Abstract
Douchi is a type of Chinese traditional fermented food that is an important source of protein and is used in flavouring ingredients. The end product is affected by the microbial community present during fermentation, but exactly how microbes influence the fermentation process remains poorly understood. We used an Illumina MiSeq approach to investigate bacterial and fungal community diversity during both douchi-koji making and fermentation. A total of 181,443 high quality bacterial 16S rRNA sequences and 221,059 high quality fungal internal transcribed spacer reads were used for taxonomic classification, revealing eight bacterial and three fungal phyla. Firmicutes, Actinobacteria and Proteobacteria were the dominant bacterial phyla, while Ascomycota and Zygomycota were the dominant fungal phyla. At the genus level, Staphylococcus and Weissella were the dominant bacteria, while Aspergillus and Lichtheimia were the dominant fungi. Principal coordinate analysis showed structural separation between the composition of bacteria in koji making and fermentation. However, multivariate analysis of variance based on unweighted UniFrac distances did identify distinct differences (p <0.05), and redundancy analysis identified two key genera that are largely responsible for the differences in bacterial composition between the two steps. Staphylococcus was enriched in koji making, while Corynebacterium was enriched in fermentation. This is the first investigation to integrate douchi fermentation and koji making and fermentation processes through this technological approach. The results provide insight into the microbiome of the douchi fermentation process, and reveal a structural separation that may be stratified by the environment during the production of this traditional fermented food.
Introduction
Douchi is a fermented food that is important for flavouring food and that has been produced in China and several other countries for thousands of years [1]. Traditional Chinese medicine also uses douchi for treating dyspepsia, restlessness, asthma, and to increase sweating [2,3]. Recent studies indicated that douchi can inhibit prostate and breast cancer [4], and it also displays anti-diabetic activity [5] and protects against osteoporosis and cardiovascular diseases [6].
Douchi is produced through the fermentation of soybean by naturally occurring microorganisms. In general, douchi is produced in two stages; an initial koji making stage, and a subsequent fermentation stage. To make douchi, black beans used as the raw material are screened, steamed at 115°C for 30 min to soften, cooled, and treated with the ‘house flora’ that initiates koji making. At this stage, a koji inoculum (5%) from a later stage of production is inoculated into the black beans and koji making is continued for 7 days until the beans are covered with a white mold. Room temperature is maintained throughout koji making, and koji is washed in order to remove mycelia and spores that could otherwise make the douchi taste bitter [7]. The black beans are then mixed with salt (5% w/w) to prevent the growth of microorganisms and transferred to fermentation tanks which are sealed to exclude oxygen. The fermentation stage lasts for 15 days at a temperature of ~55°C, and the final product is dried in an oven. During the koji making stage, Aspergillus oryzaethe is traditionally used for inoculation, while other microorganisms such as Bacillus spp. are used to produce proteases for the degradation of peptides in the subsequent fermentation stage. During koji making, these microorganisms are a source of various enzymes and metabolites for fermentation, including glutaminase, cellulose, proteases, lipases, amylases and fibrinolytic enzymes, that are needed for the degradation of key ingredients such as lipids, proteins, carbohydrates and other functional constituents [8]. Fermentation is then performed in fermentation tanks, and this stage creates the characteristic flavours and nutritional content. Since douchi fermentation is performed in a relatively uncontrolled and spontaneous manner, the results can be inconsistent, and a better understanding of the microbial community diversity during fermentation would be useful for ensuring adequate quality of the final product.
The influence of the molecular ecology and culturing method on the diversity, composition and dynamics of the microbial community have been investigated using cell cultures, colony counting, denaturing gradient gel electrophoresis [9,10], temperature gradient gel electrophoresis [11–13], microarray [14] and length heterogeneity polymerase chain reaction [15,16]. However, a complete understanding of the microbial community remains elusive [17]. Illumina MiSeq has been used to investigate many microbial ecosystems, including milk [17,18], landfill leachate treatment [19], Italian salami [20], gut [21], and cheese [22–24]. Such high-throughput sequencing approaches can provide a more comprehensive insight into microbial community diversity without the bias associated with some of the other techniques [25]. Furthermore, Illumina MiSeq has the benefit of easy miniaturization and parallelization, which dramatically decreases the cost of microbial community diversity analysis.
Although high-throughput sequencing is now widely used, until now this approach has not been employed to study microbial community diversity in the douchi fermentation process. In the present study, we used Illumina MiSeq to study microbial community diversity in the douchi fermentation process. The results should enhance our understanding of the microbiome in this traditional fermented food.
Materials and Methods
Sampling
A total of 10 samples were included in this study. Samples were collected from koji making and fermentation in the workshop of Daoxiangyuan corporation (Jiangxi province, China). All samples were taken from the same site. Samples were taken from koji making on day 1, 3, 5, and 7, and from fermentation on day 1, 3, 6, 9, 12, and 15. Samples (100 g) were collected in triplicate, mixed together to reduce errors and immediately stored at −80°C for subsequent analysis.
DNA extraction
DNA was extracted during koji making and fermentation with an OMEGA E.Z.N.A soil DNA kit purchased from Feiyang BIOTECH Co., Ltd. (Guangzhou, China) according to the manufacturer’s instructions without modifications [26]. DNA quality was monitored by 0.8% agarose gel electrophoresis, and DNA was stored at −80°C for further analysis.
Illumina MiSeq sequencing
The V4 region of the 16S rRNA was amplified using primers 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′), and the internal transcribed spacer 1 (ITS 1) region of fungi amplicons was amplified with forward (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and reverse (5′-GCTGCGTTCTTCATCGATGC-3′) primers. 5′-barcoded amplicons were generated using Ex Taq HS (TaKaRa Bio Inc., Shiga, Japan) by PCR under conditions of 2 min at 95°C, followed by 35 cycles at 95°C for 30 s, annealing at 55°C for 1 min, extension at 72°C for 1 min, and a final extension at 72°C for 10 min. Amplicons were pooled in equimolar concentrations and sequenced by an Illumina MiSeq platform and MiSeq Reagent Kit v1 (Illumina, Inc., Santiago, CA, USA) at the Beijing Genomics Institute (Shenzhen, China).
Clean data were obtained using scripts written in house as follows: (1) removal of sequences containing more than one ambiguous base (N); (2) confirmation of barcode and adaptor completeness; (3) removal of sequences shorter than 100 bp. All 250 bp pair-end sequence reads were connected using COPE software (Connecting Overlapped Pair-end, V 1.2.1) [27] to merge read pairs into tags from DNA fragments. Further data processing was performed as previously described [28–32,18] and included removal of sequencing noise using the pre.cluster tool in the MOTHUR software package v. 1.31.2 [33], and de novo chimera detection and removal in UCHIME v. 4.2. Operational taxonomic units (OTUs) were also determined using MOTHUR, with a 97% sequence identity threshold [33]. 16S rRNA reads were assigned using 16S rRNA training set 9 in the RDP database using a local BLAST search. A local BLAST search was also used to assign ITS 1 reads with the NCBI GenBank database (for all ITS 1 sequences with taxonomic annotations).
Statistical analysis
Alpha diversity including Chao 1, Simpson diversity and Shannon diversity indices, as well as observed species and phylogenetic diversity [34], were subjected to statistical analysis using the MOTHUR package. Principal coordinate analysis (PCoA), which was used to measure dissimilarity at phylogenetic distances based on UniFrac analysis, was performed with QIIME and visualized using KING [35]. Statistically significant differences between koji making and fermentation were determined using the non-parametric Mann—Whitney test and multivariate analysis of variance in MATLAB R2014a (The Math works, Natick, MA, USA) and Canoco for Windows 4.5 (Microcomputer Power, NY, USA).
