Abstract
Salinity is a severe environmental stress that greatly impairs production of crops worldwide. Previous studies have shown that GMPase plays an important role in tolerance of plants to salt stress at vegetative stage. However, the function of GMPase in plant responses to salt stress at reproductive stage remains unclear. Studies have shown that heterologous expression of rice GMPase OsVTC1-1 enhanced salt tolerance of tobacco seedlings, but the native role of OsVTC1-1 in salt stress tolerance of rice is unknown. To illustrate the native function of GMPase in response of rice to salt stress, OsVTC1-1 expression was suppressed using RNAi-mediated gene silencing. Suppressing OsVTC1-1 expression obviously decreased salt tolerance of rice varieties at vegetative stage. Intriguingly, grain yield of OsVTC1-1 RNAi rice was also significantly reduced under salt stress, indicating that OsVTC1-1 plays an important role in salt tolerance of rice at both seedling and reproductive stages. OsVTC1-1 RNAi rice accumulated more ROS under salt stress, and supplying exogenous ascorbic acid restored salt tolerance of OsVTC1-1 RNAi lines, suggesting that OsVTC1-1 is involved in salt tolerance of rice through the biosynthesis regulation of ascorbic acid. Altogether, results of present study showed that rice GMPase gene OsVTC1-1 plays a critical role in salt tolerance of rice at both vegetative and reproductive stages through AsA scavenging of excess ROS.
Introduction
GDP-d-mannose pyrophosphorylase (EC 2.7.7.13, d-mannose-1-phosphate guanylyltransferase, GMPase) catalyzes the conversion of d-mannose-1-P to GDP-d-mannose in mammals, plants, yeasts and bacteria [1]. In plants, GDP-d-mannose is used to synthesize not only structural carbohydrates but also ascorbic acid (AsA) [2–4]. Reflecting multiple metabolite pathways involving GDP-d-mannose, GMPase is of great importance in developmental processes and responses to environmental stresses [5–10].
GMPase acts as a key factor in cell division, flowering, and senescence of plants [8, 9, 11–13]. cyt1, a knockout mutant of GMPase in Arabidopsis, is embryonic lethal. Embryonic development in cyt1 is arrested at an early stage, resulting in plant tissues with severely abnormal cell walls, reflecting deficiency of GMPase [14, 15]. vtc1 is a knockdown allelic mutant of cyt1 with reduced GMPase activity [5, 16] and GDP-mannose synthesis, resulting in the deficiency of cell wall carbohydrates and protein N-glycosylation [8, 12, 17]. Inhibiting GMPase expression in tobacco results in a phenotype with smaller leaves and early flowering [9].
GMPase also plays various functions in the response of plants to biotic and abiotic stresses [9, 18–20]. Mutant vtc1-1 showed pathogen resistance through H2O2, salicylic acid, and gibberellic acid signaling pathways to regulate the expression of defense genes [21–24]. Barth [22] and Pavet [23] showed that vtc1-1 can inhibite the growth of virulent bacterial pathogen Pseudomonas syringae pv. maculicola ES4326 and virulent Oomycete Hyaloperonospora parasitica pv. Noco by enhancing the transcript and protein levels of pathogenesis-related (PR) proteins. Results of latter study suggest that the pathogen resistance mechanism of vtc1-1 probably reflects the accumulation of SA and transcripts of PR genes through H2O2 buildup [24]. In addition, GMPase is also involved in responses to abiotic stresses. For example, in tobacco, overexpression of GMPase gene increases the tolerance to low and high temperature stresses. In contrast, inhibiting GMPase expression decreased the tolerance of tobacco to temperature stresses [9, 25]. Arabidopsis mutant vtc1 shows impaired tolerance to salt stress, reflecting a dramatic decrease in the production of antioxidant AsA and deficiency in scavenging reactive oxygen species (ROS) under salt stress [19]. vtc1 allelic mutant, soz1, is also deficient in AsA and hypersensitive to both sulfur dioxide and ultraviolet B irradiation [16]. Overexpression of tomato GMPase, SlGMP3, enhances tolerance of tomato to oxidative stress [13]. These studies showed that GMPase plays an important role in the responses to environmental stresses [18, 26, 27]. However, most early studies are focused on the function of GMPases in salt tolerance of plants at vegetative stage, the role of GMPases in salt stress at reproductive stage remains unclear.
Previous study showed that there are three GMPase homologous genes, named as OsVTC1-1, OsVTC1-3 and OsVTC1-8 in rice. OsVTC1-1 and OsVTC1-3 are involved in AsA biosynthesis of rice, and OsVTC1-1 is responsible for most production of AsA in rice leaf [28]. In addition, results from Kumar [29] showed that heterologous overexpression of OsVTC1-1 improved the tolerance of yeast and tobacco to salt stress, indicating that OsVTC1-1 may play an important role in the response of rice to salt stress, but the native role of GMPases in response of rice to salt stress is not well understood. To further study the function of OsVTC1-1 in the response to salt stress in rice, we knocked down the expression of OsVTC1-1 in rice by RNA interference. In present study, we showed that OsVTC1-1 plays a critical role in the response of rice to salt stress not only at seedling stage but also at reproductive stage.
Materials and methods
Plant materials and growth conditions
Seeds of different rice varieties were germinated in a greenhouse at 30°C (16-hour light) and 22°C (8-hour dark). Japonica cultivar ZH17 (Oryza sativa L. ssp. japonica cv. Zhonghua 17) was used as wild-type (WT) sample in the present study, except where explicitly indicated. Arabidopsis seeds were maintained at 4°C for 4 days and subsequently germinated and grown in an incubator with 14-hour lighting (22°C) and 10-hour darkness (18°C). To examine the role of OsVTC1-1 in the tolerance of rice to salt stress at reproductive stage, ZH17 and OsVTC1-1 RNAi (RI) rice seedlings were transferred into a small concrete pool (length: 60 cm, width: 60 cm, and depth: 80 cm).
Genetic transformation of Arabidopsis and rice
Full-length ORF of OsVTC1-1 was cloned into the plant expression vector pCAMBIA 1307 using the Xba I and Sal I sites, and subsequently transformed into vtc1-1 using the floral dip method [30, 31]. The transgenic OsVTC1-1 overexpression lines were selected using hygromycin and confirmed through western blotting. Homozygous T3 transgenic lines were used in the present study, as previously described [28].
OsVTC1-1 knockdown rice plants were established using RNA interference (RNAi) technology. Briefly, forward and reverse OsVTC1-1 3’UTR specific sequences were cloned into pUCCRNAi vector using endonuclease. The forward and reverse OsVTC1-1 DNA fragments in pUCCRNAi vector were further cloned into plant vector pCAMBIA2300 and subsequently introduced into Zhonghua17 rice using Agrobacterium-mediated transformation. The transformed plants were selected using G418 and confirmed through PCR, as previously described [28]. The homozygous T3 transgenic rice lines RI1-1, RI1-2 and RI1-3 were used in present study.
The production of hybrid rice
To limit the expression of OsVTC1-1 in salt-tolerant local rice varieties Ningjing 16 (NJ16) and Zhongdao 13 (ZD13), we generated the hybrid rice NJ16/RI1-3 and ZD13/RI1-3 using OsVTC1-1 RNAi line RI1-3 as male parent and lines NJ16 and ZD13 as female parents, respectively. The hybrid plant lines NJ16/ZH17 and ZD13/ZH17 derived from the hybrid of ZH17 (male parent) with NJ16 and ZD13 (female parent), respectively, were used as controls. F1 plants of hybrid rice were selected using G418 (100μg/ml) for two weeks and subsequently used to analyze the transcript level of OsVTC1-1 and characterize the tolerance to salt stress.
