremia. (Blue: non-bacteremic VAP patients; Green: bacteremic VAP patients).
Table 2. Bivariate and multivariate survival analysis for mortality in patients with A. baumannii ventilator-associated pneumonia.
| Variable | Dead | Survivors | Bivariate | Multivariate | ||
|---|---|---|---|---|---|---|
| (n = 14) | (n = 43) | HR (95%CI) | p | HR (95%CI) | p | |
| Female sex | 7 (50) | 15 (34.9) | 1.22 (0.42–3.48) | 0.710 | ||
| n (%) | ||||||
| Age | 58 (43.25–76.75) | 49 (43–65) | 1.01 (0.97–1.04) | 0.580 | ||
| med (IQR) | ||||||
| Charlson Score | 2 (0.75–2.25) | 0 (0–1) | 1.27 (0.95–1.7) | 0.107 | ||
| med (IQR) | ||||||
| APACHE II | 19.5 (17.75–20.25) | 15.5 (12.25–19.5) | 1.01 (0.95–1.08) | 0.632 | ||
| med (IQR) | ||||||
| Medical patient | 8 (57.1) | 14 (32.6) | 2.27 (0.79–6.57) | 0.129 | ||
| n (%) | ||||||
| Surgical patient | 5 (35.7) | 12 (27.9) | 1.38 (0.46–4.13) | 0.563 | ||
| n (%) | ||||||
| Trauma patient | 1 (7.1) | 17 (39.5) | 0.15 (0.02–1.12) | 0.064 | ||
| n (%) | ||||||
| Septic shock | 11 (78.6) | 19 (44.2) | 2.26 (0.63–8.13) | 0.212 | ||
| n (%) | ||||||
| Acute kidney injury | 11 (78.6) | 16 (37.2) | 3.5 (0.97–12.54) | 0.055 | 3.88 (1.06–14.21) | 0.04 |
| n (%) | ||||||
| Colistin | 13 (92.9) | 40 (93.0) | 0.03 (0.002–0.47) | 0.013 | 0.02 (0.001–0.72) | 0.033 |
| n (%) | ||||||
| Bacteremia | 6 (42.9) | 9 (20.9) | 4.49 (1.52–13.3) | 0.007 | 4.67 (1.53–14.25) | 0.02 |
| n (%) | ||||||
Median ICU LOS was 17 days (8–33) for the whole sample, and 9 (6–22) vs. 21.5 (11.5–42.75) days for colonized and VAP patients, respectively (p = 0.004). Development of A. baumannii VAP (p = 0.003) and elder age (p = 0.013) were independently associated to a longer ICU stay (Table 3).
Table 3. Factors associated to the length of intensive care unit-stay (bivariate and multivariate analysis) in the whole cohort (n = 100).
| Variable | Bivariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| p | OR (95%CI) | p | OR (95%CI) | |
| VAP * | 0.007 | 1.81 (1.18–2.79) | 0.004 | 1.88 (1.23–2.88) |
| Age | 0.041 | 1.01 (1.00–1.03) | 0.010 | 1.01 (1.00–1.03) |
| Female sex | 0.097 | 1.45 (0.93–2.26) | - | - |
| Charlson Score | 0.257 | 1.45 (0.90–1.44) | - | - |
| APACHE II | 0.03 | 1.04 (1.00–1.07) | 0.47 | 1.01 (0.98–1.05) |
| Septic shock | 0.011 | 1.80 (1.15–2.81) | 0.204 | 1.35 (0.85–2.15) |
| Acute kidney injury | 0.015 | 1.84 (1.13–3.01) | 0.107 | 1.5 (0.91–2.47) |
| Bacteremia | 0.98 | 0.99 (0.50–1.97) | - | - |
* VAP: ventilator-associated pneumonia
Antimicrobial susceptibility is detailed in Table 4. Just one isolate was susceptible to imipenem. The MIC90 of colistin was 0.12mg/L. Ninety (90%) isolates were MDR.blaOXA51, which is constitutive in A. baumannii, was present in all the isolates, but just 9 of them presented the upstream sequence ISAba-1. blaOXA58 was present in 81 isolates and blaOXA40 in 18 isolates, with an imipenem MIC50 of 16 and 64 mg/L, respectively (p<0.001); none of them were MBL producers. There were no differences in antimicrobial susceptibility/resistance between isolates from colonized and VAP patients.