Nucleotide sequence accession numbers
Sequences reported in this paper are available in the SRA database under accession numbers SRX1925639–SRX1925640, SRX1925651–SRX1925658 (bacterial 16S rRNA gene sequences) and SRX1925641–SRX1925650 (fungi ITS 1 region sequences).
Results and Discussion
Abundance and diversity of members of the bacterial and fungal microbiota
Illumina MiSeq sequencing generated 181,443 high quality bacterial tags from 10 examined sample sets, with an average of 18,144 tags per sample (range = 15,944–19,846, SD = 1,246). Meanwhile, a total of 221,059 high quality ITS tags were obtained from koji making and fermentation across the entire douchi fermentation process. Sequencing results are shown in Table 1 High quality sequences were grouped into 1083 OTUs for bacteria and 88 OTUs for fungi (both at the 97% similarity level), and after removing singletons, the average number of OTUs was 115 for bacteria (range = 66–167, SD = 39.19) and 22 for fungi (range = 12–44, SD = 9), and these were subjected to further analysis.
Table 1. Sample information, microbial diversity and sequence abundance.
| Sample | Number of reads | Number of OTUs | Shannon index | Simpson index | Chao 1 index | Observed species | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacteria | Fungi | Bacteria | Fungi | Bacteria | Fungi | Bacteria | Fungi | Bacteria | Fungi | Bacteria | Fungi | |
| Koji making 1 | 17092 | 21913 | 73 | 30 | 1.05 | 1.08 | 0.5 | 0.45 | 199.43 | 98 | 101 | 30 |
| Koji making 3 | 17809 | 21469 | 49 | 12 | 0.82 | 0.92 | 0.49 | 0.54 | 96 | 12 | 65 | 12 |
| Koji making 5 | 18797 | 20365 | 66 | 13 | 0.84 | 0.75 | 0.59 | 0.69 | 240.13 | 14 | 87 | 13 |
| Koji making 7 | 16509 | 22484 | 121 | 44 | 1.47 | 0.57 | 0.39 | 0.75 | 476.79 | 86.85714 | 200 | 44 |
| Mean ± SD | 17551.75 ± 985.82 | 21557.75 ± 897.16 | 77.25 ± 30.86 | 24.75 ± 15.26 | 1.04 ± 0.30 | 0.83 ± 0.22 | 0.49 ± 0.08 | 0.61 ± 0.14 | 253.08 ± 161.00 | 52.71 ± 46.09 | 113.25 ± 59.70 | 24.75 ± 15.26 |
| Fermentation 1 | 15994 | 20900 | 141 | 15 | 1.64 | 0.7 | 0.27 | 0.7 | 461.57 | 16.5 | 222 | 15 |
| Fermentation 3 | 17731 | 22727 | 128 | 15 | 1.58 | 0.56 | 0.32 | 0.76 | 424.29 | 15.6 | 188 | 15 |
| Fermentation 6 | 19848 | 23100 | 118 | 17 | 1.35 | 1.02 | 0.36 | 0.43 | 432.11 | 20 | 161 | 17 |
| Fermentation 9 | 19047 | 23348 | 150 | 23 | 1.48 | 1.05 | 0.41 | 0.43 | 526.62 | 30 | 214 | 23 |
| Fermentation 12 | 19323 | 22510 | 137 | 22 | 1.87 | 1.04 | 0.26 | 0.45 | 530.92 | 32.5 | 208 | 22 |
| Fermentation 15 | 19293 | 21457 | 167 | 32 | 2.75 | 0.97 | 0.11 | 0.45 | 1299.78 | 67 | 367 | 32 |
| Mean ± SD | 18539.33 ± 1434.20 | 22340.33 ± 961.83 | 140.17 ± 17.13 | 20.67 ± 6.53 | 1.78 ± 0.51 | 0.89 ± 0.21 | 0.29 ± 0.10 | 0.54 ± 0.15 | 612.55 ± 339.74 | 30.27 ± 19.31 | 226.67 ± 72.15 | 20.67 ± 6.53 |
Rarefaction curves for (a) observed species and (b) Simpson diversity indices for bacteria and fungi are shown in S1 and S2 Figs, respectively. Although the rarefaction cure is not parallel with the x-axis, the Simpson diversity index reached saturation, suggesting that some additional phenotypes could be added with additional sequencing, but the great majority of microbial diversity was captured. Meanwhile, the Shannon diversity, Chao 1 and observed species indices were applied to measure bacterial and fungal sequence abundance and diversity during douchi fermentation.
Diversity indices of koji making and fermentation (S1 and S2 Tables) indicated significant differences in bacterial diversity between steps (p <0.05). In contrast, no significant differences were apparent in fungal diversity (p > 0.05).
Diversity indices indicated that the diversity of the bacterial community increased as the koji making stage progressed, consistent with a previous report [25]. However, bacterial diversity then declined during the early stages of fermentation, before gradually recovering. This suggests that bacteria were prolific during koji making, but suffered when transferred to the fermentation environment, presumably due to the high temperature, low humidity, oxygen scarcity, and high salt content [36]. These harsh conditions likely inhibited the bacteria until they adapted to, and became tolerant of, the harsh fermentation environment. Temperature, salt, humidity and submerged fermentation can all inhibit or kill microorganisms that are not tolerant to these environmental challenges, unlike the halotolerant and anaerobic microbes that can thrive during the product-making process [25]. Alternatively, interspecific competition might be a factor causing the decrease in diversity indices, and bacteria that can adapt to the fermentation environment survive and eventually thrive. In the present study, fungal diversity remained stable during both koji making and fermentation, suggesting the fungal species adapted to both environments, and could tolerate the transition between steps.