Quantitative real-time PCR (qPCR)
To examine the expression of OsVTC1-1 gene under different stress treatments, four-week-old ZH17 seedlings were sprayed with 200 mM NaCl solution, 10% (w/v) PEG6000 solution or 100 mM MV (Methyl Viologen), harvested at different time points and subsequently stored in liquid nitrogen for RNA extraction. Four-week-old seedlings were used to isolate total RNA from F1 plants NJ16/RI1-3 and ZD13/RI1-3. Total RNA was extracted using TRIzol solution (Tiangen, Beijing, China). Subsequently, approximately 2 μg of total RNA was reverse transcribed using oligo (dT) primers and M-MLV reverse transcriptase according to the manufacturer’s instructions (Toyobo, Osaka, Japan). qPCR was performed using 2X SYBR Green mix (Takara, Cat No. 330523) and iQ5 according to the manufacturer’s instructions (Bio-Rad iQ5). Primers 5’-GACCTTGCTGGGCGTGAT-3’ and 5’-GTCATAGTCCAGGGCGATGT-3’ were used for OsActin1, and primers 5’-GTCATGTGAACTAACCCTCC-3’ and 5’-GAGTTTCTTCTGGTCCTCTTG-3’ were used for OsVTC1-1. For analyzing the expression character of OsVTC1-1 under abiotic stresses, the transcript level of OsVTC1-1 under control conditions (0 h) was assigned as 1, and what results shows in figure was relative expression levels of OsVTC1-1 at others times relative to 0 h. For analyzing the expression character of OsVTC1-1 in F1 plants, the transcript level of OsVTC1-1 under ZH17 was assigned as 1, and what results shows in figure was relative expression levels of OsVTC1-1 to that in ZH17. qPCR reactions were performed in biological triplicate (data of each replicate from the average of three parallel samples)for each individual line, and threshold cycle values were quantified using relative quantification method. The expression of OsActin1 gene was used as internal standard, and the relative expression values of OsVTC1-1 were calculated using comparative Ct method [32].
Analysis of salt stress tolerance
To examine salt tolerance, wild-type, mutant (vtc1-1) and transgenic seven-day-old Arabidopsis seedlings were cultured on Murashige and Skoog (MS) plates with or without 150 mM NaCl for ten days [19, 28]. To examine the tolerance of OsVTC1-1 RI plants to salt stress, two-week-old OsVTC1-1 RI seedlings were treated with 150 mM NaCl solution for two weeks, followed by watering without NaCl for an additional ten days. Subsequently, the percentage survival rate of rice seedlings was determined (plants with green leaves represented surviving seedlings). To evaluate the role of OsVTC1-1 RI in the response of rice to salt stress at reproductive stage, two-week-old seedlings of ZH17 and OsVTC1-1 RNAi (RI) plants were transferred into a small concrete pool, grown for another four weeks under normal growth conditions, and subsequently watered with 100 mM NaCl until harvest. To examine the salt-tolerant of different rice landraces, F1 hybrid seeds of NJ16/RI1-3 and ZD13/RI1-3 were germinated and selected on G418 (100μg/ml) for two weeks and subsequently planted and grown in soil for two weeks prior to watering with 150 mM NaCl for another ten days. Moreover, seedlings were watered without NaCl for ten days; subsequently, the percentage survival rates were calculated. For treatments with both NaCl and AsA, ZH17 and OsVTC1-1 RI seeds were cultured on MS medium (without sucrose), germinated and grown at 30°C for four days. Then seedlings were transferred to MS medium (without sucrose) containing 150 mM NaCl and cultivated for another five days with or without 10μM exogenous AsA. Subsequently, phenotypes were observed.
AsA content
To measure the AsA levels, four-week-old rice seedlings were harvested and stored in liquid nitrogen. A 0.2-g sample (fresh weight) of seedlings was ground to fine powder in liquid nitrogen with mortar and pestle. The extraction and measurement of AsA were performed according to Wang et al. [33].
MDA and chlorophyll contents
To analyze malondialdehyde (MDA) and chlorophyll contents under salt stress, two-week-old rice seedlings were grown in soil and watered with or without 150 mM NaCl for ten days. To measure MDA contents, protoplasts were extracted from 0.1 g of leaf tissue in 1 ml of 0.1% (w/v) trichloroacetic acid (TCA), and the mixture was boiled at 95°C for 15 min. Subsequently, 4 ml of 20% TCA containing 0.5% thiobarbituric acid (TBA) was added, and the mixture was immediately cooled on ice. MDA content was determined after measuring the optical density at 532 and 600 nm, according to the methods of Zhang et al. [34].
To detect chlorophyll content, 0.1 g of leaf tissue was ground into powder, mixed with 100% dimethyl formamide at a ratio of 1:20 (w:v) and subsequently centrifuged at 10,000 g for 5 min. Chlorophyll content was determined after measuring the optical density at 664 and 647 nm. Total chlorophyll level (chlorophyll a + chlorophyll b) was determined according to Aono et al. [35].
O2- staining
To analyze O2.- content under salt stress, two-week-old rice seedlings were grown in soil and watered with or without 150 mM NaCl for two days. O2.- polymerizes nitrotetrazolium blue chloride (NBT) into a blue deposit. To analyze O2.- content, rice leaves were incubated in 25 mM HEPES buffer (pH 7.6) containing 1% NBT in dark at 25°C for 12 hours. Next, these leaves were destained with 80% ethanol (v/v) until chlorophyll was removed and clear blue deposits were observed.
Results
The expression of OsVTC1-1 was induced by abiotic stresses
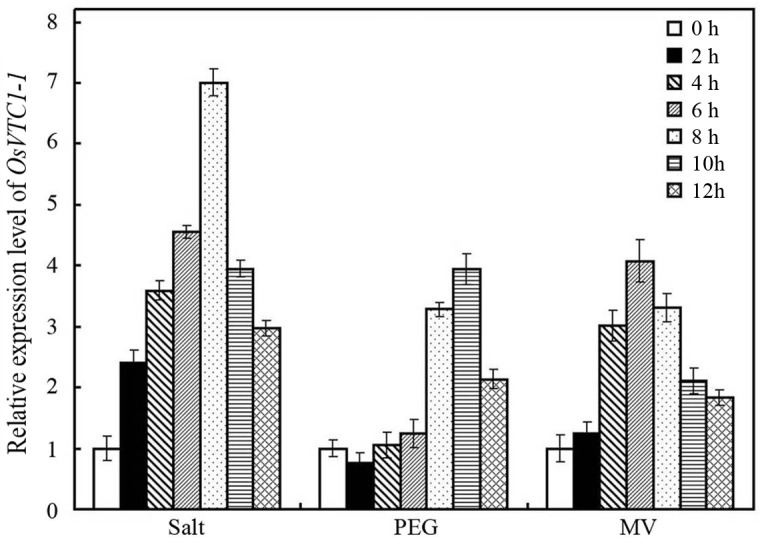
To illustrate the role of OsVTC1-1 in the response of rice to salt stress, we analyzed the expression patterns of OsVTC1-1 under abiotic stresses. The qPCR results showed that, similar to the results reported by Kumar [29], the expression OsVTC1-1 is induced through salt (NaCl) treatment (Fig 1). In addition, the expression of OsVTC1-1 was also induced after oxidative stress treatment (Methyl Viologen, MV) and osmotic stress treatment (PEG 6000), although gene expression patterns showed some difference under these treatments (Fig 1). The expression of OsVTC1-1 was rapidly induced at 2 hours after NaCl treatment and peaked at 8 hours. In contrast, the expression of OsVTC1-1 was slightly induced at 8 hours after PEG 6000 treatment and peaked at 10 hours (Fig 1). The expression of OsVTC1-1 was induced through MV within 4 hours and peaked at 6 hours (Fig 1).
Fig 1. The expression of OsVTC1-1 in plant responses to abiotic stresses.
To examine the expression patterns of OsVTC1-1 in plant responses to salt, PEG or MV, the transcript level of OsVTC1-1 was analyzed by Q-PCR. After normalizing to internal control (Actin), the transcript level of OsVTC1-1 under control conditions (0 h) was assigned as 1, and this figure shows the expression level of OsVTC1-1 at others times relative to 0 h. The experiments were repeated three times.
