Abstract
Background
The angiotensin-I converting enzyme (ACE) plays a central role in the renin-angiotensin system, acting by converting the hormone angiotensin-I to the active peptide angiotensin-II (Ang-II). More recently, ACE was shown to act as a receptor for Ang-II, and its expression level was demonstrated to be higher in melanoma cells compared to their normal counterparts. However, the function that ACE plays as an Ang-II receptor in melanoma cells has not been defined yet.
Aim
Therefore, our aim was to examine the role of ACE in tumor cell proliferation and migration.
Results
We found that upon binding to ACE, Ang-II internalizes with a faster onset compared to the binding of Ang-II to its classical AT1 receptor. We also found that the complex Ang-II/ACE translocates to the nucleus, through a clathrin-mediated process, triggering a transient nuclear Ca2+ signal. In silico studies revealed a possible interaction site between ACE and phospholipase C (PLC), and experimental results in CHO cells, demonstrated that the β3 isoform of PLC is the one involved in the Ca2+ signals induced by Ang-II/ACE interaction. Further studies in melanoma cells (TM-5) showed that Ang-II induced cell proliferation through ACE activation, an event that could be inhibited either by ACE inhibitor (Lisinopril) or by the silencing of ACE. In addition, we found that stimulation of ACE by Ang-II caused the melanoma cells to migrate, at least in part due to decreased vinculin expression, a focal adhesion structural protein.
Conclusion
ACE activation regulates melanoma cell proliferation and migration.
Introduction
The renin-angiotensin system (RAS), a peptidergic hormone system, is well known for its role in the regulation of blood pressure, electrolyte balance and vascular remodeling [1, 2, 3]. Renin cleaves angiotensinogen to produce the decapeptide angiotensin (Ang) I. Subsequently, after cleavage of two carboxy-terminal amino acids by the angiotensin I–converting enzyme (ACE), Ang-I is converted into the octapeptide Ang-II. Two distinct forms of ACE are expressed in humans: a somatic form that is abundant on the surface of lung endothelial cells and a smaller isoenzyme found exclusively in testis [4]. The activity of somatic ACE has a crucial role in catalyzing the conversion of Ang-I to the Ang-II, which modulates blood pressure, vasoconstriction, inflammation, cell proliferation and vascular rearrangement [5].
In addition to the classic participation of ACE in the above-mentioned functions, new roles for ACE have recently been described [4, 6, 7]. The canonical Ang-II pathway is mediated by activation of either AT1 or AT2 receptors, which typically mediate opposite functions [8]. However, recent findings have revealed that, despite the traditional enzymatic functions, ACE is also capable of mediating intracellular signaling. Kohlstedt and colleagues [4] showed that binding of an ACE inhibitor to ACE elicits outside-in signaling in endothelial cells, enhancing the activity of ACE-associated kinase CK2 and increasing the phosphorylation of the intracellular tail of ACE. This in turn promotes the activation of JNK as well as the accumulation of phosphorylated c-Jun in the endothelial cell nucleus that ultimately increases ACE expression in vitro and in vivo. Such mechanism suggested that ACE activation might control expression of diverse proteins besides ACE itself. Indeed, Kohlstedt et al. [6] found that binding of ramipril (ACE inhibitor) to ACE directly induces a signaling cascade that results in the activation of the transcription factor AP-1 and an increase in the expression/activity of cyclooxygenase-2 in endothelial cells. Furthermore, Guimarães et al. [7] demonstrated that ACE behaves as a receptor for Ang-II triggering Ca2+ signaling, through inositol 1,4,5-trisphosphate (InsP3) formation. Accordingly, a binding site for Ang-II was described on ACE [9]. However, the role of Ang-II-mediated signaling through ACE is still unclear. In the current study, we investigated novel roles of ACE as Ang-II receptor, demonstrating that ACE regulates cell proliferation and migration in melanoma cells.
Materials and Methods
Material and Reagents
Dulbeccos’s Modified Eagle’s Medium (DMEM), RPMI 1640 medium, penicillin, streptomycin, amphotericin and fetal bovine serum (FBS) were purchased from Gibco (Grand Island, USA). Fluo-4/AM, DAPI, secondary antibodies conjugated to Alexa-488, Alexa-633, Angiotensin-II FITC conjugate and Lipofectamine® 2000 were purchased from Life Technologies (New York, USA); rabbit IgG secondary antibody was purchased from Sigma-Aldrich, (St. Louis, USA). Polyclonal anti-GAPDH and anti-PLC antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, USA). Monoclonal anti-ACE antibody and mouse anti-Clathrin was obtained from Merck Millipore (Darmstadt, Germany). Anti-BrDU-POD kit was obtained from Calbiochem (Damstadt, Germany). Ambion Silencer kits were purchased from Life Technologies (New, York, USA). Hydromount was purchased from National Diagnostics (St. Louis, USA). Moloney murine leukemia virus was obtained from Invitrogen (California, USA), TaqMan Universal PCR Master Mix from Applied Biosystems (California, USA), enhanced chemiluminescence (ECL-plus Western Blotting Detection System) and peroxidase-conjugated antibodies were purchased from Amersham Biosciences (Buckinghamshire, UK). Hydroxyurea was purchased from Sigma-Aldrich, (St. Louis, USA), enzyme linked immunosorbent assay from Roche Applied Science (Indianapolis, IN). Angiotensin II was purchased from Sigma-Aldrich (St. Louis, USA) and Angiotensin-II FITC was purchased from Thermo Fisher Scientific (Massachusetts, USA).
Cell Culture
Chinese Hamster Ovary (CHO, kindly supplied by Dr François Alhenc-Gelas from the Institut National de la Santé et de la Recherche Médicale, Paris, France) cells were stably transfected with a plasmid containing the sequence of the human ACE (CHO-ACE), as previously reported [7]. Similarly, CHO-AT1 cells were stably transfected with the plasmid pcDNA3, containing the sequence of the human AT1. Also, we used Melan-a (murine melanocytes) and TM-5 cells (murine melanoma cells). Cells were cultured in DMEM supplemented with 10% FBS, while melan-a and TM-5 were cultured in RPMI 1640 medium supplemented with 5% FBS. Cells were incubated at 37°C in a humidified atmosphere of 95% air and 5% CO2. Medium was changed every 3 or 4 days, and cells were subcultured, between days 6 and 8, by harvesting with trypsin-EDTA. Semi-confluent (80% to 90%) cells were used in all of the studies.
Preparation of siRNA
Potential target sites within the ACE gene were selected and then searched with NCBI Blast to confirm specificity for the protein. The siRNAs for ACE and scrambled sequence were prepared by a transcriptional-based method using the Ambion Silencer kit (Life Technologies, New York, USA), according to the manufacturer’s instructions. The sense and antisense oligonucleotides of siRNA were, respectively: siRNA ACE 5’ GCA GTA CAA CTC TCT GCT A 3’ and 5’ GCG GAT CAT AAA GAA GCT T 3’; siRNA scramble 5’ GCG ATG AGT AGC ATC TCT A 3’ and 5’GCA TGC GAC GAT GAC ATA A 3’. Validated siRNAs for clathrin heavy chain were obtained from Ambion (Life Technologies, New York, USA). The sense and antisense sequences were, respectively: siRNA cla 5′ UAA UCC AAU UCG AAG ACC AAU 3′ and 5′ GUA UGA UGC UGC UAA ACU A 3′. Single wall carbon nanotubes (CNT) were used to deliver each siRNA as previously described [10, 11]. Cells were used 48 hours after siRNA treatment as indicated.
Western Blotting
CHO-ACE and TM-5 cells were harvested as described and protein content was quantified according to Bradford protein assay. For GAPDH detection, mouse monoclonal anti-GAPDH antibody was used at a dilution of 1:5000. For ACE detection, a mouse monoclonal antibody was used at dilution of 1:1000. For Clathrin detection, a rabbit monoclonal anti-Clathrin was used at a dilution of 1:1000. The antibody incubation proceeded for 2 hours at room temperature. After washing, blots were incubated with HRP conjugated goat anti-mouse or anti-rabbit IgG secondary antibody at a dilution of 1:5000 at room temperature for 1 hour. Immuno detection was carried out using enhanced chemiluminescence [12].