Comparison of bacterial communities in koji making and fermentation
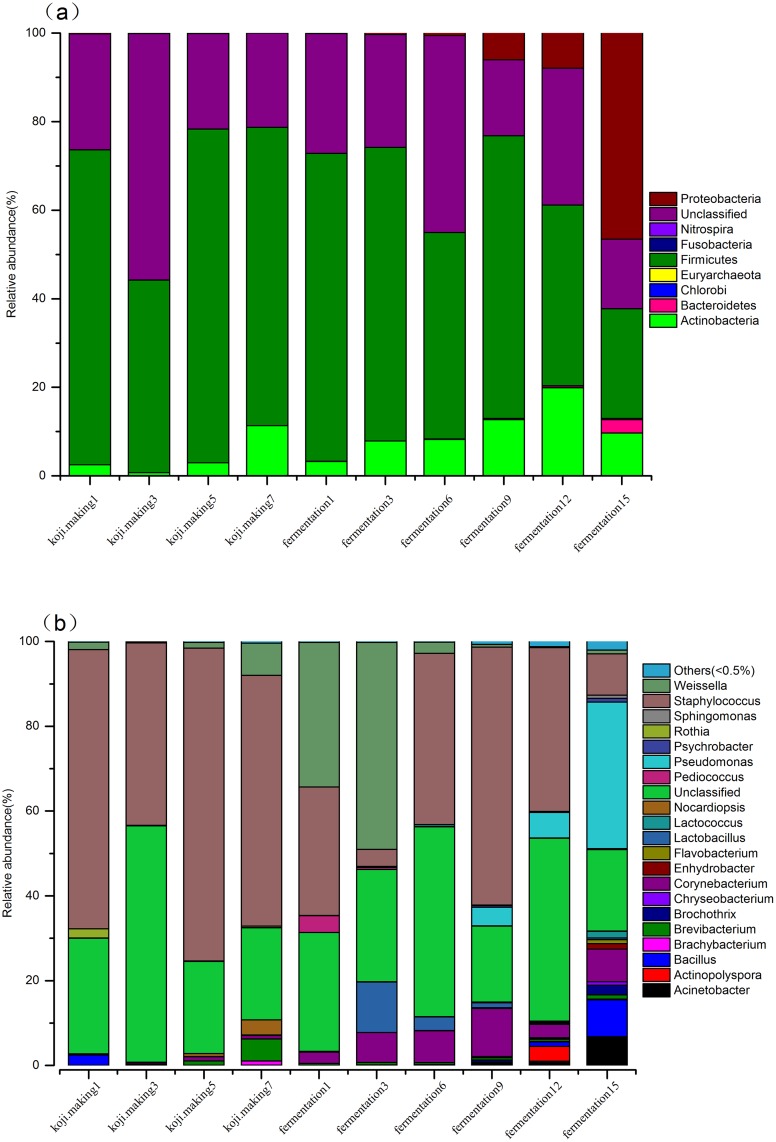
Eight bacterial phyla were identified in the koji making step, consisting of Firmicutes, Actinobacteria, Bacteroidetes and Proteobacteria, and these phyla were also detected in the fermentation step, along with four additional phyla (Chlorobi, Euryachacota, Fusobacteria and Nitrospira). Analysis of the relative abundance of bacterial phyla level throughout the entire douchi fermentation process revealed that Firmicutes, Actinobacteria and Proteobacteria were the predominant phyla in both koji making and fermentation (Fig 1a), and this finding is consistent with previous reports [17,37]. Similarly, Ascomycota and Zygomycota were the dominant fungal phyla, as was observed in previous studies [18,38]. Indeed, the majority (> 40%) of OTUs in koji making were Firmicutes, and this did not change significantly throughout the koji making step. In contrast, the proportion of Firmicutes declined sharply during fermentation, from 69.55% (day 1) to 24.76% (day 15). Proteobacteria remained rare throughout koji making, and decreased from 0.13% (day 1) to 0.01% (day 7), but during the subsequent fermentation, Proteobacteria increased sharply, from 0.08% (day 1) to 46.57% (day 15), by which time they had become a dominant genus. Actinobacteria increased during koji making and fermentation, from an initial relative abundance of 3.28% (day 1) to 19.89% (day 12). Interestingly, Actinobacteria then declined to 9.67% by day 15. The proportion of Firmicutes and Actinobacteria remained stable in koji making and the early stages of fermentation, while Proteobacteria was barely detectable in koji making but became a dominant genus in fermentation, suggesting Firmicutes and Actinobacteria adapted to the koji making and fermentation environment. The observed decrease in the later stages of fermentation might be due to interspecific completion with Proteobacteria that underwent a sharp increase to 46.57% on day 15, at that same time that the other two dominant phyla decreased markedly. Bacteroidetes, Chorobi, Euryachaeota, Fusobacteria and Nitrospira phyla were barely detectable in koji making, and only slightly more abundant during fermentation.
Fig 1. Relative abundance of bacteria at phyla (a) and genus (b) levels during koji making and fermentation.
At the genus level, 17 bacterial genera were detected during koji making, and 20 were detected during fermentation (Fig 1b). Staphylococcus and Weissella were the predominant genera in both steps, and constituted 42.59% and 9.81% of all bacterial sequences detected during the complete douchi fermentation process, respectively, consistent with previous studies [39–41]. Staphylococcus accounted for between 43.04% and 73.81% of genera present in koji making, and this genus declined dramatically during fermentation, from an initial 30.33% on day 1 to only 4.06% on day 3. Interestingly, this genus then recovered rapidly to reach 60.83% on day 9, but decreased sharply again to 9.75% by day 15. During koji making, the relative abundance of the other dominant genus, Weissella, declined sharply from an initial value of 1.75% to 0.26% on day 3, then increased rapidly to 7.57%. During fermentation, Weissella showed a similar trend to Staphylococcus, with the proportion increasing slightly from 34.05% to 48.79% between day 1 and 3. Interestingly, this genus then declined dramatically to 2.6%, but recovered to 15% by day 9, before continually declining to become a minority genus by the end of fermentation. Other major genera present in the koji making step included Brevibacterium and Nocardiopsis, each constituting more than 1% of total bacteria in this population. Acinetobacter, Bacillus, Corynebacterium, Lactobacillus and Pseudomonas were relatively abundant genera (>1%) during fermentation. The dynamics of the other genera in koji making and fermentation are shown in Fig 1b. In koji making, Staphylococcus was the dominating genus and maintained stably high levels, suggesting it is an aerobic or facultative anaerobic bacteria adapted to the koji making environment. Staphylococcus might play an important role in koji making, consistent with previous studies that revealed a role for Staphylococcus gallinarum in antibiotic activity and proteolytic capacity in the douchi fermentation process [42–44], possibly by providing a variety of amino acids or peptides for other genera to use during fermentation. Other major genera such as Brevibacterium and Nocardiopsis increased gradually and reached a maximum on day 7 in koji making, which indicated that these genera might play an important role during the later stages of koji making, consistent with the report that Brevibacterium lactofermentum produces glutamic acid [45]. Ganesh et al. reported that Nocardiopsis sp. can produce cellulolytic enzymes that enhance the flavour of douchi by degrading fiber into glucose [46]. Members of this genus also produce thermostable α-amylase [46].
During fermentation, Staphylococcus and Weissella remained the predominant genera, as was concluded in previous studies [40,47,48]. The proportion of Staphylococcus sharply decreased to 4.06% on day 3, before recovering to 40.44% then remaining a dominant genus, while the proportion of Weissella reached 48.79% but then decreased to 2.6% to become a minority genus (<1%). This indicates interspecific competition between Staphylococcus and Weissella. Ndagano et al. found that Weissella is activated by LAB in fermented food [49], and the metabolism of members of this genus, specifically that related to bacteriocins and acids, not only contributed to the formation of flavour compounds, but also inhibited the growth of pathogens with a low acid tolerance [50]. Other major genera in the fermentation step were still thriving on day 15, while the proportion of Staphylococcus and Weissella was 9.75% and 0.87%, respectively, suggesting Acinetobacter, Bacillus, Corynebacterium, Lactobacillus and Pseudomonas might play an important role during the later stages of fermentation, and metabolites secreted from these organisms might inhibit the growth of Staphylococcus and Weissella. Acinetobacter and Pseudomonas genera are able to degrade biosurfactants and hydrocarbons [51]. Among the microbiota in cheese, Lactobacillus possess some capacity for the breakdown of proteins during the fermentation process [52], and members of this genus are believed to be responsible for the distinctive sour taste and rich flavour [53]. Bacillus species play a crucial role by secreting microbial inhibitors and plasminogen during the fermentation of soybean [1], and Bacillus subtilis can enhance the angiotensin converting enzyme inhibitory effect of soybeans, as well as the anthocyanin content and reducing activity [54]. Bacillus subtilis also enhances lipases, amylases, proteases, cellulases and glutaminases that degrade lipids, proteins carbohydrates and flavonoid glycosides during the douchi fermentation process, resulting in metabolites such as organic acids, amino acids and aglycones that contribute to the taste, flavour, functionality and nutritional content [8].