Table 4. Antimicrobial susceptibility and mechanism of resistance to carbapenems in the isolates of A. baumannii.
| Antimicrobial agent | MIC range | MIC50 | MIC90 | % susceptible isolates |
|---|---|---|---|---|
| Imipenem | 0.25–256 | 16 | 128 | 1 |
| Meropenem | 0.38->8 | >8 | >8 | 1 |
| Amikacin | 0.25–64 | 8 | 32 | 78 |
| Ceftazidime | 16–256 | 64 | 128 | 0 |
| Ciprofloxacin | 0.5 | 32 | 128 | 0 |
| Colistin | 0.06–0.12 | 0.06 | 0.12 | 100 |
| Rifampicin | 1–128 | 32 | 128 | 15 |
| Sulbactam | 2–32 | 4 | 16 | 62 |
| Tigecycline | 0.06–0.5 | 0.25 | 0.25 | 100 |
| n | Imipenem MIC50 | |||
| blaOXA-58 | 81 | 16 | ||
| blaOXA-40 | 18 | 128 | ||
| ISAba-1-blaOXA51 | 9 | 128 | ||
| IMP | 0 | - | ||
| VIM | 0 | - | ||
Discussion
In this prospective cohort, A. baumannii VAP did not produce an excess of mortality with respect to the lower airways colonization by the same pathogen in critically ill patients on MV. Nonetheless, VAP patients had a longer ICU stay, which points out the morbidity that it causes among patients on mechanical ventilation. Colistin at traditional low doses was used in almost all the patients with VAP; the high susceptibility to this drug among the A. baumannii isolates (MIC90 = 0.12 mg/L) could explain the lack of differences in mortality. Inside the group of patients with VAP, bacteremia had a poorer survival rate (p = 0.02). Given that the impact on mortality of VAP is related to its severity [21], the presence of concomitant A. baumannii bacteremia, whose crude mortality in the setting of pneumonia is high [22], can be understood as a key event that warns on severity and invasiveness of the VAP. A previous surgical procedure was more frequent in VAP than in colonized patients, but this feature was not associated to mortality in the whole sample (data not shown).
The crude mortality described for any-cause VAP is usually higher, ranging between 34.5% and 84% [3,23–25]. In this context, A. baumannii VAP has been associated to higher mortality and longer LOS in ICU than VAP caused by other pathogens [21, 26–28]. For instance, in a cohort of 163 patients with VAP, A. baumannii was identified as an independent risk factor for fatal outcome (OR 3.3, CI 1.12–9.7), reaching a crude mortality of 61% [26]. Carbapenem-resistance in A. baumannii has been associated to increased VAP mortality. A matched case-control study comparing 60 patients with A. baumannii VAP and 60 controls (patients with VAP caused by other microorganisms and patients without VAP), did not report differences in mortality for the whole sample [3]. Nevertheless, the sub-analysis of the patients with VAP by imipenem-resistant A. baumannii showed differences in mortality between cases and their matched controls (44% vs. 24%). In the present study, despite having found almost a 100% of carbapenem-resistance, mortality was quite lower than that previously described for A. baumannii VAP.