Immunofluorescence
Confocal immunofluorescence was performed as described [12]. Briefly, CHO-ACE cells were seeded onto 6 well culture dishes and 24 hours later, treated with 1 μM Ang-II for the indicated times. Cells were fixed with 4% paraformaldehyde, permeabilized with PBS 1X/Triton 0.5% and blocked (PBS, BSA 10%, Triton 0.5%, goat serum 5%) for 1 hour. Cells were then incubated with anti-ACE (1:100), for 2 hours at room temperature. This was followed by incubation with DAPI and the specific secondary antibodies conjugated with Alexa-Fluor 488 or 633 (1:500) for 1 hour. Images were obtained using a Zeiss LSM 510 confocal microscope with 63X, 1.4 NA objective lens (Thornwood, USA) [10, 12].
Detection of calcium signals
Ca2+ signals were evaluated either by line-scanning or time-lapse confocal microscopy as described [13, 14]. Cells were incubated with 6 μM Fluo-4/AM for 20 min at 37°C. Coverslips containing the cells were transferred to a custom-built perfusion chamber and were observed using a 63X, 1.4 NA objective lens under a Zeiss LSM 510 confocal microscope. Fluo-4/AM was excited at 488 nm using a krypton/argon laser. Global Ca2+ transients were measured in both the nucleus and the cytosol.
Internalization assay
Internalization kinetic assays were performed as described previously (modified from Thomas et al. [15]). Briefly, the transfected cells were exposed to 0.4 nM of 3H-AngII in a receptor-binding buffer for 3 hours at 4°C. Thereafter, cells were extensively washed with ice-cold receptor-binding buffer and placed at 37°C for 2, 5, 10, 20 and 40 min. Incubations were stopped by placing the cells on ice. Acid-released and acid-resistant radioactive were separated and measured [16]. The percentage of internalized ligand at each time point was calculated from the ratio of the acid-resistant specific binding to the total (acid-resistant + acid-released) specific binding.
Measurement of BrDU incorporation
Proliferation was measured by BrdU incorporation using an enzyme-linked immunosorbent assay (Roche Applied Science, Indianapolis, IN), according to the manufacturer’s instructions.
Migration assays
Migration experiments were performed as previously described [17, 18]. TM-5 cells were grown in 12-well plates and cultured in serum-free medium for 24 hours before the experiments. The wound was achieved by scratching a pipette tip across the cell monolayer (approximately 1.3 mm in width). Hydroxyurea (1 mM) was always included in the tissue culture media to prevent cell proliferation. Cells were stimulated with 1 μM of Ang-II for the indicated times. The wound area was measured using the Northern Eclipse (Empix, Mississauga, Canada) software, and the percentage of wound closure at each time point was derived by the formula: (1 –[current wound size/initial wound size]) × 100.
Quantitative RT-PCR analysis of ACE mRNA in TM-5 and melan-a cells
Total RNA (750 ng) was reverse transcribed to cDNA using Moloney murine leukemia virus (Invitrogen, California, USA) according to the manufacturer’s instructions. The reaction product was amplified by real-time PCR on the 7000 Sequence Detection System (ABI Prism, Applied Biosystems, California, USA) using the TaqMan Universal PCR Master Mix (Applied Biosystems, California, USA). The thermal cycling conditions consisted by an initial denaturation step of 95°C for 10 minutes, 50 cycles at 95°C for 15 seconds, and 60°C for 1 minute. The experiments were performed in triplicate for each data point. The ACE mRNA abundance was quantified as a relative value compared to an internal reference - β-actin. The primers used for real-time PCR were: ACE (forward primer: 5’ TGA GAA AAG CAC GGA GGT ATC C 3’; reverse primer: 5’ AGA GTT TTG AAA GTT GCT CAC ATC A 3’); murine β-actin (GenBank accession No. NM007393), forward primer 5’ CTG GCC TCA CTG TCC ACC TT 3’ and reverse primer 5’ CGG ACT CAT CGT ACT CCT GCT T 3’. The ACE and β-actin mRNA expressions were obtained from the cycle threshold (Ct) associated with the exponential growth of the PCR products. Quantitative values for ACE mRNA expression were obtained by the parameter 2ΔCt, in which ΔCt represents the subtraction of the β-actin Ct values from the ACE receptor values.
Measurement of ACE activity using the fluorescent peptide Abz-FRK(Dnp)P-OH
ACE activity measurements in all the transfected and non-transfected cells were performed using the FRET peptide Abz-FRK(Dnp)P-OH as described [19].
In silico studies
To perform the protein-protein docking calculations, we used the structural coordinates of ACE (PDB entry 1O8A) [20] and PLCβ3 (PDB entry 3OHM) [21], obtained by X-ray crystallography. To predict the structures of the complex ACE-PLCβ3 [21] we performed a rigid-body protein-protein docking with the program ZDOCK 3.02, a freely protein docking server. The result presenting the lowest interaction energy was selected and subsequently evaluated as a function of the centroid-centroid distance in order to estimate the potential energy profile for the ACE-PLC β3 complex within the Universal classical force field [22] available in the Forcite Module of Materials Studio 5.5.
Statistical analysis
The results are expressed as mean values ± SEM, except where otherwise noted. Prism (GraphPad Prism Software, San Diego, CA) and Image J (NIH; Bethesda, MD) softwares were used for data and image analysis, respectively. Statistical significance was tested using One-way ANOVA followed by Bonferroni test, and p value < 0.05 was taken to indicate statistical significance.
Results
Binding of Ang-II to ACE leads to the complex internalization
In addition to the well-known effects of ACE as an enzyme that converts Ang-I to Ang-II [23], ACE has also been reported as a new receptor for Ang-II [7, 9]. In order to investigate the role Ang-II plays through ACE binding, we used CHO cells stably expressing somatic human ACE expression (CHO-ACE cell), in comparison with cells transfected with AT1 receptor (CHO-AT1 cell). Since it is well known that upon stimulation, AT1 receptors internalize [24, 25], we tested whether ACE undergoes a similar process upon Ang-II stimulation. In CHO-AT1 and CHO-ACE cells treated with radiolabeled Ang-II (3H-Ang-II), we found a more pronounced 3H-Ang-II internalization upon binding to ACE than to AT1 (Bmax in CHO-AT1: 69.6 ± 10.3%; Bmax in CHO-ACE: 79.6 ± 5.7%, n = 3, p<0.05), (Fig 1A). In order to check if this observation was not due to a matter of a difference on the expression level of AT1 and ACE, we performed western blotting and observed that the total expression levels of ACE and AT1 was the same (CHO-AT1: 0.46 ± 0.045 vs CHO-ACE: 0.42 ± 0.07), (Fig 1B). Similarly, the amount of internalized Ang-II-FITC on CHO-ACE was significantly higher than that on CHO-AT1 (CHO-ACE: 69.2 ± 9.3 a.u. vs CHO-AT1: 22.6 ± 1.3 a.u., n = 3, p<0.01), (Fig 1C). Cell membranes of both cells were counterstained with Wheat Germ Agglutinin (WGA). The purpose of using WGA as a probe is that it is a member of the lectin family that binds to N-acetyl-D-glucosamine and sialic acid residues found on the surface of cell membranes. It has been used extensively to stain surface of membranes [26] better than the usage of phalloindin toxins, for example, which in turn label the actin cytoskeleton, providing an indirect labeling of the membrane and not this component itself. A WGA staining of unstimulated cells is shown in order to exclude any possible membrane reorganization due to Ang-II (Fig 1D).
Fig 1. Ang-II induces ACE translocation to the nucleus.
(A) Internalization of AT1 and ACE in the presence of 4 nM 3H-Ang-II. Data are shown as mean from three independent experiments, each performed in duplicate. (B) CHO-ACE and CHO-AT1 cells present the same relative protein level of each respective receptor. (Values are mean ± S.E.M, *p<0.05, n = 63 individual experiments). (C) Representative confocal images of internalized Ang-II-FITC (1 μM) in CHO-ACE and CHO-AT1 cells after 30 seconds of Ang-II stimulation. DAPI (blue) and Wheat Germ Agglutinin (red), scale bar = 10 μm. On the right, quantification of internalized Ang-II-FITC is presented. Values are mean ± S.E.M, *p<0.05, n = 6. (D) Representative confocal images of unstimulated CHO-ACE and CHO-AT1 cells, labeled for DAPI and WGA. (E) Immunolocalization of ACE after stimulation with Ang-II (1μM), for the indicated times. ACE is shown in green, actin filaments in red, and nucleus in blue (DAPI). Right panel represents a 3D reconstruction of CHO-ACE cell after 15 minutes of incubation with Ang-II (1μM). Scale bar = 10μm. (F) Western blotting of nuclear and non-nuclear protein fractions from CHO-ACE cells, before (control) and after Ang-II (1 μM) stimulation for the indicated times. Histone-3 and GAPDH were used to shown the purification of nuclear and non-nuclear protein fractions, respectively. (G) Densitometry analysis of the western blot. Values are mean ± S.E.M., n = 3 (*** p<0.01).