Comparison of bacterial community structure during koji making and fermentation
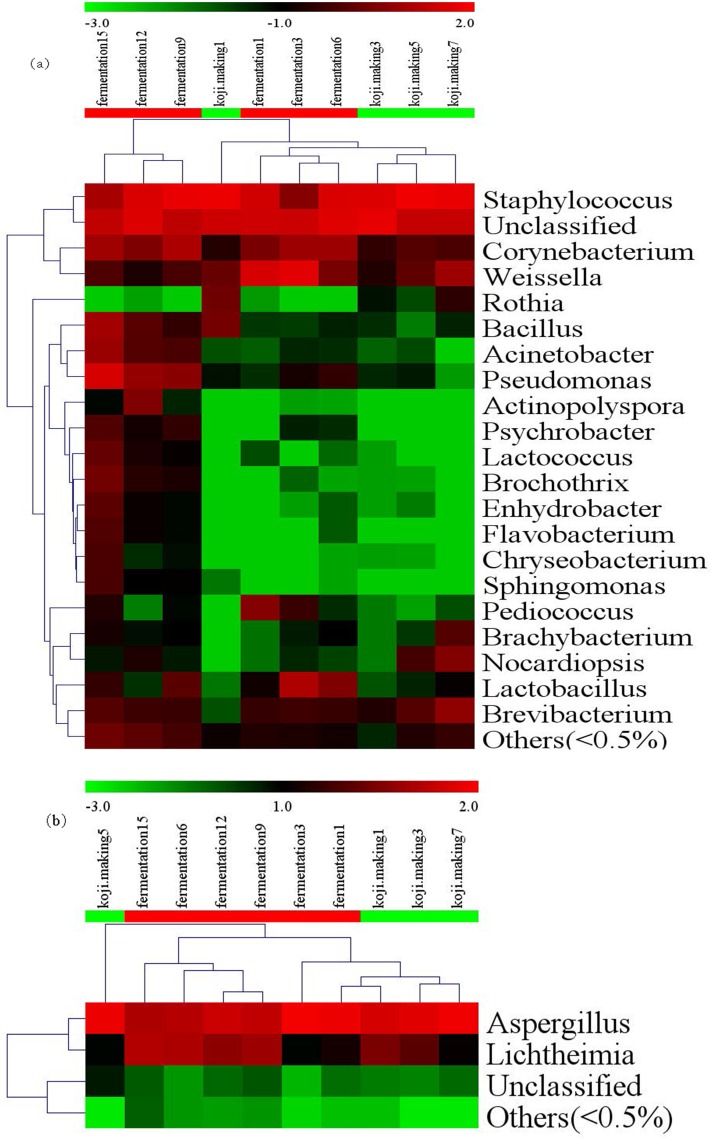
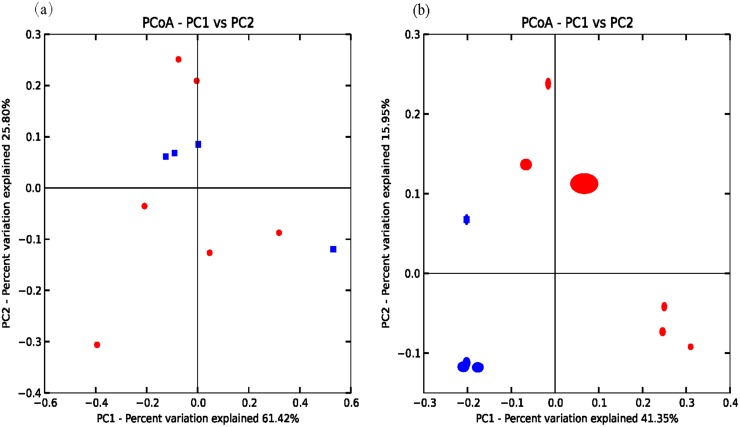
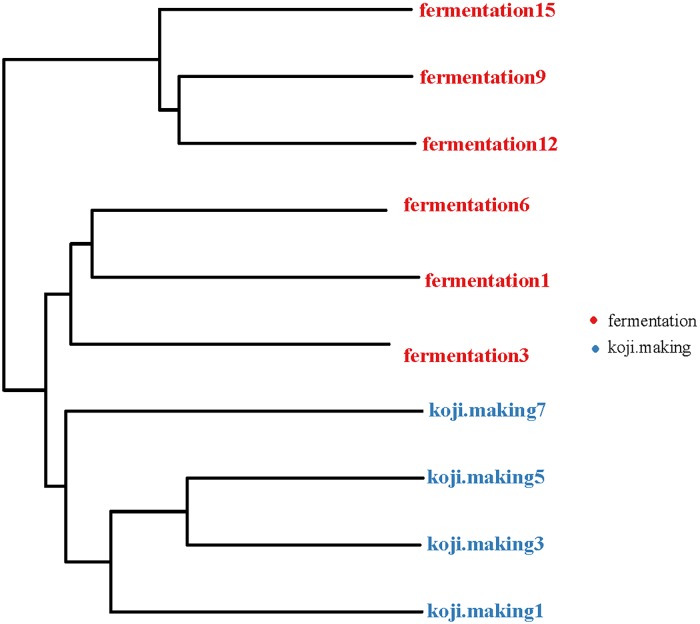
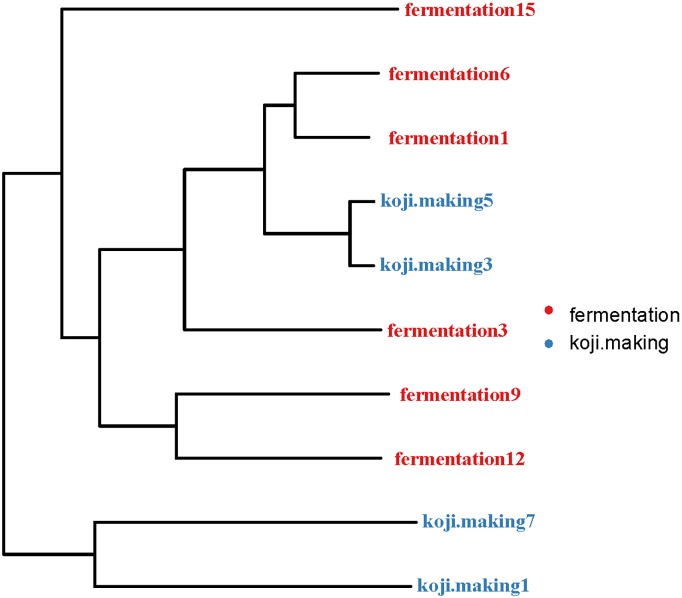
A heatmap revealed differences in genera between koji making and fermentation (Fig 2a), and PCoA and cluster analyses were performed to evaluate similarities in bacterial communities in the two stages. Both weighted (PC1 variance = 61.42%, PC2 variance = 25.80%; Fig 3a) and unweighted (PC1 variance = 41.35%, PC2 variance = 15.95%; Fig 3b) PCoA was performed. UniFrac values revealed an overlap in bacterial communities between koji making and fermentation, but multivariate analysis of variance indicated a clear, significant difference (p < 0.05) between koji making and fermentation based on the principal coordinates of the unweighted UniFrac metric. Unweighted pair-group analysis (UPGMA) using arithmetic means (Fig 4) based on unweighted UniFrac analysis also indicated a discriminative structural separation between steps, and furthermore, the bacterial communities were relatively stable during koji making, but fluctuated considerably during fermentation from day 1 to 6, and day 9 to 15.
Fig 2. Heatmap and dendrogram of abundant bacterial (a) and fungal (b) genera present in the microbial community of 10 samples from koji making and fermentation.
The heatmap plot indicates the relative abundance of genera in different samples (variables clustered on the vertical axis). The phylogenetic tree was calculated using the neighbour-joining method. The colour intensity is proportional to the relative abundance of bacterial and fungal genera.
Fig 3. Principal coordinate analysis of microbial communities based on (a) weighted and (b) unweighted UniFrac metrics of samples from koji making and fermentation.
Red and blue symbols represent samples from fermentation and koji making, respectively.