This discrepancy in the mortality could be explained by an unequal distribution in the appropriateness of antibiotic therapy among different studies. A meta-analysis on 24,186 patients from 44 observational studies [28] found that VAP was associated with higher mortality (OR 1.96, 95%CI 1.26–3.04) than their comparative groups; notwithstanding, this association was not found in VAP patients with appropriate initial treatment. The higher mortality of VAP caused by more resistant strains is probably related to the less likelihood of receiving appropriate therapy [21]. Therefore, despite the high level of resistance of the isolates included in this study, the rate of VAP patients receiving appropriate treatment (≈95%) has probably contributed to a lower mortality.
It is noteworthy that the antibiotic used for most patients in the present study was colistin, which was selected as an independent predictor of better survival. Until it became almost the only alternative, colistin was considered a last option for the treatment of A. baumannii infections due to concerns about its efficacy and safety. In a systematic review including all the studies assessing the efficacy of colistin compared to other antibiotics, the global analysis favored the comparators [29]. However, several pharmacokinetics studies have prompted that this lack of efficacy would be related to an insufficient antibiotic exposure, and higher doses have been proposed to obtain an optimal drug concentration at the infection site [7–8]. Dose-fractionation studies of colistin against A. baumannii in mouse infection models have revealed that AUC/MIC correlates with bacterial killing in vivo [30], even though its optimal value has not been well defined yet. In an A. baumannii murine pneumonia model, an AUC0-24/MIC of 158.8 was associated to a 3.78-log bacterial kill target in lungs and a reduction of 40% in mortality [31]. Karnik et al. [32] performed a PK study in patients with VAP caused by multidrug-resistant bacteria treated with 2 MU every 8 hours of colistin, the same kind of patients and colistin dosage as used in the present study, achieving an AUC0-24 of colistin of 47.1 mg.h/l. Taking into account the colistin MIC90 of 0.12 mg/L for the A. baumannii strains causing VAP in the present study, we would have achieved an average AUC0-24/MIC of 392.5 from the first colistin dose. Therefore, the low MIC90 of the included strains may explain the high effectiveness of the colistin therapy in this cohort of patients.
There is little further information on the association between MIC of colistin and effectiveness, because most clinical studies do not provide the MIC of colistin against the included strains. Markou et al. [33], in an observational study of 10 patients with A. baumannii infections, found that the MIC90 of colistin was higher among patients without clinical response to this drug (1 vs. 0.5 mg/L); however, the small sample size and the likely confounding factors preclude from assuming the impact of the MIC on the outcome. Recently, Kim et al. [34] reported a similar efficacy of tigecycline and colistin in a cohort of 70 patients with A. baumannii VAP. In this study, low tigecycline MIC was suggested as the reason for the good successful rate and survival among patients treated with tigecycline; however, unfortunately, the information regarding the precise colistin and tigecycline MIC was not reported.
In recent years, all efforts for the optimization of the treatment with colistin have been aimed at enhancing its pharmacokinetics, targeting to obtain higher levels, although safety can be of concern [35–36]. Focusing on strategies addressed to pharmacodynamics parameters related to the colistin MIC90 against the A. baumannii strains in each hospital might help to avoid over-dosage in some patients at risk of toxicity. With this strategy, the main concern would be if low dosages might select more easily resistant strains. However, this drawback seems to be ruled out by recent in vitro models, in which the emergence of resistant strains was paradoxically increased with higher colistin concentrations [37–38].
Our study has several limitations. First of all, the sample size was calculated a priori and the observed mortality of VAP patients was lower than expected. This made our sample size sub-optimal for detection of differences in mortality. Second, the method chosen for enrolling patients, periodic quantitative BAS cultures, has probably prompted the early initiation of appropriate antimicrobial therapy and influenced VAP outcome. Third, there is not a gold standard to classify patients as suffering from VAP and the use of CPIS score and other criteria, beyond the clinical ones, is a matter of debate; in fact, in the recent IDSA/ATS Guidelines for the Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia [39] the authors do not recommend any criteria, among those reviewed, to support or exclude the HAP/VAP, because of low sensitivity and specificity. However, to avoid the selection bias of the research it is necessary to use specific criteria, in spite of the problems with the sensitivity/specificity; in this context, we decided to use the CPIS score, because it includes the clinical criteria (fever, purulent sputum, new infiltrates in the X-ray, respiratory insufficiency, and leukocytosis), with different punctuation to compose the reached score value. Fourth, the possibility of ventilator-associated trachea-bronchitis or airways infection produced by other microorganisms, or the occurrence of different events including other infections after the recovery or during the ICU stay that may have influenced individual outcomes, as is common in critically ill patients, were not considered.