OsVTC1-1 plays an important role in salt tolerance of rice seedlings
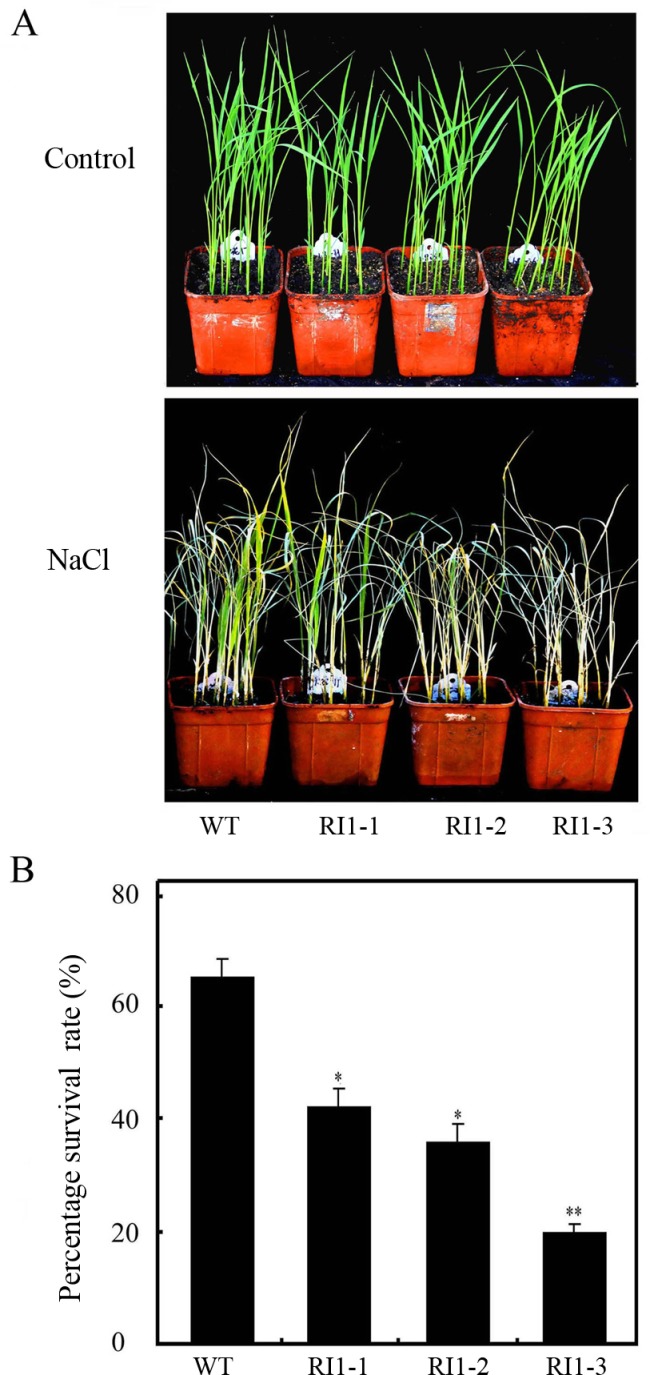
To examine the native function of OsVTC1-1 in the response of rice to salt stress, we disrupted OsVTC1-1 activity after knocking down the expression of OsVTC1-1 using RNA interference (RI) technology. Previous study showed that the expression of OsVTC1-1 is significantly decreased in OsVTC1-1 RI lines, but expressions of its homologous genes OsVTC1-3 and OsVTC1-8 are not obviously interfered [28]. Rice is sensitive to salt stress at seedling and reproductive stages [36]. First, we treated OsVTC1-1 RI seedlings with salt (NaCl) to analyze the native function of OsVTC1-1 in the response of rice to salt stress at seedling stage. When treated with 150 mM NaCl for two weeks, OsVTC1-1 RI transgenic seedlings exhibited obviously decreased tolerance to salt stress (Fig 2A). After resuming cultivation for ten days, the percentage survival rate of OsVTC1-1 RI lines also significantly decreased (Fig 2B). The percentage survival rate of wild-type rice seedlings was 64.8%, whereas that of OsVTC1-1 RI1-3, which had 15% less OsVTC1-1 transcript levels compared with wild-type, was approximately 20% [28]. This result indicated that OsVTC1-1 plays a critical role in the response of rice to salt stress at seedling stage.
Fig 2. Decreasing the expression of OsVTC1-1 impairs the tolerance of rice seedlings to salt stress.
(A), phenotypes of OsVTC1-1 RI lines under salt stress. Control indicates that rice seedlings were grown under normal conditions, and NaCl indicates that seedlings were treated with 150 mM NaCl aqueous solution. (B), the percentage survival rates (Percentage is the ratio of number of plants with green new leaves to number of total plants) of OsVTC1-1 RI plants after salt treatment in (A). WT represents ZH17 rice variety; RI1-1, RI1-2 and RI1-3 indicate independent RNA interference lines of OsVTC1-1 in ZH17 background, respectively. About 50~60 seedlings were uses in each experiment. The bars represent SE (±) of three independent assays, and the asterisk indicates results significantly different from WT (**P<0.01 and *P<0.05). Significance was evaluated using the t-test.
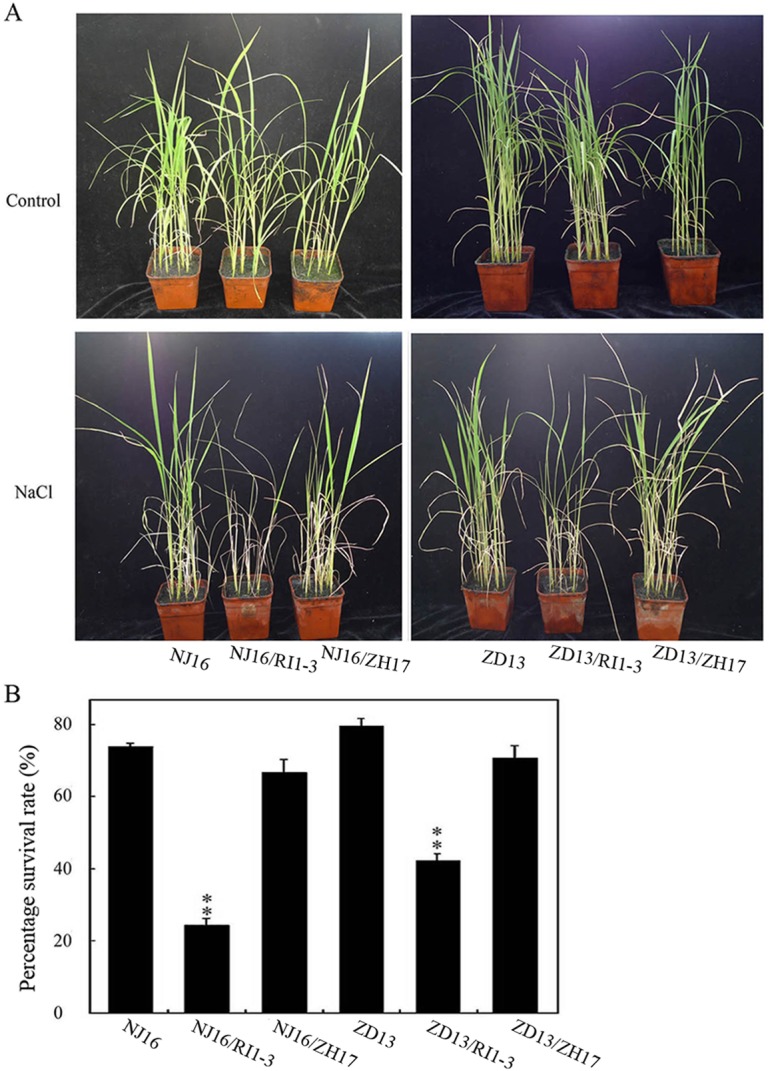
To further illustrate the role of OsVTC1-1 in the salt tolerance of rice, two salt-tolerant local varieties, DJ16 and ZD13, were selected to further examine the native function of OsVTC1-1 in the response of rice to salt stress (S1 Fig). We utilized NJ16 and ZD13 to generate hybrids with OsVTC1-1 RI1-3 plants (NJ16/RI1-3 and ZD13/RI1-3) and decrease the expression of OsVTC1-1 in NJ16 and ZD13, respectively. The plants NJ16/ZH17 and ZD13/ZH17, produced from the hybridization of ZH17 with NJ16 and ZD13, respectively, were used as controls. Expression level of OsVTC1-1 and synthesis of AsA were significantly suppressed in the F1 NJ16/RI1-3 and ZD13/RI1-3 hybrid plants (S2 Fig). We treated four-week-old F1 hybrid rice lines with 150 mM NaCl for ten days and subsequently resumed cultivation for an additional ten days. Results showed that salt tolerance of NJ16/RI1-3 and ZD13/RI1-3 plants was obviously decreased (Fig 3). After treatment with 150 mM NaCl, the percentage survival rates of NJ17/RI1-3 and ZD13/RI1-3 hybrids were 26.3% and 47.2%, respectively. In contrast, the percentage survival rates of NJ17/ZH17 and ZD13/ZH17 control plants after salt treatment were approximately 63.1% and 72.2%, respectively (Fig 3B). Above results showed that OsVTC1-1 is a general factor for examining salt tolerance of rice at seedling stage.
Fig 3. OsVTC1-1 plays an important role in the response of salt-tolerant rice varieties.