Fig 4. Cluster analysis of the bacterial microbiota in koji making and fermentation based on unweighted UniFrac distances.
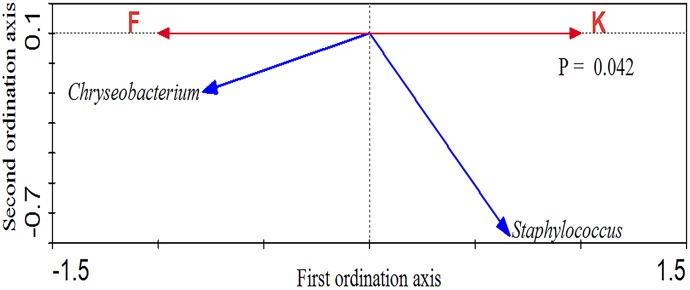
Redundancy analysis (RDA) was performed using koji making or fermentation as constrained explanatory variables and the relative abundance of all OTUs as the response variable. The significance of RDA was confirmed by a Monte Carlo Permutation test (p = 0.042; Fig 5) and 26% of the variance in OTU abundance data could be explained by the canonical axis. Two key responding OTUs were identified that were well correlated with sample scores on the canonical axis, for which at least 26% of the variability was explained by the axis (Fig 5). In the RDA ordination plot, one OTU (Staphylococcus) was enriched in koji making, while one OTU (Corynebacterium) was enriched in fermentation. Differences in microbiota could reflect differences in the environments of the two stages, and indicate a marked transition between steps [55,36,56]. In this regard, Montal et al. reported that environmental factors such as the amount of oxygen, and the time of inoculation (e.g. during the ripening process) are of great importance [57].
Fig 5. Bioplot of redundancy analysis of bacteria in koji making and fermentation.
F represents fermentation and K represents koji making.
Fungal communities present in koji making and fermentation stages of douchi fermentation
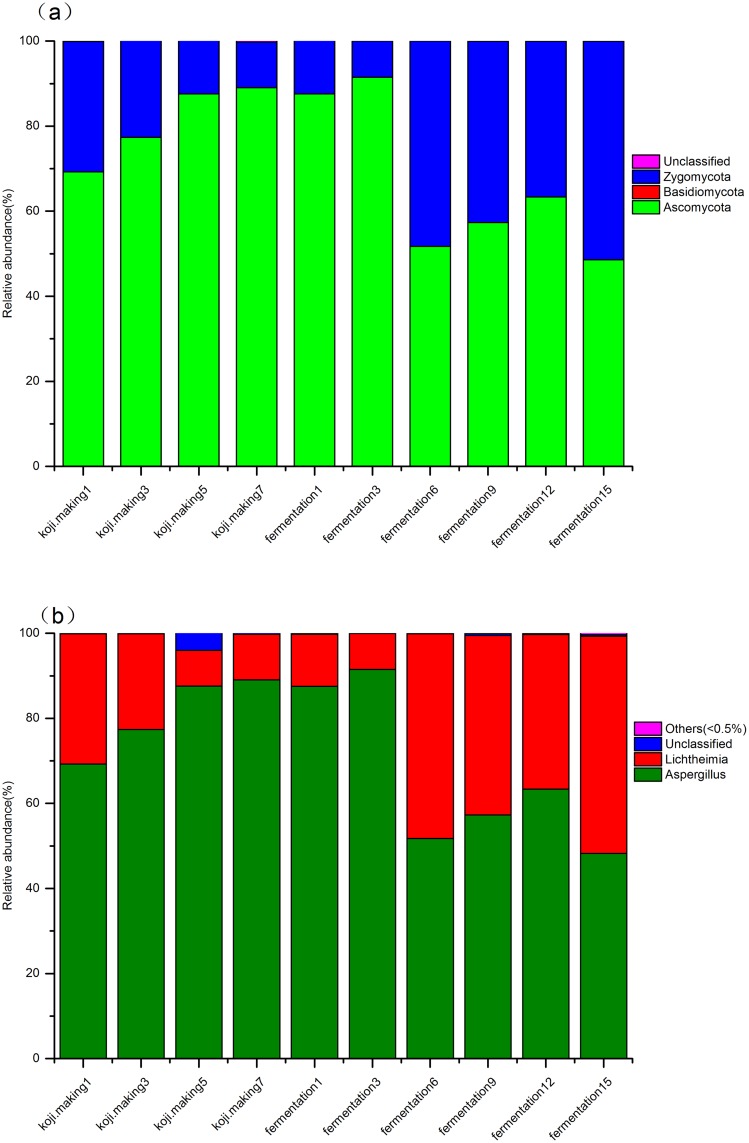
Compared with bacteria, the overall diversity of the fungal microbiota in douchi fermentation was lower. Only two and three fungal phyla were detected in koji making and fermentation, respectively. Ascomycota and Zygomycota were detected in both steps of the process, and the relative abundance of fungi at the phylum level (Fig 6a) showed that Ascomycota were dominant, accounting for 72.33%, while Zygoycota was the second most dominant phyla, accounting for 27.63%. Ascomycota and Zygomycota were the dominant fungal phyla, as was observed in previous studies [18,58]. Ascomycota and Zygomycota contributed 80.8% and 19.11% in koji making, and 66.68% and 33.31% in fermentation, respectively.
Fig 6. Relative abundance of fungi at phyla (a) and genus (b) levels during koji making and fermentation.
At the genus level, two genera, Aspergillus and Lichtheimia, dominated both koji making and fermentation, accounting for 72.29% and 27.09%, respectively (Fig 6b). Aspergillus dominated throughout koji making, increasing gradually from 69.24% on day 1 to 89.07% on day 7, and this genus continued to increase during fermentation, reaching 91.48% on day 3. However, this genus then declined to 51.76% on day 6 of fermentation, before increasing gradually to 63.33% on day 12, then decreasing to 48.26% by the end of the process. Lichtheimia was the second most abundant genus in koji making, during which members decreased from 30.63% on day 1 to 10.72% on day 7. In the early stages of fermentation, the percentage of Lichtheimia declined gradually from 12.26% on day 1 to 8.50% on day 3, then increased dramatically to 48.16% before declining to 36.40% on day 12, and finally recovering to 51.05% on day 15 to become the dominant genus at the end of the process. These two predominant genera might play an important role over the whole fermentation process. Kim et al. reported that Aspergillus oryzae secretes a large quantity of amylases and/or proteases that break down starches and proteins into sugars and peptides/amino acids that are then absorbed and utilized by yeasts and lactic acid bacteria in the fermentation stage [59]. Aspergillus oryzae may therefore play a key role in providing a suitable living habitat for fermentative microbes that are essential during the second step of the process [56]. Although Aspergillus did decline during fermentation, from a maximum of 91.48% on day 3 to 48.26% on day 15, Aspergillus oryzae remained a major genus throughout fermentation, indicating a high tolerance to the dry, high salt and high temperature conditions of the second step. Lichtheimia gradually declined in abundance during koji making, but increased during fermentation, from 12.26% on day 1 to 51.05% on day 15, suggesting this genus was adapted to the harsh environment, and indicating a key role in douchi fermentation [56], consistent with previous studies [60,61]. Burgess et al. reported that Lichtheimia can survive at temperatures of 50–60°C [62], while Garcia et al. found that Lichtheimia produce rhamnose from β-glucosidase activity during solid-state fermentation[63], which leads to the release glucose that may improve the flavour of douchi. Filamentous fungi are usually included in solid state fermentation processes due to their high production of hydrolytic enzymes and relatively high tolerance to low water activity [64].