In summary, the present study shows that MDRA. baumannii VAP treated with colistin does not have different mortality compared to lower airways colonization among patients on mechanical-ventilation. The high susceptibility to colistin among the isolated strains probably determined this similar outcome. In our opinion, this fact deserves special attention in relationship to the strategy in reaching the most appropriate treatment. Finally, bacteremic VAP showed a decreased survival in comparison to non-bacteremic VAP.
Data Availability
All relevant data are within the paper.
Funding Statement
The present study has been granted by the Instituto de SaludCarlos III (PS09/01427) and supported by Plan Nacional de I+D+i and Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía y Competitividad, Spanish Network for Research in Infectious Diseases (REIPI RD12/0015/0001) - co-financed by European Development Regional Fund "A way to achieve Europe" ERDF. RAM has a Rio Hortega grant from the Instituto de Salud Carlos III (CM14/00179). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kollef M. (1993) Ventilator-associated pneumonia. JAMA 270:1965–70. [PubMed] [Google Scholar]
- 2.Falagas ME, Rafailidis PI. Attributable mortality of Acinetobacter baumannii: no longer a controversial issue. Crit Care 2007; 11:134 10.1186/cc5911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garnacho J, Sole-Violan J, Sa-Borges M, Diaz E, Rello J. Clinical impact of pneumonia caused by Acinetobacter baumannii in intubated patients: a matched cohort study. Crit Care Med 2003; 31:2478–82. 10.1097/01.CCM.0000089936.09573.F3 [DOI] [PubMed] [Google Scholar]
- 4.Arvaniti K, Lathyris D, Ruimy R, Haidich AB, Koulourida V, Nikolaidis P, et al. The importance of the colonizatin pressure in multiresistant Acinetobacter baumannii acquisition in a Greek intensive unit. Crit Care 2012; 16 (3): R102 10.1186/cc11383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Playford EG, Craig JC, Iredell JR. Carbapenem-resistant Acinetobacter baumannii in intensive care unit patients: risk factors for acquisition, infection and their consequences. J Hosp Infect 2007; 65 (3):204–11. 10.1016/j.jhin.2006.11.010 [DOI] [PubMed] [Google Scholar]
- 6.Rodríguez-Baño J, Cisneros JM, Fernández-Cuenca F, Ribera A, Vila J, Pascual A, et al. Clinical features and epidemiology of Acinetobacter baumannii colonization and infection in Spanish hospitals. Infect Control HospEpidemiol 2004; 25:819–24. [DOI] [PubMed] [Google Scholar]
- 7.Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 2011; 55 (7): 3284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plachouras D, Karvanen M, Friberg LE, Papadomichelakis E, Antoniadou A, Tsangaris I, et al. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob Agents Chemother 2009; 53: 3430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nation RL, Garonzik SM, Li J, Thamlikitkul V, Giamarellos-Bourboulis EJ, Paterson DL, et al. Updated US and European Dose Recommendations for Intravenous Colistin: How Do They Perform? Clin Infect Dis. 2016; 62(5):552–8. 10.1093/cid/civ964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landersdorfer CB, Nation RL. Colistin: how should it be dosed for the critically ill? Semin Respir Crit Care Med. 2015; 36(1):126–35. 10.1055/s-0034-1398390 [DOI] [PubMed] [Google Scholar]
- 11.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13:818 [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic co-morbidity in longitudinal studies: development and validation. J Chron Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 13.Pugin J, Auckenthaler R, Mili N, Janssens JP, Lew PD, Suter PM. Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid. Am Rev Respir Dis 1991; 143:1121–9. 10.1164/ajrccm/143.5_Pt_1.1121 [DOI] [PubMed] [Google Scholar]
- 14.American College of Chest Physicians/Society of Critical Care Medicine. Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992; 20:864–74. [PubMed] [Google Scholar]
- 15.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11:R31 10.1186/cc5713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standards Institute. (2007) Performance standards for antimicrobial susceptibility testing-17th Informational supplement. CLSI document M100-S17. CLSI, Wayne, USA.