(A), phenotypes of F1 hybrid rice plants under salt stress. NJ16 represents salt-tolerant rice landrace Ningjing16; NJ16/RI1-3 represents F1 hybrid of Ningjing16 with OsVTC1-1 RNA interference line RI1-3; NJ16/ZH17 represents F1 plants of Ningjing16 and Zhonghua17, which were used as controls. ZD13 indicates the salt-tolerant rice landrace Zhongdao13; ZD13/RI1-3 indicates F1 plants of Zhongdao13 and OsVTC1-1 RI1-3; ZD13/ZH17 indicates F1 plants of Zhongdao13 and Zhonghua17, which were used as controls. Ningjing16 and Zhongdao13 were used as the female parents. Control indicates that plants were grown under normal conditions, and NaCl indicates that four-week rice seedlings were treated with 150 mM NaCl solution for another ten days. (B), percentage survival rates (Percentage is the ratio of number of plants with green new leaves to number of total plants) of F1 hybrid rice seedlings after salt stress treatment in (A). About 50~60 seedlings were uses in each experiment. The bars represent SD (±) of two independent assays, and the asterisk indicates results significantly different from control (**P<0.01 and *P<0.05). Significance was evaluated using the t-test.
Downregulating OsVTC1-1 expression reduces grain production in rice
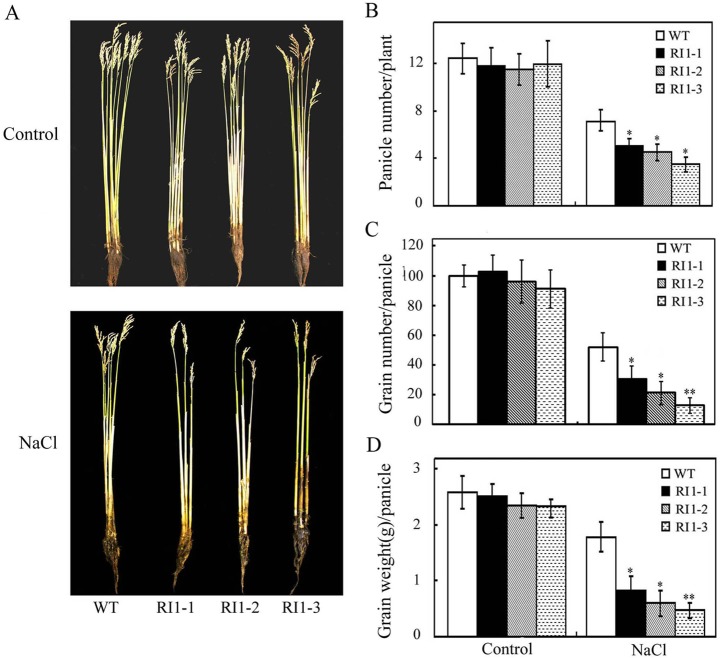
Rice yield also is severely decreased by salt stress at reproductive stage [36, 37]. To further illustrate the role of OsVTC1-1 in the tolerance of rice to salt stress, we analyzed the effect of OsVTC1-1 on rice grain production under high salt condition. Six-week-old wild-type and RI transgenic plants growing in soil were watered with 100 mM NaCl until harvest. Results showed that inhibiting the expression of OsVTC1-1 clearly reduced grain production of transgenic rice under salt treatment (Fig 4A). Under high salt conditions, OsVTC1-1 RI lines produced less grains compared with control lines, reflecting fewer panicles and less grain number per panicle compared with control plants (Fig 4B, 4C and 4D). These results further indicate that OsVTC1-1 also plays a key role in the tolerance of rice to salt stress at reproductive stage.
Fig 4. OsVTC1-1 RI lines show reduced grain production under salt stress.
(A), phenotypes of OsVTC1-1 RI lines grown under salt stress at harvest stage. (B), panicle number of OsVTC1-1 RI plants with or without salt treatment. (C), grain number of the panicle of OsVTC1-1 RI plants with or without salt treatment. (D), production of the panicle of OsVTC1-1 RI plants with or without salt treatment. Control indicates that plants were grown under normal conditions, and NaCl indicates that plants were treated with 100 mM NaCl. WT represents ZH17; RI1-1, RI1-2 and RI1-3 indicate different RI lines of OsVTC1-1 in ZH17 background. About 40~50 plants were uses in each experiment. Bars represent SE (±) of three independent assays, and the asterisk indicates that the results were significantly different from WT (**P<0.01 and *P<0.05). Significance was evaluated using the t-test.
OsVTC1-1 controls salt tolerance of rice by controlling AsA production
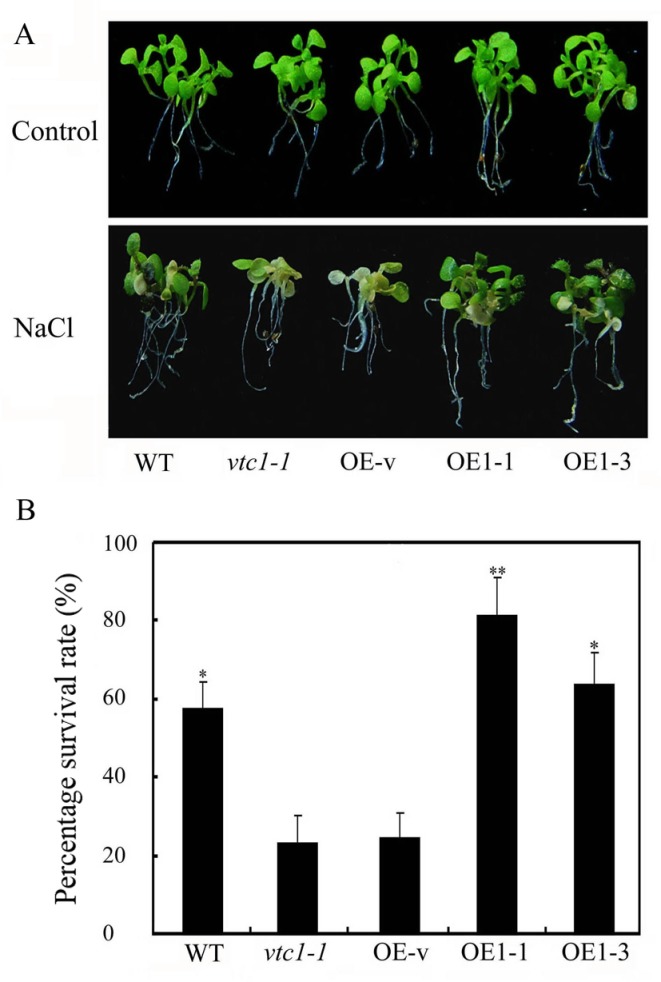
Arabidopsis mutant vtc1-1 which only has approximately one-fourth AsA content of control plants impairs salt tolerance [19]. Transgenic Arabidopsis overexpressing OsVTC1-1 (vtc1-1 background [28]) restored AsA synthesis and recovered salt tolerance (Fig 5A and 5B), suggesting that OsVTC1-1 might be involved in the response of rice to salt through the regulation of AsA synthesis.
Fig 5. Overexpression of OsVTC1-1 enhances the tolerance of Arabidopsis to salt stress.
(A), phenotypes of OsVTC1-1 overexpressing Arabidopsis under salt stress. Control indicates that plants were grown under normal conditions, and NaCl indicates that Arabidopsis seedlings were treated with 150 mM NaCl for ten days. (B), the percentage survival rates of OsVTC1-1 overexpressing lines after salt treatment in (A). OE-v represents vtc1-1 plants transformed with the pCAMBIA1307 blank vector. OE1-1 and OE1-3 indicate different overexpression lines of OsVTC1-1 in vtc1-1 background. WT represents wild-type Col-0 Arabidopsis. About 70~80 seedlings were uses in each experiment. The bars represent SE (±) of three independent assays, and the asterisk indicates results significantly different from vtc1-1 (**P<0.01 and *P<0.05). Significance was evaluated using the t-test.