Additionally, we observed that after Ang-II stimulation, ACE itself is also routed to the nucleus (Fig 1E). Nuclear and non-nuclear protein fraction, before and after Ang-II stimulation, confirmed the translocation of ACE from the cell membrane into the nuclear compartment (Nuclear fraction: Control = 0.1 ± 0.001 a.u., 5 min = 0.99 ± 0.09 a.u., 15 min = 1.08 ± 0.07 a.u.; Non-nuclear fraction: Control = 1.10 ± 0.01 a.u., 5 min = 0.94 ± 0.03 a.u., 15 min = 0.49 ± 0.04 a.u.; n = 3, p<0.01), (Fig 1F and 1G). Together, these results show that Ang-II induces internalization of ACE, and a subpopulation of these complexes translocates to the nucleus.
Phospholipase-C mediates the Ca2+ signals induced by ACE activation
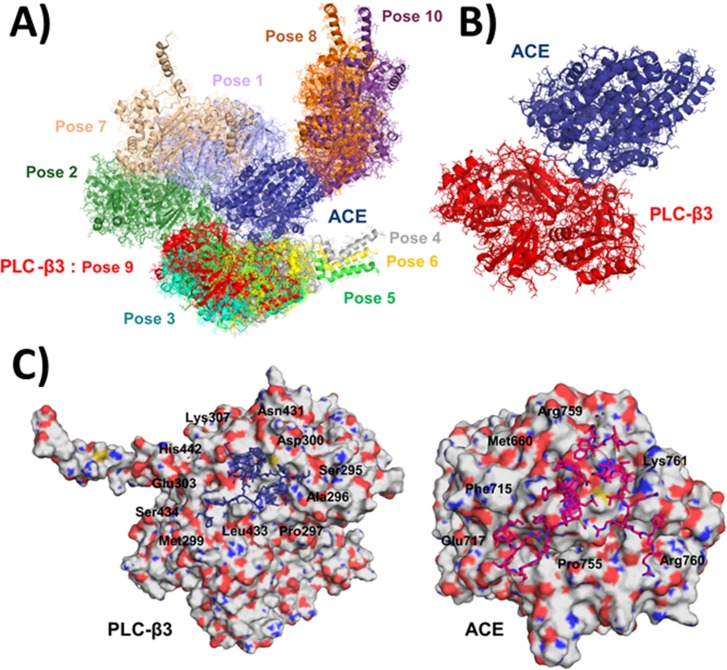
The binding of Ang-II to ACE is known to cause intracellular Ca2+ increase, through InsP3 formation [7]. We then investigated whether the membrane-associated phospholipase C (PLC) plays any role in the InsP3-mediated Ca2+ signals induced by ACE activation. Our in silico modeling studies of ACE [27] and PLC Beta 3 (β3) isoform [28] structures, suggested that there is a direct interaction between these molecules, which is independent of G-protein (Fig 2A–2D). The ten top ranked docking poses obtained with ZDOCK are presented in Fig 2A. The interaction energy obtained for the best docking pose of PLCβ3, Pose 9 (Fig 2B), possess an energy value of -194.06 kcal/mol, within the Universal classical force field. Fig 2C shows the amino acid residues located at the interface between the best docking pose of PLCβ3 (left panel) and ACE (right panel), Pose 9, explored through docking protocols.
Fig 2. Molecular interaction between ACE and PLC by computational analysis in silico.
(A) Representation of the ten top ranked docking poses for ACE (blue) with PLCβ3 protein superimposed (the color of the PLC protein pose correspond to the colors of the labels). (B) Structure of the complex between ACE (blue) and PLC-β3 (Pose 9, red). The binding energy for the best docking pose of ACE and PLC-β3, Pose 9, is -194.06 kcal/mol. C) Amino acid residues located at the interface between the best docking pose of PLC-β3 (left panel) and ACE (right panel), Pose 9, explored through docking protocols.
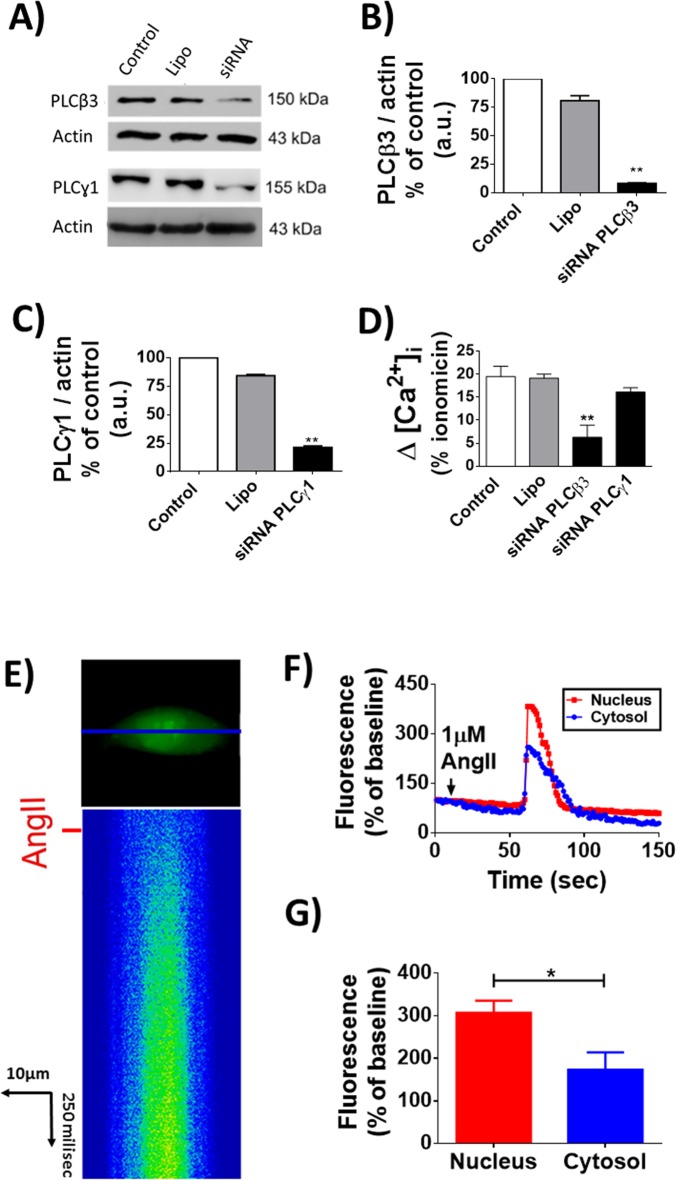
The importance of this interaction between PLC and ACE was experimentally validated by silencing of specific PLC isoforms followed by evaluation of Ang-II triggered Ca2+ signals in CHO-ACE cells. The effectiveness of silencing PLCβ3 or the PLC gamma (γ) isoforms was confirmed by western blotting (PLCβ3: Control = 100% vs siRNA PLCβ3 = 12 ± 0.15%; PLCγ: Control = 100% vs siRNA PLCγ = 24.5 ± 0.36%, n = 5, p<0.01 for each condition), (Fig 3A–3C). Silencing of PLCβ3 but not PLCγ in CHO-ACE decreased Ca2+ signaling induced by Ang-II (Control: 19.52 ± 2.2%, Lipo: 19.15 ± 0.85%, siRNA PLCβ3: 16.6 ± 1.9% and siRNA PLCγ: 16.57 ± 0.12% n = 3, p<0.01), (Fig 3D). Additionally, Ca2+ signals induced by Ang-II/ACE activation initiate and predominate in the nuclear compartment (Nucleus: 166.6 ± 43.2% vs Cytosol: 49.5 ± 15.8%, n = 3, p<0.05), (Fig 3E–3G). Together, these results indicate that upon Ang-II stimulation, ACE interacts with PLC to trigger an intracellular Ca2+ increase predominantly in the nucleus of CHO cells.
Fig 3. Effect of Ang-II on intracellular Ca2+ signaling in CHO-ACE cells.