- 17.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske C, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an International expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18:268–81. 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 18.Woodford N, Ellington MJ, Coelho JM, Turton JF, Ward ME, Brown S, et al. Multiplex PCR for genes enconding prevalent OXA carbapenemases in Acinetobacter spp. Int J Antimicrob Agent 2006; 27:351–3. [DOI] [PubMed] [Google Scholar]
- 19.Ellington MJ, Kistler J, Livermore DM, Woodford N. Multiplex PCR for rapid detection of genes encoding acquired metallo-β-lactamases. J Antimicrob Chemother 2007; 59:321–2. 10.1093/jac/dkl481 [DOI] [PubMed] [Google Scholar]
- 20.Ruiz M, Marti S, Fernandez-Cuenca F, Pascual A, Vila J. High prevalence of carbapenem-hydrolysingoxacillinases in epidemiologically related and unrelated Acinetobacter baumannii clinical isolates in Spain. Clin Microbiol Infect 2007; 13 (12): 1192–8. 10.1111/j.1469-0691.2007.01825.x [DOI] [PubMed] [Google Scholar]
- 21.Tseng CC, Liu SF, Wang CC, Tu ML, Chung YH, Lin MC, Fang WF. Impact of clinical severity index, infective pathogens, and initial empiric antibiotic use on hospital mortality in patients with ventilator-associated pneumonia. Am J Infect Control 2012; 40:648–52. 10.1016/j.ajic.2011.08.017 [DOI] [PubMed] [Google Scholar]
- 22.Liu CP, Shih SC, Wang NY, Wu AY, Sun FJ, Chow SF, et al. Risk factors of mortality in patients with carbapenem-resistant Acinetobacter baumannii bacteremia. J MicrobiolImmunol Infect. 2014 [DOI] [PubMed] [Google Scholar]
- 23.Zheng YL, Wan YF, Zhou LY, Ye ML, Liu S, Xu CQ, et al. Risk factors and mortality of patients with nosocomial carbapenem-resistant Acinetobacter baumannii pneumonia. Am J Infect Control. 2013; 41(7):e59–63. 10.1016/j.ajic.2013.01.006 [DOI] [PubMed] [Google Scholar]
- 24.Chaari A, Mnif B, Bahloul M, Mahjoubi F, Chatara K, Turki O, et al. Acinetobacter baumannii ventilator-associated pneumonia: epidemiology, clinical characteristics and risks factors. Int J Infect Dis 2013; 17 (12): e1225–8. 10.1016/j.ijid.2013.07.014 [DOI] [PubMed] [Google Scholar]
- 25.Özgür ES, Horasan ES, Karaca K, Ersöz G, Nayci-Atis S, Kaya A. Ventilator-associated pneumonia due to extensive drug-resistant Acinetobacter baumannii: risk factors, clinical features, and outcomes. Am J Infect Control 2014; 42 (2): 206–8. 10.1016/j.ajic.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 26.Kollef MH, Silver P, Murphy DM, Trovillion E. The effect of late-onset ventilator associated pneumonia in determining patient mortality. Chest 1995; 108:1655–62. [DOI] [PubMed] [Google Scholar]
- 27.Mao Y, Liu G, Wang L, Wang X, Li F. The impact of pulmonary Acinetobacter baumannii infection on the prognosis of inpatients in a neurological intensive care unit. J IntMed Res 2013; 41:1120–6. [DOI] [PubMed] [Google Scholar]
- 28.Agrafiotis M, Siempos II, Ntaidou TK, Falagas ME. Attributable mortality of ventilator-associated pneumonia: a meta-analysis. Int J Tuberc Lung Dis 2011; 15:1154–63. 10.5588/ijtld.10.0498 [DOI] [PubMed] [Google Scholar]