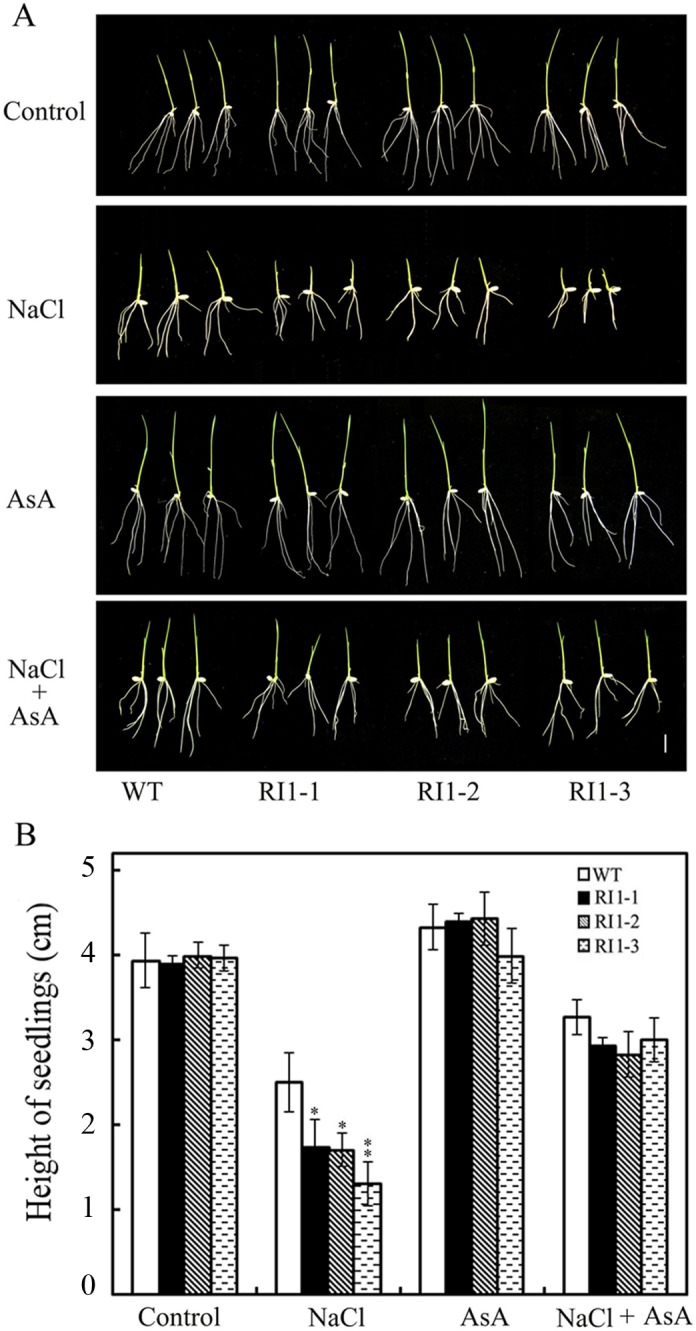
Studies have demonstrated that the synthesis of AsA plays important roles in the response of plants to salt stress [25, 38–40]. To further analyze the role of AsA in OsVTC1-1-mediated enhancement of salt tolerance in rice, we examined the effect of AsA on salt tolerance of OsVTC1-1 RI seedlings with supplying exogenous AsA. Result showed that exogenous AsA restored the tolerance of OsVTC1-1 RI rice seedlings to salt stress (Fig 6). After supplying exogenous AsA, the growth of OsVTC1-1 RI shoots was similar to that of wild-type (Fig 6B). This finding indicated that OsVTC1-1 is involved in response of rice to salt stress through the regulation of AsA biosynthesis.
Fig 6. Exogenous AsA rescues the tolerance of OsVTC1-1 RI plants to salt stress.
(A), phenotypes of OsVTC1-1 RI lines grown on MS medium with or without supplying exogenous AsA under salt treatment. (B), height of OsVTC1-1 RI lines seedlings grown on MS medium with or without supplying exogenous AsA under salt treatment. Control indicates that rice seedlings were grown on MS medium; NaCl indicates that rice seedlings were grown on MS medium with 150 mM NaCl; AsA indicates that rice seedlings were grown on MS medium with 10 μM AsA; and NaCl+ AsA represents rice seedlings grown on MS medium with 150 mM NaCl and 10 μM AsA. Above assays were repeated three times. About 50~60 seedlings were uses in each experiment. The bars represent SE (±). The asterisk indicates results significantly different from WT (**P<0.01 and *P<0.05). Significance was evaluated using the t-test.
OsVTC1-1 is involved in ROS scavenging in rice under salt stress
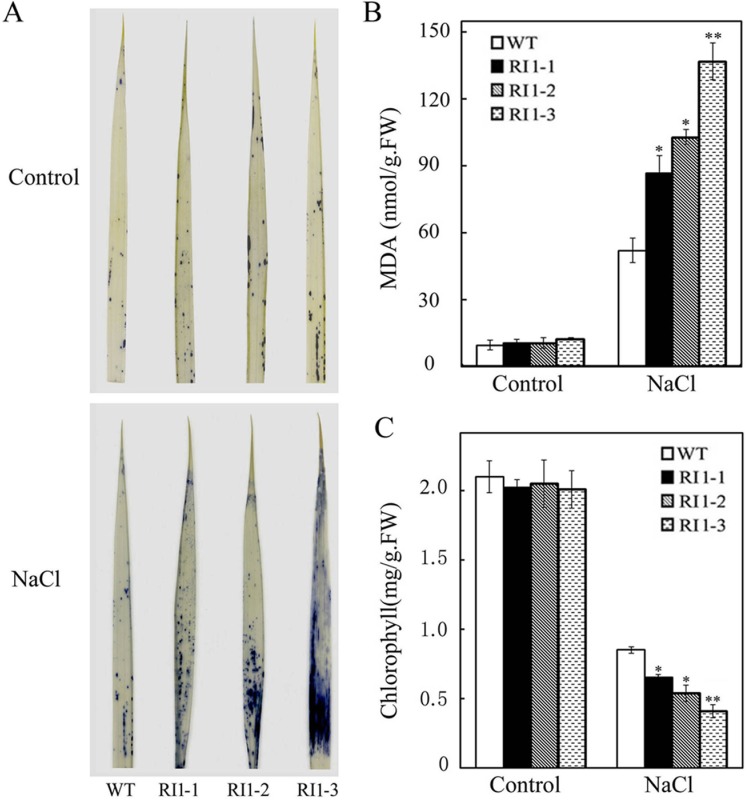
Environmental stresses, such as drought, salinity or disease, potentially harm plants through accumulation of ROS within plant cells [41, 42]. As the most abundant antioxidant in plants, AsA plays an important role in ROS scavenging to protect plants against ROS-induced damage under salt stress [25, 38, 39]. To examine the mechanism of OsVTC1-1 in response to salt stress, we analyzed ROS (O2-) content in leaves of OsVTC1-1 RI plants grown under salt stress. The data showed that the RI plants accumulated much more O2- in the leaves compared with wild-type plants (Fig 7A), indicating that inhibiting the function of OsVTC1-1 reduces ROS scavenging in rice under salt stress. The accumulation of ROS damages cellular proteins, enzymes and lipids. Malondialdehyde (MDA) is an important product of lipid peroxidation, representing the degree of oxidative damage to the plant cell [43]. The MDA content of the RI lines was clearly higher than that of wild-type (WT) plants under salt stress (Fig 7B). Consistent with higher MDA content, the chlorophyll content in the leaves of RI plants was severely reduced compared with WT plants under NaCl stress (Fig 7C). These results further suggest that OsVTC1-1 plays an important role in scavenging ROS under salt stress.
Fig 7. Downregulating expression of OsVTC1-1 impairs the capacity of rice to scavenge ROS under salt stress.
(A), O2.- content of leaves in ZH17 (WT) and OsVTC1-1 RI lines with or without salt treatment. O2.- content was determined through histological staining with NBT. (B), MDA content of WT and OsVTC1-1 RI lines with or without salt treatment. (C), chlorophyll content of WT and OsVTC1-1 RI lines with or without salt treatment. Above assays were repeated three times. About 50~60 seedlings were uses in each experiment. The bars represent SE (±). The asterisk indicates results significantly different from WT (**P<0.01 and *P<0.05). Significance was evaluated using the t-test.
Discussion
Salinity stress has an adverse effect on rice at all stages of growth [44–46]. Reproductive stage is a key period for growth and yield of rice under salt stress [45, 46]. Several studies have shown that GMPase has an important effect on tolerance of plants to salinity stress at vegetative stage, but the role of GMPase in tolerance of plants to salt stress at reproductive stage remains unclear [9, 19, 29]. Results of present study showed that GMPase OsVTC1-1 plays a critical role in salt tolerance of rice at both seedling and reproductive stages through regulating AsA biosynthesis.