(A) Western immunoblotting to confirm the silencing of PLC isoforms. Densitometric analysis are shown on (B) for PLCβ3 and (C) for PLCγ1. (D) Quantitative representation of intracellular [Ca2+] in CHO-ACE cells transfected with siRNA-PLCβ3 and PLCγ1 using Lipofectamine and stimulated with Ang-II (1μM). (E) Line scanning of Ca2+ signal in CHO-ACE. Ang-II promotes Ca2+ increase with greater intensity in the nuclear region. (F) Time course of Ca2+ signaling in the nucleus (red traces) and cytosol (blue traces). (G) Quantification of fluorescence intensity signal in the nucleus and cytosol. Values are mean ± S.E.M., (* p<0.01), 45 cells, n = 3 individual experiments.
Clathrin regulates endocytosis of ACE
Since our previous demonstrated that nucleoplasmic Ca2+ regulates cell proliferation [12, 29, 30] and the activation of ACE by Ang-II caused preferential nuclear Ca2+ increase, we investigated the role of ACE in cell growth. Ang-II stimulated CHO-ACE cells to proliferate faster compared to control non-stimulated group (CHO-ACE cells reached 1.1 ± 0.28 x 104 cells vs 1.5 x 103 ± 0.25 cells from the control group, after 15 hours of incubation in the presence of Ang-II, n = 4, p<0.05), (Fig 4A). Next, we investigated whether the nuclear translocation of ACE is necessary for the proliferative response. For that, we performed silencing of clathrin (Cla), which is involved in classic receptor endocytosis [31]. Our result showed an efficient silencing of Cla in CHO-ACE cells (siRNA SCR: 1.39 ± 0.01 vs siRNA Cla: 0.46 ± 0.025), (Fig 4B). BrDU assay showed that silencing of Cla inhibited cell proliferation stimulated by Ang-II on CHO-ACE cells (Data expressed as percentage of the control group. Ang-II: 25 ± 4.34%, siRNA Cla + Ang-II: 0 ± 5.4%, siRNA Scr + Ang-II: 20 ± 6.56%, n = 3, p<0.05), (Fig 4C). These results demonstrate that clathrin-mediated endocytosis is necessary to promote cell proliferation induced by Ang-II.
Fig 4. Cell proliferation induced by Ang-II/ACE involves clathrin- mediated internalization process.
(A) Cell growth assay of CHO-ACE cells 12, 24 and 48 hours after stimulation with Ang-II (1μM), triplicate in 3 individual experiments. (B) Western blot to confirm the silencing of clathrin (upper panel) and densitometry analysis (bottom panel). Mean ± S.E.M., n = 5 (* p<0.05). (C) BrDU incorporation is decreased in CHO-ACE cells transfected with siRNA-Cla (Clathrin) and stimulated with Ang-II (1μM). Mean ± S.E.M., n = 6 (* p<0.05).
Ang-II/ACE regulates melanoma cell proliferation and migration
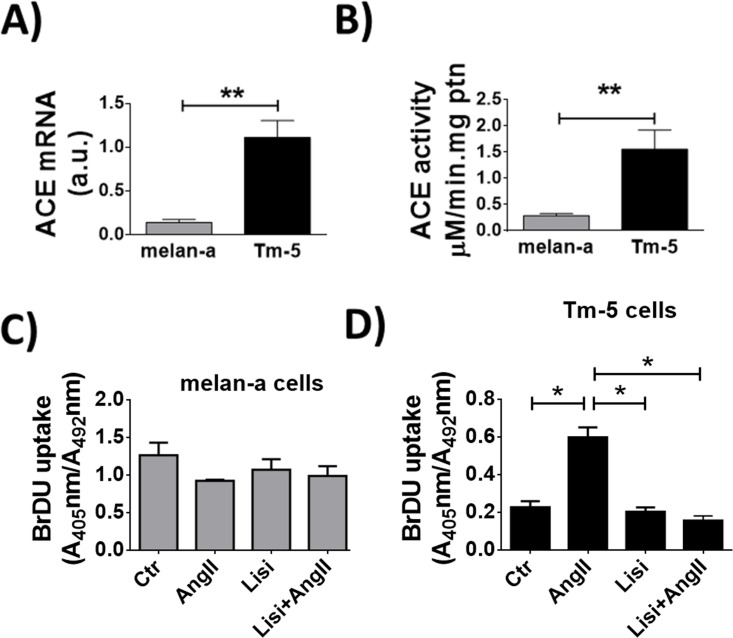
To further investigate the role of ACE on cell proliferation, we used the murine melanoma cells (TM-5), since TM-5 cells endogenously express ACE, but do not express AT1 and AT2 receptors [32]. ACE expression level is higher in TM-5 cells than in its control melanocyte counterpart, Melan-a cells (TM-5 cells: 1.1 ± 0.1 a.u. vs Melan-a cells: 0.14 ± 0.05 a.u., n = 5, p<0.01), (Fig 5A). As expected, ACE activity is also more pronounced in TM-5 compared to Melan-a (0.42 ± 0.1 in melan-a vs 1.80 ± 0.42 in TM-5, n = 3, p<0.05), (Fig 5B). No proliferation was observed in response to Ang-II stimulation in Melan-a cells (Control: 1.3± 0.2 Ang-II: 0.9 ± 0.1, Lisi: 1.2 ± 0.3, Lisi + Ang-II: 1 ± 0.2, n = 3, p = ns), (Fig 5C). Conversely, BrDU incorporation was increased in TM-5 upon Ang-II, an effect that was prevented by pre-treatment with ACE inhibitor, Lisinopril (Control: 0.2± 0.1, Ang-II: 0.6 ± 0.1, Lisi: 0.2 ± 0.1, Lisi + Ang-II: 0.19± 0.1, n = 3, p = <0.01), (Fig 5D).
Fig 5. Ang-II stimulates proliferation in melanoma cells.
(A) Real-Time PCR analysis for expression of ACE in melan-a and TM-5 cells. (B) ACE activity measured by cleavage of Abz-FRK(Dnp)P-OH in melan-a and TM-5 cells. For A and B, mean ± S.E.M, n = 6 (**p<0.01). (C-D) BrDU uptake assay 24 hours after stimulation with Ang-II (1μM) in the presence of lisinopril (1μM), showing inhibition of cell proliferation in TM-5 cells (D) but not in melan-a cells (C). Mean ± S.E.M., n = 6. (*p<0.05).
To address the potential direct involvement of ACE in TM-5 cell proliferation, ACE expression was silenced in this cell type (Control: 5.93 ± 0.2%, CNT: 5.78 ± 0.16%, siRNA SCR: 4 ± 0.1% and siRNA ACE: 0.3 ± 0.01%, n = 3, p<0.01), (Fig 6A). In cells with reduced levels of ACE, Ang-II was unable to induce TM-5 cell proliferation (Control: 147 ± 23%, siRNA SCR: 172 ± 9% and siRNA ACE: 0 ± 2%, n = 4, p<0.05), (Fig 6B), indicating that ACE activation is involved in melanoma proliferative response triggered by Ang-II.
Fig 6. ACE silencing inhibits the proliferative effect of Ang-II in melanoma cells.
(A) Western bot (upper panel) to confirm the silencing of ACE and densitometry analysis (bottom panel). Mean ± S.E.M, n = 8. (***p<0.01 compared to respective columns). (B) BrDU uptake in Tm5 cells silenced for ACE and stimulated for 24 hours with Ang-II (1μM), showing a decrease in BrDU incorporation in the absence of ACE. Mean ± S.E.M., n = 12 (*p<0.05; ns = non-significant).