- 29.Yahav D, Farbman L, Leibovici L, Paul M. Colistin: new lessons on an old antibiotic. Clin Microbiol Infect 2012; 18:18–29. 10.1111/j.1469-0691.2011.03734.x [DOI] [PubMed] [Google Scholar]
- 30.Dudhani RV, Li J, Turnidge JD Garonzik SM, Mandragos K, Shoham S, et al. In vivo pharmacodynamics of colistin against Acinetobacter baumannii in murine thigh and lung infection models. In: Abstracts of the Forty-ninth Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, 2009. Abstract A1–572, p. 41. American Society for Microbiology, Washington, DC, USA.
- 31.Pachon-Ibañez ME, Docobo-Pérez F, López-Rojas R, Domínguez-Herrera J, Jimenez-Mejias ME, Garcia-Curiel A, et al. Efficacy of rifampin and its combinations with imipenem, sulbactam and colistin in experimental models of infection caused by imipenem-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 2010; 54: 1165–72. 10.1128/AAC.00367-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karnik ND, Sridharan K, Jadhav SP, Kadam PP, Naidu RK, Namjoshi RD, et al. Pharmacokinetics of colistin in critically ill patients with multidrug-resistant Gram-negative bacilli infection. Eur J CinPharmacol 2013; 69:1429–36. [DOI] [PubMed] [Google Scholar]
- 33.Markou N, Markantonis SL, Dimitrakis E, Panidis D, Boutzouka E, Karatzas S, et al. Colistin serum concentrations after intravenous administration in critically ill patients with serious multidrug-resistant, gram-negative bacilli infections: a prospective, open-label, uncontrolled study. ClinTher 2008; 30 (1):143–51. [DOI] [PubMed] [Google Scholar]
- 34.Kim WY, Moon JY, Huh JW, Choi SH, Lim CM, Koh Y, et al. Comparable efficacy of tigecycline versus colistin therapy for multidrug-resistant and extensively drug-resistant Acinetobacter baumannii pneumonia in critically ill patients. PLos One 2016; 11 (3):e0150642 10.1371/journal.pone.0150642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forrest A, Silveira FP, Thamlikitkul V, et al. Toxicodynamics for colistin-associated changes in creatinine clearance. In: Interscience Conference on Antimicrobial Agents and Chemotherapy 2014. Washington DC, 2014.
- 36.Sorli L, Luque S, Grau S, Berenguer N, Segura C, Montero MM, et al. Trough colistin plasma level is an independent risk factor for nephrotoxicity: a prospective observational cohort study. BMC Infect Dis 2013; 13: 380 10.1186/1471-2334-13-380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsuji BT, Landersdorfer CB, Lenhard J, Cheah SE, Thamlikitkul V, Rao GG, et al. The paradoxical effect on polymyxin B: high drug exposure amplifies resistance in Acinetobacter baumannii. Antimicrob Agents Chemother 2016; 60(7):3913–20. 10.1128/AAC.02831-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheah SE, Li J, Tsuji BT, Forrest A, Bulitta JB, Nation RL. Colistin and polymyxin B dosage regimens against Acinetobacter baumannii: Differences in activity and the emergence of resistance. Antimicrob Agents Chemother 2016; 60(7):3921–33. 10.1128/AAC.02927-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016; 63(5):575–82. 10.1093/cid/ciw504 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.