Previous studies have demonstrated that GMPase is involved in response to salt stress at seedling stage [8, 9, 19, 29, 33]. A point mutation of Arabidopsis GMPase VTC1 dramatically reduces tolerance to salt stress [19]. In contrast, stable VTC1 protein in csn5b, a mutant of COP9 complex subunit CSN5B, significantly improved tolerance of Arabidopsis to salt stress [33]. Rice is an important crop species. Salinity affects rice growth in varying degrees at different development stages. Seedling and reproduction are two most sensitive stages of rice to salinity stress [36, 44–46]. Excess salt limits seedling growth and reduces tiller numbers at seedling stage, and reduces grain numbers per panicle and diminishes filled grain rate at reproductive stage, which causes severely loss of rice yield [34, 37, 44–46]. The expression of rice GMPase gene GMP1 (OsVTC1-1) is induced under salt stress ([29], Fig 1), and overexpression of OsVTC1-1 enhances salt tolerance of transgenic tobacco plants [29], indicating that expression of OsVTC1-1 is important to enhance salt tolerance of plants. In this study, knockdowning OsVTC1-1 expression impaired seedling salt tolerance of different rice varieties, including salt-tolerant rice varieties (Figs 2 and 3), suggesting that OsVTC1-1 also plays an important role in salt tolerance at vegetative stage in rice. Intriguingly, under salt stress, OsVTC1-1 RI lines showed fewer panicles, less grain numbers per panicle, and reduced grain yields compared with control rice (Fig 4), indicating that OsVTC1-1 also plays a key role in tolerance of rice to salt stress at reproductive stage. Thus, taken together, these studies reveal that GMPase plays a critical role in salt tolerance of rice at both seedling and reproductive stages.
High salinity disrupts ion and water equilibrium of plant cells, which accumulate large amounts of ROS, further impairing the activity and stability of plant proteins and resulting in serious harm to plants [42, 47–51]. For example, high salinity limits the regeneration of NADP+ in the Calvin cycle, which further damages the components of photosynthetic electron transport chain, resulting in the termination of photosynthesis due to the accumulation of ROS in plant cells under salt stress [50]. Therefore, plants need to efficiently scavenge excess ROS to protect chlorophyll and chloroplasts from damage under high salinity conditions [51]. Plants have evolved numerous mechanisms to avoid cellular damage due to ROS accumulation, including synthesis of anti-oxidants under high salinity conditions [19, 25, 27, 33–35, 49, 52–54]. In salinity stress, AsA might act as a primary antioxidant to directly scavenge ROS and indirectly clear ROS through AsA-GSH (glutathione) cycle [26, 27, 54]. Therefore, the regulation of AsA synthesis plays an important role in salt stress response [19, 33, 34, 38, 39].
In plants, AsA is synthesized through several pathways [55–57]. Among these pathways, L-galactose pathway plays a major role in plant AsA biosynthesis [26]. The biosynthesis of AsA through L-galactose pathway involves at least ten enzymes. Among these enzymes, GDP-mannose pyrophosphorylase (GMP) [2, 58], GDP-mannose-3',5'-epimerase (GME) [56], GDP-L-galactose phosphorylase (GGP) [59, 60], L-galactose-1-phosphate phosphatase (GPP) [61], L-galactose dehydrogenase (GDH) [62], and L-galactono-1,4-lactone dehydrogenase (GalLDH) [63, 64], are important enzymes that regulate AsA biosynthesis in plants. GMPase is a key enzyme of AsA biosynthesis and plays multiple important roles in the responses of plants to stresses [12, 8, 20, 26]. Previous studies have implicated GMPase in ROS scavenging in the response to salt stress in dicot plants Arabidopsis and tobacco [12, 19]. Previous study has showed that OsVTC1-1 is a key GMPase for AsA biosynthesis in rice leaves. AsA content in OsVTC1-1 RI line RI-3 was approximately 40% of that in wild-type plants [28]. In present study, application of exogenous AsA restored the growth of OsVTC1-1 RI seedlings to a level similar to that of wild-type seedlings under salt stress (Fig 6). Arabidopsis GMase mutant vtc1-1 reduces ability for ROS scavenging and salt tolerance under salt stress [2, 19]. The expression of OsVTC1-1 restored AsA content and salt tolerance of vtc1-1 ([28], Fig 5), and similar to vtc1-1, OsVTC1-1 RI plants also accumulated more ROS (O2-) and MDA than wild-type plants under salt stress ([19, 65], Fig 7), indicating that the decreased tolerance of OsVTC1-1 RI plants to salt stress might reflect impaired AsA synthesis for scavenging excess ROS induced under high salt conditions.
Supporting Information
(A), phenotypes of different rice varieties under salt stress. Control indicates that plants were grown under normal conditions; NaCl indicates that two-week rice seedlings were treated with 150 mM NaCl for 10 days. (B), the percentage survival rates of rice varieties after salt stress treatment in (A) with another ten days of re-watering without NaCl. NJ16, ZH17, Nip, Kos, YD6 and IR29 indicate rice varieties Ningjing16, Zhonghua17, Nipponbare, Koshihikari, Yangdao 6 and IR29, respectively. The bars represent SD (±) of three independent assays. About 50~60 seedlings were uses in each experiment.
(EPS)
(A), total RNA was extracted from leaves of four-week F1 OsVTC1-1 RI1-3 hybrid rice plants; subsequently, the expression of OsVTC1-1 was analyzed using qPCR. (B), AsA content of F1 OsVTC1-1 RI1-3 hybrid rice plants. NJ16 represents salt-tolerant rice landrace Ningjing16; NJ16/RI1-3 represents F1 hybrid of Ningjing16 with OsVTC1-1 RNA interference line RI1-3; and NJ16/ZH17 represents F1 plants of Ningjing16 and Zhonghua17, which were used as controls. ZD13 indicates salt-tolerant rice landrace Zhongdao13; ZD13/RI1-3 indicates F1 plants of Zhongdao13 and OsVTC1-1 RI1-3; and ZD13/ZH17 indicates F1 plants of Zhongdao13 and Zhonghua17, which were used as controls. Ningjing16 and Zhongdao13 were used as female parents. The bars represent SD (±) of three independent assays, and the asterisk indicates results significantly different from control (NJ16/ZH17 and ZD13/ZH17, respectively) (**P<0.01). Significance was evaluated using the t-test.
(EPS)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Basic Research Program of China (Grant No: 2013CB127003), the National Science Foundation of China (Grant No: 31070270) and National Transgenic Major Program (Grant No: 2015ZX08001-002 and Grant No: 2014ZX08009-15B).