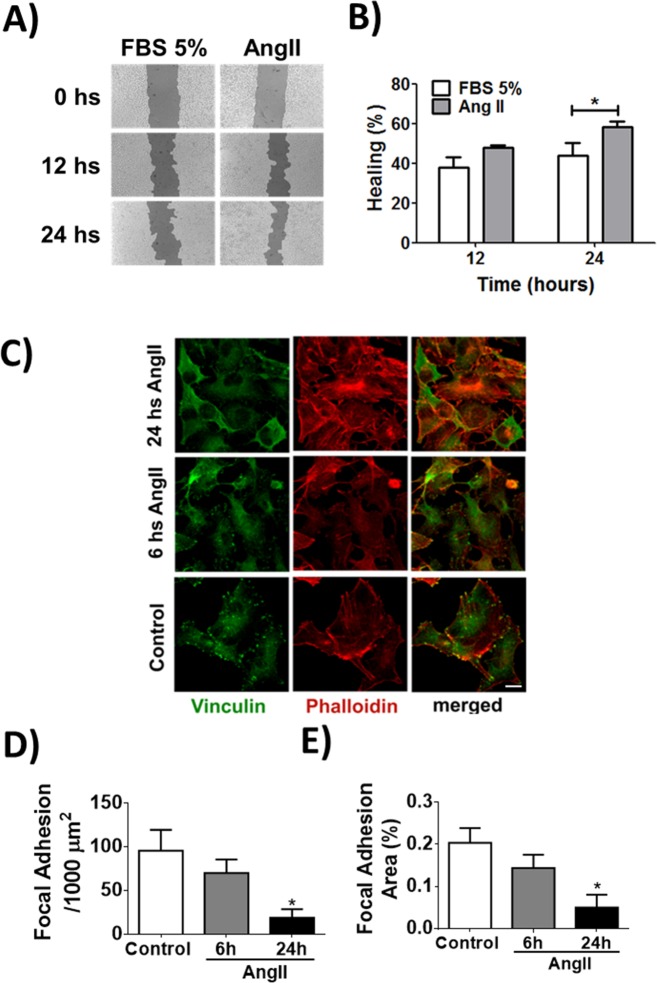
Melanoma is the most aggressive form of skin cancer [33]. When it has spread (metastatic melanoma), the prognosis is very poor. Indeed, this type of skin cancer has a great capacity of migration [33]. To investigate whether Ang-II induces migration of melanoma cells, we performed a scratch assay in the presence or absence of Ang-II. Ang-II stimulateed a faster healing process (Results expressed as the percentage of healing. FBS 12h: 37.7 ± 3.1%, Ang-II 12h: 47.9 ± 4.4%, FBS 24h: 44 ± 5.1%, Ang-II 24h: 58.2 ± 7.2%), (Fig 7A and 7B). Some recent findings showed that the filamentous (F)-actin-binding protein vinculin is required for cell polarization and migration [34], having a key role on the formation of focal adhesion points. In order to check if the increased healing observed during stimulation with Ang-II is due to alterations on the focal adhesion points, we performed immunofluorescence for vinculin. Under stimulation with Ang-II, TM-5 cells showed a decreased focal adhesion formation/1000μm2 (Control: 95.5 ± 23, Ang-II 6h: 69.9 ± 15.4, Ang-II 24h: 18.7 ± 9.8, n = 5, p<0.05), (Fig 7C–7E). Taken together, these data suggest that the reduction of focal adhesion observed in Ang-II stimulated melanocytes might contribute to the detachment and migration of melanoma cells.
Fig 7. Ang-II promotes cellular migration and reduces focal adhesion formation in melanoma cells.
(A-B) Wound healing assay using TM-5 cells stimulated with Ang-II (1μM). C) Representative confocal images of TM-5 cells double-labeled with vinculin (green) and phalloidin (red). Scale bar = 10 μm. (D-E) Quantification of the focal adhesion formation. Mean ± S.E.M., n = 6 (* p<0.05).
Discussion
The major form of somatic ACE involves two very similar protein domains (N- and C-domains) [35, 36], with the C-domain of ACE responsible for Ang-II formation [37, 38], the principal final product of the renin-angiotensin system. However, ACE is a relatively nonspecific peptidase that is capable of cleaving a wide range of substrates. Because of this, ACE and its peptide products affect many physiologic processes, involving blood pressure regulation, handling of electrolytes in the kidneys, hematopoiesis, reproduction, immune response among others [39]. Recently, evidence has been provided that ACE has kinase activity [4, 5] and also functions as a membrane receptor for Ang-II [7, 9]. Indeed, crystal structure of ACE c-domain in complex with Ang-II revealed detailed molecular interaction site between ACE and the peptide [9]. Once bound to ACE, Ang-II was shown to cause an InsP3-dependent intracellular Ca2+ increase [7]. In the current work, we showed that through ACE, Ang-II increases Ca2+ signals preferentially in the nucleus and modulates cellular functions such as proliferation and migration. We also demonstrated that ACE and Ang-II complex internalizes in a clathrin-dependent way.
Upon activation by ligand stimulation, several membrane receptors are internalized through the clathrin-coated endocytic pathway, suggesting an intranuclear signaling of both ligands and receptors [40]. For AT1 receptor, its internalization is initiated by G protein-coupled receptors (GPCRs) kinases that promote phosphorylation of aminoacid residues within the cytoplasmic tail of the receptor [41]. After that, the phosphorylated receptor can interact with arrestins (β- arrestins-1and-2), which in turn, promotes entry of the receptor into clathrin-coated pits [42]. Although we showed Cla took part on the ACE internalization, we have no evidences whether ACE interacts with β -arrestin in order to entry on clathrin-coated pits, as it has already been demonstrated for AT1 and other GPCRs [41, 43, 44, 45].
In the endosome-mediated nuclear translocation, the internalized endosome is usually directed by the nuclear transporter importin proteins, which recognize the nuclear localization signal of the proteins and direct them to the nucleus through the nuclear pore complex [46, 47]. However, the nuclear localization signal is not predicted in the entire ACE sequence [48], therefore nuclear import of ACE may require other mechanisms. Indeed, several receptors tyrosine kinases (RTKs), that lack the nuclear localization signal, have also been reported to localize in the nucleus [48]. In this aspect, we have previously described that the nuclear accumulation of the hepatocyte growth factor (HGF) receptor requires Gab1 participation [13]. Gab1 is an adaptor protein that contains a nuclear localization sequence and by binding to the target protein regulates its active import to the nucleus [49]. It is possible that a similar mechanism mediates ACE nuclear translocation. Additional questions include whether Ang-II remains bound to nuclear ACE and whether the nuclear localization of ACE may take place only at specific stages of the cell cycle. While more studies need to be done to characterize the nuclear translocation of ACE/Ang-II complex, we are now demonstrating that this internalization process is essential to cause a preferential nuclear Ca2+ increase.
Intracellular Ca2+ signals mediated by InsP3 rely on activation of PLC that causes hydrolysis of phosphatidylinositol bisphosphate (PIP2) [50]. This activation typically depends on heterotrimeric G protein subunits, but can also be triggered by protein tyrosine kinases, small G proteins, and phospholipids [51]. We now show that ACE may directly interact with PLC, and that in CHO cells, the PLCβ3 isoform, but not the PLCγ1, regulates the Ca2+ signals triggered by Ang-II/ACE pathway. One can thus speculate that the subpopulation of activated ACE that translocates to the nucleus might locally activate PLCβ3 to generate nuclear Ca2+ signals upon Ang-II stimulation. This is supported by data from other cell systems, which show expression of PLC family members not only at the plasma membrane, but also preferentially in the inner nuclear compartment [52]. Furthermore, a link between nuclear inositol lipid cycle and nuclear Ca2+ signal is well established, and the activation of this pathway has been shown to act independently from that at the plasma membrane [13, 53–58]. Moreover, it is known that mitogens such as insulin [53] and hepatocyte growth factor, once bound to their respective receptors [13] can lead to hydrolysis of the nuclear pool of PIP2, through a PLC-dependent mechanism, leading to a nuclear Ca2+ increase [50]. It is conceivable that a similar phenomenon occurs with ACE/Ang-II. However, since the selectively silencing of PLCβ3 did not completely abolish intracellular signals triggered by Ang-II and ACE interaction, we cannot exclude a partial contribution of other signaling molecules as well. Indeed, Guimarães and Alvarenga, 2011 [7] showed that either 2-APB (InsP3R antagonist) or Nifedipine (L-type voltage-gated Ca2+ channel blocker) were able to partially block ACE-evoked Ca2+ signaling in CHO-ACE cells, suggesting that, besides mobilizing intracellular Ca2+ stores, Ang-II binding to ACE also affects the opening of the voltage-gated Ca2+ channels. Therefore, the partial blockage of Ca2+ signaling due PLCβ signaling dumight be explained due the different Ca2+ pathways that are activated by Ang-II binding to ACE. Another possibility is that ACE/Ang-II links to a specific isoform of the InsP3R to trigger nuclear Ca2+ release. For instance, it is known that three InsP3 receptor isoforms exist and they have distinct sensitivity to InsP3 [50] and subcellular localizations, which would enable InsP3-mediated Ca2+ signals to occur preferentially in the nucleus, compared to the cytosol, after ACE activation [50].