Comparison of fungal community structure during koji making and fermentation
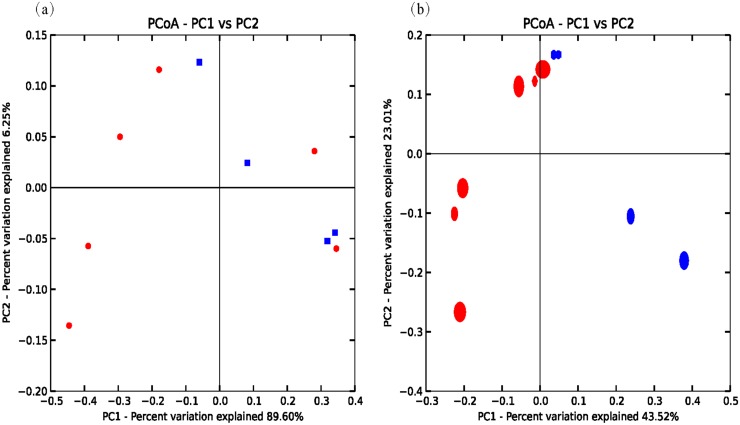
Multivariate analysis of variance of the fungal microbiota present in koji making and fermentation steps was performed using both weighted (PC1 variance = 89.6%, PC2 variance = 6.25%; Fig 7a) and unweighted (PC1 = 43.52%, PC2 = 23.01%; Fig 7b) parameters. UniFrac PCoA based on fungal microbiota communities revealed no distinct clustering pattern between koji making and fermentation (Fig 8). Similarly, multivariate analysis of variance based on unweighted UniFrac distances also indicated that there were no significant differences (p > 0.05) between fungal communities during koji making and fermentation. However, heatmap analysis did indicate differences in genera between steps, and non-parametric Mann—Whitney tests confirmed that no genera showed a significant difference (p < 0.05) between koji making and fermentation (Fig 2b).
Fig 7. Principal coordinate analysis of microbial communities based on (a) weighted and (b) unweighted UniFrac metrics of samples from koji making and fermentation.
Red and blue symbols represent samples from fermentation and koji making, respectively.
Fig 8. Cluster analysis of fungal microbiota in koji making and fermentation based on unweighted UniFrac distances.
Conclusions
In this study, bacterial and fungal diversity in the douchi fermentation process was studied by high-throughput sequencing to investigate the dynamics of the dominant genera and phyla, and to identify microorganisms that contribute to the distinct flavour and character of this traditional fermented food. This is the first study to apply this technology to studying douchi fermentation. The results provide insight into the dynamics of microbial community diversity that could be useful for improving the industrial production of douchi, and ensuring that high quality and safety are maintained.
Supporting Information
(TIF)
(TIF)
(DOCX)
(DOCX)
Acknowledgments
This work was supported by a China Agricultural Science and Technology Achievements Transformation Project (2014), by the Natural Science Foundation of Jiangxi Province (grant Nos. 20142BAB214008 and 20151BAB204003) and by the Development Foundation of the Key Lab of Protection and Utilization of Subtropical Plant Resources (grant No. YRD201405).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by China Agricultural Science and Technology Achievements Transformation Project(2014), by the Natural Science Foundation of Jiangxi Province (20142BAB214008, 20151BAB204003) and by the Development Foundation of Key Lab of Protection and Utilization of Subtropic Plant Resources(YRD201405).
References
- 1.Chen TT, Xiong SQ, Jiang SY, Wang MJ, Wu QL, Wei H. Molecular identification of microbial community in Chinese douchi during post-fermentation process. Food Science and Biotechnology. 2011; 20: 1633–1638. [Google Scholar]
- 2.Chen J, Cheng Y-Q, Yamaki K, Li L-T. Anti-α-glucosidase activity of Chinese traditionally fermented soybean (douchi). Food Chemistry. 2007; 103: 1091–1096. [Google Scholar]
- 3.Chen T, Wang M, Jiang S, Xiong S, Zhu D, Wei H. Investigation of the microbial changes during koji-making process of Douchi by culture-dependent techniques and PCR-DGGE. International Journal of Food Science & Technology. 2011; 46: 1878–1883. [Google Scholar]
- 4.Messina MJ, Persky V, Setchell KD, Barnes S. Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutrition and cancer. 1994; 21: 113–131. 10.1080/01635589409514310 [DOI] [PubMed] [Google Scholar]
- 5.Wang D, Wang L-j, Zhu F-x, Zhu J-y, Chen XD, Zou L, et al. In vitro and in vivo studies on the antioxidant activities of the aqueous extracts of Douchi (a traditional Chinese salt-fermented soybean food). Food Chemistry. 2008; 107: 1421–1428. [Google Scholar]
- 6.Ishida H, Uesugi T, Hirai K, Toda T, Nukaya H, Yokotsuka K, et al. Preventive effects of the plant isoflavones, daidzin and genistin, on bone loss in ovariectomized rats fed a calcium-deficient diet. Biological and Pharmaceutical Bulletin. 1998; 21: 62–66. [DOI] [PubMed] [Google Scholar]
- 7.Kim J-S, Chung H-Y. Components in commercial Douchi-a Chinese fermented black bean product by supercritical fluid extraction. Preventive Nutrition and Food Science. 2008; 13: 12–17. [Google Scholar]
- 8.Chen C, Xiang JY, Hu W, Xie YB, Wang TJ, Cui JW, et al. Identification of key micro-organisms involved in Douchi fermentation by statistical analysis and their use in an experimental fermentation. Journal of Applied Microbiology. 2015; 119: 1324–1334. 10.1111/jam.12917 [DOI] [PubMed] [Google Scholar]
- 9.Pires ACC, Cleary DFR, Almeida A, Cunha A, Dealtry S, Mendonca-Hagler LCS, et al. Denaturing Gradient Gel Electrophoresis and Barcoded Pyrosequencing Reveal Unprecedented Archaeal Diversity in Mangrove Sediment and Rhizosphere Samples. Applied and Environmental Microbiology. 2012; 78: 5520–5528. 10.1128/AEM.00386-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen T, Jiang S, Xiong S, Wang M, Zhu D, Wei H. Application of denaturing gradient gel electrophoresis to microbial diversity analysis in Chinese Douchi. Journal of the Science of Food and Agriculture. 2012; 92: 2171–2176. 10.1002/jsfa.5604 [DOI] [PubMed] [Google Scholar]
- 11.Mohammadi P, Hamidkhani A, Asgarani E. Comparative Analysis of Denaturing Gradient Gel Electrophoresis and Temporal Temperature Gradient Gel Electrophoresis Profiles as a Tool for the Differentiation of Candida Species. Jundishapur Journal of Microbiology. 2015; 8: e22249 10.5812/jjm.22249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filleron A, Simon M, Hantova S, Jacquot A, Cambonie G, Marchandin H, et al. tuf-PCR-temporal temperature gradient gel electrophoresis for molecular detection and identification of staphylococci: Application to breast milk and neonate gut microbiota. Journal of Microbiological Methods. 2014; 98: 67–75. 10.1016/j.mimet.2013.12.022 [DOI] [PubMed] [Google Scholar]
- 13.Cuecas A, Portillo MC, Kanoksilapatham W, Gonzalez JM. Bacterial Distribution Along a 50 degrees C Temperature Gradient Reveals a Parceled Out Hot Spring Environment. Microbial Ecology. 2014; 68: 729–739. 10.1007/s00248-014-0437-y [DOI] [PubMed] [Google Scholar]
- 14.Kyselkova M, Kopecky J, Felfoldi T, Cermak L, Omelka M, Grundmann GL, et al. Development of a 16S rRNA gene-based prototype microarray for the detection of selected actinomycetes genera. Antonie Van Leeuwenhoek International Journal of General and Molecular Microbiology. 2008; 94: 439–453. [DOI] [PubMed] [Google Scholar]