References
- 1.Smoot JW, Serif GS. Purification of GTP: alpha-D-mannose-1-phosphate guanyltransferase. Eur J Biochem. 1985; 148: 83–87. [DOI] [PubMed] [Google Scholar]
- 2.Conklin PL, Norris SR, Wheeler GL, Williams EH, Smirnoff N, Last RL. Genetic evidence for the role of GDP-mannose in plant ascorbic acid (vitamin C) biosynthesis. Proc Natl Acad Sci USA. 1999; 96: 4198–4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freshour G, Bonin CP, Reiter WD, Albersheim P, Darvill AG, Hahn MG. Distribution of fucose-containing xyloglucans in cell walls of the mur1 mutant of Arabidopsis. Plant Physiol. 2003; 131: 1602–1612. 10.1104/pp.102.016444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maeda Y, Kinoshita T. Dolichol-phosphate mannose synthase: structure, function and regulation. Biochim Biophys Acta. 2008; 1780: 861–868. 10.1016/j.bbagen.2008.03.005 [DOI] [PubMed] [Google Scholar]
- 5.Conklin PL, Williams E H and Last RL. Environmental stress sensitivity of an ascorbic acid-deficient Arabidopsis mutant. Proc Natl Acad Sci USA. 1996; 93: 9970–9974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keller R, Renz FS, Kossmann J. Antisense inhibition of the GDP-mannose pyrophosphorylase reduces the ascorbate content in transgenic plants leading to developmental changes during senescence. Plant J. 1999; 19: 131–141. [DOI] [PubMed] [Google Scholar]
- 7.Qin C, Qian W, Wang W, Wu Y, Yu C, Jiang X, et al. GDP-mannose pyrophosphorylase is a genetic determinant of ammonium sensitivity in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2008; 105: 18308–18313. 10.1073/pnas.0806168105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q, Li BH, Kronzucker HJ, Shi WM. Root growth inhibition by NH(4)(+) in Arabidopsis is mediated by the root tip and is linked to NH(4)(+) efflux and GMPase activity. Plant Cell Environ. 2010; 33: 1529–1542. 10.1111/j.1365-3040.2010.02162.x [DOI] [PubMed] [Google Scholar]
- 9.Wang HS, Zhu ZJ, Feng Z, Zhang SG, Yu C. Antisense-mediated depletion of GMPase gene expression in tobacco decreases plant tolerance to temperature stresses and alters plant development. Mol Biol Rep. 2012; 39: 10413–10420. 10.1007/s11033-012-1920-5 [DOI] [PubMed] [Google Scholar]
- 10.Mortimer JC, Yu X, Albrecht S, Sicilia F, Huichalaf M, Ampuero D, et al. Abnormal glycosphingolipid mannosylation triggers salicylic acid-mediated responses in Arabidopsis. Plant Cell. 2013; 25: 1881–1894. 10.1105/tpc.113.111500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotchoni SO, Larrimore KE, Mukherjee M, Kempinski CF, Barth C. Alterations in the endogenous ascorbic acid content affect flowering time in Arabidopsis. Plant Physiol. 2009; 149: 803–815. 10.1104/pp.108.132324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barth C, Gouzd ZA, Steele HP, Imperio RM. A mutation in GDP-mannose pyrophosphorylase causes conditional hypersensitivity to ammonium, resulting in Arabidopsis root growth inhibition, altered ammonium metabolism, and hormone homeostasis. J Exp Bot. 2010; 61: 379–394. 10.1093/jxb/erp310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang C, Ouyang B, Yang C, Zhang X, Liu H, Zhang Y, et al. Reducing AsA leads to leaf lesion and defence response in knock-down of the AsA biosynthetic enzyme GDP-D-mannose pyrophosphorylase gene in tomato plant. PLoS One. 2013; 8: e61987 10.1371/journal.pone.0061987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nickle TC, Meinke DW. cytokinesis-defective mutant of Arabidopsis (cyt1) characterized by embryonic lethality, incomplete cell walls, and excessive callose accumulation. Plant J. 1998; 15: 321–332. [DOI] [PubMed] [Google Scholar]
- 15.Lukowitz W, Nickle TC, Meinke DW, Last RL, Conklin PL, Somerville CR. Arabidopsis cyt1 mutants are deficient in a mannose-1-phosphate guanylyltransferase and point to a requirement of N-linked glycosylation for cellulose biosynthesis. Proc Natl Acad Sci USA. 2001; 98: 2262–2267. 10.1073/pnas.051625798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conklin PL, Pallanca JE, Last RL, Smirnoff N. L-ascorbic acid metabolism in the ascorbate-deficient Arabidopsis mutant vtc1. Plant Physiol. 1997; 115: 1277–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veljovic-Jovanovic SD, Pignocchi C, Noctor G, Foyer CH. Low ascorbic acid in the vtc-1 mutant of Arabidopsis is associated with decreased growth and intracellular redistribution of the antioxidant system. Plant Physiol. 2001; 127: 426–435. [PMC free article] [PubMed] [Google Scholar]
- 18.Conklin PL and Barth C. Ascorbic acid, a familiar small molecule intertwined in the response of plants to ozone, pathogens, and the onset of senescence. Plant Cell Environ. 2004; 27: 959–970. [Google Scholar]
- 19.Huang C, He W, Guo J, Chang X, Su P, Zhang L. Increased sensitivity to salt stress in an ascorbate-deficient Arabidopsis mutant. J Exp Bot. 2005; 56: 3041–3049. 10.1093/jxb/eri301 [DOI] [PubMed] [Google Scholar]
- 20.Hu T, Ye J, Tao P, Li H, Zhang J, Zhang Y, et al. Tomato HD-Zip I transcription factor, SlHZ24, modulates ascorbate accumulation through positively regulating the D-mannose/L-galactose pathway. Plant J. 2016; 85: 16–29. 10.1111/tpj.13085 [DOI] [PubMed] [Google Scholar]
- 21.Pastori GM, Kiddle G, Antoniw J, Bernard S, Veljovic-Jovanovic S, Verrier PJ, et al. Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell. 2003; 15: 939–951. 10.1105/tpc.010538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barth C, Moeder W, Klessig DF, Conklin PL. The Timing of Senescence and Response to Pathogens Is Altered in the Ascorbate-Deficient Arabidopsis Mutant vitamin c-1. Plant Physiol. 2004; 134: 1784–1792. 10.1104/pp.103.032185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pavet V, Olmos E, Kiddle G, Mowla S, Kumar S, Antoniw J, et al. Ascorbic acid deficiency activates cell death and disease resistance responses in Arabidopsis. Plant Physiol. 2005; 139: 1291–1303. 10.1104/pp.105.067686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukherjee M, Larrimore KE, Ahmed NJ, Bedick TS, Barghouthi NT, Traw MB, et al. 2010 Ascorbic acid deficiency in Arabidopsis induces constitutive priming that is dependent on hydrogen peroxide, salicylic acid, and the NPR1 gene. Mol Plant Microbe Interact. 2010; 23: 340–351. 10.1094/MPMI-23-3-0340 [DOI] [PubMed] [Google Scholar]
- 25.Wang HS, Yu C, Zhu ZJ, Yu XC. Overexpression in tobacco of a tomato GMPase gene improves tolerance to both low and high temperature stress by enhancing antioxidation capacity. Plant Cell Rep. 2011; 30: 1029–1040. 10.1007/s00299-011-1009-y [DOI] [PubMed] [Google Scholar]
- 26.Smirnoff N, Wheeler GL. Ascorbic acid in plants: biosynthesis and function. Crit Rev Biochem Mol Biol. 2000; 35: 291–314. 10.1080/10409230008984166 [DOI] [PubMed] [Google Scholar]
- 27.Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Ann Rev Plant Physiol Plant Mol Biol. 1998; 49: 249–279. [DOI] [PubMed] [Google Scholar]
- 28.Qin H, Deng Z, Zhang C, Wang Y, Wang J, Liu H, et al. Rice GDP-mannose pyrophosphorylase OsVTC1-1 and OsVTC1-3 play different roles in ascorbic acid synthesis. Plant Mol Biol. 2016; 90: 317–327. 10.1007/s11103-015-0420-0 [DOI] [PubMed] [Google Scholar]
- 29.Kumar R, Mustafiz A, Sahoo KK, Sharma V, Samanta S, Sopory SK, et al. Functional screening of cDNA library from a salt tolerant rice genotype Pokkali identifies mannose-1-phosphate guanyl transferase gene (OsMPG1) as a key member of salinity stress response. Plant Mol Biol. 2012; 79: 555–568. 10.1007/s11103-012-9928-8 [DOI] [PubMed] [Google Scholar]
- 30.Bent A. Arabidopsis thaliana floral dip transformation method. Methods Mol Biol. 2006; 343: 87–103. 10.1385/1-59745-130-4:87 [DOI] [PubMed] [Google Scholar]
- 31.Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998; 16: 735–743. [DOI] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT Method. Methods. 2001; 25: 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Yu Y, Zhang Z, Quan R, Zhang H, Ma L, et al. Arabidopsis CSN5B interacts with VTC1 and modulates ascorbic acid synthesis. Plant Cell. 2013; 25: 625–636. 10.1105/tpc.112.106880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Z, Wang J, Zhang R, Huang R. The ethylene response factor AtERF98 enhances tolerance to salt through the transcriptional activation of ascorbic acid synthesis in Arabidopsis. Plant J. 2012; 71: 273–287. 10.1111/j.1365-313X.2012.04996.x [DOI] [PubMed] [Google Scholar]