Ca2+ signals within the nucleus are particularly important in cancer cell progression [50, 59, 60]. Buffering nuclear Ca2+ arrests adenocarcinoma cells in the early phase of mitosis [61], and sensitizes head and neck cancer cells to radiotherapy [59]. Therefore, the effect of Ang-II/ACE on nuclear Ca2+ signaling might explain the observed Ang-II’s action as a mitogen, in the melanoma cell line (TM-5), a murine cell type that endogenously expresses ACE, but lack Ang-II type 1 or type 2 receptors. We further show that ACE is involved in TM-5 cell migration, another aspect of melanoma carcinogenesis. Melanoma is a common cancer in the Western world with an increasing incidence highly due to sun exposure [62]. Transformation of melanocytes into melanoma encompasses a complex interplay of both endogenous and exogenous factors and it is known that its metastasis pattern can occur during either an earlier or a later phase, being guided by genetic or phenotypic drivers [63, 64]. Although Ang-II has been reported to regulate growth, adhesion, invasion and cell migration in certain cancer cells [65], this is the first report of Ang-II-induced melanocytes proliferation and migration mediated by binding to ACE.
It is well-accepted that either ACE inhibitors or AT1 blockers are the gold standard drugs in order to manage hypertension, due their survival benefits provided on patients with heart failure, high cardiac risk profile and also proteiunuric chronic kidney disease [66]. However, a combination therapy with ACE inhibitors and Ang-II receptor blockers has been extensively explored since the monotherapy has been shown efficient in only a quite limited number of hypertensive patients [67]. Another evidence that supports the usage of a combined therapy is the fact that monotherapy with ACE inhibitor increases the concentration of circulating Ang-I and this can partially mitigate inhibition of ACE, what turns out to restore the concentration of active Ang-II towards pretreatment levels [68, 69]. In addition to that, it is known that other enzymes distinct from ACE, and therefore not blocked by ACE inhibitors, can form Ang-II [70]. Indeed, patients with mild to moderate hypertension demonstrated a more prominent decrease in diastolic blood pressure when a combination therapy was used [71, 72]. However, the long-term effects of these combination therapies on blood pressure have still been questioned since they showed no benefits in terms of the composite of cardiovascular death, myocardial infarction, stroke and hospitalization for heart failure. In fact, it caused more symptoms attributable to hypotension, increased decline in renal function and need for dialysis compared to ACE inhibitor monotherapy [73].
There are several clinical evidences showing the chemopreventive effects of ACE-blocking in cancer [74]. The first evidence for the antitumor effects of ACE blockers was demonstrated in 1998 [75] in which the relative risk of fatal, incident and female-specific cancers was lower in women on ACE inhibitors [75]. In human squamous skin cancer cells, it was observed a prominent inhibitory effect on tumor growth and angiogenesis mediated by perindopril [76]. A similar finding was observed in a cohort study performed among a high-risk group of veterans using ACE inhibitors, showing a lower incidence of keratinocyte cancer when compared to nonusers. [77]. Specifically for cutaneous melanoma, captopril presented an antitumor activity in human melanoma xenograft model [78]. Additionally, it is already known that AT1 receptor plays an important role in angiogenesis and growth of tumor cell [79]. Administration of the AT1 blocker, TCV-116, significantly decreased melanoma tumor volume in mice [80]. However, more clinical studies are still needed in order to justify the usage of ACE inhibitors or AT1 blockers for treating melanoma and other malignancies.
Taken together, our findings here suggest a novel function of ACE in the pathology of melanoma and open new paths to further studies, where ACE, as a receptor, might function as a possible therapeutic target aiming to avoid the progression of the disease.
Acknowledgments
The authors acknowledge the technical assistance of Gilson Nogueira, and assistance of Jéssica S. Malta, Luiz Orlando Ladeira, Rodrigo R. Resende and Ana Cândida from Universidade Federal de Minas Gerais. We also thank Dr. Mateus Guerra from Yale University for the critical reading of the manuscript. This work was supported by FAPEMIG, CNPq, CAPES and INCT Nanocarbono–UFMG (Brazil).
Data Availability
All relevant data are within the paper.
Funding Statement
This research was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and INCT Nanocarbono – UFMG (Brazil). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ferrario CM. The renin-angiotensin system: importance in physiology and pathology. J Cardiovasc Pharmacol. 1990; 15(3):S1–5. [PubMed] [Google Scholar]
- 2.Johnston CI, Fabris B and Jandeleit K. Intrarenal renin-angiotensin system in renal physiology and pathophysiology. Kidney Int Suppl. 1993; 42:S59 –S63. [PubMed] [Google Scholar]
- 3.Wang RF, Podos SM, Serle JB and Baltatu OC. Effect of SPP 635, a renin inhibitor, on intraocular pressure in glaucomatous monkey eyes. Experimental Eye Research. 2012; 94:146–149. 10.1016/j.exer.2011.11.019 [DOI] [PubMed] [Google Scholar]
- 4.Kohlstedt K, Brandes RP, Müller-Esterl W, Busse R, and Fleming I. Angiotensin-converting enzyme is involved in outside-in signaling in endothelial cells. Circ Res. 2004; 94:60–67. 10.1161/01.RES.0000107195.13573.E4 [DOI] [PubMed] [Google Scholar]
- 5.Fleming I. Signaling by the angiotensin-converting enzyme. Circ Res. 2006; 98:887–896. 10.1161/01.RES.0000217340.40936.53 [DOI] [PubMed] [Google Scholar]
- 6.Kohlstedt K, Busse R, and Fleming I. Signaling via the angiotensin-converting enzyme enhances the expression of cyclooxygenase-2 in endothelial cells. Hypertension. 2005; 45:126–132. 10.1161/01.HYP.0000150159.48992.11 [DOI] [PubMed] [Google Scholar]
- 7.Guimarães PB, Alvarenga EC, Siqueira PD, Paredes-Gamero EJ, Sabatiini RA, Morais RL, et al. Angiotensin II Binding to Angiotensin I-Converting Enzyme Triggers Calcium Signaling. Hypertension. 2001; 5 7(5):965–972. [DOI] [PubMed] [Google Scholar]
- 8.Fyhrquist F and Saijonmaa O. Renin-angiotensin system revisited. J. Intern. Med. 2008; 264,224–236. 10.1111/j.1365-2796.2008.01981.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masuyer G, Schwager SLU, Sturrock ED, Isaac RE and Acharya KR. Molecular recognition and regulation of human angiotensin-1 converting enzyme (ACE) activity by natural inhibitory peptides. Sci Rep. 2012; 2, 717 10.1038/srep00717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aguiar CJ, Andrade VL, Gomes ER, Alves MN, Ladeira MS, Pinheiro AC, et al. Succinate modulates Ca (2+) transient and cardiomyocyte viability through PKA-dependent pathway. Cell Calcium. 2010; 47: 37–46. 10.1016/j.ceca.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 11.Ladeira MS, Andrade VA, Gomes ER, Aguiar CJ, Moraes ER, Soares JS, et al. Highly efficient siRNA delivery system into human and murine cells using single-wallcarbon nanotubes. Nanotechnology. 2010, 21: 385101 10.1088/0957-4484/21/38/385101 [DOI] [PubMed] [Google Scholar]
- 12.Amaya MJ, Oliveira AG, Guimarães ES, Casteluber MCF, Carvalho SM, Andrade LM, et al. The insulin receptor translocates to the nucleus to regulate cell proliferation in liver. Hepatology. 2014; 59(1):274–283. 10.1002/hep.26609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomes DA, Rodrigues MA, Leite MF, Gomez MV, Varnai P, Balla T, et al. c-Met must translocate to the nucleus to initiate calcium signals. J.Biol.Chem. 2008; 283:4344–4351. 10.1074/jbc.M706550200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Echevarria W, Leite MF, Guerra MT, Zipfel WR and Nathanson MH. Regulation of calcium signals in the nucleus by a nucleoplasmic reticulum. Nat.Cell Biol. 2003; 5:440–446. 10.1038/ncb980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas WG, Baker KM, Motel TJ and Thekkumkara TJ. Angiotensin II receptor endocytosis involves two distinct regions of the cytoplasmic tail. A role for residues on the hydrophobic face of a putative amphipathic helix. J. Biol. Chem. 1995; 270 (38), 22153–22159. [DOI] [PubMed] [Google Scholar]