- 15.Balachandar D, Doud MS, Schneper L, Mills D, Mathee K. Long-Term Organic Nutrient Management Fosters the Eubacterial Community Diversity in the Indian Semi-arid Alfisol as Revealed by Length Heterogeneity-PCR. Communications in Soil Science and Plant Analysis. 2014; 45: 189–203. [Google Scholar]
- 16.Mills DK, Entry JA, Gillevet PM, Mathee K. Assessing microbial community diversity using amplicon length heterogeneity polymerase chain reaction. Soil Science Society of America Journal. 2007; 71: 572–578. [Google Scholar]
- 17.Liu WJ, Xi XX, Sudu QG, Kwok LY, Guo Z, Hou QC, et al. High-throughput sequencing reveals microbial community diversity of Tibetan naturally fermented yak milk. Annals of Microbiology. 2015; 65: 1741–1751. [Google Scholar]
- 18.Marsh AJ, O’Sullivan O, Hill C, Ross RP, Cotter PD. Sequencing-based analysis of the bacterial and fungal composition of kefir grains and milks from multiple sources. PloS one. 2013; 8: e69371 10.1371/journal.pone.0069371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie Z, Wang Z, Wang Q, Zhu C, Wu Z. An anaerobic dynamic membrane bioreactor (AnDMBR) for landfill leachate treatment: Performance and microbial community identification. Bioresource technology. 2014; 161: 29–39. 10.1016/j.biortech.2014.03.014 [DOI] [PubMed] [Google Scholar]
- 20.Polka J, Rebecchi A, Pisacane V, Morelli L, Puglisi E. Bacterial diversity in typical Italian salami at different ripening stages as revealed by high-throughput sequencing of 16S rRNA amplicons. Food Microbiology. 2015; 46: 342–356. 10.1016/j.fm.2014.08.023 [DOI] [PubMed] [Google Scholar]
- 21.Wang W, Cao J, Li JR, Yang F, Li Z, Li LX. Comparative analysis of the gastrointestinal microbial communities of bar-headed goose (Anser indicus) in different breeding patterns by high-throughput sequencing. Microbiological Research. 2016; 182: 59–67. 10.1016/j.micres.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 22.O'Sullivan DJ, Fallico V, O'Sullivan O, McSweeney PLH, Sheehan JJ, Cotter PD, et al. High-throughput DNA sequencing to survey bacterial histidine and tyrosine decarboxylases in raw milk cheeses. Bmc Microbiology. 2015; 15: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aldrete-Tapia A, Escobar-Ramirez MC, Tamplin ML, Hernandez-Iturriaga M. High-throughput sequencing of microbial communities in Poro cheese, an artisanal Mexican cheese. Food Microbiology. 2014; 44: 136–141. 10.1016/j.fm.2014.05.022 [DOI] [PubMed] [Google Scholar]
- 24.Quigley L, O'Sullivan O, Beresford TP, Ross RP, Fitzgerald GF, Cotter PD. High-Throughput Sequencing for Detection of Subpopulations of Bacteria Not Previously Associated with Artisanal Cheeses. Applied and Environmental Microbiology. 2012; 78: 5717–5723. 10.1128/AEM.00918-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen TT, Wang MJ, Jiang SY, Xiong SQ, Zhu DC, Wei H. Investigation of the microbial changes during koji-making process of Douchi by culture-dependent techniques and PCR-DGGE. International Journal of Food Science and Technology. 2011; 46: 1878–1883. [Google Scholar]
- 26.Dineen S, Aranda Rt, Anders D, Robertson J. An evaluation of commercial DNA extraction kits for the isolation of bacterial spore DNA from soil. Journal of applied microbiology. 2010; 109: 1886–1896. 10.1111/j.1365-2672.2010.04816.x [DOI] [PubMed] [Google Scholar]
- 27.Liu B, Yuan J, Yiu S-M, Li Z, Xie Y, Chen Y, et al. COPE: an accurate k-mer-based pair-end reads connection tool to facilitate genome assembly. Bioinformatics. 2012; 28: 2870–2874. 10.1093/bioinformatics/bts563 [DOI] [PubMed] [Google Scholar]
- 28.You J, Wu G, Ren F, Chang Q, Yu B, Xue Y, et al. Microbial community dynamics in Baolige oilfield during MEOR treatment, revealed by Illumina MiSeq sequencing. Applied microbiology and biotechnology. 2016; 100: 1469–1478. [DOI] [PubMed] [Google Scholar]
- 29.Li P, Liang H, Lin W-T, Feng F, Luo L. Microbiota Dynamics Associated with Environmental Conditions and Potential Roles of Cellulolytic Communities in Traditional Chinese Cereal Starter Solid-State Fermentation. Applied and Environmental Microbiology. 2015; 81: 5144–5156. 10.1128/AEM.01325-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang X, Liu L, Wen T, Zhu R, Zhang J, Cai Z. Illumina MiSeq investigations on the changes of microbial community in the Fusarium oxysporum f. sp. cubense infected soil during and after reductive soil disinfestation. Microbiological research. 2015; 181: 33–42. 10.1016/j.micres.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 31.Tang J, Iliev ID, Brown J, Underhill DM, Funari VA. Mycobiome: Approaches to analysis of intestinal fungi. Journal of immunological methods. 2015; 421: 112–121. 10.1016/j.jim.2015.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Igarashi H, Maeda S, Ohno K, Horigome A, Odamaki T, Tsujimoto H. Effect of oral administration of metronidazole or prednisolone on fecal microbiota in dogs. PloS one. 2014; 9: e107909 10.1371/journal.pone.0107909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Applied and environmental microbiology. 2009; 75: 7537–7541. 10.1128/AEM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kemp PF, Aller JY. Bacterial diversity in aquatic and other environments: what 16S rDNA libraries can tell us. FEMS Microbiology Ecology. 2004; 47: 161–177. 10.1016/S0168-6496(03)00257-5 [DOI] [PubMed] [Google Scholar]
- 35.Crawford PA, Crowley JR, Sambandam N, Muegge BD, Costello EK, Hamady M, et al. Regulation of myocardial ketone body metabolism by the gut microbiota during nutrient deprivation. Proceedings of the National Academy of Sciences. 2009; 106: 11276–11281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim J-H, Kim D-H, Ahn H-J, Park H-J, Byun M-W. Reduction of the biogenic amine contents in low salt-fermented soybean paste by gamma irradiation. Food Control. 2005; 16: 43–49. [Google Scholar]
- 37.Leite A, Mayo B, Rachid C, Peixoto R, Silva J, Paschoalin V, et al. Assessment of the microbial diversity of Brazilian kefir grains by PCR-DGGE and pyrosequencing analysis. Food microbiology. 2012; 31: 215–221. 10.1016/j.fm.2012.03.011 [DOI] [PubMed] [Google Scholar]
- 38.Ranjard L, Poly F, Lata J-C, Mougel C, Thioulouse J, Nazaret S. Characterization of bacterial and fungal soil communities by automated ribosomal intergenic spacer analysis fingerprints: biological and methodological variability. Applied and environmental microbiology. 2001; 67: 4479–4487. 10.1128/AEM.67.10.4479-4487.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicolaisen MH, Ramsing NB. Denaturing gradient gel electrophoresis (DGGE) approaches to study the diversity of ammonia-oxidizing bacteria. Journal of microbiological methods. 2002; 50: 189–203. [DOI] [PubMed] [Google Scholar]