- 35.Aono M, Kubo A, Saji H, Tanaka K, Kondo N. Enhanced tolerance to photooxidative stress of transgenic Nicotiana tabacum with high chloroplastic glutathione reductase activity. Plant Cell Physiol. 1993; 34: 129–136. [Google Scholar]
- 36.Lutts S, Kinet JM, Bouharmont J. Changes in plant response to NaCl during development of rice (Oryza sativa L.) varieties differing in salinity resistance. J Exp Bot. 1995; 46: 1843–1852. [Google Scholar]
- 37.Tuteja N. Mechanisms of high salinity tolerance in plants. Methods Enzymol. 2007; 428: 419–438. 10.1016/S0076-6879(07)28024-3 [DOI] [PubMed] [Google Scholar]
- 38.Shalata A, Neumann PM. Exogenous ascorbic acid (vitamin C) increases resistance to salt stress and reduces lipid peroxidation. J Exp Bot. 2001; 52: 2207–2211. [DOI] [PubMed] [Google Scholar]
- 39.Zhang C, Liu J, Zhang Y, Cai X, Gong P, Zhang J, et al. Overexpression of SlGMEs leads to ascorbate accumulation with enhanced oxidative stress, cold, and salt tolerance in tomato. Plant Cell Rep. 2011; 30: 389–398. 10.1007/s00299-010-0939-0 [DOI] [PubMed] [Google Scholar]
- 40.Zhang GY, Liu RR, Zhang CQ, Tang KX, Sun MF, Yan GH, et al. Manipulation of the rice L-galactose pathway: evaluation of the effects of transgene overexpression on ascorbate accumulation and abiotic stress tolerance. PLoS One. 2015; 10: e0125870 10.1371/journal.pone.0125870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamb C, Dixon RA. The Oxidative Burst in Plant Disease Resistance. Ann Rev Plant Physio Plant Mol Biol. 1997; 48: 251–275. [DOI] [PubMed] [Google Scholar]
- 42.Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010; 33: 453–467. 10.1111/j.1365-3040.2009.02041.x [DOI] [PubMed] [Google Scholar]
- 43.Tajdoost S, Farboodnia T, Heidari R. Salt pretreatment enhance salt tolerance in Zea mays L. seedlings. Pak J Biol Sci. 2007; 10: 2086–2090. [DOI] [PubMed] [Google Scholar]
- 44.Zeng L, Shannon MC. Salinity effects on the seedling growth and yield components of ric. Crop Sci. 2000; 40: 996–1003. [Google Scholar]
- 45.Walia H, Wilson C, Zeng L, Ismail AM, Condamine P, Close TJ. Genome-wide transcriptional analysis of salinity stressed japonica and indica rice genotypes during panicle initiation stage. Plant Mol Biol. 2007; 63: 609–623. 10.1007/s11103-006-9112-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rao PS, Mishra B, Gupta SR and Rathore A. Reproductive stage tolerance to salinity and alkalinity stresses in rice genotypes. Plant Breed. 2008; 127: 256–261. [Google Scholar]
- 47.Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Ann Rev Plant Biol. 2004. 55: 373–399. [DOI] [PubMed] [Google Scholar]
- 48.Lee MH, Cho EJ, Wi SG, Bae H, Kim JE, Cho JY, et al. Divergences in morphological changes and antioxidant responses in salt-tolerant and salt-sensitive rice seedlings after salt stress. Plant Physiol Biochem. 2013; 70: 325–335. 10.1016/j.plaphy.2013.05.047 [DOI] [PubMed] [Google Scholar]
- 49.Bose J, Rodrigo-Moreno A, Shabala S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J Exp Bot. 2014; 65: 1241–1257. 10.1093/jxb/ert430 [DOI] [PubMed] [Google Scholar]
- 50.Moradi F, Ismail AM. Responses of photosynthesis, chlorophyll fluorescence and ROS-scavenging systems to salt stress during seedling and reproductive stages in rice. Ann Bot. 2007; 99: 1161–1173. 10.1093/aob/mcm052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pospisil P. Production of reactive oxygen species by photosystem II. BBA-bioenergetics. 2009; 1787: 1151–1160. 10.1016/j.bbabio.2009.05.005 [DOI] [PubMed] [Google Scholar]
- 52.Yadav S, Irfan M, Ahmad A, Hayat S. Causes of salinity and plant manifestations to salt stress: a review. J Environ Biol. 2011; 32: 667–685. [PubMed] [Google Scholar]
- 53.Flowers TJ, Munns R, Colmer TD. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann Bot. 2015; 115: 419–431. 10.1093/aob/mcu217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uchendu EE, Leonard SW, Traber MG, Reed BM. Vitamins C and E improve regrowth and reduce lipid peroxidation of blackberry shoot tips following cryopreservation. Plant Cell Rep. 2010; 29: 25–35. 10.1007/s00299-009-0795-y [DOI] [PubMed] [Google Scholar]
- 55.Agius F, González-Lamothe R, Caballero JL, Muñoz-Blanco J, Botella MA, et al. Engineering increased vitamin C levels in plants by overexpression of a D-galacturonic acid reductase. Nat Biotechnol. 2003; 21: 177–181. 10.1038/nbt777 [DOI] [PubMed] [Google Scholar]
- 56.Wolucka BA, Van Montagu M. GDP-mannose 3’, 5’-epimerase forms GDP-L-gulose, a putative intermediate for the de novo biosynthesis of vitamin C in plants. J Biol Chem. 2003; 278: 47483–47490. 10.1074/jbc.M309135200 [DOI] [PubMed] [Google Scholar]
- 57.Lorence A, Chevone BI, Mendes P, Nessler CL. myo-Inositol oxygenase offers a possible entry point into plant ascorbate biosynthesis. Plant Physiol. 2004; 134: 1200–1205. 10.1104/pp.103.033936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wheeler GL, Jones MA, Smirnoff N. The biosynthetic pathway of vitamin C in higher plants. Nature. 1998; 393: 365–369. 10.1038/30728 [DOI] [PubMed] [Google Scholar]
- 59.Dowdle J, Ishikawa T, Gatzek S, Rolinski S, Smirnoff N. Two genes in Arabidopsis thaliana encoding GDP-L-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. Plant J. 2007; 52: 673–89. 10.1111/j.1365-313X.2007.03266.x [DOI] [PubMed] [Google Scholar]
- 60.Linster CL, Clarke SG. L-Ascorbate biosynthesis in higher plants: the role of VTC2. Trends Plant Sci. 2008; 13: 567–573. 10.1016/j.tplants.2008.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Conklin PL, Gatzek S, Wheeler GL, Dowdle J, Raymond MJ, Rolinski S, et al. Arabidopsis thaliana VTC4 encodes L-galactose-1-P phosphatase, a plant ascorbic acid biosynthetic enzyme. J Biol Chem. 2002; 281: 15662–15670. [DOI] [PubMed] [Google Scholar]
- 62.Gatzek S, Wheeler GL, Smirnoff N. Antisense suppression of l-galactose dehydrogenase in Arabidopsis thaliana provides evidence for its role in ascorbate synthesis and reveals light modulated l-galactose synthesis. Plant J. 2002; 30: 541–553. [DOI] [PubMed] [Google Scholar]
- 63.Tabata K, Oba K, Suzuki K, Esaka M. Generation and properties of ascorbic acid-deficient transgenic tobacco cells expressing antisense RNA for L-galactono-1,4-lactone dehydrogenase. Plant J. 2001; 27: 139–148. [DOI] [PubMed] [Google Scholar]
- 64.Alhagdow M, Mounet F, Gilbert L, Nunes-Nesi A, Garcia V, Just D, et al. Silencing of the mitochondrial ascorbate synthesizing enzyme L-galactono-1,4-lactone dehydrogenase affects plant and fruit development in tomato. Plant Physiol. 2007; 145: 1408–1422. 10.1104/pp.107.106500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frei M, Wissuwa M, Pariasca-Tanaka J, Chen CP, Südekum KH, Kohno Y. Leaf ascorbic acid level—is it really important for ozone tolerance in rice? Plant Physiol Biochem. 2012; 59: 63–70. 10.1016/j.plaphy.2012.02.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A), phenotypes of different rice varieties under salt stress. Control indicates that plants were grown under normal conditions; NaCl indicates that two-week rice seedlings were treated with 150 mM NaCl for 10 days. (B), the percentage survival rates of rice varieties after salt stress treatment in (A) with another ten days of re-watering without NaCl. NJ16, ZH17, Nip, Kos, YD6 and IR29 indicate rice varieties Ningjing16, Zhonghua17, Nipponbare, Koshihikari, Yangdao 6 and IR29, respectively. The bars represent SD (±) of three independent assays. About 50~60 seedlings were uses in each experiment.
(EPS)
(A), total RNA was extracted from leaves of four-week F1 OsVTC1-1 RI1-3 hybrid rice plants; subsequently, the expression of OsVTC1-1 was analyzed using qPCR. (B), AsA content of F1 OsVTC1-1 RI1-3 hybrid rice plants. NJ16 represents salt-tolerant rice landrace Ningjing16; NJ16/RI1-3 represents F1 hybrid of Ningjing16 with OsVTC1-1 RNA interference line RI1-3; and NJ16/ZH17 represents F1 plants of Ningjing16 and Zhonghua17, which were used as controls. ZD13 indicates salt-tolerant rice landrace Zhongdao13; ZD13/RI1-3 indicates F1 plants of Zhongdao13 and OsVTC1-1 RI1-3; and ZD13/ZH17 indicates F1 plants of Zhongdao13 and Zhonghua17, which were used as controls. Ningjing16 and Zhongdao13 were used as female parents. The bars represent SD (±) of three independent assays, and the asterisk indicates results significantly different from control (NJ16/ZH17 and ZD13/ZH17, respectively) (**P<0.01). Significance was evaluated using the t-test.
(EPS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.