- 16.Leite MF, Page EC and Ambler SK. Regulation of atrial natriuretic peptide secretion by endothelin-1: desensitization and receptor subtype. Am J Physiol. 1994; 267: H2193–203. [DOI] [PubMed] [Google Scholar]
- 17.Liang CC, Park AY and Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007; 2(2):329–333. 10.1038/nprot.2007.30 [DOI] [PubMed] [Google Scholar]
- 18.Kapoor M, Liu S, Shi-wen X, Huh K, McCann M, Denton CP, et al. GSK-3beta in mouse fibroblasts controls wound healing and fibrosis through an endothelin-1-dependent mechanism. J Clin Invest. 2008; 118(10):3279–3290. 10.1172/JCI35381 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Sabatini RA, Bersanetti PA, Farias SL, Juliano L, Juliano MA, Casarini DE, et al. Determination of angiotensin I-converting enzyme activity in cell culture using fluorescence resonance energy transfer peptides. Anal Biochem. 2007; 15:255–262. [DOI] [PubMed] [Google Scholar]
- 20.Inducible K, Ramanathan N, Sylva S, and Edward S. Crystal Structure of the Human Enzyme–Lisinopril Complex. Nature. 2003; 1429(1995): 1427–1429. [Google Scholar]
- 21.Waldo GL, Ricks TK, Hicks SN, Cheever ML, Kawano T, Tsuboi K, et al. Kinetic Scaffolding Mediated by a Phospholipase C–β and Gq Signaling Complex. Structure. 2010; 21:974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pierce BG, Hourai Y and Weng Z. Accelerating Protein Docking in ZDOCK Using an Advanced 3D Convolution Library. PLoS One; 2011; 6(9):246–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masuyer G, Yates CJ, Sturrock ED and Acharya KR. Angiotensin-I converting enzyme (ACE): structure, biological roles, and molecular basis for chloride ion dependence. Biol Chem. 2014; 395(10):1135–1149. 10.1515/hsz-2014-0157 [DOI] [PubMed] [Google Scholar]
- 24.Petrel C and Clauser E. Angiotensin II AT1 receptor constitutive activation: From molecular mechanisms to pathophysiology. Mol Cell Endocrinol. 2009; 302(2): 176–184. 10.1016/j.mce.2008.10.049 [DOI] [PubMed] [Google Scholar]
- 25.Bhuiyan MA1, Hossain M, Nakamura T, Ozaki M and Nagatomo T. Internalization of constitutively active N111G MUTANT of AT1 receptor induced by angiotensin II-receptor antagonists candesartan, losartan, and telmisartan: comparison with valsartan. J Pharmacol Sci. 2010; 112(4):459–62. [DOI] [PubMed] [Google Scholar]
- 26.Andrade LM, Geraldo JML, Gonçalves OX, Leite MTT, Catarina AM, Guimarães MM, et al. Nucleoplasmic Calcium Buffering Sensitizes Human Squamous Cell Carcinoma to Anticancer Therapy. J Cancer Sci Ther.Volume 2012; 4(5): 131–139. [Google Scholar]
- 27.Hooper NM and Turner AJ. An ACE structure. Nature structural biology. 2003; 10(3): 155–157. 10.1038/nsb0303-155 [DOI] [PubMed] [Google Scholar]
- 28.Koss H, Bunney TD, Behjati S and Katan M. Dysfunction of phospholipase C- gamma in immune disorders and cancer Trends Biochem. Sci. 2014; 39:603–611. [DOI] [PubMed] [Google Scholar]
- 29.Rodrigues MA, Gomes DA, Leite MF, Grant W, Zhang L, Lam W, et al. Nucleoplasmic calcium is required for cell proliferation. J. Biol. Chem. 2007; 282:17061–17068. 10.1074/jbc.M700490200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andrade LM, Geraldo JML, Gonçalves OX, Leite MTT, Catarina AM, Guimarães MM, et al. Nucleoplasmic Calcium Buffering Sensitizes Human Squamous Cell Carcinoma to Anticancer Therapy. J Cancer Sci Ther.Volume 2012; 4(5): 131–139. [Google Scholar]
- 31.Brodsky FM, Chen CY, Knuehl C, Towler MC and Wakeham DE. Biological basket weaving: Formation and function of clathrin-coated vesicles. Annu Rev Cell Dev Biol. 2001; 17:517–568. 10.1146/annurev.cellbio.17.1.517 [DOI] [PubMed] [Google Scholar]
- 32.Correa M, Machado J, Carneiro CR, Pesquero JB, Bader M, Travassos LR, et al. Transient inflammatory response induced by apoptotic cells is an important mediator of melanoma cell engraftment and growth. Int J Cancer. 2005; 114:356–363. 10.1002/ijc.20673 [DOI] [PubMed] [Google Scholar]
- 33.Miller AJ and Mihm MC Jr. Melanoma. N Engl J Med. 2006;355(1):51–65. 10.1056/NEJMra052166 [DOI] [PubMed] [Google Scholar]
- 34.Thievessen N, Fakhri J, Steinwachs V, Kraus RS, McIsaac L, Gao BC et al. Vinculin is required for cell polarization, migration, and extracellular matrix remodeling in 3D collagen. FASEB J. 2015; 29:4555–4567 10.1096/fj.14-268235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Acharya KR, Sturrock ED, Riordan JF and Ehlers MR. ACE revisited: a new target for structure-based drug design. Nat. Rev. Drug Discov. 2003; 2: 891–902. 10.1038/nrd1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watermeyer JM, Kroger WL, Sturrock ED and Ehlers MR. Angiotensin converting enzyme—New insights into structure, biological significance and prospects for domain-selective inhibitors. Current Enzyme Inhibition. 2009; 5:134–147. [Google Scholar]
- 37.Fuchs S, Xiao HD, Hubert C, Michaud A, Campbell DJ, Adams JW, et al. Angiotensin-converting enzyme C-terminal catalytic domain is the main site of angiotensin I cleavage in vivo. Hypertension. 2008; 51, 267–274. 10.1161/HYPERTENSIONAHA.107.097865 [DOI] [PubMed] [Google Scholar]
- 38.Van Esch JH, Tom B, Dive V, Batenburg WW, Georgiadis D, Yiotakis A, et al. Selective angiotensin-converting enzyme C-domain inhibition is sufficient to prevent angiotensin I-induced vasoconstriction. Hypertension 2005; 45:120–125. 10.1161/01.HYP.0000151323.93372.f5 [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez-Villalobos RA, Janjoulia T, Fletcher NK, Giani JF, Nguyen MT, Riquier-Brison AD, et al. The absence of intrarenal ACE protects against hypertension. J Clin Invest. 2013; 123:2011–2023. 10.1172/JCI65460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sorkin A and Goh LK. Endocytosis and intracellular trafficking of ErbBs. Exp Cell Res. 2008; 314:3093–3106. 10.1016/j.yexcr.2008.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahn S, Shenoy SK,Wei H and Lefkowitz RJ. Differential kinetic and spatial patterns of {beta}-arrestin and G-protein-mediated ERK activation by the angiotensin-II receptor. J. Biol. Chem. 2004; 279: 35518–35525. 10.1074/jbc.M405878200 [DOI] [PubMed] [Google Scholar]
- 42.Kule CE, Karoor V, Day JNE, Thomas WT, Baker KM, Dinh D, et al. Agonist-dependent internalization of the angiotensin II type one receptor (AT1): role of C-terminus phosphorylation in recruitment of h-arrestins. Regulatory Peptides. 2004; 120:141–148. 10.1016/j.regpep.2004.03.001 [DOI] [PubMed] [Google Scholar]
- 43.Touyz RM and Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol. Rev. 2000; 52,639–672. [PubMed] [Google Scholar]
- 44.Pierce KL and Lefkowitz RJ. Classical and new roles of beta-arrestins in the regulation of G-protein-coupled receptors. Nat. Rev. Neurosci. 2001; 2: 727–733. 10.1038/35094577 [DOI] [PubMed] [Google Scholar]
- 45.Drake MT, Shenoy SK and Lefkowitz RJ. Trafficking of G protein-coupled receptors. Circ. Res. 2006; 99: 570–582. 10.1161/01.RES.0000242563.47507.ce [DOI] [PubMed] [Google Scholar]
- 46.Huotari J and Helenius A. Endosome maturation. EMBO J. 2011; 30:3481–500. 10.1038/emboj.2011.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Izaurralde E. and Adam S. Transport of macromolecules between the nucleus and the cytoplasm. RNA. 1998; 4: 351–364 [PMC free article] [PubMed] [Google Scholar]