- 40.Liu C-j, Gong F-m, Li X-r, Li H-y, Zhang Z-h, Feng Y, et al. Natural populations of lactic acid bacteria in douchi from Yunnan Province, China. Journal of Zhejiang University Science B. 2012; 13: 298–306. 10.1631/jzus.B1100221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Consortium HMP. Structure, function and diversity of the healthy human microbiome. Nature. 2012; 486: 207–214. 10.1038/nature11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Furuhata K, Ishizaki N, Kawakami Y, Fukuyama M. Bacterial contamination of stock solutions in storage cases for contact lens, and the disinfectant-resistance of isolates. Biocontrol science. 2010; 15: 81–85. [DOI] [PubMed] [Google Scholar]
- 43.Jeyaram K, Singh WM, Premarani T, Devi AR, Chanu KS, Talukdar N, et al. Molecular identification of dominant microflora associated with ‘Hawaijar’—a traditional fermented soybean (Glycine max (L.)) food of Manipur, India. International journal of food microbiology. 2008; 122: 259–268. 10.1016/j.ijfoodmicro.2007.12.026 [DOI] [PubMed] [Google Scholar]
- 44.Kim TW, Lee J-H, Park M-H, Kim H-Y. Analysis of bacterial and fungal communities in Japanese-and Chinese-fermented soybean pastes using nested PCR—DGGE. Current microbiology. 2010; 60: 315–320. 10.1007/s00284-009-9542-4 [DOI] [PubMed] [Google Scholar]
- 45.Momose H, Takagi T. Glutamic acid production in biotin-rich media by temperature-sensitive mutants of Brevibacterium lactofermentum, a novel fermentation process. Agricultural and Biological Chemistry. 1978; 42: 1911–1917. [Google Scholar]
- 46.Saratale GD, Oh SE. Production of thermotolerant and alkalotolerant cellulolytic enzymes by isolated Nocardiopsis sp. KNU. Biodegradation. 2011; 22: 905–919. 10.1007/s10532-010-9450-0 [DOI] [PubMed] [Google Scholar]
- 47.Justé A, Malfliet S, Waud M, Crauwels S, De Cooman L, Aerts G, et al. Bacterial community dynamics during industrial malting, with an emphasis on lactic acid bacteria. Food microbiology. 2014; 39: 39–46. 10.1016/j.fm.2013.10.010 [DOI] [PubMed] [Google Scholar]
- 48.Koort J, Coenye T, Santos EM, Molinero C, Jaime I, Rovira J, et al. Diversity of Weissella viridescens strains associated with “Morcilla de Burgos”. International journal of food microbiology. 2006; 109: 164–168. 10.1016/j.ijfoodmicro.2006.01.021 [DOI] [PubMed] [Google Scholar]
- 49.Ndagano D, Lamoureux T, Dortu C, Vandermoten S, Thonart P. Antifungal activity of 2 lactic acid bacteria of the Weissella genus isolated from food. Journal of food science. 2011; 76: M305–M311. 10.1111/j.1750-3841.2011.02257.x [DOI] [PubMed] [Google Scholar]
- 50.Zhao J, Dai X, Liu X, Chen H, Tang J, Zhang H, et al. Changes in microbial community during Chinese traditional soybean paste fermentation. International journal of food science & technology. 2009; 44: 2526–2530. [Google Scholar]
- 51.Van Hamme JD, Singh A, Ward OP. Recent advances in petroleum microbiology. Microbiology and molecular biology reviews. 2003; 67: 503–549. 10.1128/MMBR.67.4.503-549.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Georgieva R, Iliev I, Haertlé T, Chobert J-M, Ivanova I, Danova S. Technological properties of candidate probiotic Lactobacillus plantarum strains. International Dairy Journal. 2009; 19: 696–702. [Google Scholar]
- 53.Luckow T, Delahunty C. Which juice is ‘healthier’? A consumer study of probiotic non-dairy juice drinks. Food Quality and Preference. 2004; 15: 751–759. [Google Scholar]
- 54.Juan M-Y, Wu C-H, Chou C-C. Fermentation with Bacillus spp. as a bioprocess to enhance anthocyanin content, the angiotensin converting enzyme inhibitory effect, and the reducing activity of black soybeans. Food microbiology. 2010; 27: 918–923. 10.1016/j.fm.2010.05.009 [DOI] [PubMed] [Google Scholar]
- 55.Chen TT, Jiang SY, Xiong SQ, Wang MJ, Zhu DC, Wei H. Application of denaturing gradient gel electrophoresis to microbial diversity analysis in Chinese Douchi. Journal of the Science of Food and Agriculture. 2012; 92: 2171–2176. 10.1002/jsfa.5604 [DOI] [PubMed] [Google Scholar]
- 56.Chen TT, Wang MJ, Li SJ, Wu QL, Wei H. Molecular Identification of Microbial Community in Surface and Undersurface Douchi During Postfermentation. Journal of Food Science. 2014; 79: M653–M658. 10.1111/1750-3841.12417 [DOI] [PubMed] [Google Scholar]
- 57.Montel M-C, Buchin S, Mallet A, Delbes-Paus C, Vuitton DA, Desmasures N, et al. Traditional cheeses: rich and diverse microbiota with associated benefits. International journal of food microbiology. 2014; 177: 136–154. 10.1016/j.ijfoodmicro.2014.02.019 [DOI] [PubMed] [Google Scholar]
- 58.Ezeokoli OT, Gupta AK, Mienie C, Popoola TO, Bezuidenhout CC. PCR-denaturing gradient gel electrophoresis analysis of microbial community in soy-daddawa, a Nigerian fermented soybean (Glycine max (L.) Merr.) condiment. International journal of food microbiology. 2016; 220: 58–62. 10.1016/j.ijfoodmicro.2016.01.003 [DOI] [PubMed] [Google Scholar]
- 59.Kim T-W, Lee J-H, Kim S-E, Park M-H, Chang HC, Kim H-Y. Analysis of microbial communities in doenjang, a Korean fermented soybean paste, using nested PCR-denaturing gradient gel electrophoresis. International journal of food microbiology. 2009; 131: 265–271. 10.1016/j.ijfoodmicro.2009.03.001 [DOI] [PubMed] [Google Scholar]
- 60.Shen Z, Ruan Y, Chao X, Zhang J, Li R, Shen Q. Rhizosphere microbial community manipulated by 2 years of consecutive biofertilizer application associated with banana Fusarium wilt disease suppression. Biology and Fertility of Soils. 2015; 51: 553–562. [Google Scholar]
- 61.Zheng X-W, Yan Z, Nout MR, Smid EJ, Zwietering MH, Boekhout T, et al. Microbiota dynamics related to environmental conditions during the fermentative production of Fen-Daqu, a Chinese industrial fermentation starter. International journal of food microbiology. 2014; 182: 57–62. 10.1016/j.ijfoodmicro.2014.05.008 [DOI] [PubMed] [Google Scholar]
- 62.Burgess SA, Lindsay D, Flint SH. Thermophilic bacilli and their importance in dairy processing. International journal of food microbiology. 2010; 144: 215–225. 10.1016/j.ijfoodmicro.2010.09.027 [DOI] [PubMed] [Google Scholar]
- 63.Garcia NFL, da Silva Santos FR, Gonçalves FA, da Paz MF, Fonseca GG, Leite RSR. Production of β-glucosidase on solid-state fermentation by Lichtheimia ramosa in agroindustrial residues: Characterization and catalytic properties of the enzymatic extract. Electronic Journal of Biotechnology. 2015; 18: 314–319. [Google Scholar]
- 64.Yovita Siti P (2005) Fungal Mats in Solid-State Fermentation. Tesis doctoral, Universidad de Wageningen Holanda. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.