- 48.Lucero HA, Kintsurashvili E, Marketou ME and Gavras H. Cell Signaling, Internalization, and Nuclear Localization of the Angiotensin Converting Enzyme in Smooth Muscle and Endothelial Cells. J Biol Chem. 2010; 285(8): 5555–5568. 10.1074/jbc.M109.074740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gu H and Neel BG. The “Gab” in signal transduction. Trends Cell Biol. 2003; 13: 122–130. [DOI] [PubMed] [Google Scholar]
- 50.Oliveira AG, Guimaraes ES, Andrade LM, Menezes GB and Leite MF. Decoding Calcium Signaling Across the Nucleus. Physiology. 2014; 29: 361–368. 10.1152/physiol.00056.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kadamur G and Ross EM. Mammalian Phospholipase C. Annual Review of Physiology. 2013; 75: 127–154. 10.1146/annurev-physiol-030212-183750 [DOI] [PubMed] [Google Scholar]
- 52.Kim CG, Park D and Rhee SG. The role of carboxyl-terminal basic amino acids in Gq alpha-dependent activation, particulate association, and nuclear localization of phospholipase C-beta1. J Biol Chem. 1996; 271: 21187–21192. [DOI] [PubMed] [Google Scholar]
- 53.Rodrigues MA, Gomes DA, Andrade VA, Leite, MF and Nathanson MH. Insulin induces calcium signals in the nucleus of rat hepatocytes. Hepatology. 2008; 48:1621–1631. 10.1002/hep.22424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Divecha N, Banc H and Irvine RF. The polyphosphoinositide cycle exists in the nuclei of Swiss 3T3 cells under the control of a receptor (for IGF-I) in the plasma membrane, and stimulation of the cycle increases nuclear diacylglycerol and apparently induces translocation of protein kinase C to the nucleus. EMBO J. 1991; 10: 3207–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martelli AM, Gilmour RS, Bertagnolo V, Neri LM, Manzoli L and Cocco L. Nuclear localization and signalling activity of phosphoinositidase C beta in Swiss 3T3 cells. Nature. 1992; 358: 242–245. 10.1038/358242a0 [DOI] [PubMed] [Google Scholar]
- 56.York JD, Satz E and Majerus PW. Inositol Polyphosphate 1-Phosphatase Is Present in the Nucleus and Inhibits DNA Synthesis J. Biol. Chem. 1994; 269: 19992–19999. [PubMed] [Google Scholar]
- 57.Cocco L, Martelli AM, Gilmour RS, Ognibene A, Manzoli FA and Irvine RF. Rapid changes in phospholipid metabolism in the nuclei of Swiss 3T3 cells induced by treatment of the cells with insulin-like growth factor I. Biochem Biophys Res Commun. 1988; 154(3):1266–1272. [DOI] [PubMed] [Google Scholar]
- 58.Manzoli L, Billi AM, Gilmour RS, Martelli AM, Matteucci A, Rubbini S, et al. Phosphoinositide signaling in nuclei of friend-cells-tiazofurin down-regulates phospholipase-C beta (1). Cancer Res. 1995; 55:2978–2980 [PubMed] [Google Scholar]
- 59.Andrade LM, Geraldo JM, Goncalves OX, Meite MT, Anderson MC, Yokoo S, et al. Nucleoplasmic Calcium Buffering Sensitizes Human Squamous Cell Carcinoma To Anticancer Therapy. Journal of Cancer Science & Therapy. 2012; 4: 131–139. [Google Scholar]
- 60.Rodrigues MA, DA, Nathanson MH and Leite MF. Nuclear Calcium signaling: a cell within a cell. Brazilian Journal of Medical and Biological Research. 2009; 42:17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rodrigues MA, Gomes DA, Leite MF, Grant W, Zhang L, Lan W, et al. Nucleoplasmic calcium is required for cell proliferation. The Journal of Biological Chemistry. 2007; 282: 17061–17068. 10.1074/jbc.M700490200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013; 499: 214–218. 10.1038/nature12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Damsky WE, Theodosakis N and Bosenberg M. Melanoma metastasis: new concepts and evolving paradigms. Oncogene. 2014; 33:2413–2422. 10.1038/onc.2013.194 [DOI] [PubMed] [Google Scholar]
- 64.Klein CA. Parallel progression of primary tumours and metastases. Nat. Rev. Cancer. 2009; 9:302–312. 10.1038/nrc2627 [DOI] [PubMed] [Google Scholar]
- 65.Rodrigues-Ferreira S, Abdelkarim M, Dillenburg-Pilla P, Luissint AC, di-Tomasso A, Deshayes F. et al. Angiotensin-II facilitates breast cancer cell migration and metastasis. PLoS One. 2012; 7: 35667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sica DA. Combination ACE inhibitor and angiotensin receptor blocker therapy–future considerations. J Clin Hypertens (Greenwich). 2007; 9(1):78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taddei S. Combination therapy in hypertension: what are the best options according to clinical pharmacology principles and controlled clinical trial evidence? Am J Cardiovasc Drugs. 2015; 15(3): 185–94. 10.1007/s40256-015-0116-5 [DOI] [PubMed] [Google Scholar]
- 68.Ménard J, Guyene TT, Chatellier G, Kleinbloesem CH and Bernadet P. Renin release regulation during acute renin inhibition in normal volunteers. Hypertension. 1991; 18(3):257–265. [DOI] [PubMed] [Google Scholar]
- 69.van den Meiracker AH, Man in 't Veld AJ, Admiraal PJ, Ritsema van Eck HJ, Boomsma F, Derkx FH, et al. Partial escape of angiotensin converting enzyme (ACE) inhibition during prolonged ACE inhibitor treatment: does it exist and does it affect the antihypertensive response? J Hypertens. 1992; 10(8):803–812. [PubMed] [Google Scholar]
- 70.Caughey GH. Mast cell tryptases and chymases in inflammation and host defense. Immunol Rev. 2007; 217:141–54. 10.1111/j.1600-065X.2007.00509.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Azizi M, Linhart A, Alexander J, Goldberg A, Menten J, Sweet C, et al. Pilot study of combined blockade of the renin-angiotensin system in essential hypertensive patients. J Hypertens. 2000; 18:1139–1147. [DOI] [PubMed] [Google Scholar]
- 72.Stergiou GS, Skeva II, Baibas NM, Roussias LG, Kalkana CB, Achimastos AD, et al. Additive hypotensive effect of angiotensin-converting enzyme inhibition and angiotensin-receptor antagonism in essential hypertension. J Cardiovasc Pharmacol. 2000; 35:937–941. [DOI] [PubMed] [Google Scholar]
- 73.Ritter JM. Dual blockade of the renin-angiotensin system with angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs). Br J Clin Pharmacol. 2011; 71(3): 313–315. 10.1111/j.1365-2125.2011.03918.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.George AJ, Thomas WG and Hannan RD. The renin-angiotensin system and cancer: old dog, new tricks. Nat Rev Cancer. 2010; 10(11): 745–759. 10.1038/nrc2945 [DOI] [PubMed] [Google Scholar]
- 75.Lever AF, Hole DJ, Gillis CR, McCallum IR, McInnes GT, MacKinnon PL, et al. Do inhibitors of angiotensin-I-converting enzyme protect against risk of cancer? Lancet. 1998; 352, 179–184. 10.1016/S0140-6736(98)03228-0 [DOI] [PubMed] [Google Scholar]
- 76.Yasumatsu R, Nakashima T, Masuda M, Ito A, Kuratomi Y, Nakagawa T, et al. Effects of the angiotensin–I converting enzyme inhibitor perindopril on tumor growth and angiogenesis in head and neck squamous cell carcinoma cells. J Cancer Res Clin Oncol. 2004; 130(10): 567–573. 10.1007/s00432-004-0582-7 [DOI] [PubMed] [Google Scholar]
- 77.Christian JB, Lapane KL, Hume AL, Eaton CB and Weinstock MA. Association of ACE inhibitors and angiotensin receptor blockers with keratinocyte cancer prevention in the randomized VATTC trial. J Natl Cancer Inst. 2008; 100 (17): 1223–1232. 10.1093/jnci/djn262 [DOI] [PubMed] [Google Scholar]
- 78.De Groot-Besseling RR, Ruers TJ, van Kraats AA, Poelen GJ, Ruiter DJ, de Waal RM, et al. Anti-tumor activity of a combination of plasminogen activator and captopril in a human melanoma xenograft model. Int J Cancer. 2004; 112(2): 329–334. 10.1002/ijc.20400 [DOI] [PubMed] [Google Scholar]
- 79.Deshayes F and Nahmias C. Angiotensin receptors: a new role in cancer? Trends Endocrinol Metab. 2005; 16(7): 293–299. 10.1016/j.tem.2005.07.009 [DOI] [PubMed] [Google Scholar]
- 80.Egami K, Murohara T, Shimada K, Shitani S, Sugaya T, Ishii M, et al. Role of host angiotensin-II type 1 receptor in tumor angiogenesis and growth. J Clin Invest. 2003; 112(1):67–75. 10.1172/JCI16645 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.